Abstract
In the past two decades, several newly emerging and reemerging viral respiratory pathogens including several influenza viruses (avian influenza and pandemic influenza), severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV), have continued to challenge medical and public health systems. Thereafter, the development of cost-effective, broad-spectrum antiviral agents is the urgent mission of both virologists and pharmacologists. Current antiviral developments have focused targets on viral entry, replication, release, and intercellular pathways essential for viral life cycle. Here, we review the current literature on challenges and prospects in the development of these antivirals.
Keywords: respiratory virus, antiviral, entry, replication, pathway
Respiratory infections are a major cause of morbidity and mortality for humans, and most of these infections are caused by respiratory viruses.1 Between 1997 and 2015, several unprecedented epizootic avian influenza viruses (e.g., H5N1, H7N9, and H10N8) crossed the species barrier to cause human death.2 3 4 5 6 From severe acute respiratory syndrome coronavirus (SARS-CoV) to recent Middle East respiratory syndrome coronavirus (MERS-CoV), coronaviruses also pose a major threat to medical and public health systems.7 Moreover, human adenovirus-14, human metapneumovirus, enterovirus D68, bunyaviruses, and common respiratory viruses (respiratory syncytial virus, parainfluenza viruses, rhinoviruses, and human influenza) are all gangsters of respiratory disease.1 8 The continual emergence of these new and reemerging viral threats emphasizes the unpredictability of these pathogens, and brings challenges to the development of control strategies.
Although vaccination remains a milestone for the prevention of viral infections, a homologous vaccine was not available during the first pandemic wave of most newly emerging viruses.9 10 Furthermore, continuous antigenic drifting and antigenic shifting of virus can lead to the escape of recognition by immune system and inefficacy to protect the host from a novel antigenic variant.11 Thus, antivirals with novel pharmacological activities are highly needed. Several antiviral agents have been developed. Some of the agents are undergoing clinical trials and some are at different stages of preclinical development, but the vast majority have not yet been approved for clinical use.12 13 Several challenges have blocked the development of these antivirals as well as its application.13 In this review, we focus on the problems and prospects of these antivirals which were developed to control the newly emerging and reemerging respiratory viruses with pandemic potential.
Diagnostics and Measurements of Virus in the Development of Antivirals
A respiratory viral infection is difficult to differentiate from other respiratory viral infections by clinical symptoms alone.14 15 Thus, a rapid and reliable laboratory diagnostic result is required before clinical therapy. For the rapid viral antigen detection tests, traditional solid-phase immunoassay and immunofluorescence have a moderate sensitivity.15 16 But their specificities to low prevalence disease are limited. The viral diagnostics were significantly promoted by the development of nucleic acids amplification techniques (NAAT).10 15 This method enables the detection of low concentrations of viral nucleic acid.15 Moreover, NAAT are the only available method for most newly emerging respiratory viruses as traditional tests lack efficient cell-culture system or antigen detection methods.17 Currently, automated and multiplex NAAT have been developed to make it easier to obtain laboratory diagnostic results.18 These techniques are anticipated to be used for the cost-effective assessment of the antivirals for respiratory virus.
The Targets of Antivirals in Viral Replication Cycle
A series of antivirals have been discovered and developed to control the respiratory virus infection. Some of these agents target the essential steps of viral infection, including viral attachment, endocytosis, replication, assembly, budding, and release (Fig. 1). The others directly target the virus or the intracellular pathway essential for viral replication.
Fig. 1.
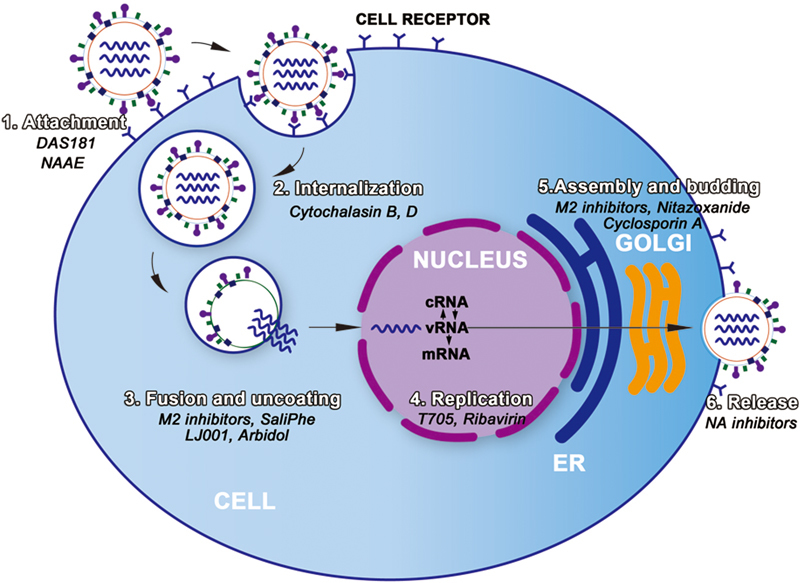
The replication cycle of virus and agents for the treatment of respiratory viruses.
Viral Attachment Inhibitory
The attachment process is a critical step for viruses to gain entry into cells; blocking the interactions between viral-specific surface proteins and cell-receptors is a promising strategy in developing novel antivirals.19 The sialidase and amphiregulin glycosaminoglycan recombinant construct DAS181 (Fludase®, Ansun Biopharma Inc., San Diego, CA), can hydrolyze both α2,3- and α2,6-linked sialic acid receptors on the respiratory epithelium cell surface.20 Thus, it can efficiently block the attachment of human and avian influenza virus.21 DAS181 have been confirmed to have antiviral activity on H1N1, H3N2, and H5N1 both in vitro and in vivo.21 Moreover, a phase II clinical trial confirmed DAS181 can significantly reduce viral load and viral shedding, compared with the placebo group (p = 0.002 and p = 0.006 for the multiple-dose and single-dose groups, respectively).22 DAS181 is advancing in clinical development now, with prospects for treatment in human and avian influenza infections. N-(2-aminoethyl)-1-aziridine-ethanamine (NAAE) is a small molecule which inhibits interactions between the SARS-Cov spike protein and its cell surface receptor, angiotensin converting enzyme 2 (ACE2).23 However, inhibition of ACE2 by NAAE also has a potential risk for hypertension.24 Hence, if the viral receptor is also important to cell function, agents that utilize this mechanism might cause side effects to the host too.
Several human monoclonal antibodies have been developed to block the viral attachment to cells. The human monoclonal antibody CR6261 can protect ferrets 100% from lethal challenges from the highly pathogenic avian H5N1 virus with both prophylactic and therapeutic administration, compared with 20% survival in the control group (p < 0.001).25 Motavizumab and palivizumab have been confirmed to prevent participants from respiratory syncytial virus infection.26 27 But an independent clinical trial showed that motavizumab treatment has no effect on the duration of hospitalization, severity of illness measures, or wheezing episodes at 12-month follow-up among children infected with respiratory syncytial virus, when compared with placebo treatment.28 Despite the inconsistent clinical data to human monoclonal antibodies, the high costs of these biologics can also be a formidable problem to the therapy of these infections.29
Viral Endocytosis Inhibitor
Pharmacologic inhibition of the endocytosis process of viral envelope with cell membrane is also a promising strategy to develop antivirals.30 The endocytosis process of respiratory virus can be subdivided into three steps: internalization, fusion, and uncoating.31 And the inhibition of this process can be achieved through the following mechanisms: (1) blocking the pathways that facilitate viral internalization; (2) suppression of acidification in the endosome; (3) reducing membrane curvature and fluidity; (4) interfering with the conformational changes of viral protein required for fusion; and (5) viral uncoating inhibition. Numerous broad-spectrum antivirals have been developed for these purposes.32
Both dynamin-dependent and dynamin-independent pathways are considered as the routes of influenza cell internalization.33 The inhibitors to these pathway including Cytochalasin B and D, jasplakinolide, and latruncul A are all specific inhibitors to the internalization of influenza.34 There are accumulated evidence to confirm that activation of PI3K promotes virus internalization.35 The interplay of influenza virus and receptor tyrosine kinases (RTKs) of host cell can promote this pathway and facilitate viral uptake.36 However, one should be cautious about potential benefit of RTK inhibitors as potential antiviral agents, as a limited number of RTKs can be manipulated by chemical inhibitors or siRNAs at the same time.36
For most enveloped viruses, the low-pH environment of endosome is not only triggers the fusion between endosomes and virions, but also causes dissociation of viral ribonucleoprotein complexes (vRNPs) from the capsid, which allows for its subsequent import into the nucleus and the start of viral replication.37 38 Thus, blocking endosomal acidification can also interfere with viral infection. Saliphenylhalamide, concanamycin A, archazolid B, diphyllin, and bafilomycin A1 are all endosomal acidification inhibitors which may abrogate influenza viral infection through inhibiting cellular vacuolar ATPase (vATPase) or vacuolar-type proton (V-H+) pump of endosome.39 40 41 Among them, saliphenylhalamide (SaliPhe) can effectively inhibit several wild type of influenza viruses, including pandemic swine-origin influenza virus, highly pathogenic H5N1 avian influenza virus, and oseltamivir-resistant seasonal H1N1 influenza virus.41 Moreover, it protected 62.5% of mice against a lethal challenge of a mouse-adapted influenza strain.41 Thus, these endosomal acidification inhibitors with even better selective indices may become candidates for the treatment of viral infections in the future.
Membrane curvature and fluidity is critical for viral-endosomal membrane fusion.42 LJ001 is a lipophilic thiazolidine derivative that inhibits membrane curvature and fluidity by O2-mediated lipid oxidation, and LJ001 exhibits broad-spectrum antiviral activity against enveloped viruses, including influenza virus, bunyaviruses, Ebola and Rift Valley fever viruses, HIV-1, and so on.43 LJ001 has led to a new class of membrane-targeted photosensitizers (oxazolidine-2,4-dithiones) with increased potencies in antiviral research.
Arbidol is another broad-spectrum antiviral with antifusion activity by stabilizing the surface glycoprotein of influenza virus (hemagglutinin, HA) in a low-pH form.44 This agent has proved to be active against several subtypes of avian influenzas, such as H5N1, H9N2, H2N2, and H6N1 in vitro.2 44 45 46 GS-5806 and MDT-637 are all small-molecule inhibitors that interfere with RSV fusion through interaction with the F protein of respiratory syncytial virus, and some of them are now in phase I development.47 48 It should be noted that the entry of several highly pathogenic viruses (such as SARS-Cov and Ebola virus) into host cells depends on cathepsin L to facilitate the fusion of viral envelope and cellular membrane after viral attachment.49 Thus, small molecules to prevent cathepsin L cleavage would be anticipated to ameliorate severe respiratory viral infection.49
There are also agents targeted to the uncoating process.50 This process of influenza requires coordinated action of the viral proteins M2 and M1.50 M2 is a tetramer presented in virions possessing selective H+ ion-channel activity that allows for the uncoating of virus particles in the endosome.50 51 There are two commercially available M2 inhibitors, amantadine and rimantadine, which have been widely used in the treatment of both human and avian influenza infections for many years. Unfortunately, most of the current human and avian influenza viruses are amantadine- or rimantadine-resistant due to drug overuse.52 53 54 However, Wang et al offer a glimmer of hope to the development of M2 inhibitors.55 They discovered a novel M2 proton channel inhibitor which is effective against both the wild-type and the most prevalent drug-resistant mutant (S31N) from influenza A virus.
Viral Replication Inhibitor
As RNA polymerase is essential for the replication of RNA viruses, an inhibitor specific for viral RNA polymerase would exhibit antiviral activity and does not affect mRNA synthesis or protein translation of the host cells.56 Favipiravir (T705) is one of these agents; it can selectively inhibit the viral RNA polymerase of several influenza strains, respiratory syncytial virus, and Ebola virus.57 58 Moreover, favipiravir was effective in protecting mice from the lethal infection of highly pathogenic influenza H5N1 virus.59 It is now in phase II clinical trials in the United States. Novel compounds (such as THC19, ASN2, etc.) have been developed to inhibit the polymerase of influenza viruses.60 Moreover, inhibitors such as BCX4430, ALS-8176, and JMN3–003 exhibit broad-spectrum antiviral activity against various respiratory viruses, including influenza viruses, respiratory syncytial virus, paramyxoviruses, coronaviruses, and bunyaviruses.61 62 63 That makes them particularly attractive to researchers.
The target of ribavirin is inosine 5′-monophosphate (IMP) dehydrogenase involved in viral RNA synthesis and cellular biosynthesis of GTP.64 Ribavirin exhibits efficient anti-H5N1 avian influenza activity in vitro with IC50 ranging from 6 to 22 μmol/L.65 Treatment with ribavirin at 75 mg·kg−1·d−1 (po, bid × 8 d) protected 70% of mice from lethal H5N1 infection.65 66 However, the clinical efficacy of ribavirin toward influenza was limited by the mode of delivery.67 Aerosolized ribavirin exhibits effective inhibitory activity against both influenza viruses and respiratory syncytial viruses in clinical observations, while orally administered ribavirin did not.67 Moreover, the clinical utility of ribavirin is restrained by the risk of hemolytic anemia and teratogenicity.64 A prodrug of ribavirin, viramidine, is anticipated to solve these problems.68 This prodrug has a similar antiviral activity as ribavirin but is less toxic. This drug is now in Phase III development for hepatitis C virus (HCV) treatment.68
Dihydroorotate dehydrogenase (DHODH) is a key enzyme essential for replication of both viral RNA and DNA by the biosynthesis of de novo pyrimidine to generate uracil.69 Inhibitors (such as compound A3, D282, etc.) targeting DHODH have been found to inhibit the replication of both RNA and DNA respiratory viruses (such as influenza virus and adenovirus).70 71
Proteases or proteinases of virus are also the attractive targets for the development of antiviral agents.7 72 The papain-like protease (PLP) is the nsp3 protein which takes part in the formation of the ORF1a polyprotein during the SARS-CoV replication.73 NSC158362, an nsp3-dependent protease inhibitor, has exhibited antireplication acitivity against SARS-CoV, but not for influenza virus in vitro.74 Helicases of virus are proteins to unpackage the duplex oligonucleotides into single strands.75 Bananin and SSYA10–001 are helicase inhibitors which have been confirmed to be efficient inhibitors against the SARS-CoV replication in vitro with IC50 values in the range 0.5 to 3 μM.76 77
Viral Assembly, Budding and Release Inhibitor
Since several respiratory viruses replicate their genome in the nucleus of infected cells, their newly synthesized vRNA, together with RNA polymerase, and nucleoprotein, in the form of vRNPs, have to export to the cytoplasm before assembly.78 79 This process depends on a series of pathways, such as a Crm1-dependent pathway.80 Leptomycin B is a potent inhibitor of Crm1-mediated export, and leptomycin B treatment can lead to a decrease in the export of influenza A vRNPs from the nucleus.80 However, leptomycin B is toxic in vivo, which limits its further therapeutic use.81 Verdinexor is a novel selective vRNP-exportation inhibitor targeted to the Crm-1 homolog exportin-1, and verdinexor effectively inhibits the replication of various influenza stains, including pandemic H1N1 influenza virus, highly pathogenic H5N1 and newly emerged H7N9 avian influenza viruses.81 Moreover, both prophylactic and therapeutic administration of verdinexor can effectively protect mice from influenza infections.81 Thus, verdinexor is a promising candidate which deserves further clinical investigation.
Nitazoxanide is an approved antiparasitic agent which can also prevent the HA transport of influenza virus to cell surface by interfering HA trafficking between endoplasmic reticulum and the Golgi complex.82 Moreover, nitazoxanide is confirmed to be a broad-spectrum antiviral against both RNA and DNA viruses in vitro.83 Currently, nitazoxanide is in clinical development for the treatment of influenza.
The influenza virus budding event is initiated by the deformation of the membrane caused by the clustering of HA and NA.79 Then, M1 binds to the cytoplasmic tails of HA and NA and recruits the vRNPs and M2 to form the interior structure of the emerging virion at the site of virus budding.79 Subsequently, M2 alters the membrane curvature at the neck of the budding virus to cause membrane scission and initiate the release of the progeny virion.79 Thus, M2 inhibitors mentioned above may also inhibit the budding of influenza virus. Cyclophilin inhibitors such as Cyclosporin A can also inhibit the propagation of influenza virus by interfering the assembly or budding of influenza virus besides interfering with viral RNA synthesis in A549 cells.84 85
In the final release process of influenza virus, NA enables progeny virus to be cleaved from its receptor and spread to other cells.86 Thus, the NA inhibitors can prevent the influenza from spreading further by blocking the release of progeny virion.87 Four commercial NA inhibitors (oseltamivir, zanamivir, peramivir, and laninamivir) have been approved for use in humans.2 Among these NA inhibitors, laninamivir has an extremely long persisting time in the lungs, increasing the prospect of the long-acting antiviral that can effectively prevent influenza infection with a single dose.88 The emergence of NA-inhibitor-resistant influenza strains can be a substantial challenge to antiviral therapy in the future.89 Although there is no direct evidence to confirm that the development of resistance have correlations to the wide use of oseltamivir, the resistant viruses would acquire more chances of transmissibility under drug-selection pressure compared with the drug-sensitive strains.11
Signal-Transduction Inhibitors
Aside from agents that directly target viral proteins, current antiviral strategies also focus on the intracellular pathways essential for viral replication.90 91 The NF-κB, Raf/MEK/ERK, PI3K/Akt, or PKC signaling pathways might be promising targets for antiviral approaches.90 91 92 93 Since these pathways are also critical for cytokine and interferon synthesis during viral infection, inhibitors targeting these cascades may inhibit not only viral replication, but also the subsequent inflammation of viral infection.90 91 Moreover, compounds for these targets have an extremely low risk of emergence of drug-resistant variants.90 91 92 93 Thus, inhibitors of intracellular pathways are promising candidates as treatment against respiratory viruses, and further development should be pursued.
Herbs
Herbs may also be a potential choice for patients hospitalized with respiratory viruses. Several Chinese herbal prescriptions were recommended and authorized by the Chinese government during the 2004 SARS-CoV, 2009 H1N1 influenza, and 2013 H7N9 avian influenza pandemics.94 95 These herbal prescriptions commonly contain herbal medicines including Isatis tinctoria L., Lonicera japonica Thunb., Saposhnikovia divaricata (Turcz) Schischek, Bupleurum chinense DC., Forsythia suspensa (Thunb.) Vahl, and Citrus reticulata Blanco,94 95 which are considered to have heat-clearing and detoxifying properties according to the theoretical system of Traditional Chinese Medicine. The antiviral mechanism of these herbal medicines has not been fully elucidated; however, some data suggest that the active components exhibit actual antiviral activity.96 97 98 99 Additionally, we have reported that extracts from Jiawei-Yupingfeng-Tang, a traditional Chinese herbal formula, exhibit antirespiratory viral activity.100 We also have confirmed that a green tea–derived polyphenol, epigallocatechin gallate (EGCG) can inhibit influenza virus by its antioxidant activity.101 These data suggest that herbal medicine may be a reservoir for antiviral development. Nevertheless, the current confirmation of herbal therapy is still insufficient owing to a lack of data from randomized and controlled clinical trials.
Conclusion
Persistent outbreaks of respiratory viral infections all over the world suggest that respiratory viral pathogens continue to be the most important threats to human health. Because of the the similarity of clinical symptoms to respiratory viral infections, rapid and sensitive diagnoses are essential for early viral identification and subsequently cost-effective monitoring of antivirals in clinical trials. The automated and multiplex NAAT may help to solve these problems, and this method is anticipated to generalize its applications in the future. There are still many challenges and unanswered questions about the development of antivirals. Several potent agents have been developed to target viral proteins or cellular factors essential for virus replication. However, they generally have yet to pass through clinical trials to show antiviral evidence in humans. On the other hand, the existing antiviral therapies also face the challenges of drug resistance and nonspecific side effects. Therefore, there is an urgent need for novel drugs and combination therapies. Efforts should be made to design and develop new antivirals that specifically target the basic steps of viral life cycle, including attachment, endocytosis, replication, assembly, budding, release, and cellular processes. The efficacy, viral resistance, mutual effect, and toxicity of combined usage of antivirals need to be clarified.
Acknowledgments
We acknowledge research funding from the National Nature Science Foundation of China (No. 81403163 and 81402404) and Yi Chang Scientific and Technological Bureau (No. A14301–04 and A14301–10).
Footnotes
All three authors contributed equally to this work.
References
- 1.Jartti T, Jartti L, Ruuskanen O, Söderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med. 2012;18(3):271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 2.Liu Q, Liu D Y, Yang Z Q. Characteristics of human infection with avian influenza viruses and development of new antiviral agents. Acta Pharmacol Sin. 2013;34(10):1257–1269. doi: 10.1038/aps.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirst M, Astell C R, Griffith M. et al. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg Infect Dis. 2004;10(12):2192–2195. doi: 10.3201/eid1012.040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peiris M, Yam W C, Chan K H, Ghose P, Shortridge K F. Influenza A H9N2: aspects of laboratory diagnosis. J Clin Microbiol. 1999;37(10):3426–3427. doi: 10.1128/jcm.37.10.3426-3427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris M, Yuen K Y, Leung C W. et al. Human infection with influenza H9N2. Lancet. 1999;354(9182):916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 6.Su S, Bi Y, Wong G, Gray G C, Gao G F, Li S. Epidemiology, evolution and recent outbreaks of avian influenza viruses in China: A Review. J Virol. 2015;89(17):8671–8676. doi: 10.1128/JVI.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2015;89(17):8671–8676. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osterhaus A D New respiratory viruses of humans Pediatr Infect Dis J 200827(10, Suppl):S71–S74. [DOI] [PubMed] [Google Scholar]
- 9.Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17(3):295–300. doi: 10.1016/j.chom.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knipe D M, Howley P M. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. Fields virology. 6th ed. [Google Scholar]
- 11.Webster R G, Govorkova E A. Continuing challenges in influenza. Ann N Y Acad Sci. 2014;1323:115–139. doi: 10.1111/nyas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simões E A, DeVincenzo J P, Boeckh M. et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J Infect Dis. 2015;211 01:S1–S20. doi: 10.1093/infdis/jiu828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J D, Meng W, Wang X J, Wang H C. Broad-spectrum antiviral agents. Front Microbiol. 2015;6:517. doi: 10.3389/fmicb.2015.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambon M C, Stockton J D, Clewley J P, Fleming D M. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358(9291):1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 15.Henrickson K J Advances in the laboratory diagnosis of viral respiratory disease Pediatr Infect Dis J 200423(1, Suppl)S6–S10. [DOI] [PubMed] [Google Scholar]
- 16.Principi N, Esposito S. Antigen-based assays for the identification of influenza virus and respiratory syncytial virus: why and how to use them in pediatric practice. Clin Lab Med. 2009;29(4):649–660. doi: 10.1016/j.cll.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Ruiz M, Pedrosa-Corral I, Sanbonmatsu-Gámez S, Navarro-Marí M. Laboratory detection of respiratory viruses by automated techniques. Open Virol J. 2012;6:151–159. doi: 10.2174/1874357901206010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dundas N E, Ziadie M S, Revell P A. et al. A lean laboratory: operational simplicity and cost effectiveness of the Luminex xTAG™ respiratory viral panel. J Mol Diagn. 2011;13(2):175–179. doi: 10.1016/j.jmoldx.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edinger T O Pohl M O Stertz S Entry of influenza A virus: host factors and antiviral targets J Gen Virol 201495(Pt 2):263–277. [DOI] [PubMed] [Google Scholar]
- 20.Hedlund M, Aschenbrenner L M, Jensen K, Larson J L, Fang F. Sialidase-based anti-influenza virus therapy protects against secondary pneumococcal infection. J Infect Dis. 2010;201(7):1007–1015. doi: 10.1086/651170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls J M, Moss R B, Haslam S M. The use of sialidase therapy for respiratory viral infections. Antiviral Res. 2013;98(3):401–409. doi: 10.1016/j.antiviral.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss R B, Hansen C, Sanders R L, Hawley S, Li T, Steigbigel R T. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis. 2012;206(12):1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huentelman M J, Zubcevic J, Hernández Prada J A. et al. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44(6):903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 24.Danilczyk U, Penninger J M. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98(4):463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 25.Friesen R H, Koudstaal W, Koldijk M H. et al. New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets. PLoS ONE. 2010;5(2):e9106. doi: 10.1371/journal.pone.0009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagos R, DeVincenzo J P, Muñoz A. et al. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)-specific humanized monoclonal antibody, when administered to RSV-infected children. Pediatr Infect Dis J. 2009;28(9):835–837. doi: 10.1097/INF.0b013e3181a165e4. [DOI] [PubMed] [Google Scholar]
- 27.Han Y M, Seo H J, Choi S H. et al. Effect of Prophylactic Palivizumab on Admission Due to Respiratory Syncytial Virus Infection in Former Very Low Birth Weight Infants with Bronchopulmonary Dysplasia. J Korean Med Sci. 2015;30(7):924–931. doi: 10.3346/jkms.2015.30.7.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramilo O, Lagos R, Sáez-Llorens X. et al. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J. 2014;33(7):703–709. doi: 10.1097/INF.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 29.Hegele R G. Respiratory syncytial virus therapy and prophylaxis: have we finally turned the corner? Eur Respir J. 2011;38(2):246–247. doi: 10.1183/09031936.00012011. [DOI] [PubMed] [Google Scholar]
- 30.Plemper R K. Cell entry of enveloped viruses. Curr Opin Virol. 2011;1(2):92–100. doi: 10.1016/j.coviro.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosset F L, Lavillette D. Cell entry of enveloped viruses. Adv Genet. 2011;73:121–183. doi: 10.1016/B978-0-12-380860-8.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teissier E, Penin F, Pécheur E I. Targeting cell entry of enveloped viruses as an antiviral strategy. Molecules. 2011;16(1):221–250. doi: 10.3390/molecules16010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries E, Tscherne D M, Wienholts M J. et al. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011;7(3):e1001329. doi: 10.1371/journal.ppat.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Whittaker G R. Role of the actin cytoskeleton during influenza virus internalization into polarized epithelial cells. Cell Microbiol. 2007;9(7):1672–1682. doi: 10.1111/j.1462-5822.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 35.Ehrhardt C, Marjuki H, Wolff T. et al. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol. 2006;8(8):1336–1348. doi: 10.1111/j.1462-5822.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 36.Eierhoff T, Hrincius E R, Rescher U, Ludwig S, Ehrhardt C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6(9):e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Sieben C, Ludwig K. et al. pH-Controlled two-step uncoating of influenza virus. Biophys J. 2014;106(7):1447–1456. doi: 10.1016/j.bpj.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia N K, Guttman M, Ebner J L, Lee K K. Dynamic changes during acid-induced activation of influenza hemagglutinin. Structure. 2015;23(4):665–676. doi: 10.1016/j.str.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochiai H, Sakai S, Hirabayashi T, Shimizu Y, Terasawa K. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antiviral Res. 1995;27(4):425–430. doi: 10.1016/0166-3542(95)00040-s. [DOI] [PubMed] [Google Scholar]
- 40.Chen H W, Cheng J X, Liu M T. et al. Inhibitory and combinatorial effect of diphyllin, a v-ATPase blocker, on influenza viruses. Antiviral Res. 2013;99(3):371–382. doi: 10.1016/j.antiviral.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller K H, Kainov D E, El Bakkouri K. et al. The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections. Br J Pharmacol. 2011;164(2):344–357. doi: 10.1111/j.1476-5381.2011.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vigant F, Lee J, Hollmann A. et al. A mechanistic paradigm for broad-spectrum antivirals that target virus-cell fusion. PLoS Pathog. 2013;9(4):e1003297. doi: 10.1371/journal.ppat.1003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf M C, Freiberg A N, Zhang T. et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci U S A. 2010;107(7):3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leneva I A, Russell R J, Boriskin Y S, Hay A J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Boriskin Y S, Leneva I A, Pécheur E I, Polyak S J. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15(10):997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 46.Burtseva E I, Shevchenko E S, Beliakova N V. et al. [Monitoring of the sensitivity of epidemic influenza virus strains isolated in Russia to etiotropic chemical agents] Vopr Virusol. 2009;54(5):24–28. [PubMed] [Google Scholar]
- 47.DeVincenzo J P, Whitley R J, Mackman R L. et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371(8):711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 48.Douglas J L, Panis M L, Ho E. et al. Small molecules VP-14637 and JNJ-2408068 inhibit respiratory syncytial virus fusion by similar mechanisms. Antimicrob Agents Chemother. 2005;49(6):2460–2466. doi: 10.1128/AAC.49.6.2460-2466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elshabrawy H A, Fan J, Haddad C S. et al. Identification of a broad-spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high-throughput screening assay. J Virol. 2014;88(8):4353–4365. doi: 10.1128/JVI.03050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu R, Liu L A, Wei D. Drug inhibition and proton conduction mechanisms of the influenza a M2 proton channel. Adv Exp Med Biol. 2015;827:205–226. doi: 10.1007/978-94-017-9245-5_13. [DOI] [PubMed] [Google Scholar]
- 51.Wei C, Pohorille A. M2 proton channel: toward a model of a primitive proton pump. Orig Life Evol Biosph. 2015;45(1–2):241–248. doi: 10.1007/s11084-015-9421-x. [DOI] [PubMed] [Google Scholar]
- 52.Ilyushina N A, Govorkova E A, Webster R G. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology. 2005;341(1):102–106. doi: 10.1016/j.virol.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y, Hu B, Wen X. et al. Evolution analysis of the matrix (M) protein genes of 17 H9N2 chicken influenza viruses isolated in northern China during 1998-2008. Virus Genes. 2009;38(3):398–403. doi: 10.1007/s11262-009-0339-0. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC) . Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(16):433–435. [PubMed] [Google Scholar]
- 55.Wang J, Ma C, Wang J. et al. Discovery of novel dual inhibitors of the wild-type and the most prevalent drug-resistant mutant, S31N, of the M2 proton channel from influenza A virus. J Med Chem. 2013;56(7):2804–2812. doi: 10.1021/jm301538e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez-Frandsen A, Alfonso R, Nieto A. Influenza virus polymerase: Functions on host range, inhibition of cellular response to infection and pathogenicity. Virus Res. 2015;209:23–38. doi: 10.1016/j.virusres.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Furuta Y, Gowen B B, Takahashi K, Shiraki K, Smee D F, Barnard D L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata T, Lefor A K, Hasegawa M, Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep. 2015;9(1):79–81. doi: 10.1017/dmp.2014.151. [DOI] [PubMed] [Google Scholar]
- 59.Kiso M, Takahashi K, Sakai-Tagawa Y. et al. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci U S A. 2010;107(2):882–887. doi: 10.1073/pnas.0909603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada K, Koyama H, Hagiwara K. et al. Identification of a novel compound with antiviral activity against influenza A virus depending on PA subunit of viral RNA polymerase. Microbes Infect. 2012;14(9):740–747. doi: 10.1016/j.micinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Warren T K, Wells J, Panchal R G. et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508(7496):402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang G, Deval J, Hong J. et al. Discovery of 4′-chloromethyl-2′-deoxy-3′,5′-di-O-isobutyryl-2′-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J Med Chem. 2015;58(4):1862–1878. doi: 10.1021/jm5017279. [DOI] [PubMed] [Google Scholar]
- 63.Krumm S A, Ndungu J M, Yoon J J. et al. Potent host-directed small-molecule inhibitors of myxovirus RNA-dependent RNA-polymerases. PLoS ONE. 2011;6(5):e20069. doi: 10.1371/journal.pone.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brochot E, François C, Castelain S. et al. A new tool to study ribavirin-induced haemolysis. Antivir Ther. 2012;17(7):1311–1317. doi: 10.3851/IMP2308. [DOI] [PubMed] [Google Scholar]
- 65.Sidwell R W, Bailey K W, Wong M H, Barnard D L, Smee D F. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antiviral Res. 2005;68(1):10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Ilyushina N A, Hay A, Yilmaz N, Boon A C, Webster R G, Govorkova E A. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother. 2008;52(11):3889–3897. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith C B, Charette R P, Fox J P, Cooney M K, Hall C E. Lack of effect of oral ribavirin in naturally occurring influenza A virus (H1N1) infection. J Infect Dis. 1980;141(5):548–554. doi: 10.1093/infdis/141.5.548. [DOI] [PubMed] [Google Scholar]
- 68.Gish R G. Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006;57(1):8–13. doi: 10.1093/jac/dki405. [DOI] [PubMed] [Google Scholar]
- 69.Evans D R, Guy H I. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem. 2004;279(32):33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann H H, Kunz A, Simon V A, Palese P, Shaw M L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Natl Acad Sci U S A. 2011;108(14):5777–5782. doi: 10.1073/pnas.1101143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smee D F, Hurst B L, Day C W. D282, a non-nucleoside inhibitor of influenza virus infection that interferes with de novo pyrimidine biosynthesis. Antivir Chem Chemother. 2012;22(6):263–272. doi: 10.3851/IMP2105. [DOI] [PubMed] [Google Scholar]
- 72.Graham A C, Temple R M, Obar J J. Mast cells and influenza a virus: association with allergic responses and beyond. Front Immunol. 2015;6:238. doi: 10.3389/fimmu.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frieman M, Basu D, Matthews K. et al. Yeast based small molecule screen for inhibitors of SARS-CoV. PLoS ONE. 2011;6(12):e28479. doi: 10.1371/journal.pone.0028479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adedeji A O, Sarafianos S G. Antiviral drugs specific for coronaviruses in preclinical development. Curr Opin Virol. 2014;8:45–53. doi: 10.1016/j.coviro.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valiente-Echeverría F, Hermoso M A, Soto-Rifo R. RNA helicase DDX3: at the crossroad of viral replication and antiviral immunity. Rev Med Virol. 2015;25(5):286–299. doi: 10.1002/rmv.1845. [DOI] [PubMed] [Google Scholar]
- 76.Tanner J A, Zheng B J, Zhou J. et al. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem Biol. 2005;12(3):303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adedeji A O, Singh K, Kassim A. et al. Evaluation of SSYA10-001 as a replication inhibitor of severe acute respiratory syndrome, mouse hepatitis, and Middle East respiratory syndrome coronaviruses. Antimicrob Agents Chemother. 2014;58(8):4894–4898. doi: 10.1128/AAC.02994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu G, Xiang Y, Guo C, Pei Y, Wang Y, Kitazato K. Cofilin-1 is involved in regulation of actin reorganization during influenza A virus assembly and budding. Biochem Biophys Res Commun. 2014;453(4):821–825. doi: 10.1016/j.bbrc.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 79.Rossman J S, Lamb R A. Influenza virus assembly and budding. Virology. 2011;411(2):229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chase G P, Rameix-Welti M A, Zvirbliene A. et al. Influenza virus ribonucleoprotein complexes gain preferential access to cellular export machinery through chromatin targeting. PLoS Pathog. 2011;7(9):e1002187. doi: 10.1371/journal.ppat.1002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perwitasari O, Johnson S, Yan X. et al. Verdinexor, a novel selective inhibitor of nuclear export, reduces influenza a virus replication in vitro and in vivo. J Virol. 2014;88(17):10228–10243. doi: 10.1128/JVI.01774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossignol J F, La Frazia S, Chiappa L, Ciucci A, Santoro M G. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284(43):29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rossignol J F. Thiazolides: a new class of antiviral drugs. Expert Opin Drug Metab Toxicol. 2009;5(6):667–674. doi: 10.1517/17425250902988487. [DOI] [PubMed] [Google Scholar]
- 84.de Wilde A H, Li Y, van der Meer Y. et al. Cyclophilin inhibitors block arterivirus replication by interfering with viral RNA synthesis. J Virol. 2013;87(3):1454–1464. doi: 10.1128/JVI.02078-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamamoto I, Harazaki K, Inase N, Takaku H, Tashiro M, Yamamoto N. Cyclosporin A inhibits the propagation of influenza virus by interfering with a late event in the virus life cycle. Jpn J Infect Dis. 2013;66(4):276–283. doi: 10.7883/yoken.66.276. [DOI] [PubMed] [Google Scholar]
- 86.Gasparini R, Amicizia D, Lai P L, Bragazzi N L, Panatto D. Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part II: Future compounds against influenza virus. J Prev Med Hyg. 2014;55(4):109–129. [PMC free article] [PubMed] [Google Scholar]
- 87.Varghese J N, Smith P W, Sollis S L. et al. Drug design against a shifting target: a structural basis for resistance to inhibitors in a variant of influenza virus neuraminidase. Structure. 1998;6(6):735–746. doi: 10.1016/s0969-2126(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 88.Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubo S. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother. 2009;53(1):186–192. doi: 10.1128/AAC.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dharan N J, Gubareva L V, Meyer J J. et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301(10):1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 90.Planz O. Development of cellular signaling pathway inhibitors as new antivirals against influenza. Antiviral Res. 2013;98(3):457–468. doi: 10.1016/j.antiviral.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Ludwig S. Targeting cell signalling pathways to fight the flu: towards a paradigm change in anti-influenza therapy. J Antimicrob Chemother. 2009;64(1):1–4. doi: 10.1093/jac/dkp161. [DOI] [PubMed] [Google Scholar]
- 92.Droebner K, Pleschka S, Ludwig S, Planz O. Antiviral activity of the MEK-inhibitor U0126 against pandemic H1N1v and highly pathogenic avian influenza virus in vitro and in vivo. Antiviral Res. 2011;92(2):195–203. doi: 10.1016/j.antiviral.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Mazur I, Wurzer W J, Ehrhardt C. et al. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol. 2007;9(7):1683–1694. doi: 10.1111/j.1462-5822.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 94.Guidelines for Management of Avian H7N9 Influenza Infection. 2013. http://www.moh.gov.cn/ewebeditor/uploadfile/2013/04/20130410212136993.doc http://www.moh.gov.cn/ewebeditor/uploadfile/2013/04/20130410212136993.doc
- 95.Guidelines for Management of Pandemic . (H1N1) 2009 Influenza. 2009. http://www.moh.gov.cn/mohwsyjbgs/s9990/200910/43111.shtml http://www.moh.gov.cn/mohwsyjbgs/s9990/200910/43111.shtml
- 96.Shang X, Pan H, Li M, Miao X, Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2011;138(1):1–21. doi: 10.1016/j.jep.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.WHO monographs on selected medicinal plants. 1999. http://apps.who.int/medicinedocs/en/d/Js2200e/ http://apps.who.int/medicinedocs/en/d/Js2200e/
- 98.Li C, Dai Y, Zhang S X. et al. Quinoid glycosides from Forsythia suspensa. Phytochemistry. 2014;104:105–113. doi: 10.1016/j.phytochem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 99.Xu J J, Wu X, Li M M. et al. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of citrus reticulata ‘Chachi’. J Agric Food Chem. 2014;62(10):2182–2189. doi: 10.1021/jf404310y. [DOI] [PubMed] [Google Scholar]
- 100.Liu Q, Lu L, Hua M. et al. Jiawei-Yupingfeng-Tang, a Chinese herbal formula, inhibits respiratory viral infections in vitro and in vivo. J Ethnopharmacol. 2013;150(2):521–528. doi: 10.1016/j.jep.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 101.Ling J X, Wei F, Li N. et al. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacol Sin. 2012;33(12):1533–1541. doi: 10.1038/aps.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]


