Abstract
Thrombotic microangiopathy (TMA) is a rare but severe complication of tumors and their chemotherapeutic treatment. We report on two patients with chemotherapy-induced TMA who were successfully treated with a short course of the terminal complement inhibitor eculizumab. Both patients quickly achieved remission of microangiopathic hemolytic anemia and recovery of renal function. After withdrawal of eculizumab, remission was stable over an observation period of 47 months and 15 months, respectively. Our data show that eculizumab is effective in treating chemotherapy-induced TMA. Discontinuation of eculizumab is feasible once the complement-activating condition is controlled and the trigger is eliminated. Additional studies need to determine the optimal duration of complement-directed therapies and validate effective monitoring strategies after discontinuation of such therapy.
Keywords: thrombotic microangiopathy, complement, eculizumab, chemotherapy, remission, aHUS
Introduction
Thrombotic microangiopathy (TMA) encompasses a group of disorders presenting with microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and ischemic organ damage, most frequently of the kidneys and the central nervous system [1, 2].
Atypical hemolytic uremic syndrome (aHUS) is a complement-mediated TMA caused by dysregulation of the alternative complement pathway. At least 50% of patients have an underlying inherited or acquired complement abnormality, exacerbated by complement-activating conditions, like infections, drugs, pregnancy, or cancer [3, 4].
Drug-induced TMA has been reported with antineoplastic agents including gemcitabine, docetaxel, and doxorubicin [5, 6, 7]. Direct cytotoxic and immune-mediated endothelial damage have been proposed as underlying pathologies [5]. While immune-mediated damage generally shows acute onset within 2 – 3 weeks of drug exposure, the clinical manifestation of cytotoxic damage is either acute or slowly progressive with cumulative dose-dependent toxicity [5, 8, 9]. Distinguishing cancer-related TMA as a consequence of cancer itself from cases of chemotherapy-induced TMA can be challenging. However, metastatic disease is more common in cancer-related TMA, whereas in chemotherapy-induced TMA, little or no active malignancy is detectable [10]. While discontinuation of the offending drug and supportive care are the primary treatment options in drug-induced TMA, in some cases this intervention is unable to limit the already dysregulated complement activity and requires therapeutic complement inhibition.
Eculizumab, approved as therapy for aHUS in 2011, is a humanized monoclonal antibody that binds to the complement component C5, preventing its cleavage into C5a and ultimately the formation of the membrane attack complex (SC5b-9) [11].
While the contributory role of complement dysregulation in drug-induced TMA is increasingly acknowledged, data on the efficacy of eculizumab, the duration of such therapy, and the incidence and type of detected complement abnormalities are sparse.
Here, we report on two patients with chemotherapy-induced TMA, who were successfully managed with temporary eculizumab therapy and remained relapse free for a follow-up of 47 and 15 months, respectively.
Table 1. Studies of chemotherapy-induced thrombotic microangiopathy treated with eculizumab.
| Patients (n) |
Drug | Previous therapy | Doses of eculizumab (range) / duration of treatment | Median follow-up (range) | Improved renal outcome | Genetic analysis | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Gemcitabine | DW + TPE + Steroids + RTX | 4 / 3 weeks | 17.5 weeks | Yes | NT | Starck [34] 2014 |
| 1 | Mitomycin C | DW + TPE | 8 / 3 months | 18 months | Yes | NT | Faguer [35] 2013 |
| 1 | Cisplatin | DW | Not reported / 4 months | Not reported | Yes, relapse 2 months after stop of eculizumab | CD46 mutation | Gilbert [36] 2013 |
| 4 | Gemcitabine | DW + TPE in 1 patient DW + steroids in 1 patient DW in 2 patients |
6.25 (5 – 8) / not reported | Not reported | Yes | NT | Al-Ustwani [37] 2014 |
| 1 | Gemcitabine | DW + TPE | 4 / not reported | 11 weeks | No | ND | Tsai [38] 2014 |
| 1 | Gemcitabine | DW + Steroids | 6 / 7 weeks | 3 months | No | NT | Karkowsky [39] 2015 |
| 1 | Gemcitabine | DW + TPE | 7 / 10 weeks | Not reported | Yes | NT | Rogier [16] 2016 |
| 1 | Gemcitabine | DW + TPE | 7 / 8 weeks | 3 months | Yes | NT | Lopez [40] 2017 |
| 8 | Gemcitabine | DW | 4.5 (3 – 22) / not reported | Not reported | Yes | NT | Grall [41] 2016 |
| 7 | Gemcitabine Dasatinib Bevacizumab Bleomycin |
DW DW + TPE (2 patients) |
Not reported / 14 weeks (2 – 24 weeks), 1 ongoing |
Not reported | Yes | NT | Weitz/Deloughery [42] 2018 |
| 2 | Gemcitabine Carfilzomib |
DW + TPE | Not reported | 42.5 weeks (33-52) | Yes | NT | Gosain [43] 2017 |
| 1 | Gemcitabine | DW + TPE | 20 / 9 months | 17 months | Yes | NT | Krishnappa [44] 2018 |
DW = offending drug withdrawn; TPE = therapeutic plasma exchange; RTX = rituximab; NT = not tested; ND = not detected.
Case reports
Patient 1
A 52-year-old woman admitted with acute onset of altered mental status, bloody diarrhea, and anuric acute kidney injury. Six months prior to admission, chemotherapy with docetaxel, doxorubicin, and cyclophosphamide was started for invasive ductal breast cancer. The last dose of chemotherapy was administered 3 days before symptoms started. Admission laboratory showed serum creatinine of 480 µmol/L, Coombs-negative hemolytic anemia with schistocytes on peripheral blood smear and thrombocytopenia of 64/µL. Lactate dehydrogenase (LDH) was 1,658 IU/mL and haptoglobin < 0.10 g/L. Coagulation tests were within the normal range, international normalized ratio (INR) 1.3 and partial thromboplastin time (PTT) 35 seconds, ruling out disseminated intravascular coagulation. Shiga toxin producing E. coli associated hemolytic uremic syndrome (STEC-HUS) was excluded by negative stool cultures for Shiga toxin-producing E. coli strains. Stool cultures for shigella, salmonella, campylobacter, and yersiania as well as PCR for Clostridium difficile were negative. Thrombotic thrombocytopenic purpura (TTP) was excluded by ADAMTS13 activity of 37% of control values. No ADAMTS13 antibodies were detected.
Shortly after admission, she developed seizures with respiratory failure requiring intubation. With the diagnosis of TMA, therapeutic plasma exchanges (TPE) were started. Daily TPE over 12 days and steroid therapy showed no effect on clinical symptoms and hemolysis. She had persistent seizures and required renal replacement therapy. Eculizumab was eventually initiated 20 days after admission. Immediately after the first dose of eculizumab, we observed a rapid and dramatic improvement of neurological symptoms. Renal replacement therapy could be discontinued 2 weeks later. Eculizumab was administered 6 times over a period of 5 weeks (Figure 1A). Breast-conserving surgery was performed 7 weeks after termination of eculizumab, followed by radiation therapy. During 47 months of follow-up, renal function continued to improve (eGFR 68 mL/min), and no relapse of TMA has occurred (Figure 1A) (Table 2).
Figure 1. A: Case 1, induction therapy with 6 doses of eculizumab. Serum creatinine and thrombocytes from admission to last follow-up (week 211). Breast-conserving surgery was performed 7 weeks after withdrawal of eculizumab, followed by radiation therapy 3 months later. TPE = therapeutic plasma exchange; CVVHD = continuous veno-venous hemodialysis; HD = hemodialysis. B: Case 2, induction therapy with 8 doses of eculizumab. Serum creatinine and thrombocytes from admission to last-follow up (week 42). FFP = fresh frozen plasma.
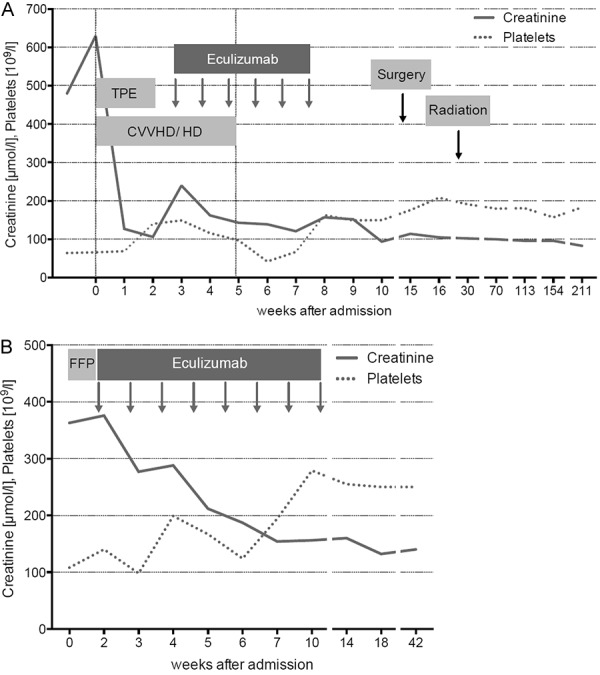
Table 2. Summary case report 1 and 2.
| Case | Age/ Gender | CAC | Organ involvement/ Presentation | Genetic | Eculizumab duration/doses | Creatinine (µmol/L) at the end of eculizumab | Creatinine (µmol/L) at last follow-up | Relapse | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 y/F | Docetaxel Doxorubicin | Kidney/acute CNS/acute Lungs/acute |
CFH polymorphism synonymous variant c.1419 G>A, p.Ala4773 Ala |
5 weeks/6 | 120 | 83 | No | 47 |
| 2 | 57 y/F | Gemcitabine | Kidney/chronic Severe hypertension |
CFH polymorphism Synonymous variant c.1419 G>A p.Ala473 Ala |
10 weeks/8 | 154 | 140 | No | 15 |
CAC = complement activating condition; CNS = central nervous system; CFH = complement factor H.
Next-generation sequencing identified a homozygous polymorphism in complement factor H (CFH) gene (synonymous variant c.1419 G>A, p.Ala473Ala). CFH autoantibodies were not detected.
Patient 2
A 57-year-old woman admitted with hypertensive urgency, progressive decline of renal function, and MAHA. She was diagnosed with pancreatic cancer 30 months prior to admission and since then treated with gemcitabine and Nab-paclitaxel. Chemotherapy had been discontinued 6 weeks earlier when a decline in renal function, hemolytic anemia, and mild thrombocytopenia (130/µL) was first noted.
Admission laboratory showed serum creatinine of 363 µmol/L (eGFR 11 mL/min), Coombs negative hemolytic anemia with schistocytes on peripheral blood smear, and thrombocytopenia of 108/µL. LDH was 760 IU/mL, haptoglobin < 0.10 g/L, and coagulation tests were within the normal range (INR 1, PTT 31 seconds). Stool cultures were negative for Shiga toxin-producing E. coli, and ADAMTS13 activity was 61% of control values. Urinalysis showed proteinuria with a protein/creatinine ratio of 0.8 g/g. Several plasma infusions were given without improvement of hemolysis or renal function. The patient received eculizumab 7 days after admission, followed by prompt resolution of hemolysis and improvement of renal function. Eculizumab was discontinued after a total of 8 doses over a period of 10 weeks. During 15 months of follow-up, renal function remained stable (eGFR 36 mL/min), and no relapse of TMA occurred (Figure 1B) (Table 2). The patient died of pancreatic cancer 18 months after initial hospital admission.
Next-generation sequencing identified a heterozygous polymorphism in CFH gene (synonymous variant c.1419 G>A, p.Ala473 Ala).
Discussion
We report on two patients with chemotherapy-induced TMA, persistent after discontinuation of the culprit drug and TPE/plasma infusion. In both patients, the clinical response to therapy with eculizumab was prompt and remission stable after cessation of treatment. Treatment with eculizumab was well tolerated, and no adverse events were reported.
Due to limited clinical experience, the optimal strategy for treatment of chemotherapy-induced TMA, especially the role of eculizumab, is not yet clear. Discontinuation of the offending drug and supportive care are the primary treatment options. Due to the long turnaround time for us to receive the results of the ADAMTS13 activity plasma infusions (case #2) and therapeutic plasma exchange (case #1) was initially used.
In agreement with others we found that TPE/plasma infusion was not effective in patients with chemotherapy-induced TMA [12, 13, 14, 15, 16, 17, 18].
Patient 1 presented, among other symptoms, with bloody diarrhea. However, the presence of diarrhea is not sufficient to exclude other forms of TMA as ~ 30 – 40% of aHUS and TTP cases involve gastrointestinal symptoms, including bloody diarrhea [19, 20].
Genetic mutations leading to dysregulation of the alternative complement pathway or autoantibodies against complement regulatory proteins are identified in ~ 50% of aHUS patients [4]. However, genetic variants in complement regulatory proteins are also detected in patients with secondary, e.g., chemotherapy-induced, TMA [3]. In such patients, the underlying dysregulation of the alternative complement system may be unmasked by the applied drug, acting as a complement-activating trigger. Given the incomplete penetrance of the genetic defects, complement-activating conditions play an important role for the development of TMA [3, 21, 22].
In most patients with TMA, a complement-activating trigger can be identified, and in 28% of patients with an activating trigger, a genetic risk mutation can be found [3].
The largest group of aHUS-associated mutations occurs in the CFH gene, and more than 60% of these mutations are clustered within the C-terminal recognition region [23, 24, 25]. CFH is a central regulator of the alternative pathway of complement by acting as a cofactor to factor I in the breakdown and inactivation of C3b [26].
In both of our patients, we identified a polymorphism in the CFH gene (c.1419 G>A). This variant has a high allele frequency in the general population, and its isolated occurrence seems not to be associated with an increased aHUS risk [27].
The mechanism of gemcitabine-related TMA appears to be dose-related with a reported incidence of 1%. Both immune-mediated and cytotoxic injury have been proposed as underlying pathophysiology [12, 28]. Cytotoxic damage is the assumed mechanism in the few described cases of doxorubicin- and docetaxel-related TMA [5, 6, 29].
To date, several cases of the use of eculizumab in chemotherapy-induced TMA have been reported, most of them regarding gemcitabine (summarized in Table 1). Before the availability of eculizumab, a case series reported ~ 29 patients with suspected gemcitabine-related TMA. Despite discontinuation of gemcitabine, 7 (24%) patients progressed to end-stage renal disease (ESRD), and 3 (10%) patients developed chronic renal failure [30]. These reports and our observation support induction therapy with eculizumab in cases of persisting TMA.
Eculizumab is approved for lifelong therapy of aHUS. However, the possible side effects, especially the risk of meningococcal infection, the inconvenience of a bi-monthly application, and the significant costs have prompted interest in alternative dosing schedules and complete discontinuation. A recent review analyzed data from unpublished cases, published case reports, clinical trials, and the Global aHUS Registry regarding patient outcomes after eculizumab discontinuation [31]. Of the case reports, a subsequent TMA manifestation was observed in 31% (16/52) of patients after eculizumab discontinuation. Data from five clinical trials documented a relapse in 20% (12/61) of patients after cessation of therapy with eculizumab with a median follow-up of 24 weeks. Terminal renal failure occurred in 5% (3/61) of the patients. Of note, relapse risk was independent of an identified genetic mutation, high-risk polymorphism, or autoantibody status. Data from the Global aHUS Registry found a relapse in 16% (12/76) of patients. In the cases described above, disease recurrence was unpredictable in both timing and severity [31].
The French aHUS Registry described a relapse rate after eculizumab discontinuation in 31% (12/38) of the patients [32]. The risk of recurrence was higher in the presence of complement gene variants. The highest risk was associated with CFH variants, whereas no relapse was seen in patients without identified mutations or negative CFH autoantibodies. In case of relapse, early reinstitution (≤ 48 hours) of eculizumab resulted in rapid hematologic remission and a return of serum creatinine to baseline level [32].
While current evidence suggests a relapse rate after eculizumab discontinuation of ~ 30%, there is little available clinical data for estimating the risk of relapse in chemotherapy-induced TMA [29].
In 2017, the KDIGO controversies conference published recommendations for best treatment strategies in aHUS. No evidence was currently seen to support lifelong therapy in all aHUS patients. The consensus suggested that eculizumab withdrawal could be considered on an individual and risk-stratified basis after a minimum treatment duration of 6 – 12 months to ensure recovery of endothelial damage [33]. Important risk factors for TMA relapses constitute an identified genetic mutation, former TMA episodes, or concomitant permanent or likely recurrent complement-activating condition. Close monitoring of renal function and hematological parameters after eculizumab withdrawal is mandatory; however, there are no evidence-based data about the reliability of a specific parameter and the optimal frequency of testing [33].
In summary, our report supports the role of complement-directed therapy with eculizumab as an effective therapeutic option in the management of refractory chemotherapy-induced TMA. In our opinion, eculizumab discontinuation is feasible in carefully selected patients after permanent removal of the complement-activating condition. Further studies are needed to elucidate the role of genetic variants in complement-regulatory proteins in chemotherapy-induced TMA and to define parameters predictive of complement activation and likely TMA recurrence. Until then, the decision to withdraw eculizumab has to be made on an individual basis.
Funding
None.
Conflict of interest
B.S.: Consultant/Speaker Honoraria from Alexion, Amgen, Novartis, Astellas, Boehringer Ingelheim, Vifor Pharma, Astra-Zeneka, Janssen. Grants from Alexion, Sanofi, Pfizer.
C.B. is an employee of Limbach and holds a part-time faculty appointment at the University of Freiburg. His research lab receives support from the Deutsche Forschungsgemeinschaft (DFG) DFG BE 3910/8-1 and DFG BE 3910/9-1, the Collaborative Research Center (SFB) KIDGEM 1140 and from the Federal Ministry of Education and Research (BMBF, 01GM1903I and 01GM1903G). He received speaker honoraria from Alexion and PTC Therapeutics.
The other authors do not have a conflict of interest.
References
- 1. George JN Nester CM Syndromes of thrombotic microangiopathy. N Engl J Med. 2014; 371: 654–666. [DOI] [PubMed] [Google Scholar]
- 2. Moake JL Thrombotic microangiopathies. N Engl J Med. 2002; 347: 589–600. [DOI] [PubMed] [Google Scholar]
- 3. Noris M Caprioli J Bresin E Mossali C Pianetti G Gamba S Daina E Fenili C Castelletti F Sorosina A Piras R Donadelli R Maranta R van der Meer I Conway EM Zipfel PF Goodship TH Remuzzi G Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010; 5: 1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campistol JM Arias M Ariceta G Blasco M Espinosa L Espinosa M Grinyó JM Macía M Mendizábal S Praga M Román E Torra R Valdés F Vilalta R Rodríguez de Córdoba S An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2015; 35: 421–447. [DOI] [PubMed] [Google Scholar]
- 5. Al-Nouri ZL Reese JA Terrell DR Vesely SK George JN Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015; 125: 616–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reese JA Bougie DW Curtis BR Terrell DR Vesely SK Aster RH George JN Drug-induced thrombotic microangiopathy: Experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015; 90: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwa M Baumgartner R Shavit L Barash I Michael J Puzanov I Kopolovic J Rosengarten O Blank S Curtin JP Gabizon A Muggia F Is renal thrombotic angiopathy an emerging problem in the treatment of ovarian cancer recurrences? Oncologist. 2012; 17: 1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izzedine H Perazella MA Thrombotic microangiopathy, cancer, and cancer drugs. Am J Kidney Dis. 2015; 66: 857–868. [DOI] [PubMed] [Google Scholar]
- 9. Izzedine H Perazella MA Anticancer Drug-Induced Acute Kidney Injury. Kidney Int Rep. 2017; 2: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murgo AJ Thrombotic microangiopathy in the cancer patient including those induced by chemotherapeutic agents. Semin Hematol. 1987; 24: 161–177. [PubMed] [Google Scholar]
- 11. Legendre CM Licht C Muus P Greenbaum LA Babu S Bedrosian C Bingham C Cohen DJ Delmas Y Douglas K Eitner F Feldkamp T Fouque D Furman RR Gaber O Herthelius M Hourmant M Karpman D Lebranchu Y Mariat C Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013; 368: 2169–2181. [DOI] [PubMed] [Google Scholar]
- 12. Leal F Macedo LT Carvalheira JB Gemcitabine-related thrombotic microangiopathy: A single-centre retrospective series. J Chemother. 2014; 26: 169–172. [DOI] [PubMed] [Google Scholar]
- 13. Gore EM Jones BS Marques MB Is therapeutic plasma exchange indicated for patients with gemcitabine-induced hemolytic uremic syndrome? J Clin Apher. 2009; 24: 209–214. [DOI] [PubMed] [Google Scholar]
- 14. Shaz BH Linenberger ML Bandarenko N Winters JL Kim HC Marques MB Sarode R Schwartz J Weinstein R Wirk A Szczepiorkowski ZM Category IV indications for therapeutic apheresis: ASFA fourth special issue. J Clin Apher. 2007; 22: 176–180. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz J Winters JL Padmanabhan A Balogun RA Delaney M Linenberger ML Szczepiorkowski ZM Williams ME Wu Y Shaz BH Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013; 28: 145–284. [DOI] [PubMed] [Google Scholar]
- 16. Rogier T Gerfaud-Valentin M Pouteil-Noble C Taleb A Guillet M Noel A Broussolle C Sève P [Clinical efficacy of eculizumab as treatment of gemcitabine-induced thrombotic microangiopathy: A case report]. Rev Med Interne. 2016; 37: 701–704. [DOI] [PubMed] [Google Scholar]
- 17. Cavero T Rabasco C López A Román E Ávila A Sevillano Á Huerta A Rojas-Rivera J Fuentes C Blasco M Jarque A García A Mendizabal S Gavela E Macía M Quintana LF María Romera A Borrego J Arjona E Espinosa M Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 2017; 32: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryu H Kang E Park S Park S Lee K Joo KW Lee H A case of gemcitabine-induced thrombotic microangiopathy in a urothelial tumor patient with a single kidney. Kidney Res Clin Pract. 2015; 34: 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaefer F Ardissino G Ariceta G Fakhouri F Scully M Isbel N Lommelé Å Kupelian V Gasteyger C Greenbaum LA Johnson S Ogawa M Licht C Vande Walle J Frémeaux-Bacchi V Blasco M Cresseri D Generolova G Webb N Hirt-Minkowski P Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018; 94: 408–418. [DOI] [PubMed] [Google Scholar]
- 20. Schönermarck U Ries W Schröppel B Pape L Dunaj-Kazmierowska M Burst V Mitzner S Basara N Starck M Schmidbauer D Mellmann A Dittmer R Jeglitsch M Haas CS Relative incidence of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome in clinically suspected cases of thrombotic microangiopathy. Clin Kidney J. 2019; 1–9. [DOI] [PMC free article] [PubMed]
- 21. Laurence J Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol. 2016; 14: 2–15. [PubMed] [Google Scholar]
- 22. Caprioli J Noris M Brioschi S Pianetti G Castelletti F Bettinaglio P Mele C Bresin E Cassis L Gamba S Porrati F Bucchioni S Monteferrante G Fang CJ Liszewski MK Kavanagh D Atkinson JP Remuzzi G Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006; 108: 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caprioli J Bettinaglio P Zipfel PF Amadei B Daina E Gamba S Skerka C Marziliano N Remuzzi G Noris M The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol. 2001; 12: 297–307. [DOI] [PubMed] [Google Scholar]
- 24. Neumann HP Salzmann M Bohnert-Iwan B Mannuelian T Skerka C Lenk D Bender BU Cybulla M Riegler P Königsrainer A Neyer U Bock A Widmer U Male DA Franke G Zipfel PF Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J Med Genet. 2003; 40: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saunders RE Goodship TH Zipfel PF Perkins SJ An interactive web database of factor H-associated hemolytic uremic syndrome mutations: insights into the structural consequences of disease-associated mutations. Hum Mutat. 2006; 27: 21–30. [DOI] [PubMed] [Google Scholar]
- 26. Parente R Clark SJ Inforzato A Day AJ Complement factor H in host defense and immune evasion. Cell Mol Life Sci. 2017; 74: 1605–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caprioli J Castelletti F Bucchioni S Bettinaglio P Bresin E Pianetti G Gamba S Brioschi S Daina E Remuzzi G Noris M Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003; 12: 3385–3395. [DOI] [PubMed] [Google Scholar]
- 28. Saif MW Xyla V Makrilia N Bliziotis I Syrigos K Thrombotic microangiopathy associated with gemcitabine: rare but real. Expert Opin Drug Saf. 2009; 8: 257–260. [DOI] [PubMed] [Google Scholar]
- 29. Román E Mendizábal S Jarque I de la Rubia J Sempere A Morales E Praga M Ávila A Górriz JL Secondary thrombotic microangiopathy and eculizumab: A reasonable therapeutic option. Nefrologia. 2017; 37: 478–491. [DOI] [PubMed] [Google Scholar]
- 30. Glezerman I Kris MG Miller V Seshan S Flombaum CD Gemcitabine nephrotoxicity and hemolytic uremic syndrome: report of 29 cases from a single institution. Clin Nephrol. 2009; 71: 130–139. [DOI] [PubMed] [Google Scholar]
- 31. Macia M de Alvaro Moreno F Dutt T Fehrman I Hadaya K Gasteyger C Heyne N Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome. Clin Kidney J. 2017; 10: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fakhouri F Fila M Provôt F Delmas Y Barbet C Châtelet V Rafat C Cailliez M Hogan J Servais A Karras A Makdassi R Louillet F Coindre JP Rondeau E Loirat C Frémeaux-Bacchi V Pathogenic Variants in Complement Genes and Risk of Atypical Hemolytic Uremic Syndrome Relapse after Eculizumab Discontinuation. Clin J Am Soc Nephrol. 2017; 12: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodship TH Cook HT Fakhouri F Fervenza FC Frémeaux-Bacchi V Kavanagh D Nester CM Noris M Pickering MC Rodríguez de Córdoba S Roumenina LT Sethi S Smith RJ Alpers CE Appel GB Ardissino G Ariceta G Arici M Bagga A Bajema IM Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017; 91: 539–551. [DOI] [PubMed] [Google Scholar]
- 34. Starck M, Wendtner CM Use of eculizumab in refractory gemcitabine-induced thrombotic microangiopathy. Br J Haematol. 164: 888-902. [DOI] [PubMed] [Google Scholar]
- 35. Faguer S Huart A Frémeaux-Bacchi V Ribes D Chauveau D Eculizumab and drug-induced haemolytic-uraemic syndrome. Clin Kidney J. 2013; 6: 484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilbert RD Stanley LK Fowler DJ Angus EM Hardy SA Goodship TH Cisplatin-induced haemolytic uraemic syndrome associated with a novel intronic mutation of CD46 treated with eculizumab. Clin Kidney J. 2013; 6: 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al Ustwani O Lohr J Dy G Levea C Connolly G Arora P Iyer R Eculizumab therapy for gemcitabine induced hemolytic uremic syndrome: case series and concise review. J Gastrointest Oncol. 2014; 5: E30–E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai HM Kuo E Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol. 2014; 2014: 295323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karkowsky RM Parikh KMD Eculizumab for gemcitabine-induced hemolytic uremic syndrome: A novel therapy for an emerging condition. Med Forum. 2015; 16: 8. [Google Scholar]
- 40. Lopez Rubio ME Rodado Martínez R Illescas ML Mateo Bosch E Martinez Díaz M de la Vara Inesta L Cabezuelo B Morales Albuja ME Lucas Guillén E Jimeno García L Gemcitabine-induced hemolytic-uremic syndrome treated with eculizumab or plasmapheresis: two case reports. Clin Nephrol. 2017; 87: 100–106. [DOI] [PubMed] [Google Scholar]
- 41. Grall M Provôt F Coindre J-P Pouteil-Noble C Guerrot D Benhamou Y Bordessoule D Veyradier A Coppo P Grange S Efficacy of eculizumab in gemcitabine-induced thrombotic microangiopathy: Experience of the French thrombotic microangiopathies reference centre. Blood. 2016; 128: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weitz IC Deloughery T Effective treatment of chemotherapy induced atypical Haemolytic Uraemic Syndrome: a case series of 7 treated patients. Br J Haematol. 2018; 183: 136–139. [DOI] [PubMed] [Google Scholar]
- 43. Gosain R Gill A Fuqua J Volz LH Kessans Knable MR Bycroft R Seger S Gosain R Rios JA Chao JH Gemcitabine and carfilzomib induced thrombotic microangiopathy: Eculizumab as a life-saving treatment. Clin Case Rep. 2017; 5: 1926–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krishnappa V Gupta M Shah H Das A Tanphaichitr N Novak R Raina R The use of eculizumab in gemcitabine induced thrombotic microangiopathy. BMC Nephrol. 2018; 19: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


