Abstract
Objective
To examine the differences in the adverse drug reaction (ADR) profile of antipsychotic and antidepressant agents between pediatric and adult patients in studies submitted to the Food and Drug Administration (FDA) during the drug development process.
Study design
Clinical trials in adult and pediatric patients were conducted by sponsors as part of the drug development programs for antipsychotic and antidepressant agents, and ADR information was collected as part of those trials and submitted to the FDA. Data collection was conducted by reviewing publicly available FDA-authored reviews and FDA-approved product labels for 10 drugs with an antipsychotic or an antidepressant indication from 2007 to 2017.
Results
There were 308 drug and ADR combinations for the 10 drugs and drug combinations with 113 (36.7%) having a significantly different incidence in pediatric patients compared with adults. Sixty-eight (60.2%) of these ADRs had a significantly higher incidence in pediatric patients than in adults. Sedation was higher in 6 of the 10 drugs and drug combinations with risk differences ranging from 9.6 to 36.6%.
Conclusions
This analysis indicates that there were significant differences between the pediatric and adult safety profiles of antipsychotic and antidepressant drugs. Sedation was the major ADR associated with the use of atypical antipsychotic drugs in pediatric patients. Clinicians caring for children should consider the ADR profile when prescribing antipsychotics and antidepressants in pediatric patients.
Many children living in the US experience a mental health disorder each year, and surveillance during the 1994–2011 period indicates that the prevalence of these conditions is increasing.1 With the advent of the Best Pharmaceuticals for Children Act in 1997, many medications studied for mental health disorders in adults were also studied in children. Two of the most commonly studied of these medications are the antipsychotics and antidepressants.
Antipsychotics are studied in adults for schizophrenia, bipolar mixed and manic episodes, bipolar depression, and treatment resistant depression. In pediatric patients, antipsychotics have been studied for bipolar mixed and manic, schizophrenia, bipolar depression, irritability and aggression in autism, and Tourette syndrome.2 Antidepressants are studied in adults for major depression, obsessive compulsive disorder, social phobia, panic disorder, post-traumatic stress disorder, eating disorder, and generalized anxiety disorder, but carry a risk of suicidality.3 Antidepressants are studied in youth for major depression, obsessive compulsive disorder, social phobia, post-traumatic stress disorder, and generalized anxiety disorder, but similarly carry a risk of suicidality.4
As the Food and Drug Administration (FDA) pediatric trials of antipsychotic and antidepressant medication have grown in scope since the late 1990s, the use of these drugs in children has also increased. Several recent large epidemiologic studies have examined the rates and prevalence of antipsychotic and antidepressant medications in the US and across the world. The prevalence of antidepressant use in youth is complicated by the inclusion of the boxed warning on antidepressants and suicidality in youth in 2004, which moved clinicians and families away from using those medications. Trends in antidepressant use have recently started to rebound from lows in the 2003–2005 period.
Most clinical trials for psychotropic medications range between 3 weeks (bipolar mania trial) and 8 weeks (major depression trial) in length. Acute adverse drug reactions (ADRs) and discontinuation rates attributable to ADRs are important factors in both short- and long-term treatment outcomes. One example of differences among age groups is the incidence of suicidality with the use of antidepressants vs placebo. The incidence of suicidality is increased in youth up to the age of 25 years, hence, the boxed warning,4 even in patients aged 26–65 years of age, but is lower with the use of drug compared with placebo in adults over age 65 years.3,5The evaluation of ADRs is a critical part of new drug evaluation, and the definition of ADRs in Investigational New Drug applications is provided in the Code of Federal Regulations.6
In this study, the investigators wanted to examine the incidence of ADRs in the overlapping categories of FDA-approved indications between children and adults. This direct comparison by indication can allow us to determine whether the incidence of ADRs is similar or different based on age. Although adult safety studies are often informative regarding the types of ADRs that may be expected in children, our previous experience has suggested that the incidence of ADRs may be different between children and adults.7 If ADRs are greater in children and adolescents than in adults with the agents used to treat depression or schizophrenia, these ADRs should be recognized and managed by pediatric practitioners. Therefore, the objective of this study was to compare the incidence of ADRs observed during drug development in adults with those observed in children and adolescents for approved antipsychotic and antidepressant drugs.
Methods
Data collection was conducted by reviewing publicly available FDA-authored reviews,8,9 which are available for all products studied in children since 2002, and FDA-approved product labels with an approved pediatric mental health indication from 2007 to 2017. All approved drug products for these years that have the same indication for use in adults and children were reviewed.
The incidence of ADRs and the size of the patient population were taken from the above resources. These ADRs were a summary from the actual clinical trials, and generally a threshold is set so as to only report those ADRs above the incidence threshold. The thresholds for the incidence of reporting ADRs, which were either ≥2% or ≥5%, for all included drugs are presented in Table I. For the purpose of this analysis, the threshold incidence (2%) was imputed for any ADR below the threshold, thereby taking a conservative approach to examining risk differences (RDs) between adults and children. A 2% reporting threshold for ADRs means that the ADRs reported occurred in at least 2% of patients. For the 3 instances where a 5% threshold was used (escitalopram, risperidone for schizophrenia, risperidone for bipolar I mania), a similarly conservative approach was taken in that the 5% incidence for pediatric patients was not imputed when the adult incidence was below 5%.
Table I.
Summary of indications, doses, number of patients, and thresholds for ADR reporting
| Drug names | Indication for pediatric patient | Indication for adult patient | Dose for pediatric patients | Dose for adult patients | Age of pediatric patients | Patient number in pediatric clinical trials | Patient number in adult clinical trials | Threshold for adverse event report for pediatric clinical trials | Threshold for adverse event report for adult clinical trials |
|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole | Schizophrenia, bipolar mania, autistic disorder, Tourette disorder | Schizophrenia and bipolar mania | ≥2 mg/d | ≥2 mg/d | 6–18 y | 732 (pooled) | 1843 (pooled) | ≥2% | ≥2% |
| Duloxetine | General anxiety disorder, major depressive disorder | General anxiety disorder, major depressive disorder | 30–120 mg/d | 60–120 mg | 7–17 y | 476 | 4797 (pooled) | ≥2% | ≥2% |
| Paliperidone | Schizophrenia | Schizophrenia | 1.5–12 mg/d | 3–12 mg/d | 12–17 y | 150 (pooled) | 850 (pooled) | ≥2% | ≥2% |
| Lurasidone 40 mg | Schizophrenia | Schizophrenia | 40 mg | 40 mg | 13–17 y | 110 | 487 | ≥2% | ≥2% |
| Lurasidone 80 mg | Schizophrenia | Schizophrenia | 80 mg | 80 mg | 13–17 y | 104 | 538 | ≥2% | ≥2% |
| Escitalopram | Major depressive disorder | Major depressive disorder | 10 mg, 20 mg | 10–20 mg/d | 12–17 y | 234 | 715 | ≥5% | ≥2% |
| Risperidone (bipolar I mania) | Bipolar I mania | Bipolar I mania | 0.5–6 mg/d | 1–6 mg/d | 10–17 y | 111 | 448 | ≥5% | ≥2% |
| Risperidone (schizophrenia) | Schizophrenia | Schizophrenia | 1–6 mg/d | 2–16 mg/d | 13–17 y | 106 | 564 | ≥5% | ≥2% |
| Asenapine | Bipolar I mania | Bipolar I mania | 2.5 mg, 5 mg, 10 mg | 5 mg, 10 mg | 10–17 y | 302 | 620 | ≥2% | ≥2% |
| Quetiapine | Schizophrenia, bipolar mania | Schizophrenia, bipolar mania | 400–800 mg/d | 75–800 mg/d | 10–17 y | 340 | 719 (pooled) |
≥2% | ≥2% |
| Olanzapine/fluoxetine | Bipolar I depression | Bipolar I depression and depression | 3/25–12/ 50 mg/d | 6/25–12/ 50 mg/d | 10–17 y | 170 | 771 | ≥2% | ≥2% |
| Olanzapine | Schizophrenia, bipolar I disorder (manic or mixed episodes) | Schizophrenia, bipolar I disorder (manic or mixed episodes) | ≥2.5 mg/d | ≥2.5 mg/d | 13–17 y | 179 | 905 | ≥2% | ≥2% |
The definitions of ADRs are from the Common Terminology Criteria for Adverse Events v 4.0, Medical Dictionary for Regulatory Activities (MedDRA) terms, and some specified combinations of ADR terminology as defined in the label.10 The grouped ADRs were defined as a group of ADRs that were represented by one ADR term, and are listed in Table II (available at www.jpeds.com). The grouped ADRs were analyzed for all medications to provide consistency. For example, sedation in some clinical trial ADR reports included sedation, somnolence, hypersomnolence, hypersomnia, and lethargy. Extrapyramidal symptoms were collectively grouped and were the largest group of ADRs.
Table II.
Grouped ADRs
| Grouped ADRs | Contents |
|---|---|
| Abdominal pain | Abdominal tenderness, upper abdominal pain, lower abdominal pain, abdominal discomfort, gastrointestinal |
| Bipolar disorder/mania | Bipolar disorder, bipolar disorder I, mania |
| Dyspepsia | Dyspepsia, gastroesophageal reflux disease |
| Edema | Edema, peripheral edema, pitting edema, generalized edema, eyelid edema, joint swelling, eye swelling, face edema, gravitational edema, localized edema, periorbital edema, swelling, swelling face, |
| Fatigue | Fatigue, lethargy, asthenia |
| Gynecomastia | Gynecomastia, breast swelling |
| Hepatic enzyme increased | Alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, transaminases increased, liver function test abnormal, gamma-glutamyl transferase increased |
| Hyperinsulinemia | Hyperinsulinemia, blood insulin increased |
| Hypertension | Hypertension, blood pressure increased |
| Insomnia | Initial insomnia, top insomnia, early morning awakening, insomnia |
| Oral hypoesthesia | Oral hypoesthesia, oral paresthesia, oral dysesthesia |
| Respiratory tract infection | Lower respiratory tract infection, respiratory tract infection, viral respiratory tract infection, upper respiratory tract infection, viral upper respiratory tract infection, nasopharyngitis, influenza, viral infection |
| Rhinitis | Rhinitis, allergic rhinitis, rhinorrhea, nasal congestion |
| Sedation | Hypersomnia, sedation, somnolence, hypersomnolence, lethargy |
| Tachycardia | tachycardia, sinus tachycardia, increased heart rate |
| Extrapyramidal symptoms | akathisia, akinesia, blepharospasm, bradykinesia, cogwheel rigidity, dystonia, dyskinesia, drooling, extrapyramidal disorder, hypokinesia, hypertonia, laryngospasm, muscle rigidity, musculoskeletal stiffness, muscle spasms, involuntary muscle contractions, muscle contracture, myoclonus, masked facies, muscle tightness, nuchal rigidity, oculogyric crisis, oculogyration, oromandibular dystonia, Parkinsonian rest tremor, Parkinsonian gait, Parkinsonism, Parkinson disease, psychomotor retardation, restlessness, tongue disorder, torticollis, trismus, tongue paralysis, tongue spasm, tremor |
The ADR risk difference (RD) and associated CI for these medications were calculated. The observed RD is equal to risk in the pediatric population minus the risk in the adult population. The RD is calculated from the frequency of the ADR in the selected labels with the unit of risk as a percentage. The analysis was performed for individual agents, and the agents were also grouped by mechanism of action for analysis.
The statistical calculation was performed using the Miettinen-Nurminen score interval as implemented in the R package PropCIs (R Project for Statistical Computing, www.r-project.org).11 The RD was demonstrated using a heatmap generated by the R package Heatmap.12 The R code and data for this analysis are available upon request.
Results
In total, 414 products had pediatric reviews between 2007 and 20179; there were only 10 drug products and combinations that were indicated in mental health disorders in children and had matching pediatric and adult approved indications (antipsychotics and antidepressants). One drug had ADR evaluations for 2 separate doses, and 1 drug had separate ADR evaluations for 2 different indications, making a total of 12 comparisons (Table I). The 10 drugs and drug combinations that were analyzed are listed in Table III (available at www.jpeds.com). For lurasidone, there were 2 dosages, 40 mg and 80 mg, which were included separately. For risperidone, 2 indications were studied and included bipolar depression and schizophrenia. The 12 medications, dosages, and indications included in this analysis are listed in Table I.
Table III.
Medication list included in this study
| Brand names | Generic names | Class |
|---|---|---|
| Abilify | Aripiprazole | Atypical19 antipsychotic agent |
| Cymbalta | Duloxetine | Selective serotonin and norepinephrine reuptake inhibitor antidepressant agent |
| Invega | Paliperidone | Atypical antipsychotic agent |
| Latuda | Lurasidone | Atypical antipsychotic agent |
| Lexapro | Escitalopram | Selective serotonin reuptake inhibitor antidepressant agent |
| Risperdal | Risperidone | Atypical antipsychotic agent |
| Saphris | Asenapine | Atypical antipsychotic agent |
| Seroquel | Quetiapine | Atypical antipsychotic agent |
| Symbyax | Olanzapine/fluoxetine | Combination of atypical antipsychotic agent and selective serotonin reuptake inhibitor antidepressant agent |
| Zyprexa | Olanzapine | Atypical antipsychotic agent |
The summary of clinical trials from which the ADRs were reported in FDA drug labels is listed in Table I. The age range of the pediatric patients was 6–18 years old, and the majority of pediatric patients in the clinical trials were adolescents (12–18 years old).
There were 308 drug-ADR combinations with 113 (36.7%) for which a 95% CI for the RD did not contain 0, thus, indicating a significantly different incidence in children compared with adults (Figure 1; available at www.jpeds.com). In 68 out of the 113 (60.2%), a significantly higher incidence of the ADR was found in children than in adults. Forty-five out of 113 (39.8%) ADRs had a significantly lower incidence in pediatric patients than in adults, comprising 14.6% of the total ADRs.
Figure 1.
The number of significant ADRs (or RDs; risk differences) for each medication. ADRs higher in adults (black column); ADRs higher in pediatric patients (white column).
The significant RDs >5% (pediatric > adult) or <−5% (pediatric < adult) are listed by physiologic systems in Figure 2. The ADRs with a significant positive RD, where the risk of an ADR in pediatric patients was higher than in adults, are listed in Table IV. The most significant ADRs were in the central nervous system and gastrointestinal systems.
Figure 2.
Summary of ADRs with significant RDs for all medications with any significant RD >5% or RD <−5%. RD = risk in pediatric patients minus risk in adult patients, so that positive RDs represent pediatrics > adults, and negative RDs represent adults > pediatrics. Classification of RD: RD >10% (red); RD 5%−10% (orange); RD 0%−5% (yellow); RD −5%−0% (blue); RD −10% to −5% (green); RD <−10% (purple).
Table IV.
ADRs that were significantly higher in pediatric patients than in adults
| Drugs | Age range of pediatric patients | Patient number in pediatric clinical trials | Patient number in adult clinical trials | ADRs (pediatric patients > adults) |
|---|---|---|---|---|
| Aripiprazole | 6–18 y | 732 (pooled) | 1843 (pooled) | Abdominal pain, diarrhea, salivary hypersecretion, fatigue, pyrexia, decreased appetite, increased appetite, sedation |
| Duloxetine | 7–17 y | 476 | 4797 (pooled) | Abdominal pain, vomiting, weight increased, decreased appetite, headache, oropharyngeal pain |
| Paliperidone | 12–17 y | 150 (pooled) | 850 (pooled) | Sedation |
| Lurasidone 40 mg | 13–17 y | 110 | 487 | Respiratory tract infection |
| Lurasidone 80 mg | 13–17 y | 104 | 538 | Respiratory tract infection |
| Escitalopram | 12–17 y | 234 | 715 | Abdominal pain, vomiting, inflicted injury, respiratory tract infection, headache, menstrual cramps |
| Risperidone (bipolar I disorder) | 10–17 y | 111 | 448 | Blurred vision, abdominal pain, diarrhea, dyspepsia, nausea, vomiting, fatigue, increased appetite, dizziness, sedation, pharyngolaryngeal pain |
| Risperidone (schizophrenia) | 13–17 y | 106 | 564 | Salivary hypersecretion, extrapyramidal disorder, sedation, |
| Asenapine | 10–17 y | 302 | 620 | Blurred vision, abdominal pain, oral hypoesthesia, irritability, dehydration, dyspnea, fatigue, increased appetite, myalgia, headache, dysmenorrhea, sedation, anger, pharyngolaryngeal pain, rhinitis, rash, tachycardia |
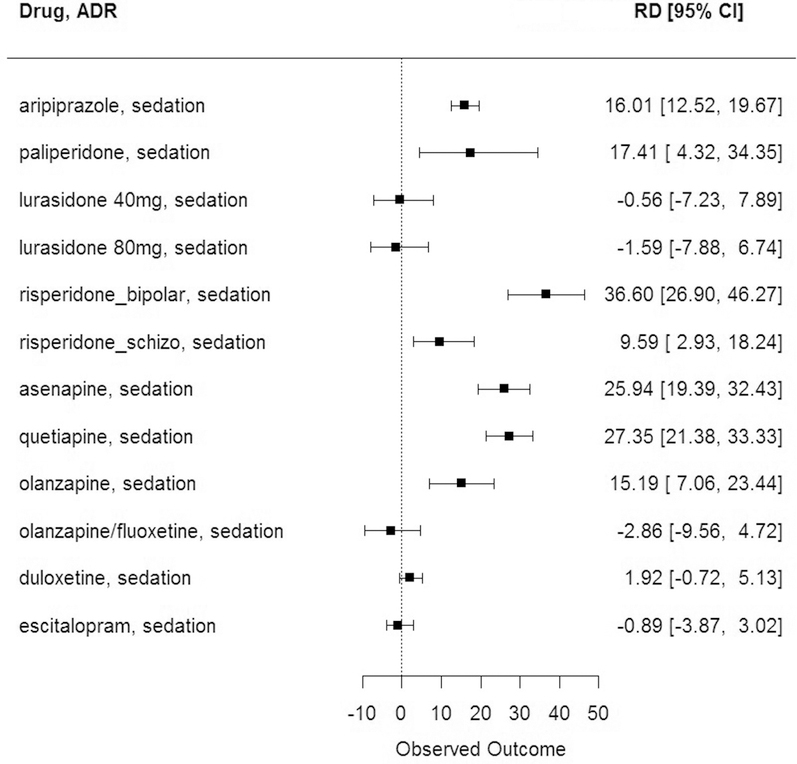
The largest 5 RDs of ADRs with pediatric incidence higher than adults were sedation with risperidone for bipolar disorder treatment (RD = 36.6%, 95% CI 26.9%, 46.3%), followed by weight increase with olanzapine (RD = 28.1%, 95% CI 21.7%, 35.3%), sedation with quetiapine (RD = 27.4%, 95% CI 21.4%, 33.3%), sedation with asenapine (RD = 25.9%, 95% CI 19.4%, 32.4%), and fatigue with risperidone for bipolar treatment (RD = 22.32%, 95% CI 15.1%, 31.2%). The 12 CIs for sedation are shown in Figure 3. The largest 5 RDs of ADRs with a pediatric incidence higher than in adults are shown in Figure 4 (available at www.jpeds.com).
Figure 3.
Sedation for each medication. RD is represented as the 95% CI.
Figure 4.
Top 5 adverse reactions with a greater frequency in pediatric patients than in adults.
The leading 5 RDs of ADRs with a pediatric occurrence lower than in adults were extrapyramidal symptoms with risperidone for bipolar treatment (RD = −26.0%, 95% CI −33.9%, −16.6%), insomnia with risperidone for schizophrenia (RD = −24.9%, 95% CI −29.9%, −18.2%), extrapyramidal symptoms with lurasidone 80 mg (RD = −24.4%, 95% CI −30.4%, −16.6%), headache with quetiapine (RD = −19.2%, 95% CI −22.6%, −15.9%), and agitation with quetiapine (RD = −18.3%, 95% CI −21.6%, −15.0%). The leading 5 RDs of ADRs with pediatric incidence lower than adults are shown in Figure 5 (available at www.jpeds.com).
Figure 5.
Top 5 adverse reactions with a lower frequency in pediatric patients than in adults.
The drug products with the largest number of significant RDs were aripiprazole (n = 21), asenapine (n = 18), olanzapine (n = 14), risperidone for bipolar treatment (n = 12), and duloxetine (n = 12). The drugs with the most significant RDs, which were higher in children than in adults, were paliperidone (100%; 1 out of 1), risperidone for bipolar treatment (91.7%; 11 out of 12), and asenapine (88.9%; 16 out of 18). The drugs with the most significant RDs, and had lower ADRs in children than in adults, were lurasidone 40 mg (75%; 3 out of 4), lurasidone 80 mg (66.7%; 2 out of 3), olanzapine (57.1%; 8 out 14), and aripiprazole (57.1%; 12 out of 21).
Sedation, respiratory tract infection, abdominal pain, and increased appetite were the ADRs with the largest number of significantly higher RDs for the majority of the investigated medications. The ADRs that had significantly different RDs between antipsychotic agents and antidepressant agents were sedation and extrapyramidal symptoms (Figure 2). Most antipsychotic medications had significantly higher RDs for sedation and lower RDs for extrapyramidal symptoms.
The total patient number of children involved in these clinical trials was 3014, and the total number of adults in these clinical trials was 13 257. The current information known about the mechanisms of action of these agents is listed in Table V (available at www.jpeds.com). The primary indications of the antipsychotic agents were schizophrenia and bipolar mania, and the primary indications of the antidepressant agents were major depressive disorder and general anxiety disorder. The indication of the combination drug was bipolar I depression.
Table V.
Mechanism of action of the antipsychotic and antidepressant drugs included
| Drug names | Mechanism of action |
|---|---|
| Aripiprazole | Unknown. The efficacy could be mediated through a combination of partial agonism at dopamine type 2 receptors and serotonin 5-HT1A receptor and antagonist activity at 5-HT2A receptor. |
| Duloxetine | Unknown. The mechanisms of the antidepressant, central pain inhibitory and anxiolytic actions of are believed to be related to its potentiation of serotoninergic and noradrenergic activity in the central nervous system |
| Paliperidone | Unknown. The efficacy in schizophrenia has been proposed to be mediated through a combination of central dopamine type 2 and serotonin 5-HT2A receptor antagonism. |
| Lurasidone | Unknown. The efficacy in schizophrenia and bipolar depression could be mediated through a combination of central dopamine type 2 receptor and serotonin 5-HT2A receptor antagonism. |
| Escitalopram | The mechanism is presumed to be linked to potentiation of serotonergic activity in the central nervous system resulting from its inhibition of central nervous system reuptake of serotonin (5-HT). |
| Risperidone | Unknown. The mechanism could be mediated through a combination of antagonism of dopamine type 2 receptor and serotonin 5-HT2 receptor. |
| Asenapine | Unknown. The efficacy in schizophrenia could be mediated through a combination of antagonism of 5-HT2A and D2 receptors |
| Quetiapine | Unknown. The efficacy in schizophrenia could be mediated through a combination of dopamine type 2 receptor and serotonin 5-HT2A receptor. |
| Olanzapine/fluoxetine | Unknown. The mechanism has been proposed that the activation of 3 monoaminergic neural systems (serotonin, norepinephrine, and dopamine) is responsible for its enhanced antidepressant effect. |
| Olanzapine | Unknown. The mechanism has been proposed that this drug’s efficacy in schizophrenia is mediated through a |
| combination of antagonism at dopamine type 2 receptor and serotonin 5-HT2A receptor. | |
Discussion
There were statistically significant differences between the pediatric and adult safety profiles with antipsychotic and antidepressant drugs in drug development studies. Over one-third of the ADRs had significantly different RDs in pediatric patients when compared with the adult population, with 68 out of 113 (60.2%) of these ADRs having a significantly higher incidence in pediatric patients than in the adult population. Among antipsychotic and antidepressant agents in this study, most medications had significantly higher RDs in pediatric patients than in the adult population. Only the combination agent olanzapine/fluoxetine had a similar RD profile in pediatric patients and adults.
In clinical trials, ADRs are collected in several different ways: based on changes in vital signs and laboratory measures, based on specific rating scales to capture ADRs (ie, movement scales and suicidality rating scales), and finally based on spontaneous reporting of ADRs. One difference in these sources of ADRs is that pediatric trials provide spontaneous reports from both the subject and the caregiver thereby allowing for a potentially increased rate of ADRs in pediatric patients because of higher rates of spontaneous reporting. Drug development studies are unlikely to detect all long-term adverse events, increasing the importance of prolonged pharmacovigilance using systems such as the FDA adverse event reporting system (FAERS).
In a study examining antipsychotic agents in the FAERS data base comparing youth, adult, and geriatric populations, the most common ADR in adults was diabetes, in youth it was behavioral problems, and in geriatric patients it was neurologic symptoms.13 The European Medicines Agency used a spontaneous reporting system similar to FAERS and the proportional reporting ratio to determine whether the association between enalapril and nephrotoxicity was different in children and adults as reflected in the ADRs.14 The European Medicines Agency’s pediatric query can highlight an imbalance in drug-event pair for a drug used in children compared with adults and did show a much higher rate of nephrotoxicity in children. Our current study did not use spontaneous reporting as in FAERS, but rather used matched indications in formal drug development studies to examine the different risks among 2 of the most commonly used groups of psychiatric medications, antidepressants and antipsychotics, in pediatric vs adult populations.
The ADRs observed within each physiologic category were consistently observed with the atypical antipsychotic15 and antidepressant agents. This finding continues to support the present understanding that adult ADRs are informative regarding pediatric ADRs, but that the incidence of ADRs in children may be significantly different in varying age groups. The reason for this phenomenon is most likely related to the mechanism of the ADR and the sensitivity of these different age groups to the ADR.
The cause of many ADRs is the off-target effect of these drugs. The atypical antipsychotic agents are all antagonists of the dopamine type 2 receptor and the serotonin 5-hydroxytryptamine (5-HT) receptor. At the same time, these drugs also bind to receptors such as histamine type 1, adrenergic, dopamine type 1, and cholinergic receptors. Those off-target effects produce a series of ADRs, which are consistent with the observations documented during drug development. Sedation in particular was a major problem with the use of the atypical antipsychotic drugs in children (Figure 3). Antidepressant agents and the combination agent did not have higher RDs with sedation because these medications work through different mechanisms without histamine type 1 receptor binding. Other ADRs in this analysis with significantly higher RDs in children than in adults were weight gain, respiratory tract infection, tachycardia, and agitation. These observations were similar to a study from the US poison control centers, which indicated that drowsiness/lethargy, tachycardia, and agitation were the most frequently observed clinical toxic effects of the atypical antipsychotic agents for ingestion in pediatric patients under 6 years of age.16
Another important ADR with a higher RD in children than in adults was weight gain. In this study, olanzapine had a higher RD (28.1%, 95% CI 21.7%, 35.3%) with weight gain and a higher RD (21.2%, 95% CI 15.1%, 27.9%) for increasing appetite in pediatric patients. In 1 review of atypical antipsychotic drugs, olanzapine was associated with more weight gain (6–13 pounds) than other atypical antipsychotic agents, and with an increased risk of new-onset type II diabetes mellitus.17 Other studies have also associated new-onset type II diabetes mellitus or diabetic ketoacidosis in children and adolescents with the use of atypical antipsychotic agents.18 In our study, although olanzapine was the only medication that demonstrated a significantly higher RD in pediatric patients for weight gain, there were several medications such as aripiprazole, risperidone for bipolar treatment, asenapine, and quetiapine that had higher RDs in pediatric patients for increasing appetite.
There are limitations to this type of analysis. A limited number of products which met the criteria of approval in pediatric patients for a mental health disorder, with a matching indication in adults, were identified. In an effort to use only publicly available information, we were unable to subset age groups within the pediatric patient population. Also, although these drug development studies are conducted in different institutions for pediatric patients than adults, the general construct of the studies for drug development through a single sponsor provides the best opportunity for comparison of ADRs. Although pediatric dosing is generally devised based upon matching the exposure (area under the plasma concentration vs time curve) to the adult drug exposure, some differences in drug exposure may occur.19 Additionally, because missing ADR incidence rates in the publicly available data were imputed based on threshold values, it is possible that the actual number of significant differences between incidence rates is greater than the number obtained above. The authors accepted this potential conservative bias in reporting to provide a timely report to the medical community.
A major concern is that the primary ADR for children was sedation. Sedation, somnolence, and lethargy ADRs are associated with negative effects including learning disabilities in children.20–22 Additional important ADRs included weight gain, fatigue, orthostatic hypotension, and tachycardia, which are all likely to decrease the tolerance of and adherence to these medications in the pediatric patient population. Given the limitation of unbalanced treatment experience between adult and pediatric patients, the long-term consequences of this difference in ADRs in pediatric patients are presently unknown,17 but should be shared with pediatric healthcare providers and be assessed in future pharmacovigilance programs and drug development studies of new antipsychotic and antidepressant drugs in pediatric patients. ■
Glossary
- ADR
Adverse drug reaction
- FAERS
FDA adverse event reporting system
- FDA
Food and Drug Administration
- RD
Risk difference
Footnotes
The opinions expressed in this article are those of the authors and should not be interpreted as the position of the US Food and Drug Administration. The authors declare no conflicts of interest.
References
- 1.Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, et al. Mental health surveillance among children—United States, 2005–2011. MMWR 2013;62(Suppl):1–35. [PubMed] [Google Scholar]
- 2.Murthy S, Mandl KD, Bourgeois F. Analysis of pediatric clinical drug trials for neuropsychiatric conditions. Pediatrics 2013;131:1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 2009;339:b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332–9. [DOI] [PubMed] [Google Scholar]
- 5.Miller M, Swanson SA, Azrael D, Pate V, Sturmer T. Antidepressant dose, age, and the risk of deliberate self-harm. JAMA Intern Med 2014;174:899–909. [DOI] [PubMed] [Google Scholar]
- 6.Code of Federal Regulations: Investigational New Drug Application Safety Reporting. 21CFR312.32. 2017. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.32. Accessed August 4, 2018.
- 7.Momper JN, Chang Y, Jackson M, Schuette P, Seo S, Younis I, et al. Adverse event detection and labeling in pediatric drug development: antiretroviral drugs. Ther Innov Reg Sci 2015;49:302–9. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Drugs@FDA Database. https://www.fda.gov/drugs/informationondrugs/ucm135821.htm. Accessed August 4, 2018.
- 9.US Food and Drug Administration. Pediatrics. https://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/default.htm. Accessed August 4, 2018.
- 10.US Food and Drug Administration. FDA Online Label Repository. https://labels.fda.gov/. Accessed January 11, 2019.
- 11.Scherer R Various confidence interval methods for proportions, in Package ‘PropCIs’, Scherer R, Editor. 2018. https://cran.r-project.org/web/packages/PropCIs/PropCIs.pdf. Accessed January 11, 2019.
- 12.Day A. Heatmap with more sensible behavior, in Package ‘heatmap. plus’, A Day, Editor. 2015. https://cran.r-project.org/web/packages/heatmap.plus/heatmap.plus.pdf. Accessed January 11, 2019.
- 13.Sagreiya H, Chen YR, Kumarasamy NA, Ponnusamy K, Chen D, Das AK. Differences in antipsychotic-related adverse events in adult, pediatric, and geriatric populations. Cureus 2017;9:e1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake KV, Saint-Raymond A, Zaccaria C, Domergue F, Pelle B, Slattery J. Enhanced paediatric pharmacovigilance at the European Medicines Agency: a novel query applied to adverse drug reaction reports. Paediatr Drugs 2016;18:55–63. [DOI] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Future Research Needs for First- and Second Generation Antipsychotics for Children and Young Adults. https://www.ncbi.nlm.nih.gov/books/NBK84660/pdf/Bookshelf_NBK84660.pdf. Accessed August 9, 2018. [PubMed]
- 16.Stassinos G, Klein-Schwartz W. Comparison of pediatric atypical antipsychotic exposures reported to U.S. poison centers. Clin Toxicol 2017;55:40–5. [DOI] [PubMed] [Google Scholar]
- 17.Mcdonagh M, Peterson K, Carson S, Fu R, Thakurta S. Drug Class Review: Atypical Antipsychotic Drugs: Final Update 3 Report. Portland (OR): Oregon Health & Science University. 2010. https://www.ncbi.nlm.nih.gov/books/NBK50583/pdf/Bookshelf_NBK50583.pdf. Accessed March 6, 2018. [PubMed]
- 18.Jin H, Meyer JM, Jeste DV. Phenomenology of and risk factors for new-onset diabetes mellitus and diabetic ketoacidosis associated with atypical antipsychotics: an analysis of 45 published cases. Ann Clin Psychiatry 2002;14:59–64. [DOI] [PubMed] [Google Scholar]
- 19.Mulugeta Y, Barrett JS, Nelson R, Eshete AT, Mushtaq A, Yao L, et al. Exposure matching for extrapolation of efficacy in pediatric drug development. J Clin Pharmacol 2016;56:1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng KH, Chong D, Wong CK, Ong HT, Lee CY, Lee BW, et al. Central nervous system side effects of first- and second-generation antihistamines in school children with perennial allergic rhinitis: a randomized, double-blind, placebo-controlled comparative study. Pediatrics 2004;113:e116–21. [DOI] [PubMed] [Google Scholar]
- 21. Ten Eick AP, Blumer JL, Reed MD. Safety of antihistamines in children. Drug Saf 2001;24:119–47. [DOI] [PubMed] [Google Scholar]
- 22.Pipan M, Wang PP, Penna RT. Cognitive development considerations for long-term safety exposures in children In: Mulberg AE, et al. , eds. Pediatric drug development: concepts and applications. Hoboken, NJ: John Wiley & Sons Ltd; 2013, p. 355–82. [Google Scholar]