Abstract
Objectives:
The impact of immunosuppression on post-operative outcomes has primarily been studied in patients undergoing joint replacement surgery. We aimed to evaluate the impact of biologics and glucocorticoids on outcomes after other major surgeries.
Methods:
This retrospective cohort study used Medicare data 2006–2015 to identified adults with RA undergoing hip fracture repair, abdominopelvic surgery (cholecystectomy, hysterectomy, hernia, appendectomy, colectomy), or cardiac surgery (coronary artery bypass graft, mitral/aortic valve). Logistic regression with propensity-score based inverse probability weighting compared 90-day mortality and 30-day readmission in patients receiving methotrexate (without a biologic or targeted synthetic disease modifying anti-rheumatic drug [tsDMARD]), a tumor necrosis factor inhibitor (TNFi), or a non-TNFi biologic/tsDMARD <8 weeks before surgery. Similar analyses evaluated associations between glucocorticoids and outcomes.
Results:
We identified 10,777 eligible surgeries: 3585 hip fracture, 5025 abdominopelvic, and 2167 cardiac surgeries. Compared to patients receiving methotrexate, there was no increase in the risk of 90-day mortality or 30-day readmission among patients receiving a TNFi [mortality aOR 0.83(0.67–1.02), readmission aOR 0.86(0.75–0.993)] or non-TNFi biologic/tsDMARD [mortality aOR 0.78(0.49–1.22), readmission aOR 1.02(0.78–1.33)]. Analyses stratified by surgery category were similar. Risk of mortality and readmission were higher with 5–10mg/day of glucocorticoids [mortality aOR 1.41(1.08–1.82), readmission aOR 1.26(1.05–1.52)] or >10mg/day [mortality aOR 1.64(1.02–2.64), readmission aOR 1.60(1.15–2.24)] versus no glucocorticoids, although results varied when stratifying by surgery category.
Conclusions:
Recent biologic or tsDMARD use was not associated with a greater risk of mortality or readmission after hip fracture, abdominopelvic, or cardiac surgery compared to methotrexate. Higher dose glucocorticoids were associated with greater risk.
Keywords: rheumatoid arthritis, biologics, surgery, perioperative management, infection
INTRODUCTION
Patients with rheumatoid arthritis (RA) and other autoimmune conditions are frequently treated with immunosuppression, which can increase the risk of infection. Infections are of particular concern in patients undergoing major surgery, in which post-operative complications such as pneumonia and wound infections can have substantial morbidity.
Perioperative immunosuppression management has been studied primarily in patients with RA undergoing arthroplasty. Patients with RA are at higher risk of post-operative infections after arthroplasty, with disease activity, comorbidities, and immunosuppression all potentially contributing.[1–5] Conventional disease modifying anti-rheumatic drugs (csDMARDs) such as methotrexate have not been associated with increased post-operative risk.[6] The risk with biologic therapies remains uncertain, although studies have suggested that stopping a biologic before arthroplasty does not reduce infection risk and have shown no differences in risk across different biologics [7–9]. Several studies, however, have demonstrated increased infection risk and readmission after arthroplasty with even modest glucocorticoid doses.[1,4,10–12]
Few studies, however, have evaluated the contribution of specific immunosuppressive therapies to outcomes after other types of surgery.[13,14] These surgeries have different post-operative risks than arthroplasty and are often non-elective, with no opportunity for holding medications before surgery.
The goal of this study was to evaluate associations between biologic use and adverse outcomes in patients with RA undergoing common major surgical operations other than arthroplasty, including hip fracture, abdominopelvic, and cardiac surgeries. A secondary goal was to evaluate associations between glucocorticoids and adverse outcomes.
METHODS
Using Medicare claims data 2006–2015 we identified adults ≥18 years old with RA based on ≥2 International Classifications of Diseases (ICD-9) provider diagnoses ≥7 days apart, use of a DMARD, and absence of psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease diagnoses in the year before surgery. Included patients underwent one of the following surgeries between January 1st 2007 and August 31st 2015 based on primary ICD-9 procedure codes from inpatient hospitalizations using established definitions: hip fracture repair, abdominopelvic surgeries (cholecystectomy, hysterectomy, hernia surgery, appendectomy, colectomy for diverticular disease), and cardiac surgeries (coronary artery bypass graft [CABG], mitral or aortic valve surgery). We required that surgery occur by hospital day 3 for hip fracture, hysterectomy, hernia surgery, and appendectomy and hospital day 7 for the other surgeries to avoid surgeries in which an outcome may have occurred prior to surgery or in which the surgical procedure might be the results of a complicated hospitalization, rather than the main indication for hospitalization. More than 90% of surgeries fell within these windows. We also applied other restrictions to ensure surgeries were performed for specific indications and were not part of a larger procedure (online supplementary Table S1). We further subdivided certain applicable surgeries (cholecystectomy, hernia, colectomy, CABG, and valve surgery) into elective and non-elective surgery, as post-operative risks are different after elective and non-elective surgery. All elective surgeries were required to occur by hospital day 2 with no admission through the emergency department, transfer from another acute care hospital, or admission status of “emergent.” Hip fracture repair and appendectomy were all considered non-elective, and all included hysterectomies were elective.
We required 1 year of continuous enrollment in Medicare A, B, and D prior to surgery (baseline) (online supplementary Figure S1). We excluded patients with a diagnosis of malignancy (except non-melanoma skin cancer) or HIV in the past year, patients receiving multiple different biologics/targeted synthetic DMARDs (tsDMARDs) within 6 months before surgery, and patients with another major surgery within 6 months. Patients could contribute multiple surgeries if >6 months apart.
Exposures
To evaluate associations between immunosuppression use and post-operative outcomes, we classified patients in mutually exclusive groups: 1) methotrexate prescription fill <8 weeks before surgery with no biologic/tsDMARD <6 months before surgery (to avoid recent biologic discontinuations), 2) TNFi prescription/infusion <8 weeks before surgery with or without methotrexate, or 3) non-TNFi biologic prescription/infusion or tsDMARD prescription fill <8 weeks before surgery (16 weeks for rituximab), with or without methotrexate. All groups could receive other csDMARDs (hydroxychloroquine, sulfasalazine, or leflunomide).
We separately evaluated associations between glucocorticoids and outcomes. Glucocorticoid dose in the 90 days prior to surgery was based on oral prescriptions for prednisone, prednisolone, and methylprednisolone, using prescribed dose in prednisone equivalents and days supply to determine each daily dose and truncating prescriptions if a new prescription was filled before the prescription end date. To account for differences between prescription instructions and patient behavior (e.g. a 30 day prescription taken over 90 days), we averaged the daily dose over 90 days, categorizing the average daily dose as ≤5mg, >5 to 10mg, or >10mg/day).
In secondary analyses, rather than compare biologic users to non-users, we directly evaluated the timing of biologics before surgery similar to previous studies [7,8]. Here we evaluated only patients receiving the two most common infusions (infliximab or intravenous abatacept), since the timing of treatment for infusions can be precisely determined in this data source. We evaluated whether the time between the last infusion and surgery (<4 weeks, ≥4 to <8 weeks, or ≥8 weeks to 6 months) was associated with different risk of post-operative outcomes.
Outcomes
The primary outcomes of interest were 1) mortality within 90 days after surgery (including the index hospitalization) or 2) readmission to an acute care hospital within 30 days after discharge. Readmission was identified among patients discharged to home, home health care, acute rehabilitation, or a skilled nursing facility, considering re-hospitalizations within 1 day of discharge to be continuations of the initial hospitalization and not counting hospitalizations with a primary diagnosis indicating rehabilitation.[15] Reasons for readmission were based on primary discharge diagnosis codes classified using Chronic Conditions Warehouse software.[16,17]
Secondary outcomes included post-operative pneumonia and wound complications [18] based on diagnosis codes from the index hospitalization or any acute care hospitalizations within 30 days after surgery (online supplementary Table S1) because of specific concerns about these outcomes with biologics and glucocorticoids.[19–21]
Covariates
Covariates measured at baseline included demographics, zip-code based median household income from the American Community Survey 2009–2013,[22] disability status, skilled nursing facility residence, surgery type, admission type (elective, urgent, emergent), and hospital day of surgery. We identified comorbid conditions, an adaption of the Charlson comorbidity index[23], and measures of healthcare utilization (outpatient visits, emergency department visits, hospitalizations, hospitalized infection) during the baseline period. Medication use based on filled prescriptions ≤90 before surgery days included non-steroidal anti-inflammatories, opioids, non-methotrexate csDMARDs, and antibiotics. Number of previous biologics was determined using all data prior to the surgery date (mean 4 years).
Statistical analysis
Associations between exposures and outcomes were evaluated using logistic regression using propensity score-derived inverse probability weights to balance all measured covariates across exposure groups, as described below. Cluster robust standard errors accounted for patients contributing multiple surgeries.[24] Analyses were first conducted in the overall population and then reanalyzed (with re-calculated propensity scores) separately for each surgery category (hip fracture, abdominopelvic, and cardiac).
Propensity scores were generated based on the probability of being in each treatment group (methotrexate, TNFi, non-TNFi biologic/tsDMARD) using multinomial logistic regression models with all covariates of interest including a squared term for age (to account for non-linearity) and average glucocorticoid dose before surgery (included variables in online supplementary Table S2).[10,25,26] Propensity scores were used to create stabilized inverse probability treatment weights truncated at the 1st and 99th percentile.[26–29] Balance of covariates across treatment categories was assessed compared to the reference group (methotrexate), with standardized mean difference ≤0.1 indicating good balance. Unbalanced covariates were added as covariates to weighted models. Propensity scores and stabilized inverse probability treatment weights were re-calculated in analyses with readmission as the outcome and in analyses of specific surgery categories. Discharge status was considered an intermediate variable and was not included in readmission propensity scores. To address the possibility that methotrexate treated patients with previous biologic treatment >6 months before surgery were more ill, we conducted a sensitivity analysis excluding patients with previous biologic use.
The same method was used to generate inverse probability treatment weights in glucocorticoid analyses, also including methotrexate use, biologic use, and time of last biologic or methotrexate before surgery in propensity score models.
In secondary analyses, associations between infliximab timing or intravenous abatacept timing and outcomes were assessed using multivariable logistic regression models with potential confounders included as covariates.
Analyses were performed using Stata 15.1 (StataCorp). The protocol was approved by the University of Pennsylvania institutional review board. Preliminary results were previously presented.[30]
RESULTS
We identified 10,777 surgeries among 10,483 patients meeting inclusion and exclusion criteria (Figure 1). Mean age was 72 and 80% of patients were female (Table 1, online supplementary Table S2). RA treatment before surgery was methotrexate without a biologic/tsDMARD in 6194 (57%), TNFi in 3523 (33%) (709 adalimumab, 88 certolizumab, 972 etanercept, 46 golimumab, 1708 infliximab), and non-TNFi biologic/tsDMARD in 1060 (10%) (701 abatacept, 203 rituximab, 127 tocilizumab, 29 tofacitinib). Surgery type was hip fracture repair in 33%, abdominopelvic surgery in 47% (most commonly cholecystectomy), and cardiac surgery in 20%. Patients receiving a TNFi or non-TNFi biologic/tsDMARD were younger, had more outpatient visits, were more likely to have received prior biologics or be disabled, and were less likely to have been in a skilled nursing facility.. After applying inverse probability weights, patient characteristics were well balanced between methotrexate and TNFi groups with only slight residual imbalance in the non-TNFi biologic/tsDMARD group (online supplementary Table S2).
Figure 1: Cohort Selection.
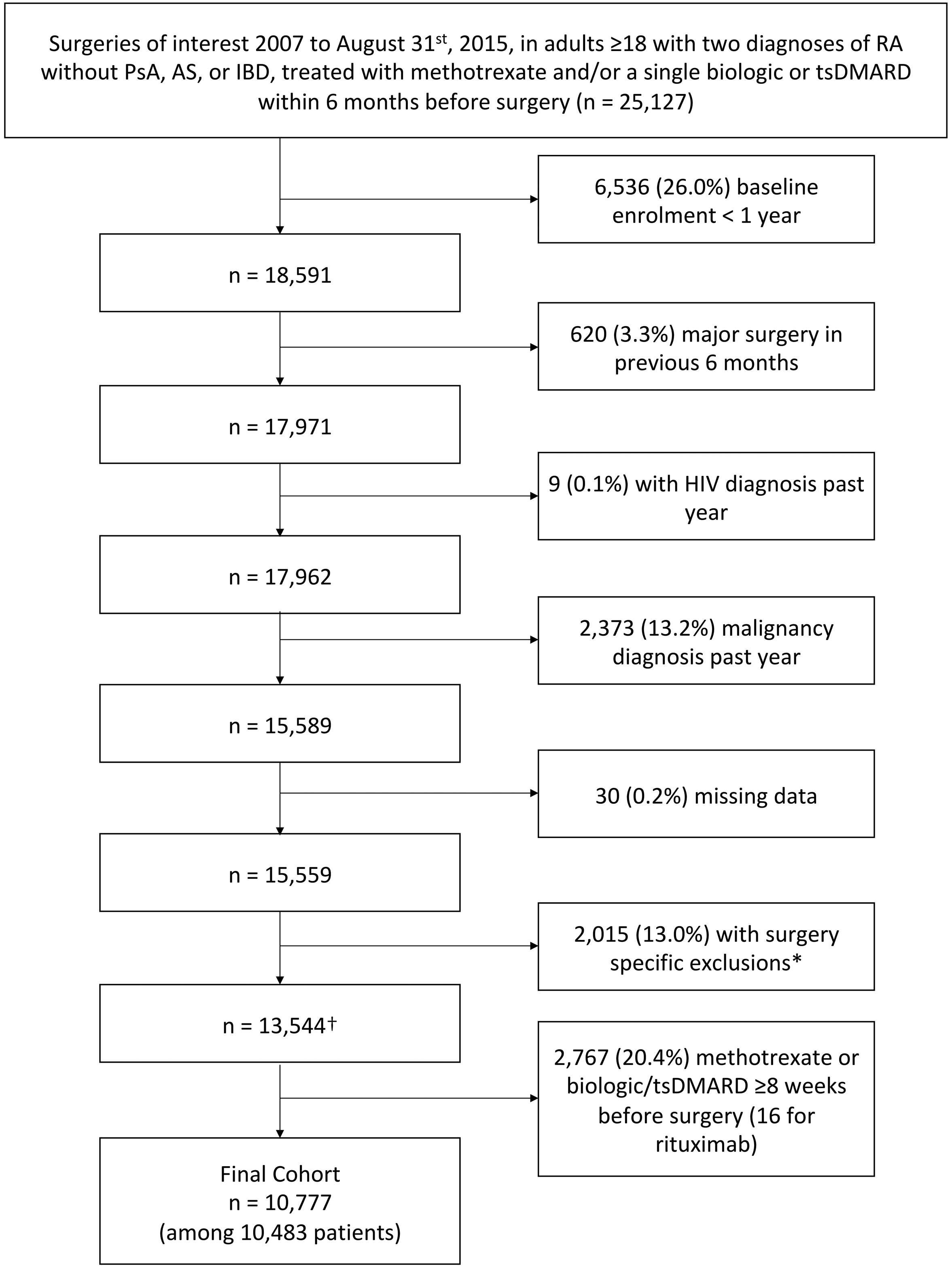
Table 1:
Select cohort characteristics
| Methotrexate (n = 6194) |
TNF (n = 3523) |
SMD pre |
SMD post | Non-TNF biologic/tsDMARD (n = 1060) |
SMD pre |
SMD post | Total | |
|---|---|---|---|---|---|---|---|---|
| 74.2 +/− 10.7 | 70.0 +/− 11.6 | −0.37 | −0.01 | 70.3 +/− 11.3 | −0.36 | -0.12 | 72.5 +/− 11.3 | |
| Female | 4907 (79.2) | 2852 (81.0) | 0.04 | 0.01 | 867 (81.8) | 0.07 | 0.05 | 8626 (80.0) |
| Race | ||||||||
| White | 5040 (81.4) | 2648 (75.2) | −0.15 | −0.01 | 831 (78.4) | −0.07 | −0.06 | 8519 (79.0) |
| Black | 397 (6.4) | 252 (7.2) | 0.03 | 0.00 | 62 (5.8) | −0.02 | 0.06 | 711 (6.6) |
| Hispanic | 371 (6.0) | 343 (9.7) | 0.14 | 0.00 | 68 (6.4) | 0.02 | −0.02 | 782 (7.3) |
| Other/Unknown | 386 (6.2) | 280 (7.9) | 0.07 | 0.02 | 99 (9.3) | 0.12 | 0.05 | 765 (7.1) |
| Disability | ||||||||
| Not disabled | 4250 (68.6) | 1977 (56.1) | −0.26 | 0.00 | 602 (56.8) | −0.25 | −0.09 | 6829 (63.4) |
| Disabled age <65 | 872 (14.1) | 868 (24.6) | 0.27 | 0.00 | 236 (22.3) | 0.21 | 0.04 | 1976 (18.3) |
| Disabled age ≥65 | 1072 (17.3) | 678 (19.2) | 0.05 | 0.00 | 222 (20.9) | 0.09 | 0.07 | 1972 (18.3) |
| Year 2011–2015* | 3307 (53.4) | 1849 (52.5) | −0.04 | 0.01 | 749 (70.7) | 0.39 | 0.22 | 5905 (54.8) |
| Surgery type | ||||||||
| Hip fracture | 2213 (35.7) | 1050 (29.8) | −0.13 | −0.01 | 322 (30.4) | −0.11 | −0.05 | 3585 (33.3) |
| Cholecystectomy elective | 210 (3.4) | 151 (4.3) | 0.05 | −0.01 | 36 (3.4) | 0.00 | −0.02 | 397 (3.7) |
| Cholecystectomy non-elective | 1020 (16.5) | 660 (18.7) | 0.06 | 0.02 | 191 (18.0) | 0.04 | 0.00 | 1871 (17.4) |
| Hysterectomy | 397 (6.4) | 349 (9.9) | 0.13 | 0.00 | 80 (7.5) | 0.05 | 0.02 | 826 (7.7) |
| Hernia elective | 282 (4.6) | 151 (4.3) | −0.01 | 0.00 | 47 (4.4) | −0.01 | 0.03 | 480 (4.5) |
| Hernia non-elective | 216 (3.5) | 148 (4.2) | 0.04 | 0.00 | 30 (2.8) | −0.04 | 0.03 | 394 (3.7) |
| Appendectomy | 180 (2.9) | 129 (3.7) | 0.04 | 0.01 | 48 (4.5) | 0.09 | 0.01 | 357 (3.3) |
| Colectomy elective | 160 (2.6) | 69 (2.0) | −0.04 | −0.01 | 25 (2.4) | −0.01 | 0.00 | 254 (2.4) |
| Colectomy non-elective | 251 (4.1) | 135 (3.8) | −0.01 | 0.01 | 60 (5.7) | 0.08 | −0.01 | 446 (4.1) |
| CABG elective | 229 (3.7) | 155 (4.4) | 0.04 | 0.00 | 37 (3.5) | −0.01 | −0.01 | 421 (3.9) |
| CABG non-elective | 523 (8.4) | 252 (7.2) | −0.05 | 0.00 | 83 (7.8) | −0.02 | −0.04 | 858 (8.0) |
| Valve elective | 273 (4.4) | 133 (3.8) | −0.03 | 0.01 | 47 (4.4) | 0.00 | 0.03 | 453 (4.2) |
| Valve non-elective | 240 (3.9) | 141 (4.0) | 0.01 | 0.00 | 54 (5.1) | 0.06 | 0.08 | 435 (4.0) |
| Average glucocorticoid dose | ||||||||
| None | 3248 (52.4) | 1984 (56.3) | 0.08 | 0.00 | 488 (46.0) | −0.13 | 0.00 | 5720 (53.1) |
| ≤5 | 1851 (29.9) | 962 (27.3) | −0.06 | −0.01 | 324 (30.6) | 0.02 | −0.04 | 3137 (29.1) |
| 5–10 | 837 (13.5) | 442 (12.5) | −0.03 | 0.00 | 180 (17.0) | 0.10 | 0.01 | 1459 (13.5) |
| >10 | 258 (4.2) | 135 (3.8) | −0.02 | 0.01 | 68 (6.4) | 0.10 | 0.05 | 461 (4.3) |
| Previous biologics | ||||||||
| 0 | 5486 (88.6) | 2949 (83.7) | −0.14 | −0.02 | 390 (36.8) | −1.27 | -0.11 | 8825 (81.9) |
| 1 | 543 (8.8) | 448 (12.7) | 0.13 | 0.01 | 461 (43.5) | 0.86 | 0.07 | 1452 (13.5) |
| >1 | 165 (2.7) | 126 (3.6) | 0.05 | 0.03 | 209 (19.7) | 0.56 | 0.05 | 500 (4.6) |
| NSAIDs | 1600 (25.8) | 957 (27.2) | 0.03 | 0.00 | 255 (24.1) | −0.04 | −0.03 | 2812 (26.1) |
| Opioids | 2986 (48.2) | 1860 (52.8) | 0.09 | 0.00 | 612 (57.7) | 0.19 | 0.15 | 5458 (50.6) |
| Charlson Score | 3.0 +/− 2.8 | 2.7 +/− 2.8 | −0.09 | 0.01 | 3.1 +/− 2.9 | 0.04 | 0.03 | 2.9 +/− 2.8 |
| Extra-articular RA | 127 (2.1) | 92 (2.6) | 0.04 | 0.00 | 42 (4.0) | 0.11 | 0.08 | 261 (2.4) |
| Diabetes | 1541 (24.9) | 834 (23.7) | −0.03 | 0.00 | 288 (27.2) | 0.05 | 0.02 | 2663 (24.7) |
| Asthma/COPD | 1346 (21.7) | 717 (20.4) | −0.03 | 0.00 | 208 (19.6) | −0.05 | 0.03 | 2271 (21.1) |
| Hospitalizations past year | ||||||||
| 0 | 3925 (63.4) | 2365 (67.1) | 0.08 | 0.00 | 698 (65.8) | 0.05 | −0.04 | 6988 (64.8) |
| 1–2 | 1454 (23.5) | 758 (21.5) | −0.05 | 0.00 | 231 (21.8) | −0.04 | 0.02 | 2443 (22.7) |
| >2 | 815 (13.2) | 400 (11.4) | −0.06 | 0.00 | 131 (12.4) | −0.02 | 0.02 | 1346 (12.5) |
| Hospitalized infection past year | 1062 (17.1) | 500 (14.2) | −0.08 | 0.00 | 153 (14.4) | −0.07 | 0.03 | 1715 (15.9) |
| Outpatient visits | 14.5 +/− 9.5 | 16.1 +/− 9.3 | 0.17 | 0.01 | 19.5 +/− 10.2 | 0.50 | 0.21 | 15.5 +/− 9.6 |
| Skilled nursing facility stay past year | 963 (15.5) | 313 (8.9) | −0.21 | −0.01 | 74 (7.0) | −0.27 | −0.05 | 1350 (12.5) |
Mean +− standard deviation or N (%) shown. Full cohort characteristics shown in online supplementary Table S2. Standardized mean differences (SMD) are shown compared to the methotrexate-treated group, either before (pre) or after (post) inverse probability weighting, with SMD > 0.1 indicating imbalance. Characteristics with SMD > 0.1 after weighting are shown in bold and were added as covariates to weighted models.
Year modeled as continuous and SMD shown indicates standardized difference in means across groups
COPD = chronic obstructive pulmonary disease
Death occurred within 90 days of surgery in 577 (5.4%) patients and readmission within 30 days of discharge in 1282 (12.8%) of 9,977 surgeries with qualifying discharge. Outcome rates varied substantially by surgery type and were highest after non-elective colectomy or valve surgery and lowest after hysterectomy (Table 2). The most common reasons for readmission were complication of the procedure (18.6%), septicemia (6.6%), and congestive heart failure (6.0%) (online supplementary Table S3).
Table 2:
Outcomes by surgery type
| N | 90-day mortality | 30-day readmission* | Pneumonia | Wound complication | |
|---|---|---|---|---|---|
| 3,585 | 240 (6.7%) | 350/3142 (11.1%) | 206 (5.7%) | 47 (1.3%) | |
| Abdominopelvic surgery | |||||
| Appendectomy | 357 | ≤ 11 | 32/346 (9.2%) | ≤ 11 | ≤ 11 |
| Cholecystectomy elective | 397 | ≤ 11 | 38/394 (9.6%) | 14 (3.5%) | ≤ 11 |
| Cholecystectomy non-elective | 1,871 | 60 (3.2%) | 239/1794 (13.3%) | 109 (5.8%) | 40 (2.1%) |
| Colectomy elective | 254 | ≤ 11 | 35/253 (13.8%) | ≤ 11 | 15 (5.9%) |
| Colectomy non-elective | 446 | 68 (15.2%) | 85/374 (22.7%) | 65 (14.6%) | 63 (14.1%) |
| Hernia elective | 480 | ≤ 11 | 48/480 (10.0%) | ≤ 11 | 26 (5.4%) |
| Hernia non-elective | 394 | 21 (5.3%) | 42/374 (11.2%) | 28 (7.1%) | 17 (4.3%) |
| Hysterectomy | 826 | ≤ 11 | 49/823 (6.0%) | ≤ 11 | 14 (1.7%) |
| Cardiac surgery | |||||
| CABG elective | 421 | ≤ 11 | 62/411 (15.1%) | 20 (4.8%) | ≤ 11 |
| CABG non-elective | 858 | 55 (6.4%) | 127/759 (16.7%) | 87 (10.1%) | 49 (5.7%) |
| Valve elective | 453 | 24 (5.3%) | 93/442 (21.0%) | 24 (5.3%) | 15 (3.3%) |
| Valve non-elective | 435 | 63 (14.5%) | 82/385 (21.3%) | 47 (10.8%) | 14 (3.2%) |
| Total | 10,777 | 577 (5.4%) | 1282/9977 (12.8%) | 627 (5.8%) | 328 (3.0%) |
Cell sizes ≤ 11 suppressed.
Readmission within 30 days of discharge reported among patients discharged to home, home health, skilled nursing facility, or inpatient rehabilitation
CABG = coronary artery bypass graft
There was no significant difference in 90-day mortality among patients receiving a TNFi [aOR 0.83 (0.67–1.02)] or non-TNFi biologic/tsDMARD [aOR 0.78 (0.49–1.22)] versus patients receiving methotrexate without a biologic/tsDMARD (Table 3). Compared to methotrexate, the risk of 30-day readmission was somewhat lower in patients receiving a TNFi [aOR 0.86 (0.75–0.993)] and was similar in those receiving a non-TNFi/tsDMARD [aOR 1.02 (0.78–1.33)]. Results were similar in sensitivity analyses excluding patients with previous biologic use (online supplementary Table S4).
Table 3:
Associations between biologics/tsDMARDs and glucocorticoids with mortality and readmission
| 90-Day Mortality | 30-Day Readmission | ||||||
|---|---|---|---|---|---|---|---|
| N | Mortality N (%) | Unadjusted OR (95% CI) | Propensity weighted OR (95% CI) | Readmission N (%)* | Unadjusted OR (95% CI) | Propensity weighted OR (95% CI) | |
| Methotrexate | 6194 | 401 (6.5%) | Reference | Reference | 781/5690 (13.7%) | Reference | Reference |
| TNF | 3523 | 134 (3.8%) | 0.57 (0.47–0.70) | 0.83 (0.67–1.02) | 367/3295 (11.1%) | 0.79 (0.69–0.90) | 0.86 (0.75–0.993) |
| Non-TNF biologic/tsDMARD | 1060 | 42 (4.0%) | 0.60 (0.43–0.82) | 0.78 (0.49–1.22) | 134/992 (13.5%) | 0.98 (0.81–1.20) | 1.02 (0.78–1.33) |
| Glucocorticoids† | |||||||
| None | 5720 | 252 (4.4%) | Reference | Reference | 596/5329 (11.2%) | Reference | Reference |
| ≤5mg/day | 3137 | 181 (5.8%) | 1.33 (1.09–1.62) | 1.20 (0.98–1.48) | 386/2893 (13.3%) | 1.22 (1.07–1.40) | 1.10 (0.96–1.27) |
| 5–10mg/day | 1459 | 106 (7.3%) | 1.70 (1.34–2.15) | 1.41 (1.08–1.82) | 216/1336 (16.2%) | 1.53 (1.29–1.81) | 1.26 (1.05–1.52) |
| >10mg/day | 461 | 38 (8.2%) | 1.95 (1.37–2.78) | 1.64 (1.02–2.64) | 84/419 (20.0%) | 1.99 (1.54–2.57) | 1.60 (1.15–2.24) |
Propensity weighted odds ratios (OR) from inverse probability weighted logistic regression models with separate analyses for methotrexate vs. biologics/tsDMARDs and for glucocorticoids. Propensity score models include all covariates shown in online supplementary Table S2. Glucocorticoid propensity score models also include use of methotrexate or type of biologic/tsDMARD and time of last prescription/infusion before surgery. Unbalanced covariates are included in weighed models.
Readmission within 30 days of discharge reported among patients discharged to home, home health, skilled nursing facility, or inpatient rehabilitation
Glucocorticoid dose is the average daily dose in the 3 months prior to surgery.
TNF = tumor necrosis factor inhibitor, tsDMARD = targeted synthetic disease modifying anti-rheumatic drug
Unadjusted 90-day mortality and 30-day readmission was greater for patients receiving higher doses of glucocorticoids (Table 3). Results from adjusted analyses were attenuated but still showed significantly higher risk of 90-day mortality among patients receiving an average of 5–10mg/day [aOR 1.41 (1.08–1.82)] or >10mg/day of glucocorticoids [aOR 1.64 (1.02–2.64)], and significantly higher risk of 30-day readmission among patients receiving 5–10mg/day [aOR 1.26 (1.05–1.52)] or >10mg/day of glucocorticoids [aOR 1.60 (1.15–2.24)] versus no glucocorticoids.
When hip, abdominopelvic, and cardiac surgeries were evaluated independently, we found no significant differences in 90-day mortality or 30-day readmission with a TNFi or non-TNFi biologic/tsDMARD versus methotrexate for any of the surgery categories (Figure 2). Associations between glucocorticoid use and adverse outcomes varied by surgery category (Figure 3). Associations with mortality were most pronounced among patients undergoing cardiac surgery although 30-day readmission was not significantly different across glucocorticoid doses in these patients. In contrast, patients undergoing hip fracture repair had no significant difference in 90-day mortality across glucocorticoid doses, but did have greater risk of 30-day readmission with higher glucocorticoid doses.
Figure 2: Associations of biologic use with mortality and readmission by surgery type.
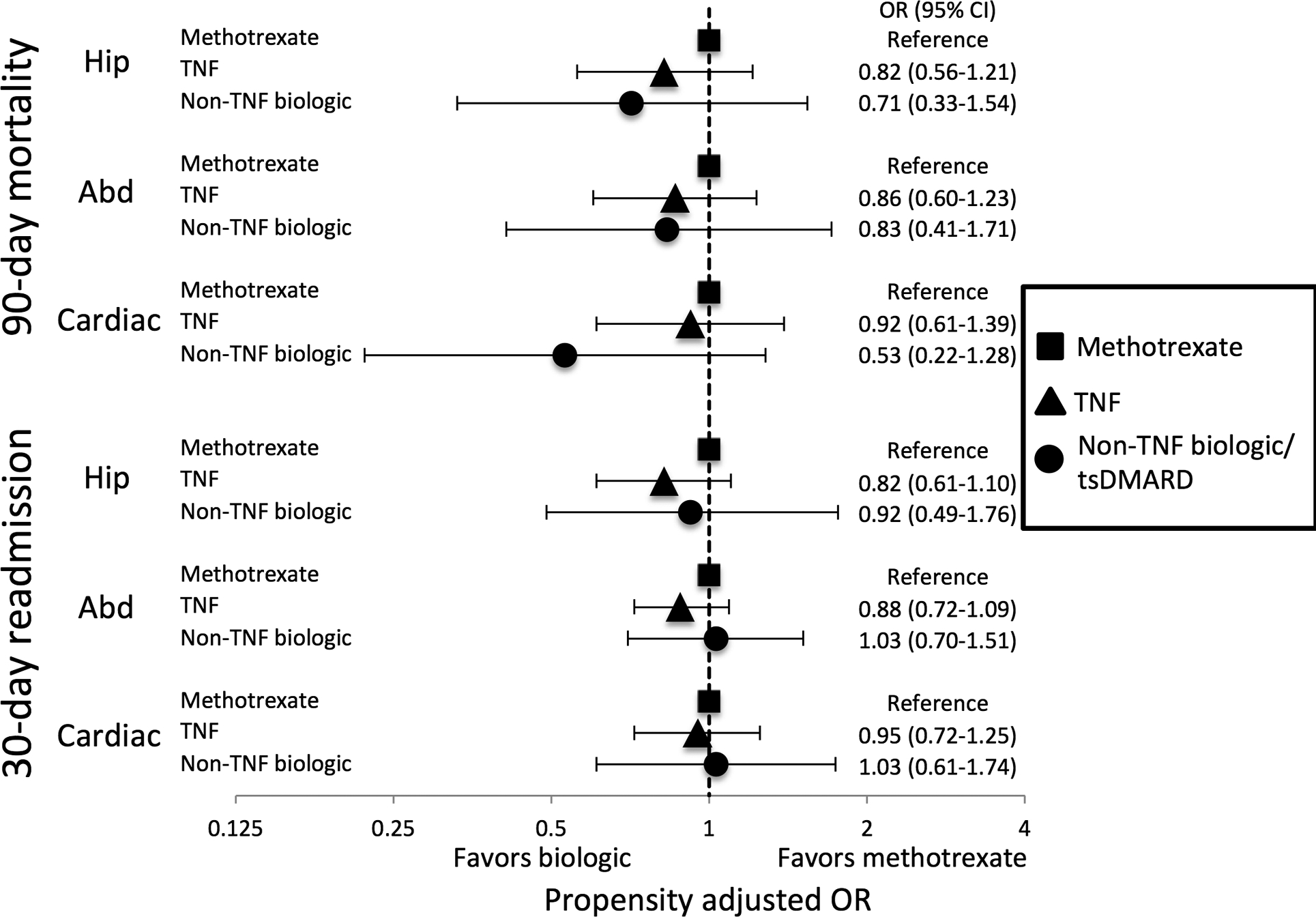
Odds ratios from inverse probability weighted logistic regression models. Propensity score models include the same variables as shown in online supplementary Table S2 and are re-calculated for each surgery type with unbalance covariates added to weighted models. Hip = hip fracture surgery, Abd = abdominopelvic surgery, Cardiac = cardiac surgery, TNF = tumor necrosis factor inhibitor, tsDMARD = targeted synthetic disease modifying anti-rheumatic drug
Figure 3: Associations of glucocorticoid use with mortality and readmission by surgery type.
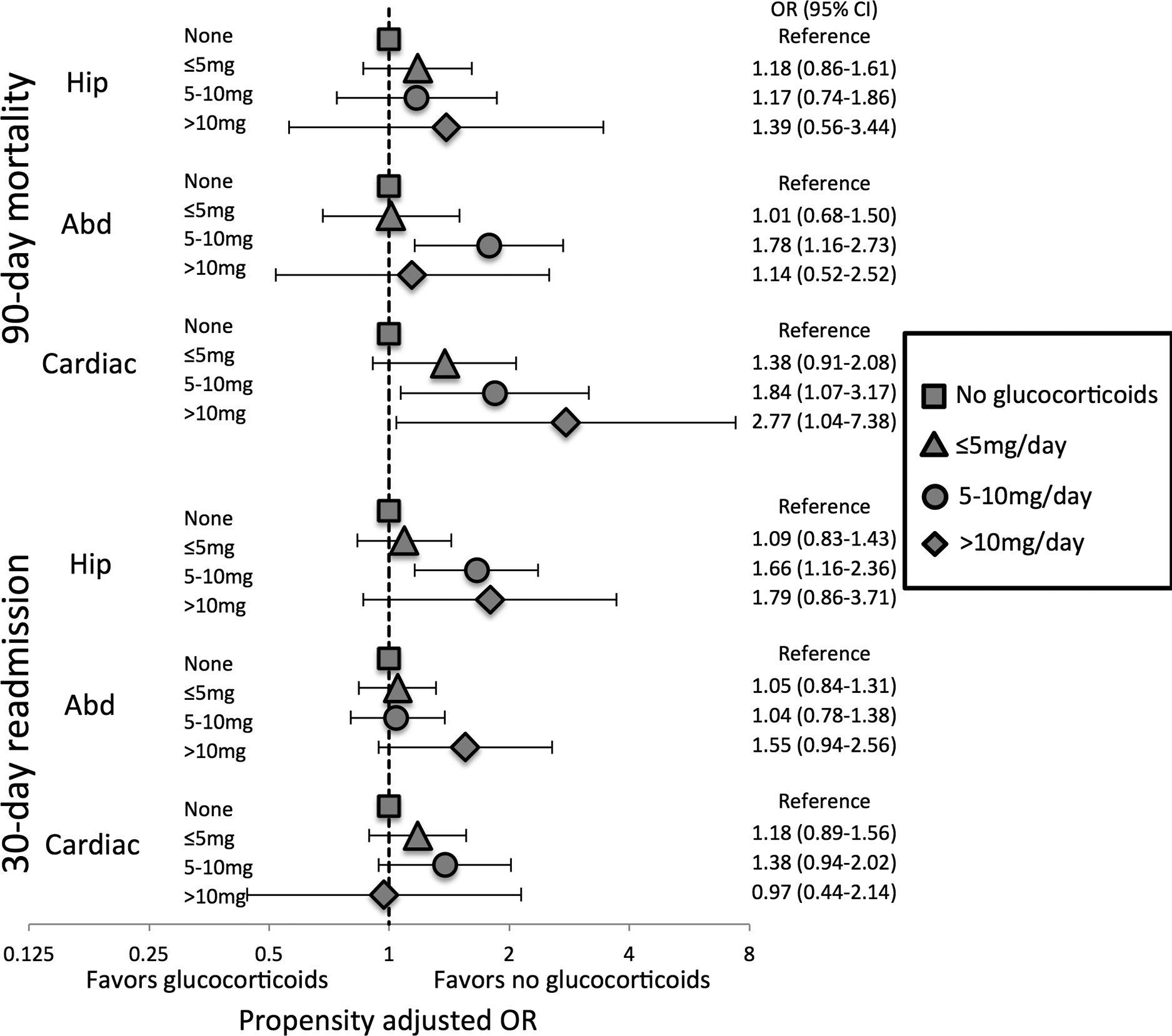
Odds ratios from inverse probability weighted logistic regression models. Propensity score models include the same variables as shown in Table 2 and are re-calculated for each surgery type with unbalance covariates added to weighted models. Hip = hip fracture surgery, Abd = abdominopelvic surgery, Cardiac = cardiac surgery
We found no significant difference in the risk of secondary outcomes of hospitalized pneumonia or hospitalized wound complication within 30 days of surgery in patients receiving a TNFi or non-TNFi biologic/tsDMARD versus patients receiving methotrexate (online supplementary Table S5). Higher dose glucocorticoids were associated with greater risk of wound complications [aOR 1.72 (1.24–2.39) for 5–10mg/day, aOR 1.68 (0.97–2.94) for >10mg/day].
Among 2,208 patients who received infliximab and 827 who received intravenous abatacept within 6 months of surgery, there was no significant difference in the risk of mortality or readmission in patients who received their last infusion <4 weeks before surgery compared to those who received the last infusion 4 to < 8 weeks or ≥8 weeks before surgery after adjusting for potential confounders (online supplementary Table S6).
DISCUSSION
In this large observational study of patients with RA undergoing hip fracture, abdominopelvic, or cardiac surgery we found no increased risk of 90-day mortality, 30-day readmission, wound complications, or post-operative pneumonia versus patients receiving methotrexate without a biologic/tsDMARD. Conversely, even low dose glucocorticoid use (5–10mg/day) was associated with greater risk of adverse outcomes.
Although previous studies of perioperative immunosuppression in patients undergoing the surgeries we examined are sparse, [14] our results showing no significant increase in adverse outcomes in patients receiving a biologic are in line with data from studies of joint replacement surgery. These studies showed no difference in post-operative risk in patients who stopped versus continued biologics before surgery.[7–9] Interestingly, in our study biologic treated patients had lower rates of adverse outcomes than patients receiving methotrexate without a biologic before adjusting for potential confounders. Although biologic treated patients may have more severe RA (in our study biologic treated patients were more likely to be disabled), biologic treated patients also tend to be younger and have fewer comorbidities [31], as we found here. The small differences in risk favoring biologic treated patients after adjusting for confounders likely reflects some residual confounding rather than risks associated with methotrexate or protective effects of biologics – reassuringly we found no difference in outcomes in patients receiving a biologic with versus without methotrexate (data not shown).
As we previously observed for arthroplasty [7,8], the lack of association between biologic use and adverse outcomes in this study suggests that holding biologics before the surgeries we examined may not be necessary. We required that all biologic-treated patients receive an infusion or fill a prescription for their biologic within 8 weeks of surgery (16 weeks of rituximab), excluding patients without evidence of recent biologic exposure. Although some patients may still have stopped their biologic before surgery, the majority of our surgeries were non-elective surgeries, in which patients would not have the opportunity to stop treatment. Additionally, we directly evaluated biologic timing among patients receiving infliximab or intravenous abatacept, for whom we could precisely identify timing based on infusion dates, and found no evidence that patients who received infliximab or intravenous abatacept within 4 weeks of surgery had greater risk of mortality or readmission compared to patients with a longer time between infusion and surgery. Nonetheless, our results should be interpreted cautiously since we cannot rule out the possibility of small benefits with biologic interruptions on the outcomes we studied or impacts on the frequency or severity of other post-operative outcomes.
Our results suggest that necessary non-elective surgeries need not be delayed in patients receiving biologic therapy, since delaying these surgeries has its own consequences. For elective surgery, a common practice has been to hold biologics before surgery. While our results show no obvious benefit to treatment interruptions, we also did not find obvious risks with short interruptions. Prolonged interruptions beyond one interval are likely not warranted. When deciding on shorter interruptions (e.g. one dosing interval) clinicians should consider the risks and benefits, including the potential need for glucocorticoids if patients have a disease flare.
In analyses with all surgery types combined, we found a dose-dependent association between glucocorticoid use and mortality, readmission, and wound complications. The difference between unadjusted and adjusted results did suggest substantial confounding; we cannot definitively establish causality and the potential for residual confounding remains. Additionally, results were more variable when stratified by surgery type. Whether these results reflect differences in glucocorticoid risk in different surgery types or whether these results are due to chance in these smaller subgroups is uncertain. Glucocorticoids have been associated with poor wound healing [32] and previous studies demonstrated a dose-dependent association between glucocorticoid use and adverse outcomes in the non-operative setting [33–35] and after orthopedic surgery [7,10]. We averaged glucocorticoid dose in the 90 days prior to surgery and could not evaluate dose changes immediately before surgery or doses during the hospitalization, but our results suggest that glucocorticoid use in the months before surgery, especially >10mg/day, should remain a concern in patients undergoing major surgery and may be a more important contributor to infection than more targeted immunosuppression.
Several limitations are inherent to this type of observational study. Outcomes from claims data may miss or misclassify specific post-operative complications [36]; we instead focused on mortality and readmission which can be accurately identified in claims data. We evaluated post-operative pneumonia and wound complications but could not fully assess morbidity or minor complications (e.g. delayed wound healing) not leading to hospitalization. Although typically biologic-users would be expected to have more severe disease, we could not measure disease activity, which could be less well controlled in some non-biologic treated patients. We also excluded patients with recent changes in therapy who might have the highest disease activity. We cannot rule out the possibility that patients receiving biologics were selected differently for surgery (especially elective surgery) or were treated or monitored differently after surgery. Additionally, biologics may be avoided completely in patients who are frail or have comorbidities [31] or survivorship bias could lead to a healthier biologic group, although we controlled for multiple comorbidities and measures of healthcare utilization and attained good balance for measured covariates between exposure groups. Glucocorticoid results were more variable and there is even greater potential for unmeasured or residual confounding in these analyses. Some contributors to post-operative outcomes such as surgeon experience, severity of the presentation, and difficulty of the surgery could not be directly measured, although these factors would not be expected to differ in biologic users versus non-users. Finally, although we analyzed surgeries in groups that would be susceptible to similar types of complications, numbers were not sufficient to evaluate specific surgeries (e.g. appendectomy, cholecystectomy) separately.
In conclusion, neither use of biologics nor biologic timing before surgery were associated with increased mortality or readmission after hip fracture, abdominopelvic, or cardiac surgery. Higher dose glucocorticoids were associated with adverse outcomes after these surgeries, suggesting that minimizing glucocorticoids should be a focus of perioperative medicine.
Supplementary Material
Key messages:
Studies of the risk of immunosuppression in patients undergoing surgery most commonly involve patients undergoing elective arthroplasty, but few studies have evaluated other major surgery
In this study, patients with rheumatoid arthritis receiving biologics did not have a greater risk of post-operative infection after hip fracture, abdominopelvic, or cardiac surgery compared to patients receiving methotrexate without a biologic
Glucocorticoids were associated with a dose-dependent increase in the risk of post-operative infection
Prolonged interruptions in biologics before major surgery is likely not required, but minimizing glucocorticoids before surgery should be a focus of perioperative management
Funding information:
Michael George is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases 1K23AR073931-01. Joshua Baker is supported by a VA Clinical Science Research and Development Merit Award (I01 CX001703).
Footnotes
Competing Interests: Michael George has received a research grant from Bristol-Myers Squibb and the National Institutes of Health and consulting fees from AbbVie. Joshua Baker has received consulting fees from Bristol-Myers Squibb and Gilead. Kevin Winthrop has received research grants from Pfizer and Bristol-Myers Squibb, and consulting fees from Pfizer, AbbVie, UCB, Lilly, Galapagos, GSK, Roche, and Gilead. Evo Alemao is an employee of Bristol-Myers Squibb. Jeffrey Curtis has received research grants from the Patient Centered Outcomes Research Institute and research grants and consulting fees from Bristol-Myers Squibb, Amgen, AbbVie, Corrona, Janssen, Lilly, Myriad, Pfizer, UCB, and Regeneron.
Data sharing statement: Data is not publically available but is available through the Centers for Medicare & Medicaid Services. Statistical code is available from the authors upon request.
Patient and public involvement: This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret results. Patients were not invited to contribute to manuscript writing or editing for readability or accuracy.
References
- 1.Cordtz RL, Zobbe K, Højgaard P, et al. Predictors of revision, prosthetic joint infection and mortality following total hip or total knee arthroplasty in patients with rheumatoid arthritis: a nationwide cohort study using Danish healthcare registers. Ann Rheum Dis 2018;77:281–8. doi: 10.1136/annrheumdis-2017-212339 [DOI] [PubMed] [Google Scholar]
- 2.Stundner O, Danninger T, Chiu Y-L, et al. Rheumatoid Arthritis vs Osteoarthritis in Patients Receiving Total Knee Arthroplasty: Perioperative Outcomes. The Journal of Arthroplasty 2014;29:308–13. doi: 10.1016/j.arth.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi B, Escott B, Shah PS, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis & Rheumatism 2012;64:3839–49. doi: 10.1002/art.37690 [DOI] [PubMed] [Google Scholar]
- 4.Salt E, Wiggins AT, Rayens MK, et al. Moderating effects of immunosuppressive medications and risk factors for post-operative joint infection following total joint arthroplasty in patients with rheumatoid arthritis or osteoarthritis. Semin Arthritis Rheum 2017;46:423–9. doi: 10.1016/j.semarthrit.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnaser EA, Browne JA, Padgett DE, et al. Perioperative Complications in Patients With Inflammatory Arthropathy Undergoing Total Hip Arthroplasty. J Arthroplasty 2016;31:2286–90. doi: 10.1016/j.arth.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 6.Grennan DM, Gray J, Loudon J, et al. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis 2001;60:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George MD, Baker JF, Hsu JY, et al. Perioperative Timing of Infliximab and the Risk of Serious Infection After Elective Hip and Knee Arthroplasty. Arthritis Care Res (Hoboken) 2017;69:1845–54. doi: 10.1002/acr.23209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George MD, Baker JF, Winthrop K, et al. Timing of Abatacept Before Elective Arthroplasty and Risk of Postoperative Outcomes. Arthritis Care Res (Hoboken) Published Online First: 11 February 2019. doi: 10.1002/acr.23843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou Zahr Z, Spiegelman A, Cantu M, et al. Perioperative use of anti-rheumatic agents does not increase early postoperative infection risks: a Veteran Affairs’ administrative database study. Rheumatol Int 2015;35:265–72. doi: 10.1007/s00296-014-3121-0 [DOI] [PubMed] [Google Scholar]
- 10.George MD, Baker JF, Winthrop K, et al. Risk of Biologics and Glucocorticoids in Patients With Rheumatoid Arthritis Undergoing Arthroplasty: A Cohort StudyPerioperative Risk With Immunosuppression in Rheumatoid Arthritis. Annals of Internal Medicine Published Online First: 21 May 2019. doi: 10.7326/M18-2217 [DOI] [PubMed] [Google Scholar]
- 11.Michaud K, Fehringer EV, Garvin K, et al. Rheumatoid arthritis patients are not at increased risk for 30-day cardiovascular events, infections, or mortality after total joint arthroplasty. Arthritis Res Ther 2013;15:R195. doi: 10.1186/ar4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilson M, Gossec L, Mariette X, et al. Risk factors for total joint arthroplasty infection in patients receiving tumor necrosis factor α-blockers: a case-control study. Arthritis Research & Therapy 2010;12:R145. doi: 10.1186/ar3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims SM, Kao AM, Spaniolas K, et al. Chronic immunosuppressant use in colorectal cancer patients worsens postoperative morbidity and mortality through septic complications in a propensity-matched analysis. Colorectal Dis 2019;21:156–63. doi: 10.1111/codi.14432 [DOI] [PubMed] [Google Scholar]
- 14.Waterman M, Xu W, Dinani A, et al. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut 2013;62:387–94. doi: 10.1136/gutjnl-2011-301495 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services (CMS). 2016 Procedure-Specific Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Readmission Measures. Elective Primary Total Hip Arthroplasty (THA) and/or Total Knee Arthroplasty (TKA) – Version 5.0. 2016https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html (accessed 9 Jan 2018).
- 16.HCUP Clinical Classifications Software (CCS) for ICD-9-CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp#download (accessed 17 Jul 2019).
- 17.Lawson EH, Hall BL, Louie R, et al. Association between occurrence of a postoperative complication and readmission: implications for quality improvement and cost savings. Ann Surg 2013;258:10–8. doi: 10.1097/SLA.0b013e31828e3ac3 [DOI] [PubMed] [Google Scholar]
- 18.Van Arendonk KJ, Tymitz KM, Gearhart SL, et al. Outcomes and costs of elective surgery for diverticular disease: a comparison with other diseases requiring colectomy. JAMA Surg 2013;148:316–21. doi: 10.1001/jamasurg.2013.1010 [DOI] [PubMed] [Google Scholar]
- 19.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 2006;54:628–34. doi: 10.1002/art.21568 [DOI] [PubMed] [Google Scholar]
- 20.Galloway JB, Mercer LK, Moseley A, et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2013;72:229–34. doi: 10.1136/annrheumdis-2011-201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aberra FN, Lewis JD, Hass D, et al. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology 2003;125:320–7. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Census Bureau, 2009–2013. 5-Year American Community Survey. https://www.census.gov/programs-surveys/acs (accessed 3 Oct 2015).
- 23.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64:749–59. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron AC, Miller DL. A Practitioner’s Guide to Cluster-Robust Inference. J Human Resources 2015;50:317–72. doi: 10.3368/jhr.50.2.317 [DOI] [Google Scholar]
- 25.Spreeuwenberg MD, Bartak A, Croon MA, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Med Care 2010;48:166–74. doi: 10.1097/MLR.0b013e3181c1328f [DOI] [PubMed] [Google Scholar]
- 26.Feng P, Zhou X-H, Zou Q-M, et al. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med 2012;31:681–97. doi: 10.1002/sim.4168 [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 2013;32:3388–414. doi: 10.1002/sim.5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med 2010;29:2137–48. doi: 10.1002/sim.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–64. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George MD, Baker JF, Winthrop K, et al. Risk of Serious Infection with Long-Term Use of Low-Dose Glucocorticoids in Patients with Rheumatoid Arthritis [abstract]. Arthritis & Rheumatology 2019;71 (suppl 10).https://acrabstracts.org/abstract/risk-of-serious-infection-with-long-term-use-of-low-dose-glucocorticoids-in-patients-with-rheumatoid-arthritis/. Accessed (accessed 25 Nov 2019). [Google Scholar]
- 31.George MD, Sauer BC, Teng C-C, et al. Biologic and Glucocorticoid Use after Methotrexate Initiation in Patients with Rheumatoid Arthritis. J Rheumatol 2019;46:343–50. doi: 10.3899/jrheum.180178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg 2013;206:410–7. doi: 10.1016/j.amjsurg.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 33.Dixon WG, Abrahamowicz M, Beauchamp M-E, et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis 2012;71:1128–33. doi: 10.1136/annrheumdis-2011-200702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis JR, Winthrop K, O’Brien C, et al. Use of a baseline risk score to identify the risk of serious infectious events in patients with rheumatoid arthritis during certolizumab pegol treatment. Arthritis Res Ther 2017;19:276. doi: 10.1186/s13075-017-1466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. doi: 10.1136/annrheumdis-2016-210457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg SM, Popa MR, Michalek JA, et al. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery 2008;144:662–7; discussion 662–667. doi: 10.1016/j.surg.2008.06.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


