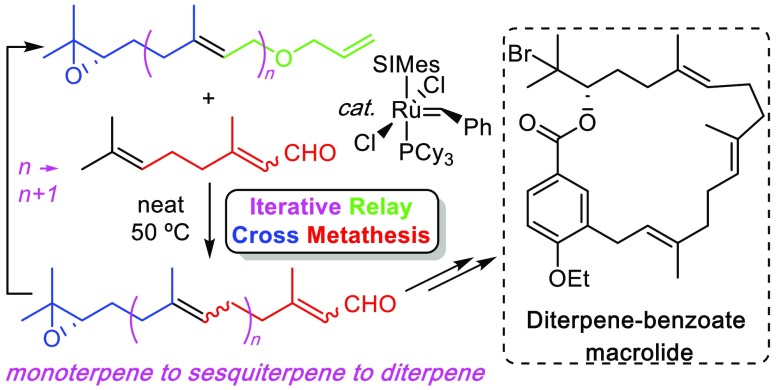
Abstract
We report a relay cross metathesis (ReXM) reaction for the construction of terpenoids in an iterative protocol. The protocol features the cross metathesis of a relay-actuated Δ6,7-functionalized C10-monoterpenoid alcohol with C10-monoterpenoid citral to form a C15-sesquiterpene. Subsequent functional group manipulation allows for the method to be repeated in an iterative fashion. The method is used for the synthesis of a diterpene-benzoate macrolide of biogenetic relevance to the bromophycolide family of natural products.
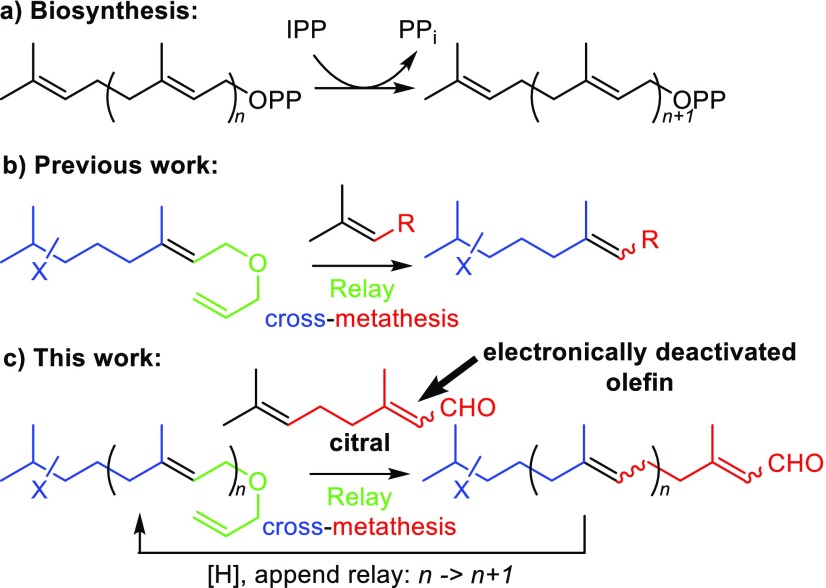
Terpenoids, consisting of “head-to-tail” and “head-to-head” arrangements of five-carbon isoprene units, are a diverse and very large class of linear and (poly)cyclic naturally occurring biomolecules with more than 40,000 distinct chemical structures, thereby accounting for approximately 60% of known natural products.1 They mediate vital biological functions including light harvesting and photo-oxidative protection, lipid membrane modulation, electron transport, intercellular signaling as hormones, and interspecies defense among others.2 Traditional herbal remedies from plants have utilized the medicinal benefits of terpenoids for centuries,1 with the subsequent development of terpenoid derivatives (e.g., steroidal medicines) as blockbuster drugs in the 20th century through to the present day.3 While a comprehensive account of their biogenesis is beyond the scope of this document, it is important to note that (poly)cyclic terpenoids all arise from their linear precursors.4 Nature assembles these linear precursors by enzyme-mediated sequential addition of C5 units of isopentenyl pyrophosphate (IPP) to (C5)n-terpenyl pyrophosphates in the mevalonate pathway (Figure 1a).5 However, despite the long-term recognition that these linear compounds are essentially C5-repeating isoprene units, a general and iterative chemical protocol for their synthesis—using naturally occurring, terpenoid building blocks—does not exist.6 We recently reported an olefin cross metathesis reaction between relay-actuated Δ6,7-functionalized monoterpenoid alcohols with trisubstituted alkenes as partner olefins to form new trisubstituted alkenes (Figure 1b).7 We now report that the use of readily available and nonexpensive citral as the partner olefin—a monoterpenoid with two electronically distinguishable alkenes—allows for the iterative construction of terpenoids in line with the above aims (Figure 1c).8 Furthermore, we report the application of this method for the synthesis of a diterpene-benzoate macrolide of biogenetic relevance to the bromophycolide natural product family.
Figure 1.
(a) Terpene biosynthesis via sequential addition of C5 units of isopentenyl pyrophosphate (IPP). (b) Previously reported cross metathesis reaction between relay-actuated Δ6,7-functionalized monoterpenoid alcohols with trisubstituted alkenes to form new trisubstituted alkenes. (c) Relay cross metathesis for the iterative construction of terpenoids.
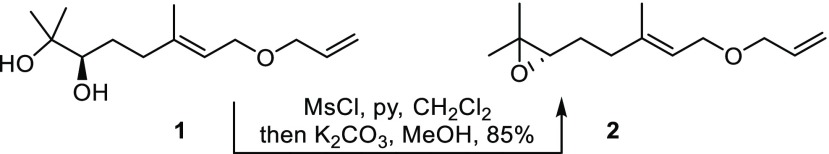
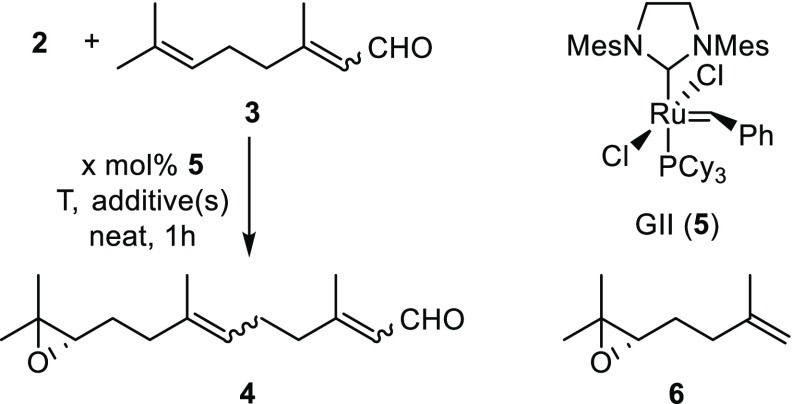
To commence our investigations enantiopure epoxide 2, as a relay-actuated Δ6,7-functionalized monoterpenoid derivative, was prepared from diol 1(7) using the method of Corey et al. (Scheme 1).9 Using our previously identified conditions (10 mol % ruthenium benzylidene precatalyst 5,10 alkene [5 equiv], 50 °C, 1 h),7 attempted relay cross metathesis reaction11 between epoxide 2 and citral (3)12,13 to give C15-sesquiterpenoid 4 using 5 was unsuccessful (Table 1, entry 1). Further attempts with 10 equiv of 3 (entry 2) or at room temperature (entry 3) or with 2 mol % catalyst loading (entry 4) also failed. In these attempts, truncated olefin 6 was observed in the 1H NMR spectra of the crude reaction mixtures, along with a triplet with a characteristic coupling constant of 9.6 Hz that we attributed to 2,3-dihydrofuran,14 implicating ruthenium hydride-induced isomerization of the expected 2,5-dihydrofuran byproduct.
Scheme 1. Synthesis of Relay-Modified Δ6,7-Functionalized Monoterpenoid 2.
Table 1. ReXM of Relay-Actuated Δ6,7-Functionalized Monoterpenoid 2 with Citral (3) Using GII Catalyst (5)a.
| entry | equiv of 3 | mol % 5 | T (°C) | additive(s) (mol %) | % yieldb |
|---|---|---|---|---|---|
| 1 | 5 | 10 | 50 | 0c | |
| 2 | 10 | 10 | 50 | 0c | |
| 3 | 5 | 10 | RT | 0c | |
| 4 | 5 | 2 | 50 | 0c | |
| 5 | 5 | 10 | 50 | pBQ (20) | 0c |
| 6 | 5 | 10 | 50 | AcOH (20) | 64 |
| 7 | 5 | 10 | 70 | AcOH (20) | 19 |
| 8 | 5 | 10 | RT | AcOH (20) | 0c |
| 9 | 10 | 10 | 50 | AcOH (20) | 30 |
| 10 | 5 | 2 | 50 | AcOH (20) | 4 |
| 11 | 5 | 10 | 50 | AcOH (20), CuI (15) | 68 |
| 12 | 5 | 20 | 50 | AcOH (20) | 80 |
| 13 | 5 | 20 | 50 | AcOH (20), CuI (30) | 84 |
| 14 | 5 | 20 | 50 | AcOH (40), CuI (30) | 88 |
| 15 | 5 | 10d | 50 | AcOH (20) | 14 |
Reactions conducted on a 0.25 mmol scale.
Isolated yields of sesquiterpene 4 after chromatography; E/Z ratio determined as ca. 3:1 at the newly formed olefin (Δ6,7) and as ca. 2–3:1 at the α,β-unsaturated aldehyde by 1H NMR and assigned on the basis of characteristic 13C NMR shielded methyl resonances for E-isomers (see the Supporting Information).
Purification not attempted due to complex mixtures of products.
Hoveyda–Grubbs II catalyst employed.
Known hydride scavengers 1,4-benzoquinone (pBQ, entry 5) and AcOH (entry 6) were therefore explored as possible additives for the reaction.15 Pleasingly, the use of AcOH was beneficial, and C10-monoterpene epoxide 2 now underwent smooth ReXM with C10-monoterpene citral (3) to provide C15-sesquiterpene 4 in good yield (entry 6). The effect of temperature (entries 7 and 8), equivalents of citral (3) (entry 9), and catalyst loading (entry 10) were also explored, with lower yields obtained. Further addition of CuI16 (entry 11) was found to be beneficial, as was increasing the catalyst loading (20 mol %, entry 12). Increasing quantities of added CuI and AcOH (entries 13–14) resulted in a higher yield, providing a final optimized yield of 88% for this challenging transformation. We note that the use of Grubbs II catalyst (5) is important in this process: the use of the Hoveyda–Grubbs II catalyst17 (entry 15) under conditions that worked well (cf. entry 6) for catalyst 5 surprisingly gave only a low yield of product from a complex product mixture.
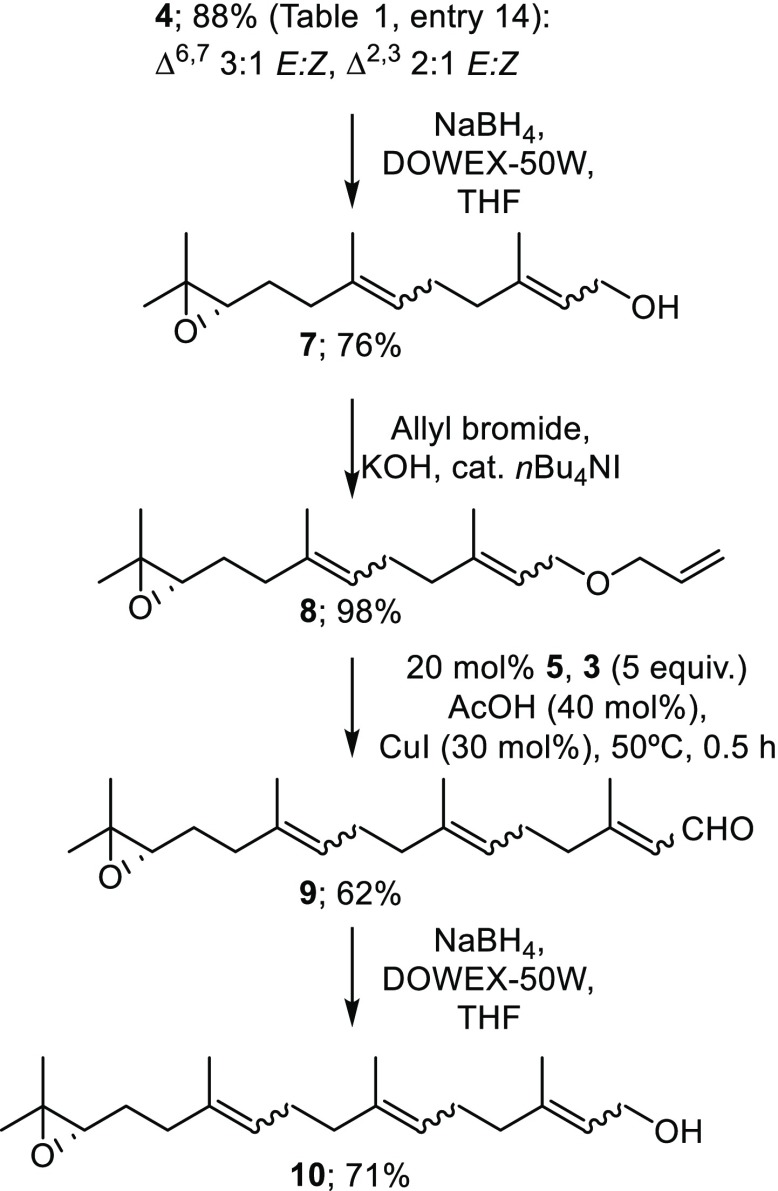
We then turned our attention to demonstrating that the protocol is suitable for iteration (Scheme 2). Accordingly, aldehyde 4 was reduced18 to alcohol 7 and O-allylated19 to provide C15-relay metathesis substrate 8. A second ReXM with citral (3), using the optimized conditions developed above, now produced C20-diterpene 9,20 which could be readily reduced to the C20-alcohol 10 as the first step of a further iteration.
Scheme 2. Iterative Reduction-Allylation-ReXM.
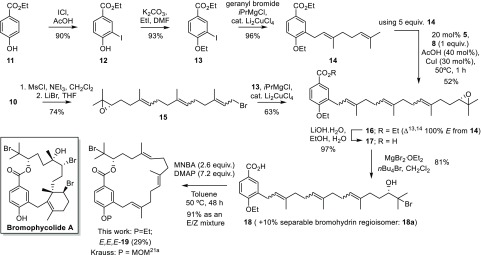
With the ability to synthesize enantiopure, Δ14,15 regioselectively functionalized geranylgeraniol 10, we now targeted diterpene-benzoate macrolide 19 (P = Et), pertinent as a putative biogenetic precursor of the bioactive bromophycolide halogenated natural product family (Scheme 3).21 Accordingly, aryl iodide 13 was produced in a two-step sequence from ethylparaben 11. Alcohol 10 was converted to its bromide 15 and coupled to aryl iodide 13 to give diterpene-benzoate 16. Alternatively, taking advantage of our previous observation7 that prenylbenzene was unreactive to the ReXM conditions, geranyl benzoate 14 was combined in excess (5 equiv) with relay sesquiterpenoid 8 to also provide diterpene-benzoate 16 in good yield. Subsequent ester hydrolysis gave acid 17 and regioselective epoxide ring-opening with bromide gave bromohydrin 18, which was readily separated away from its minor bromohydrin regioisomer 18a. With the scene now set for macrocyclization, we anticipated that the inseparable E/Z alkene isomers that had built up in the ReXM iteration sequence22 would become chromatographically distinguishable upon conversion to conformationally constrained rings. Much to our delight, Shiina macrolactonization23 of seco acid 18 proceeded with high conversion of substrate (91%) and provided (E,E,E)-macrocycle 19 (P = Et) as the major macrocyclic component (29%) which was readily separable from the other more polar Z-olefin containing macrocycles.24
Scheme 3. Synthesis of Diterpene-Benzoate Macrolide 19 (P = Et) Pertinent to the Bromophycolide Family of Natural Products (cf. Structure of Bromophycolide A, Boxed, Bottom Left).
MNBA = 2-methyl-6-nitrobenzoic anhydride; DMAP = 4-dimethylaminopyridine.
In conclusion, we have demonstrated the use of a relay cross metathesis reaction between a relay-actuated Δ6,7-functionalized monoterpenoid and citral as readily available, inexpensive and naturally occurring building blocks. This methodology allows the unprecedented construction of terpenoids in a C5n to C5(n+1) fashion (from a monoterpene, to a sesquiterpene, to a diterpene) via an iterative ReXM-reduction-relay installation sequence. Although the iterative protocol necessarily gives rise to geometrical mixtures of products because of the current limitations of olefin metathesis catalysts for the formation of geometrically pure trisubstituted olefins, we have used the method to construct an enantiomerically and geometrically pure diterpene-benzoate macrolide of relevance to bioactive substances from marine organisms. The method reported should allow for the synthesis of myriad bespoke terpenes.25,26
Acknowledgments
We thank CSIRO and Imperial College London for a studentship (to K.A.B.) and the EPSRC (Grant No. EP/P030742/1 to D.C.B.) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c00935.
General experimental section; experimental details and characterizing data for compounds; comparison of 1H and 13C NMR shifts of E,E,E-19 (P = Et) vs E,E,E-19 (P = MOM); copies of 1H and 13C spectra for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Firn R.Nature’s Chemicals: The Natural Products that Shaped Our World; Oxford University Press: Oxford, 2010. [Google Scholar]
- Breitmaier E.Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; Wily-VCH: Weinheim, 2006. [Google Scholar]
- For a pictorial “Top pharmaceuticals”, see: http://njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster (2019-06-11).
- Barton D. H. R.; Meth-Cohn O.; Nakanishi K.. Isoprenoids Including Cartenoids and Steroids. In Comprehensive Natural Product Chemistry; Cane D. E., Ed.; Pergamon: Elmsford, NY, 1999; Vol. 2. [Google Scholar]
- Miziorko H. M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a solid-phase synthesis of solanesol, see:; a Yu X.; Wang S.; Chen F. Solid-Phase Synthesis of Solanesol. J. Comb. Chem. 2008, 10, 605–610. 10.1021/cc800069t. [DOI] [PubMed] [Google Scholar]; For the use of organometallic methodology using specialisediodoalkene fragments, see:; b Negishi E.-i.; Liou S.-Y.; Xu C.; Huo S. A Novel, Highly Selective, and General Methodology for the Synthesis of 1,5-Diene Containing Oligoisoprenoids of All Possible Geometrical Combinations Exemplified by an Iterative and Convergent Synthesis of Coenzyme Q10. Org. Lett. 2002, 4, 261–264. 10.1021/ol010263d. [DOI] [PubMed] [Google Scholar]
- Bahou K. A.; Braddock D. C.; Meyer A. G.; Savage G. P.; Shi Z.; He T. A Relay Strategy Actuates Pre-Existing Trisubstituted Olefins in Monoterpenoids for Cross Metathesis with Trisubstituted Alkenes. J. Org. Chem. 2020, and references cited therein 10.1021/acs.joc.0c00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent review on metathesis of terpenes, see:; Bruneau C.; Fischmeister C.; Mandelli D.; Carvalho W.; dos Santos E.; Dixneuf P.; Sarmento Fernandes L. Transformations of Terpenes And Terpenoids Via Carbon-Carbon Double Bond Metathesis. Catal. Sci. Technol. 2018, 8, 3989–4004. 10.1039/C8CY01152D. [DOI] [Google Scholar]
- Corey E. J.; Noe M. C.; Shieh W.-C. A Short and Convergent Enantioselective Synthesis of (3S)-2,3-Oxidosqualene. Tetrahedron Lett. 1993, 34, 5995–5998. 10.1016/S0040-4039(00)61710-0. [DOI] [Google Scholar]
- Scholl M.; Ding S.; Lee C. W.; Grubbs R. H. Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands. Org. Lett. 1999, 1, 953–956. 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- For the original relay strategy as applied to relay ring closing metathesis, see:; Hoye T. R.; Jeffrey C. S.; Tennakoon M. A.; Wang J.; Zhao H. Relay Ring-Closing Metathesis (RRCM): A Strategy for Directing Metal Movement Throughout Olefin Metathesis Sequences. J. Am. Chem. Soc. 2004, 126, 10210–10211. 10.1021/ja046385t. [DOI] [PubMed] [Google Scholar]
- The use of citral (3) as an E/Z enal mixture should be unimportant in the subsequent iteration, since the same ruthenium alkylidene should be formed after initial relay metathesis from either of the original Δ2,3 geometries of, e.g., farnesol allyl ether 8. The experiments previously conducted with nerol vs geraniol derived O-allyl epoxides7 are consistent with this expectation.
- Citral (3) has recently been reported as a cross metathesis partner with terminal alkenes:; Sapkota R. R.; Jarvis J. M.; Schaub T. M.; Talipov M. R.; Arterburn J. B. Bimolecular Cross-Metathesis of a Tetrasubstituted Alkene with Allylic Sulfones. ChemistryOpen 2019, 8, 201–205. 10.1002/open.201800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- However, despite the characteristic J value, the chemical shift of 4.12 ppm does not match the literature chemical shift of 4.30 ppm (CDCl3). A referee suggested that this might instead be due to R2C=CHCH2CH2OCH=[Ru] (from ring-opening of 2,3-dihydrofuran).
- Hong S. H.; Sanders D. P.; Lee C. W.; Grubbs R. H. Prevention of Undesirable Isomerization during Olefin Metathesis. J. Am. Chem. Soc. 2005, 127, 17160–17161. 10.1021/ja052939w. [DOI] [PubMed] [Google Scholar]
- Voigtritter K.; Ghorai S.; Lipshutz B. H. Rate Enhanced Olefin Cross Metathesis Reactions: The Copper Iodide Effect. J. Org. Chem. 2011, 76, 4697–4702. 10.1021/jo200360s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber S. B.; Kingsbury J. S.; Gray B. L.; Hoveyda A. H. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. 10.1021/ja001179g. [DOI] [Google Scholar]
- Zeynizadeh B.; Shirini F. Mild and Efficient Reduction of α,β,-Unsaturated Carbonyl Compounds, α-Diketones and Acyloins with Sodium Borohydride/Dowex1-x8 System. Bull. Korean Chem. Soc. 2003, 24, 295–298. [Google Scholar]
- Rao H. S.; Senthilkumar S. P. A Convenient Procedure for the Synthesis of Allyl and Benzyl Ethers from Alcohols and Phenols. Proc. - Indian Acad. Sci., Chem. Sci. 2001, 113, 191–196. 10.1007/BF02704069. [DOI] [Google Scholar]
- After the second iteration, it was no longer possible to determine the three E:Z ratios at Δ2,3, Δ6,7, and Δ10,11 in compound 9 by NMR methods.
- The synthesis of macrocycle 19, as a MOM-protected phenol, has previously been reported:; a Lin H.; Pochapsky S. S.; Krauss I. J. A Short Asymmetric Route to the Bromophycolide A and D Skeleton. Org. Lett. 2011, 13, 1222–1225. 10.1021/ol200099n. [DOI] [PMC free article] [PubMed] [Google Scholar]; For the original isolation of bromophycolides A–C, see:; b Kubanek J.; Prusak A. C.; Snell T. W.; Giese R. A.; Hardcastle K. I.; Fairchild C. R.; Aalbersberg W.; Raventos-Suarez C.; Hay M. E. Antineoplastic Diterpene-Benzoate Macrolides from the Fijian Red Alga Callophycus serratus. Org. Lett. 2005, 7, 5261–5264. 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]; For isolations of other members of the family, see:; c Kubanek J.; Prusak A. C.; Snell T. W.; Giese R. A.; Fairchild C. R.; Aalbersberg W.; Hay M. E. Bromophycolides C-I from the Fijian Red Alga Callophycus serratus. J. Nat. Prod. 2006, 69, 731–735. 10.1021/np050463o. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lane A. L.; Stout E. P.; Lin A.-S.; Prudhomme J.; Le Roch K.; Fairchild C. R.; Franzblau S. G.; Hay M. E.; Aalbersberg W.; Kubanek J. Antimalarial Bromophycolides J-Q from the Fijian Red Alga Callophycus serratus. J. Org. Chem. 2009, 74, 2736–2742. 10.1021/jo900008w. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Lin A.-S.; Stout E. P.; Prudhomme J.; Le Roch K.; Fairchild C. R.; Franzblau S. G.; Aalbersberg W.; Hay M. E.; Kubanek J. Bioactive Bromophycolides R-U from the Fijian Red Alga Callophycus serratus. J. Nat. Prod. 2010, 73, 275–278. 10.1021/np900686w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- On the basis of an approximate 3:1 E:Z alkene ratio for each of the two ReXM iterations and an approximate 2:1 E:Z alkene ratio for olefin derived from the α,β,-unsaturated portion of citral (3), we estimate (E,E,E)-16 to be the major component of the mixture at 37%.
- Shiina I.; Kubota M.; Oshiumi H.; Hashizume M. An Effective Use of Benzoic Anhydride and Its Derivatives for the Synthesis of Carboxylic Esters and Lactones: A Powerful and Convenient Mixed Anhydride Method Promoted by Basic Catalysts. J. Org. Chem. 2004, 69, 1822–1830. 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
- The olefin geometry of 19 was established as E,E,E- by the presence of characteristic (shielded) 13C NMR resonances at δ 16.5, 15.9, and 15.0 ppm (for E-olefins) and the absence of 13C NMR resonances between 22 and 24 ppm (for Z-olefins). See, e.g., for E- and Z-8-(3,3-dimethyloxiran-2-yl)-6-methyloct-5-en-2-one:; Watanabe Y.; Laschat S.; Budde M.; Affolter O.; Shimada Y.; Urlacher V. B. Oxidation of Acyclic Monoterpenes by P450 BM-3 Monooxygenase: Influence of the Substrate E/Z-isomerism on Enzyme Chemo- and Regioselectivity. Tetrahedron 2007, 63, 9413–9422. 10.1016/j.tet.2007.06.104. [DOI] [Google Scholar]
- 1H NMR, 13C NMR, IR, and MS data are available via a data repository as Bahou K. A.; Braddock D. C.. Imperial College HPC Data Repository, 2019. 10.14469/hpc/6103 (accessed 2020-01-29). [DOI]
- The first version of this article was deposited to the ChemRxiv preprint server on June 13, 2019. 10.26434/chemrxiv.8267837.v1. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 1H NMR, 13C NMR, IR, and MS data are available via a data repository as Bahou K. A.; Braddock D. C.. Imperial College HPC Data Repository, 2019. 10.14469/hpc/6103 (accessed 2020-01-29). [DOI]