Abstract
Adenoviruses (AdVs) are DNA viruses that typically cause mild infections involving the upper or lower respiratory tract, gastrointestinal tract, or conjunctiva. Rare manifestations of AdV infections include hemorrhagic cystitis, hepatitis, hemorrhagic colitis, pancreatitis, nephritis, or meningoencephalitis. AdV infections are more common in young children, due to lack of humoral immunity. Epidemics of AdV infection may occur in healthy children or adults in closed or crowded settings (particularly military recruits). The disease is more severe and dissemination is more likely in patients with impaired immunity (e.g., organ transplant recipients, human immunodeficiency virus infection). Fatality rates for untreated severe AdV pneumonia or disseminated disease may exceed 50%. More than 50 serotypes of AdV have been identified. Different serotypes display different tissue tropisms that correlate with clinical manifestations of infection. The predominant serotypes circulating at a given time differ among countries or regions, and change over time. Transmission of novel strains between countries or across continents and replacement of dominant viruses by new strains may occur. Treatment of AdV infections is controversial, as prospective, randomized therapeutic trials have not been conducted. Cidofovir is the drug of choice for severe AdV infections, but not all patients require treatment. Live oral vaccines are highly efficacious in reducing the risk of respiratory AdV infection and are in routine use in the military in the United States, but currently are not available to civilians.
Keywords: adenovirus, respiratory viral infections, serotypes, cidofovir
Adenovirus
In 2011, we published a comprehensive review of adenovirus (AdV) infections in this journal1; this article updates new developments since that review. AdVs most often infect the upper or lower respiratory tracts, conjunctiva, or gastrointestinal (GI) tract.1 2 3 4 More than 80% of diagnosed AdV infections occur in children < 4 years old (due to lack of humoral immunity).2 3 5 6 Immunosuppressed persons2 7 8 9 are more susceptible.3 10 11 12 13 14 15 High baseline immunity against AdV (IgG titer of ≥ 1:32) confers substantial protection.16 AdV infections may occur in healthy children3 10 11 12 13 or adults in closed or crowded settings (particularly military recruits).17 18 19 20 21 The vast majority of cases are self-limited. However, the clinical spectrum is broad, and dissemination or pneumonia can be fatal, both in immunocompetent22 23 and immunocompromised patients.2 9 24 25 26 27 28
Virology
Human AdVs are a group of double-stranded nonenveloped DNA viruses belonging to the genus Mastadenovirus of the Adenoviridae family.29 30 Currently, 51 serotypes, and over 70 genotypes defined by bioinformatics analysis of complete genomic sequences and designated with consecutive numbers (52, 53, 54, etc.) have been described and classified within 7 species (HAdV-A through HAdV-G).31 32 33 34 35 36 37 Species A, B, C, D, E, and F circulate globally, and have been implicated in outbreaks of infection in humans.1 Different genome types (or genomic variants) can be distinguished within the same serotype by restriction enzyme analysis of genomic DNA.38 39 40 Approximately one-third of the described serotypes are associated with human disease.24 26 29 31 41 42 43 44 Different serotypes display different tissue tropisms that correlate with clinical manifestations of infection2 26 31 33 (discussed in detail in the next sections).
Epidemiology
AdVs may cause epidemics of febrile respiratory illness (FRI), pharyngoconjunctival fever,45 keratoconjunctivitis (KC),46 47 48 49 or gastroenteritis and diarrheal illness.50 51 52 53 54 55 56 57 58 59 60 61 Severe or disseminated AdV infections may occur in immunocompromised hosts7 9 62 63 64 and rarely in immunocompetent patients.23 65
Most epidemics occur in the winter or early spring,6 but infections occur throughout the year with no clear seasonality.2 Infection can result from exposure to infected individuals (inhalation of aerosolized droplets, conjunctival inoculation, fecal oral spread),1 2 66 67 acquisition from exogenous sources (e.g., pillows, linens, lockers, guns),68 69 or reactivation.2 26 Incubation period ranges from 2 to 14 days.2 Importantly, latent AdV may reside in lymphoid tissue,7 70 renal parenchyma,71 or other tissues for years; reactivation may occur in severely immunosuppressed patients.7 70 71 Asymptomatic carriage of AdV may persist for weeks or months.31 72 73 Epidemics may spread rapidly among closed populations16 17 20 33 40 44 68 74 75 76 (e.g., hospitals,6 67 77 neonatal nurseries,78 psychiatric77 79 or long-term care facilities,48 66 80 job training centers,21 boarding schools or dormitories,81 a children's home,82 orphanages,83 public swimming pools84 85). In institutionalized settings, infection control measures and cohorting may be essential to limit spread.66 67 86 AdV is resistant to many disinfectants87 but 95% ethanol solution is an effective disinfectant.73
Clinical Features of Adenovirus Infection
Respiratory Tract Involvement
AdV accounts for at least 5 to 10% of pediatric and 1 to 7% of adult respiratory tract infections (RTIs).2 31 Typical symptoms of AdV RTI include fever, pharyngitis, tonsillitis, cough, and sore throat.3 19 GI symptoms may be present concomitantly, particularly in children.3 13 19 88 In immunocompetent patients, symptoms usually abate spontaneously (within 2 weeks) and infection induces type-specific immunity.2 Pneumonia occurs in up to 20% of newborns and infants,3 10 12 88 89 but is uncommon in immunocompetent adults.2 16 17 77 79 90 91 However, fatalities due to AdV pneumonia have been described in previously healthy children10 or adults.19 23 65 79 90 In immunocompromised persons, dissemination and/or severe respiratory failure develop in 10 to 30% of cases2 9 27 38 and fatality rates for severe AdV pneumonia may exceed 50%2 9 90 (Fig. 1).
Fig. 1.
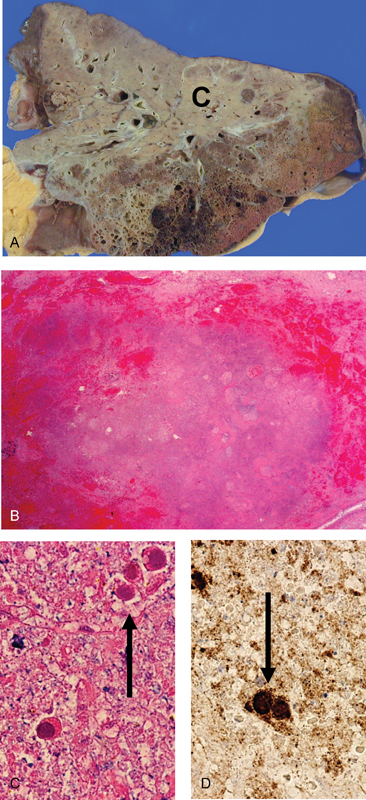
Fatal case of adenovirus pneumonia. (A) Gross lung with pale, consolidated region, C. (B) Histopathology showing hemorrhagic necrotic lung tissue (hematoxylin and eosin [H & E] stain ×40. (C) High magnification showing three cells with intranuclear inclusions (arrow) (H & E) stain ×400. (D) Immunohistochemical staining for adenovirus showing positive staining of the intranuclear inclusions in two cells (arrow) (immunoperoxidase ×400). (Reproduced with permission from Lynch et al.1)
In children, long-term respiratory sequela of AdV RTI include bronchiectasis, bronchiolitis obliterans, and hyperlucent lung.92 93 94 AdVs have a propensity to establish latent or persistent infection within the upper95 and lower respiratory tracts.96 Persistent AdV infection in children may elicit chronic neutrophilic inflammation within the airways, protracted bacterial bronchitis and bronchiectasis.97 98 99 HAdVs (particularly types 1–5, 7, 14, and 21) have been associated with small airways dysfunction96 and bronchiectasis in children94 98 and chronic obstructive pulmonary disease in adults.100 101 These various studies suggest that HAdV is not an innocent bystander in the lower airways, but may play a role in the pathogenesis of chronic suppurative endobronchial and lung disease.
Keratoconjunctivitis
Manifestations of ocular AdV infection include: epidemic KC (EKC), pharyngoconjunctival fever, and nonspecific conjunctivitis.49 102 103 104 105 106 The most common serotypes associated with EKC are AdV-8, -19, and -37,49 103 105 106 107 108 109 110 111 112 but other serotypes (e.g., AdV-3, -4, -7, -11, and -14) can also cause conjunctivitis.46 47 105 106 108 113 114 Outbreaks of EKC can occur in hospitals or outpatient clinics,102 103 115 chronic care facilities,66 116 and closed settings.117 Nosocomial transmission has been noted in eye clinics or hospitals via environmental contamination (ophthalmic instruments, eyedrops).103 115 118 Rigorous sterilization of instruments and strict infection control were essential to curb epidemics.103 115 The recently described genotypes 53, 54, and 56 of species HAdV-D have been reported in association with outbreaks of EKC.119 120 121 122 123 124
Gastrointestinal Manifestations
AdV infections can cause GI symptoms even when the primary site of involvement is the respiratory tract (particularly in young children).3 13 88 125 Some serotypes (notably AdV-40 and -41) have an affinity for the GI tract,50 53 54 57 with predominant symptoms of gastroenteritis or diarrhea.126 Rare complications include hemorrhagic colitis,2 27 127 hepatitis,27 128 129 130 131 cholecystitis,132 and pancreatitis.133 134
Urinary Tract Involvement
AdV may cause urinary tract infections (UTIs),135 particularly among hematopoietic stem cell transplant (HSCT)71 136 137 138 139 and solid organ transplant (SOT) recipients.140 141 142 143 Typical manifestations include dysuria, hematuria, hemorrhagic cystitis (HC), and renal allograft dysfunction.141 142 144 145 Most AdV UTIs (including HC) are self-limiting43 71 140 144 but fatal or dialysis-dependent renal failure,146 147 148 fatal dissemination,149 150 necrotizing tubulointerstitial nephritis,148 151 or obstructive uropathy151 have been described. Most common serotypes associated with HC include: AdV-11, -34, -35, -3, -7, and -21.2 142 144 148 The diagnosis may be confirmed by culture or polymerase chain reaction (PCR) in urine, or serology.2 137 142 Renal biopsy may demonstrate viral infection of tubular epithelial cells, with “smudge cells” and intranuclear inclusions.147 148 AdV urethritis has also been described.152
Disseminated Disease
Disseminated AdV infections are rare among immunocompetent hosts, but dissemination occurs in 10 to 30% of HSCT recipients with AdV infection.2 25 26 38 153 154 155 Diagnosis is made by PCR in blood150 and/or detection (or recovery) of AdV from more than one site. Among HSCT recipients with symptomatic AdV disease, fatality rates range from 12 to 70%.25 153 156 157 158 Case fatality rates for AdV pneumonia may exceed 50%.27 90
Rare Manifestations
Rare manifestations of AdV infections include: encephalitis159 160 161 162 163; meningitis162 164 165; myocarditis and cardiomyopathy166 167;mononucleosis-like syndromes168; pulmonary dysplasia169; intestinal intussusception in children170; sudden infant death.171
Specific Patient Populations at Risk
Adenovirus Infections in Immunocompetent Persons
Epidemics of AdV respiratory infection may occur in healthy children (particularly < 4 years old)3 10 11 12 13 172 or adults in closed settings (particularly the military).17 19 20 21 173 The vast majority of cases are self-limited; disseminated and fatal infections are rare in immunocompetent hosts.19 90
Adenovirus Infections in Military Recruits
AdV accounts for > 50% of FRI and pneumonia cases among unvaccinated military recruits,16 17 20 33 68 69 173 not only in the United States19 40 74 but globally.44 75 Military recruits are especially vulnerable during basic training, owing to crowding and stresses.19 In a survey of eight military training sites in the United States from 2004 to 2009, > 21,000 cases of FRI or pneumonia were detected; AdV was implicated in 63.6%; influenza, in only 6.6%.76 Peak illness rates occur during weeks 3 to 5 of training.20 In a prospective study of 271 new military recruits in training, 25% developed an acute FRI due to AdV-4 over a 6-week period; all FRIs occurred among recruits with an initial AdV antibody titer of < 1:4.69 Serum antibodies to AdV-4 were present in 34% at enrollment, and 97% by 6 weeks.69 Following completion of basic training, recruits are dispersed to secondary sites, paving the way for epidemic spread.86 Historically, serotypes AdV-7 and -4 predominated as a cause of FRIs in the military in the United States.16 17 40 Beginning in 1971, all recruits in the United States military were vaccinated with live enteric-coated AdV-4 and -7 vaccines.174 Following this strategy, the incidence of AdV infections in the military setting plummeted.174 In 1995, the sole manufacturer of the AdV vaccines ceased production; existing supplies were completely depleted by 1999.19 In 1996, the last year AdV vaccines were given to recruits year round, AdV-21 was the most prevalent type, implicated in 58% of AdV infections; AdV-4 and -7 were each implicated in only 4%.175 The lack of availability of vaccines led to re-emergence of epidemics of AdV infections in military facilities in the United States.19 20 40 74 176 177 178 Surveillance of U. S. recruits in training cited > 73,000 AdV infections from 1999 to 2004; serotype 4 accounted for > 95% of AdV infections.20 In a large surveillance study of eight military recruit training centers in the United States from 2000 to 2011, AdV-4 was implicated in 80% of AdV infections; the remaining 20% comprised AdV-14, -21, -3, and -7.175 In 2006 and 2007, a novel strain of AdV-14 emerged as a cause of FRIs in recruits at a U. S. Air Force base,33 and became the predominant strain in the military.
Beginning in October 2011, after a 12-year hiatus, the administration of live nonattenuated oral vaccines against AdV-4 and -7 to U. S. military recruits was resumed.179 From 1996 to 2013, FRI surveillance was performed at eight military training centers in the United States.175 During the 2 years after reintroduction of the vaccine, AdV burden declined 100-fold (from 5.8 to 0.02 cases per 1,000 person weeks, p < 0.001).175 Although the percentage of type 14 increased following reintroduction of the vaccine, the mean annual number of AdV-14 infections decreased (from 610 in 2000 to 2011 to 44 in 2013).175 Continuing to vaccinate all incoming recruits will reduce cases among trainees, and may reduce transmission to other geographical locations and to civilians.175 Future surveillance studies will monitor AdV infection rates and pay attention to emergence of AdV types not targeted by the vaccines.
Hematopoietic Stem Cell Transplant Recipients
The incidence of AdV infections among HSCT recipients is highly variable (range, 3–47%).2 4 25 26 27 28 42 153 154 155 156 180 181 182 183 184 The incidence is much higher among allogeneic (range, 5–47%)4 compared with autologous (range, 2.5–14%)185 186 187 HSCT recipients. Higher rates of AdV infections reflect prospective studies with regular (often weekly) sampling of plasma for AdV DNA (by PCR).153 188 The incidence is 2 to 3.5 times higher in children (> 20%) compared with < 10% in adults.38 181 182 189 190 Additional risk factors for AdV infections among HSCT recipients include: allogeneic HSCT4 38 182; graft versus host disease (GVHD)2 25 27 28 153 154 156 182 191; severe T-cell depletion28 38 191; human leukocyte antigen (HLA) mismatch.38 192 Infection can reflect primary infection (e.g., community or nosocomial acquisition)73 or reactivation of latent infection.70 73
AdV in HSCT recipients is usually detected within 100 days of transplant.38 193 The disease is usually localized (e.g., urinary tract, gastroenteritis, upper or lower respiratory tract) but dissemination occurs in 10 to 30% of cases.28 38 181 189 In this context, mortality rates are high.38 Among 76 adult HSCT recipients with symptomatic AdV infections, mortality rate was 26%.182 Mortality rates were higher among patients with pneumonia (73%) and disseminated disease (61%).182 Severe lymphopenia,2 38 severe GVHD,28 182 isolation from more than one site,38 and high AdV viral loads in plasma194 195 correlate with higher mortality. In one study of 123 consecutive pediatric allogeneic HSCT recipients, 12.3% developed symptomatic AdV infections.183 Overall survival was much worse in patients with AdV infections (15.4%) compared with noninfected subjects (50%; p < 0.03). In multivariate analysis, the most important risk factor for mortality was AdV infection (hazard ratio, 3.15; p < 0.001).183 However, prognosis may be good, particularly when the viral load is low. A retrospective study in pediatric HSCT recipients detected AdV in blood (by PCR) in 11/26 (42%); viremia cleared in 7 (63%) without antiviral therapy.43 In another study of 116 adult HSCT recipients who had weekly screening for AdV in blood by PCR, 14 (12.1%) developed AdV viremia.193 Only five were treated with cidofovir (CDV); only one died as a result of AdV infection. In another study of pediatric HSCT recipients, weekly sampling of plasma PCR identified 57 patients with AdV infections; 8 (14%) patients had disseminated disease. All 57 patients were treated with intravenous CDV; clinical and microbiological cure was achieved in 56 (98%). One patient died of AdV pneumonia.188 Quantification of AdV DNA load by real-time PCR in plasma of HSCT recipients may identify patients at high risk for dissemination189 194 or assess response to therapy.189 194 However, indications for, and duration of therapy, with CDV are controversial.
Solid Organ Transplant Recipients
The incidence of AdV infections among SOT recipients is 5 to 22%, usually within the first 6 months posttransplantation.2 4 38 156 196 197 AdV infections have been noted in liver,198 199 renal,140 142 146 200 201 202 heart,196 203 204 intestinal,205 206 and lung207 208 209 transplant recipients. Among SOT recipients, risk factors for AdV include: pediatric age4 38 198; donor-positive/recipient-negative AdV status38; receipt of antilymphocyte antibodies.38 In a prospective study, AdV viremia (by PCR) was detected within 12 months of transplant in 19/263 (7.3%) SOT recipients including: liver, 10/121 (8.3%); kidney, 6/92 (6.5%); heart, 3/45 (6.7%).196 At the time of viremia, 11 (58%) were asymptomatic. All recovered spontaneously without sequela. In a retrospective review of 484 pediatric liver transplant recipients, 49 (10%) developed AdV infections; 9 died of invasive AdV infection.198 In another retrospective review of 191 adult liver transplant recipients, 11 (5.8%) had AdV infection, and 2 AdV-associated deaths were documented.199 Clinical manifestations of AdV infection are protean, but the primary site of disease in SOT recipients is often related to the transplanted organ.38 210 In liver transplant recipients, AdV typically causes hepatitis, jaundice, and hepatomegaly.38 In renal transplant patients, HC is the principal symptom; further, AdV may target the renal allograft, leading to graft failure.142 146 200 In pediatric heart transplant recipients, the presence of AdV in posttransplant endomyocardial biopsies increased the risk for graft loss and posttransplant coronary artery disease.211 212 213 In a cohort of 383 lung transplant recipients (LTRs), only 4 AdV infections were identified; incidence was 3/40 (8%) among pediatric LTR and 1/268 (0.4%) among adult LTR.207 However, all four developed severe hemorrhagic, necrotizing AdV pneumonia; all died within 45 days of transplant. In another study of 19 pediatric LTR, 8 developed AdV, resulting in 2 early deaths, as well as late graft loss and obliterative bronchiolitis.200 A case of fatal AdV pneumonia in an adult LTR 4 years posttransplant was described.214 Although AdV can cause fatal infections in SOT recipients, indications for treatment with CDV for mild infections have not been established. AdV viremia may be asymptomatic, and may clear spontaneously.196 Routine PCR surveillance is not recommended in adult SOT recipients. Further, treatment (with CDV) should be reserved for symptomatic patients or those with pneumonia or disseminated infection.
Human Immunodeficiency Virus Infection
AdV infections occur in 12 to 28% of human immunodeficiency virus (HIV)-infected patients.215 216 In one prospective study of 63 HIV+ patients, 18 (28%) developed AdV infections within 1 year (17% if CD4 count was > 200/mm3 vs. 38% if the CD4 count was < 200/mm3).216 In Nigeria, 39% of 184 HIV-infected patients had serological evidence for AdV infection.217 The GI tract is involved in > 90%, but most patients are asymptomatic or have mild symptoms (e.g., diarrhea).216 UTIs occur in up to 20% of AIDS patients,218 but HC is rare.38 Serotype D is associated with GI infection whereas UTIs are usually caused by serotypes B or D.216 AdV (particularly serotypes 1 to 3) may cause fatal cases in HIV-infected patients.38 197 Since the availability of highly active antiretroviral therapy, AdV disease is uncommon in HIV/AIDS patients until immune system deterioration occurs.38
Congenital Immunodeficiency Syndromes
AdV infection may complicate congenital immunodeficiency disorders such as severe combined immunodeficiency syndrome, common variable immunodeficiency, agammaglobulinemia, immunoglobulin A deficiency, and others.38 64 197 219 In patients with severe immunodeficiency, AdV tends to cause severe and recurrent pulmonary infections, disseminated disease, and even death.38
Importance of Serotypes
Globally, serotypes 1 to 5, 7, 21, and 41 are most commonly associated with human disease (Table 1). Different serotypes display different tissue tropisms and clinical manifestations of infection.2 26 31 33 Among children, the most common AdV serotypes associated with RTI are types 1 to 7 and an intertypic recombinant H11F14 designated as genotype 55.31 220 In adults, serotypes most often implicated in FRI include: AdV-1 to 7, -21, and -14.10 16 17 18 24 33 40 41 75 221 222 AdV-55 was implicated in outbreaks of FRI in China,81 Singapore,44 the Middle East,223 United States,21 and South America.224 AdV-11 may cause UTIs or HC in children or transplant recipients.33 38 Other serotypes associated with HC include: AdV-7, -33, -34, and -35.33 225 AdV-8, -19, and -37 are frequent causative agents of KC.31 105 226 Gastroenteritis is most frequently associated with infection by enteric AdV-40 and -41,9 227 but has also been reported in association with AdV-12, -18, and -31,9 and AdV-5232 infection. AdV-5, -31, -34, -35, and -39 have been implicated in infections in immunocompromised patients43 51 180 220 228 (particularly HSCT2 43 128 229 230 or SOT142 231 recipients). Hepatitis has been reported associated with infection by serotypes 1 to 3, 5, and 7.38 225
Table 1. Adenovirus serotype according to geographic region.
| Country | 1 | 2 | 3 | 4 | 7 | 21 | 41 |
|---|---|---|---|---|---|---|---|
| United States (2004–2007) (civilians)161 | 17.7% | 24.3% | 34.6% | 4.8% | 3.0% | 2.0% | 1.7% |
| United States (2004–2007) (military)161 | NA | NA | 2.6% | 92.8% | NA | 2.4% | NA |
| Toronto (2007–2008)5 | 18% | 26% | 46% | 4.8% | NA | 5.5% | NA |
| Korea (1991–2007)31 | 9.2% | 11.2% | 37% | 3.9% | 23.3% | NA | NA |
| Taiwan (1981–1989)11 | NA | 6% | 68% | 0% | 3% | NA | NA |
| Taiwan (2000)11 | NA | 6% | 36% | 28% | 21% | NA | NA |
| Taiwan (2001)11 | NA | 15% | 2% | 52% | 1% | NA | NA |
| Taiwan (2004–2005)3 | 4.1% | 6.4% | 87.2% | 0.6% | NA | NA | NA |
| United Kingdom (1982–1996)248 | 12.1% | 18.6% | 14.9% | NA | NA | NA | 10.9% |
Abbreviation: NA, not applicable.
Source: Reproduced with permission from Lynch et al.1
Molecular Characterization of Adenovirus
Different genome types within serotypes have been identified by restriction enzyme analysis,39 40 232 multiplex PCR techniques targeting fiber genes or hexon genes233 or sequencing of the fiber genes29 234 and hexon genes.29 31 235 The widely used genome typing system was proposed and modified by Li et al.12 236 The prototype AdV strain is designated “p”; other genome types within the serotype are designated “a” through “k” based on their distinct BamHI digestion profiles. Genome types may be further distinguished by restriction pattern with additional selected enzymes (e.g., AdV-7p, AdV-7p1, etc.).12 40 80 This system has been used to correlate intraserotypic genetic variability with geographic distribution and pathogenic potential.40
Whole Genome Sequencing and Designation of Viruses Described by Bioinformatics Analysis of Complete Genomic Sequences
Rapidly advancing sequencing technologies at affordable costs have allowed relatively easy access to complete genomic sequence data for human AdV strains expanding the information on the genetic makeup of several viruses of medical importance and contributing to a better understanding of AdV evolution.35 237 238 239 240
Novel genomes representing cases of intertypic recombination or viruses with truly novel hexon, penton base or fiber genes have been under consideration as candidate new types and designated with numbers consecutive to the original set of 51 used to designate HAdV serotypes. The criteria for designation remain a matter of active debate.241
Global Epidemiology
The predominant serotypes detected in association with disease differ among different countries or regions, and change over time.3 12 31 40 86 242 243 244 245 Transmission of novel strains between countries or across continents and replacement of dominant serotypes by new strains may occur.33 246
Serotypes 1 to 7 account for > 80% of AdV infections in infants and children.31 247 The most common serotypes reported in the United States,161 Canada,5 the United Kingdom,248 Taiwan,11 and South Korea31 are displayed in Table 1. Striking differences in distribution of serotypes have been noted in civilian and military populations161 (Table 1).
In South America, AdV-7 has been a predominant strain associated with RTI requiring hospitalization in many countries.10 224 In Brazil, AdV-7 was the predominant serotype for decades, but an outbreak of AdV-3 occurred in 2000.10 In Asia, AdV-3 and -7 have been the predominant serotypes associated with RTI in children.3 11 12 13 249
Documented changes in relative prevalence of serotypes and genomic variants among geographic regions underscore the potential for new strains to emerge and replace existing strains.10 11 12 40 65 244 246 250 251 252 For interested readers, we discussed the epidemiology and temporal changes in circulating genomic variants globally in greater detail in a review in 2011.1
Epidemiology and Characteristics of Specific Serotypes
Given the large number of AdV serotypes, a discussion of each serotype is beyond the scope of this review. However, we will discuss a few of the commonly detected serotypes (e.g., AdV-1, -2, -3, -4, -7, and -21), additional serotypes associated with specific clinical syndromes (e.g., AdV-8, -37, -40, -41, and -55) and the recent emergence of AdV-14 in the United States.
Adenovirus Serotypes 1 and 2
Serotypes AdV-1 and -2 (both species C) are common causes of acute FRI worldwide, but appear to be less virulent than AdV-711 224 246 or -3.88 224 However, a nosocomial outbreak of severe pneumonia in immunocompetent hosts due to AdV-1 was recently described in France.253 The prevalence of AdV-1 and -2 varies among different geographic regions and populations. In the United States (2004–2006), AdV-1 and -2 accounted for 17.6 and 24.3% of AdV clinical respiratory isolates among civilians (children or adults), respectively, but only 0.4 and 0.4% among military recruits.161 The prevalence of these serotypes at other sites is variable: that is, Toronto, Canada (2007–2008), AdV-1 (18%); AdV-2 (26%)5; United Kingdom (1982–1996), AdV-1 (12.1%); AdV-2 (18.6%)248; Buenos Aires (1984–1988); AdV-1 (10%); AdV-2 (20%)246; Seoul, Korea (1990–98); AdV-1 (9.2%); AdV-2 (11.2%).88
Adenovirus Serotype 3
Globally, AdV-3 is among the most common serotypes implicated in AdV infections in children and adults.3 84 161 251 AdV accounted for 13% of AdV respiratory isolates reported to the World Health Organization from 1967 to 197684 and remains a cause of endemic and epidemic infections3 5 19 161 248 (Table 1). In the United States and southern Ontario from 2004 to 2006, AdV-3 accounted for 34.6% of AdV RTI in civilians but only 2.6% among military trainees.161 The prevalence of AdV-3 at other sites is variable: that is, Toronto, Canada (2007–2008), (46%)5; United Kingdom (1982–1996), (14.9%)248; Seoul, Korea (1990–1998), (15%)88; Seoul, Korea (1991–2007), (37.0%).31 In Taiwan, AdV-3 was the predominant serotype in 1981–1989 (68%) and 1990–1998 (44%) but decreased to 2% of respiratory isolates in 2001 (largely replaced by AdV-4 and -711). During an outbreak of respiratory AdV infections in children from November 2004 to February 2005 in Taiwan, AdV-3 was implicated in 87.5% of the cases.3 AdV-3 may cause fatal pneumonias in immunocompetent children249 254 and adults.65 AdV-3 and a recombinant strain of AdV-3/7 were responsible for an outbreak of FRIs (including two fatalities) in children in Portugal in 2004.254
Adenovirus Serotype 4
AdV-4 is a cause of sporadic infections in civilians5 and has been implicated in epidemic outbreaks of FRI or pneumonia in civilian11 255 and military18 20 74 177 populations. In civilian populations, AdV-4 was implicated in 4.8% of AdV RTI in the United States (2004–2006)161; 1% in Toronto, Canada (2007–2008)5; 3.9% (pediatric isolates) in South Korea (1991–1997).31 In Taiwan, AdV-4 accounted for 29% of pediatric respiratory isolates from 1981–2001, and became the predominant serotype (52%) in 2001.11 Until recently, AdV-4 was the most common serotype associated with FRI in military recruits in the United States.18 80 177 256 The strategy of vaccinating all military recruits against AdV-4 and -7 beginning in 1971174 257 eliminated both serotypes as causes of epidemic of FRI in the military for more than two decades.80 After the vaccine was depleted, an outbreak of AdV-4 occurred at an Army basic training site in 1997.74 Over the next several years, AdV-4 spread to multiple secondary sites.20 From 1999 to 2004, AdV-4 accounted for > 95% of AdV FRI among military recruits in the United States.20 By 2006 to 2007 the emerging AdV-14 largely replaced AdV-4 as a cause of AdV FRI among military recruits in the United States.33 After a 12-year interruption in vaccination the original vaccine formulation was reintroduced in October of 2011 resulting in a dramatic decline in the rates of AdV-associated febrile illness among recruits in training.175
Adenovirus Serotype 7
Globally, AdV-7 was the third most common serotype reported to the World Health Organization from 1967 through 1976, following AdV-1 and -284 and remains one of the leading serotypes detected in association with disease globally.31 40 258 AdV-7 infections manifest as FRI, pharyngoconjunctival fever, bronchitis, necrotizing bronchiolitis, or pneumonia.40 224 259 AdV-7 appears to be more virulent than other serotypes.11 88 224 242 249 260 261 262 Fatal pneumonias may occur in immunocompetent children6 224 250 263 264 and adults.23 265
Epidemic AdV-7 infections have been reported in the United States,6 264 266 Canada,263 Latin America,224 267 Australia,268 Israel,243 Korea,75 88 Japan,242 259 China,12 262 the Philippines,261 and globally.161 243 Outbreaks typically occur in closed settings (e.g., military barracks18 39; chronic care facilities80; hospitals6 78 269 270). In the late 1960s, AdV-7 and -4 accounted for most cases of FRI among military recruits in the United States.80 256 Following routine vaccination of military recruits in the United States beginning in 1971,174 257 no epidemics of FRI were attributed to AdV-7 or -4 from 1984 through 1994.80 However, in 1997 (after the vaccine supply was depleted), an epidemic (> 500 cases) of AdV FRI in a U. S. Navy training site was attributed to serotypes AdV-7 (70%) and AdV-3 (24%), respectively.19 Since 2007, AdV-7 has largely disappeared as a cause of FRI in U. S. military settings (possibly replaced by AdV-14).33
The prevalence of AdV-7 varies according to geographic regions and over time, and depends on strain genome type, herd immunity in the region, and epidemiological settings.6 80 161 264 In the United States from 2004 to 2006, AdV-7 accounted for only 5/581 (0.9%) of clinical AdV respiratory isolates in military facilities and 48/1,653 (2.9%) isolates in civilian settings.161 By contrast, AdV-7 was a prominent cause of FRI in South America in the 1990s224 267 and Asia.12 13 31 88 242 258 AdV-7 has been recently reported in association with severe disease in several provinces of China.262 265 271 AdV-7 was the leading cause of death due to AdV pneumonia in South America in the 1980s and 1990s.224 267 In a study of 165 AdV RTIs in children in Argentina and Uruguay, AdV-7 accounted for 62.2% of isolates and was responsible for 17 of 18 fatalities.224 The prevalence of AdV-7 as a cause of AdV FRI is Asia is variable, ranging from < 1242 to > 60%.75 In Seoul, Korea from 1990 to 1998, AdV-7 accounted for 41% of RTI (followed by AdV-3 [15%] and AdV-2 [15%]).88 From 1991 to 2007 in Seoul, AdV-7 accounted for 23.3% of pediatric respiratory AdV isolates, second only to AdV-3 (37.0%) (Table 1).31 In a survey of 200 military recruits in South Korea in 2006, 122 recruits (61%) developed AdV infections; all 122 isolates were AdV-7.75 In Taiwan, AdV-7 emerged as the predominant serotype (45%) in 1999 to 2000, but fell drastically to 1% in 2001 (replaced by AdV-4).11 In Beijing, China, AdV-7 and -3 were the most common serotypes causing pneumonia from 1958 to 1990.12
At least 27 genome types of AdV-7 have been identified by restriction enzyme fragment analysis80; shifts or replacement of predominant genome types may occur.40 161 243 244 In some cases, new genomic variants exhibit an apparent heightened virulence or transmissibility compared with earlier strains. For interested readers, the epidemiology, global shifts, and changing genotypes of AdV-7 were discussed in detail in our previous review.1
Adenovirus Serotype 8
AdV-8 accounts for < 1% of AdV infections,5 31 88 161 but is a common cause of EKC.88 105 107 111 116 272 273 In four studies in Asia and the Middle East, AdV-8 accounted for 64 to 79% of EKC due to AdV.105 106 109 117 In a neonatal intensive care unit in Turkey, cases of conjunctivitis due to AdV-8 were linked to a contaminated eyelid speculum.272
Adenovirus Serotype 11
AdV-11 is relatively uncommon, but may cause hemorrhagic conjunctivitis45 46 47 81 and FRI (including pneumonia) in immunocompetent patients and HC in immunocompromised patients.21 81 In the United States from 2004 to 2006, AdV-11 accounted for < 1% of AdV RTI in military recruits and civilians161; in Toronto, Canada, AdV-11 was not detected among 96 clinical respiratory AdV isolates (Table 1). AdV-11 comprised 3.4% of 741 pediatric respiratory isolates from Korea from 1991 to 2007.31 Outbreaks of AdV-11 FRIs were described in South America,224 United States,21 274 Asia,44 81 the Middle East,223 and globally. AdV-11 may cause UTI, including HC, in organ transplant recipients (particularly children).2 71 139 275
Adenovirus Serotype 14
AdV-14 was first isolated in the Netherlands in 1955 during an outbreak of acute respiratory disease (ARD) among military recruits.33 Subsequent outbreaks of ARD were described in Great Britain in 1955,276 Uzbekistan in 1962,33 and Czechoslovakia in 1963.33 Apart from sporadic cases in the Netherlands in the early 1970s, no cases of AdV-14 infections were reported globally between the 1960s and 2004.13 33 AdV-14 had never been identified in North America before 2006.41 Beginning in March 2006, outbreaks of FRI due to AdV-14 (several hundred cases) were noted in several military bases in the United States68 86 274 277 and among health care workers.68 By 2007, outbreaks in civilian populations were documented in at least 15 states.24 33 221 222 278 The severity of FRIs was variable, but fatal pneumonias were reported.24 33 68 221 278 By 2007, AdV-14 had replaced AdV-4 as the dominant serotype on U. S. military bases.41 274 Analysis of 99 isolates recovered from patients (military and civilian) with AdV FRI between December 2003 and June 2009 from different geographic locations confirmed that all isolates were identical.33 These isolates represented a new genomic type designated AdV-14p1 (formerly known as 14a).33 The complete genetic sequence of AdV-14p1 indicates a close relationship to AdV-11a, suggesting recombination between AdV-14 and -11 strains.41 AdV-14p1 was implicated in outbreaks of severe pneumonias in the United States33 and Ireland.279 AdV-14p1 has an increased potential for high attack rates and rates of transmission, owing to the lack of herd immunity.41
Adenovirus Serotype 21
AdV-21 was associated with epidemics of FRIs in military recruits in the Netherlands in the 1960s,280 but only sporadic cases were reported over the next two decades.281 In 1984 and 1985, outbreaks of AdV-21 infections in children in the Netherlands and Germany were published.281 AdV-21 has been associated with pharyngitis and conjunctivitis282 and FRI228 but is uncommon.31 In the United States from 2004 to 2006, AdV-21 accounted for 2.0 and 2.4% of AdV RTI in civilians and military recruits, respectively.161 In Toronto, Canada (2007–2008), AdV-21 accounted for 5.5% of clinical respiratory AdV isolates. By contrast, AdV-21 was never isolated in 741 pediatric respiratory isolates from Korea from 1991 to 2007.31 Interestingly, Adv-21 may be less transmissible than other AdV serotypes.283 However, a highly virulent strain of AdV-21 was associated with severe pneumonia cases in Germany34 and neurological284 and cardiac285 manifestations in Malaysia. Similar strains were found to circulate in the United States over the last 3 decades39 with no apparent association with severe disease among the infected young adults.
Adenovirus Serotype 31
AdV-31 may cause gastroenteritis in healthy children, and has been associated with severe (sometimes) fatal infections in HSCT recipients.28 157 286 287 288 Nosocomial transmission (seven cases) in a pediatric SCT unit was described.288
Adenovirus Serotype 37
AdV-37 accounts for < 1% of AdV infections,5 31 88 161 but may cause EKC.88 103 105 106 107 108 109
Adenovirus of Species F (Serotypes 40 and 41)
AdV of species F (serotypes 40 and 41) typically cause gastroenteritis and diarrheal illness in children.50 51 52 53 54 55 56 57 58 59 60 61 Fatalities may occur as a result of dehydration in infants.50 51 In immunocompromised hosts, fatal dissemination may occur.73 289 Epidemics have been cited in schools56 and hospitals.73 Endogenous reactivation originating from AdV persistent in mucosal lymphoid cells may occur.70 Nosocomial transmission may occur due to high AdV levels in feces.73 Shedding of these viruses may be prolonged in immunosuppressed patients.73
Adenovirus Genotype 55
Infections due to AdV-55 of species B are rare, but this virus has been implicated in outbreaks of severe pneumonia and acute respiratory distress syndrome in China since 2006.89 91 290 291 This type is an intertypic recombinant with an AdV-11-like hexon gene and an AdV-14-like fiber gene.240 Several reports describing cases of respiratory infection by this unique AdV under other designations (AdV-11, 14–11 or genome type 11a, depending on the typing approach) can be found in the literature.44 292 293 294
Diagnosis of Adenovirus Infection
AdV can be detected in affected sites (e.g., nasopharyngeal aspirates, swabs, washings, bronchoalveolar lavage, urine, stool, blood) by direct or indirect immunofluorescence, conventional or shell vial cultures, or PCR.31 Viral cultures by conventional techniques are the gold standard, but could be insensitive for certain samples (e.g., blood) and may take up to 21 days to develop the cytopathic effect.2 31 Biopsy of involved tissues may reveal AdV nuclear inclusions2; immunohistochemical stains may identify the AdV hexon antigen in tissue.146 PCR of AdV DNA in plasma, urine, or other clinical specimens is currently the most frequently used approach to establish the diagnosis,2 194 and is highly sensitive for disseminated disease.295 296 Quantification of the viral load using real-time PCR is a useful marker to assess response to therapy.189 295 Among transplant recipients, serial PCR assays of blood and stool weekly may detect AdV disease before the onset of symptoms, and facilitate early “preemptive” therapy.26 153 188 196 In one study of 138 pediatric allogeneic SCT recipients, AdV was detected in stool samples at median of 11 days before AdV viremia.297 The role of routine surveillance is controversial although it has been increasingly used in high-risk patients (particularly HSCT recipients2). Quantitative viral loads may not correlate with clinical presentation or disease severity.43
Molecular typing is not routinely performed on AdV-positive clinical specimens in clinical diagnostic laboratories but has been the focus of several recently reported studies investigating the epidemiology of AdV-associated disease. Serological tests may be useful in epidemiological investigations, but are of limited practical value in individual patients.38 Determination of serotype by seroneutralization with reference sera is laborious and time-consuming and currently only performed at a few reference public health laboratories around the world. PCR-based techniques targeting the fiber genes233 or hypervariable regions of the hexon235 298 and/or sequencing of hexon genes allow definitive identification of the type/species.29 31 Molecular typing by PCR amplification and sequencing of both hexon and fiber genes has proved to be extremely valuable for the identification of intertypic recombinants.299 300
Therapy
No antiviral drug has been approved to treat AdV.38 Prospective randomized controlled trials are lacking.14 CDV, a cytosine nucleotide analogue that inhibits DNA polymerase, has the greatest in vitro activity against AdV among currently available antiviral agents301 302 303 and is the preferred therapeutic agent.2 CDV is available only intravenously.2 Regimens (dosing, frequency, and duration) are variable. Standard doses include 5 mg/kg every 1 to 2 weeks38 188 or 1 mg/kg twice weekly.38 158 188 Duration of therapy is variable (weeks to months) and depends upon clinical response and persistence or eradication of AdV.158 188 CDV is generally well tolerated,153 188 304 but adverse effects include nephrotoxicity, myelosuppression, and uveitis.2 38 Hydration and probenecid may minimize nephrotoxicity.2 143 153 201 209 Careful monitoring of renal function (serum creatinine, proteinuria) is critical. Hexadecyloxy propyl-CDV or brincidofovir (CMX001), an orally active lipophilic form of CDV, has potent activity against AdV in vitro305 and in animal models,306 307 with anecdotal successes in small clinical series.308 309 Compared with CDV, CMX001 appears be less nephrotoxic.310 An open-label phase 3 trial to assess safety and efficacy of CMX001 for treating AdV infections in immunosuppressed patients is in progress (Clinical Trials.gov identifier: NCT02087306).
Numerous nonrandomized studies in HSCT and SOT recipients documented favorable responses to CDV.25 26 28 153 158 188 192 209 231 311 312 313 314 315 316 317 Three studies of allogeneic HSCT recipients with AdV infections cited improvement with CDV in 20/29 (69%),311 10/14 (77%),318 and 8/10 (80%) patients, respectively.192 However, given the lack of controlled trials, indications for, and efficacy of CDV remain controversial.27 Interpretation of these studies is confounded by heterogeneous patient populations, differing extent and sites of disease, and degree of immunosuppression or immune reconstitution.38 Intravenous immunoglobulin has been used (together with CDV), but data are insufficient to assess efficacy.25 316
Immune reconstitution plays a critical role in controlling AdV infection.38 Increases in lymphocyte counts or CD4 counts were associated with clearance of AdV infection319 320 and improved survival.320 321 Serotypic-specific neutralizing antibodies correlate with clearance of AdV.38 320 Reduction of immunosuppression,146 153 immune reconstitution of HSCT recipients,25 38 or donor leukocyte infusions28 may have adjunctive roles to treat serious or recalcitrant AdV infections. T cells are important to eradicate AdV. Adoptive transfer of AdV antigen-specific T cells may reconstitute immunity against AdV.322 323 In a recent clinical trial of HSCT recipients with AdV disease refractory to therapy, ex vivo adoptive T-cell transfer with predominantly TH-1 phenotype was highly effective in clearing viremia and markedly reduced mortality.324
Importantly, not all patients with AdV infections or viremia require treatment.2 14 43 201 High-mortality rates in retrospective studies in part reflect that virtually all patients had symptomatic AdV infections. Prospective studies in SOT196 or HSCT43 recipients using plasma PCR at regular intervals noted that up to 58% were asymptomatic at the time of viremia, and spontaneous resolution without sequela was common. In a cohort of SOT recipients with AdV viremia, all 19 recovered spontaneously without sequela.196 Similarly, in a cohort of 26 pediatric HSCT recipients, 11 (42%) developed AdV viremia that cleared without therapy in 7 (64%).43 Two children died as a result of AdV infections. Antiviral treatment should be considered for the following indications: disseminated (≥ 2 sites) disease; pneumonia; high viral loads in blood; virulence or tropism of the viral strain; persistent severe lymphopenia or immune deficits. Further, “preemptive” therapy may have a role in viremic but asymptomatic organ transplant recipients at high risk for dissemination. Prospective, randomized trials are needed to elucidate indications for therapy in both symptomatic and asymptomatic patients with AdV infections.
Vaccines
Oral vaccines against AdV types 4 and 7 developed for the U. S. military in 197120 were depleted by 1999.20 Produced by a new manufacturer, and after a new round of clinical trials179 the same live nonattenuated vaccine formulation for AdV-4 and -7 was successfully reintroduced for military use in the United States in October 2011.39 Importantly, antibodies to AdV-4 and -7 may cross protect against other serotypes (e.g., AdV-3 and -14).86 274 325
References
- 1.Lynch J P III, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011;32(4):494–511. doi: 10.1055/s-0031-1283287. [DOI] [PubMed] [Google Scholar]
- 2.Ison M G. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43(3):331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 3.Chang S Y, Lee C N, Lin P H. et al. A community-derived outbreak of adenovirus type 3 in children in Taiwan between 2004 and 2005. J Med Virol. 2008;80(1):102–112. doi: 10.1002/jmv.21045. [DOI] [PubMed] [Google Scholar]
- 4.Sandkovsky U, Vargas L, Florescu D F. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep. 2014;16(8):416. doi: 10.1007/s11908-014-0416-y. [DOI] [PubMed] [Google Scholar]
- 5.Yeung R, Eshaghi A, Lombos E. et al. Characterization of culture-positive adenovirus serotypes from respiratory specimens in Toronto, Ontario, Canada: September 2007-June 2008. Virol J. 2009;6:11. doi: 10.1186/1743-422X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell L S, Taylor B, Reimels W, Barrett F F, Devincenzo J P. Adenovirus 7a: a community-acquired outbreak in a children's hospital. Pediatr Infect Dis J. 2000;19(10):996–1000. doi: 10.1097/00006454-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kojaoghlanian T, Flomenberg P, Horwitz M S. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13(3):155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 8.Chemaly R F, Ghosh S, Bodey G P. et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(5):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y-J, Boeckh M, Englund J A. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28(2):222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 10.Moura P O, Roberto A F, Hein N. et al. Molecular epidemiology of human adenovirus isolated from children hospitalized with acute respiratory infection in São Paulo, Brazil. J Med Virol. 2007;79(2):174–181. doi: 10.1002/jmv.20778. [DOI] [PubMed] [Google Scholar]
- 11.Lin K H, Lin Y C, Chen H L. et al. A two decade survey of respiratory adenovirus in Taiwan: the reemergence of adenovirus types 7 and 4. J Med Virol. 2004;73(2):274–279. doi: 10.1002/jmv.20087. [DOI] [PubMed] [Google Scholar]
- 12.Li Q G, Zheng Q J, Liu Y H, Wadell G. Molecular epidemiology of adenovirus types 3 and 7 isolated from children with pneumonia in Beijing. J Med Virol. 1996;49(3):170–177. doi: 10.1002/(SICI)1096-9071(199607)49:3<170::AID-JMV3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen H L, Chiou S S, Hsiao H P. et al. Respiratory adenoviral infections in children: a study of hospitalized cases in southern Taiwan in 2001–2002. J Trop Pediatr. 2004;50(5):279–284. doi: 10.1093/tropej/50.5.279. [DOI] [PubMed] [Google Scholar]
- 14.Matthes-Martin S, Feuchtinger T, Shaw P J. et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011) Transpl Infect Dis. 2012;14(6):555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 15.Breuer S, Rauch M, Matthes-Martin S, Lion T. Molecular diagnosis and management of viral infections in hematopoietic stem cell transplant recipients. Mol Diagn Ther. 2012;16(2):63–77. doi: 10.1007/BF03256431. [DOI] [PubMed] [Google Scholar]
- 16.Kolavic-Gray S A, Binn L N, Sanchez J L. et al. Large epidemic of adenovirus type 4 infection among military trainees: epidemiological, clinical, and laboratory studies. Clin Infect Dis. 2002;35(7):808–818. doi: 10.1086/342573. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez J L, Binn L N, Innis B L. et al. Epidemic of adenovirus-induced respiratory illness among US military recruits: epidemiologic and immunologic risk factors in healthy, young adults. J Med Virol. 2001;65(4):710–718. doi: 10.1002/jmv.2095. [DOI] [PubMed] [Google Scholar]
- 18.Kajon A E, Moseley J M, Metzgar D. et al. Molecular epidemiology of adenovirus type 4 infections in US military recruits in the postvaccination era (1997-2003) J Infect Dis. 2007;196(1):67–75. doi: 10.1086/518442. [DOI] [PubMed] [Google Scholar]
- 19.Ryan M A, Gray G C, Smith B, McKeehan J A, Hawksworth A W, Malasig M D. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin Infect Dis. 2002;34(5):577–582. doi: 10.1086/338471. [DOI] [PubMed] [Google Scholar]
- 20.Russell K L, Hawksworth A W, Ryan M A. et al. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999-2004. Vaccine. 2006;24(15):2835–2842. doi: 10.1016/j.vaccine.2005.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) . Civilian outbreak of adenovirus acute respiratory disease—South Dakota, 1997. MMWR Morb Mortal Wkly Rep. 1998;47(27):567–570. [PubMed] [Google Scholar]
- 22.Zarraga A L, Kerns F T, Kitchen L W. Adenovirus pneumonia with severe sequelae in an immunocompetent adult. Clin Infect Dis. 1992;15(4):712–713. doi: 10.1093/clind/15.4.712. [DOI] [PubMed] [Google Scholar]
- 23.Dudding B A, Wagner S C, Zeller J A, Gmelich J T, French G R, Top F H Jr. Fatal pneumonia associated with adenovirus type 7 in three military trainees. N Engl J Med. 1972;286(24):1289–1292. doi: 10.1056/NEJM197206152862403. [DOI] [PubMed] [Google Scholar]
- 24.Louie J K, Kajon A E, Holodniy M. et al. Severe pneumonia due to adenovirus serotype 14: a new respiratory threat? Clin Infect Dis. 2008;46(3):421–425. doi: 10.1086/525261. [DOI] [PubMed] [Google Scholar]
- 25.Neofytos D, Ojha A, Mookerjee B. et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant. 2007;13(1):74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 26.Zheng X, Lu X, Erdman D D. et al. Identification of adenoviruses in specimens from high-risk pediatric stem cell transplant recipients and controls. J Clin Microbiol. 2008;46(1):317–320. doi: 10.1128/JCM.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symeonidis N, Jakubowski A, Pierre-Louis S. et al. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transpl Infect Dis. 2007;9(2):108–113. doi: 10.1111/j.1399-3062.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 28.Bordigoni P, Carret A S, Venard V, Witz F, Le Faou A. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32(9):1290–1297. doi: 10.1086/319984. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Erdman D D. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch Virol. 2006;151(8):1587–1602. doi: 10.1007/s00705-005-0722-7. [DOI] [PubMed] [Google Scholar]
- 30.Henquell C, Boeuf B, Mirand A. et al. Fatal adenovirus infection in a neonate and transmission to health-care workers. J Clin Virol. 2009;45(4):345–348. doi: 10.1016/j.jcv.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Choi E H, Lee H J. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991-2007) J Med Virol. 2010;82(4):624–631. doi: 10.1002/jmv.21701. [DOI] [PubMed] [Google Scholar]
- 32.Jones M S II, Harrach B, Ganac R D. et al. New adenovirus species found in a patient presenting with gastroenteritis. J Virol. 2007;81(11):5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kajon A E, Lu X, Erdman D D. et al. Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J Infect Dis. 2010;202(1):93–103. doi: 10.1086/653083. [DOI] [PubMed] [Google Scholar]
- 34.Hage E, Huzly D, Ganzenmueller T, Beck R, Schulz T F, Heim A. A human adenovirus species B subtype 21a associated with severe pneumonia. J Infect. 2014;69(5):490–499. doi: 10.1016/j.jinf.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Robinson C M, Singh G, Lee J Y. et al. Molecular evolution of human adenoviruses. Sci Rep. 2013;3:1812. doi: 10.1038/srep01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hage E, Gerd Liebert U, Bergs S, Ganzenmueller T, Heim A. Human mastadenovirus type 70: a novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J Gen Virol. 2015;96(9):2734–2742. doi: 10.1099/vir.0.000196. [DOI] [PubMed] [Google Scholar]
- 37.Kajon A E, Echavarria M, de Jong J C. Designation of human adenovirus types based on sequence data: an unfinished debate. J Clin Virol. 2013;58(4):743–744. doi: 10.1016/j.jcv.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Echavarría M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21(4):704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajon A E, Hang J, Hawksworth A. et al. Molecular Epidemiology of Adenovirus Type 21 Respiratory Strains Isolated From US Military Trainees (1996-2014) J Infect Dis. 2015;212(6):871–880. doi: 10.1093/infdis/jiv141. [DOI] [PubMed] [Google Scholar]
- 40.Erdman D D, Xu W, Gerber S I. et al. Molecular epidemiology of adenovirus type 7 in the United States, 1966-2000. Emerg Infect Dis. 2002;8(3):269–277. doi: 10.3201/eid0803.010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houng H S, Gong H, Kajon A E. et al. Genome sequences of human adenovirus 14 isolates from mild respiratory cases and a fatal pneumonia, isolated during 2006-2007 epidemics in North America. Respir Res. 2010;11:116. doi: 10.1186/1465-9921-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebner K, Rauch M, Preuner S, Lion T. Typing of human adenoviruses in specimens from immunosuppressed patients by PCR-fragment length analysis and real-time quantitative PCR. J Clin Microbiol. 2006;44(8):2808–2815. doi: 10.1128/JCM.00048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walls T, Hawrami K, Ushiro-Lumb I, Shingadia D, Saha V, Shankar A G. Adenovirus infection after pediatric bone marrow transplantation: is treatment always necessary? Clin Infect Dis. 2005;40(9):1244–1249. doi: 10.1086/429235. [DOI] [PubMed] [Google Scholar]
- 44.Kajon A E, Dickson L M, Metzgar D, Houng H S, Lee V, Tan B H. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol. 2010;48(4):1438–1441. doi: 10.1128/JCM.01928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama M, Miyazaki C, Ueda K. et al. Pharyngoconjunctival fever caused by adenovirus type 11. Pediatr Infect Dis J. 1992;11(1):6–9. doi: 10.1097/00006454-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Yin-Murphy M, Lim K H, Chua P H. Adenovirus type 11 epidemic conjunctivitis in Singapore. Southeast Asian J Trop Med Public Health. 1974;5(3):333–341. [PubMed] [Google Scholar]
- 47.Tai F H, Chu S, Chi W H, Wei H Y, Hierholzer J C. Epidemic haemorrhagic conjunctivitis associated with adenovirus type 11 in Taiwan. Southeast Asian J Trop Med Public Health. 1974;5(3):342–349. [PubMed] [Google Scholar]
- 48.James L, Vernon M O, Jones R C. et al. Outbreak of human adenovirus type 3 infection in a pediatric long-term care facility—Illinois, 2005. Clin Infect Dis. 2007;45(4):416–420. doi: 10.1086/519938. [DOI] [PubMed] [Google Scholar]
- 49.Ishiko H, Aoki K. Spread of epidemic keratoconjunctivitis due to a novel serotype of human adenovirus in Japan. J Clin Microbiol. 2009;47(8):2678–2679. doi: 10.1128/JCM.r00313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filho E P, da Costa Faria N R, Fialho A M. et al. Adenoviruses associated with acute gastroenteritis in hospitalized and community children up to 5 years old in Rio de Janeiro and Salvador, Brazil. J Med Microbiol. 2007;56(Pt 3):313–319. doi: 10.1099/jmm.0.46685-0. [DOI] [PubMed] [Google Scholar]
- 51.Madisch I, Wölfel R, Harste G, Pommer H, Heim A. Molecular identification of adenovirus sequences: a rapid scheme for early typing of human adenoviruses in diagnostic samples of immunocompetent and immunodeficient patients. J Med Virol. 2006;78(9):1210–1217. doi: 10.1002/jmv.20683. [DOI] [PubMed] [Google Scholar]
- 52.Hársi C M, Rolim D P, Gomes S A. et al. Adenovirus genome types isolated from stools of children with gastroenteritis in São Paulo, Brazil. J Med Virol. 1995;45(2):127–134. doi: 10.1002/jmv.1890450203. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda S, Kuwayama M, Takao S, Shimazu Y, Miyazaki K. Molecular epidemiology of subgenus F adenoviruses associated with pediatric gastroenteritis during eight years in Hiroshima Prefecture as a limited area. Arch Virol. 2006;151(12):2511–2517. doi: 10.1007/s00705-006-0816-x. [DOI] [PubMed] [Google Scholar]
- 54.Sdiri-Loulizi K, Gharbi-Khelifi H, de Rougemont A. et al. Molecular epidemiology of human astrovirus and adenovirus serotypes 40/41 strains related to acute diarrhea in Tunisian children. J Med Virol. 2009;81(11):1895–1902. doi: 10.1002/jmv.21586. [DOI] [PubMed] [Google Scholar]
- 55.Magwalivha M, Wolfaardt M, Kiulia N M, van Zyl W B, Mwenda J M, Taylor M B. High prevalence of species D human adenoviruses in fecal specimens from Urban Kenyan children with diarrhea. J Med Virol. 2010;82(1):77–84. doi: 10.1002/jmv.21673. [DOI] [PubMed] [Google Scholar]
- 56.Gonçalves G, Gouveia E, Mesquita J R. et al. Outbreak of acute gastroenteritis caused by adenovirus type 41 in a kindergarten. Epidemiol Infect. 2011;139(11):1672–1675. doi: 10.1017/S0950268810002803. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Shimizu H, Doan L T. et al. Characterizations of adenovirus type 41 isolates from children with acute gastroenteritis in Japan, Vietnam, and Korea. J Clin Microbiol. 2004;42(9):4032–4039. doi: 10.1128/JCM.42.9.4032-4039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marie-Cardine A, Gourlain K, Mouterde O. et al. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin Infect Dis. 2002;34(9):1170–1178. doi: 10.1086/339807. [DOI] [PubMed] [Google Scholar]
- 59.Soares C C, Volotão E M, Albuquerque M C. et al. Prevalence of enteric adenoviruses among children with diarrhea in four Brazilian cities. J Clin Virol. 2002;23(3):171–177. doi: 10.1016/s1386-6532(01)00220-7. [DOI] [PubMed] [Google Scholar]
- 60.Cunliffe N A, Booth J A, Elliot C. et al. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis. 2010;16(1):55–62. doi: 10.3201/eid1601.090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iturriza Gómara M, Simpson R, Perault A M. et al. Structured surveillance of infantile gastroenteritis in East Anglia, UK: incidence of infection with common viral gastroenteric pathogens. Epidemiol Infect. 2008;136(1):23–33. doi: 10.1017/S0950268807008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King J C Jr Community respiratory viruses in individuals with human immunodeficiency virus infection Am J Med 1997102(3A):19–24., discussion 25–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wigger H J, Blanc W A. Fatal hepatic and bronchial necrosis in adenovirus infection with thymic alymphoplasia. N Engl J Med. 1966;275(16):870–874. doi: 10.1056/NEJM196610202751603. [DOI] [PubMed] [Google Scholar]
- 64.Dagan R, Schwartz R H, Insel R A, Menegus M A. Severe diffuse adenovirus 7a pneumonia in a child with combined immunodeficiency: possible therapeutic effect of human immune serum globulin containing specific neutralizing antibody. Pediatr Infect Dis. 1984;3(3):246–251. doi: 10.1097/00006454-198405000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Barker J H, Luby J P, Sean Dalley A, Bartek W M, Burns D K, Erdman D D. Fatal type 3 adenoviral pneumonia in immunocompetent adult identical twins. Clin Infect Dis. 2003;37(10):e142–e146. doi: 10.1086/379127. [DOI] [PubMed] [Google Scholar]
- 66.Buffington J, Chapman L E, Stobierski M G. et al. Epidemic keratoconjunctivitis in a chronic care facility: risk factors and measures for control. J Am Geriatr Soc. 1993;41(11):1177–1181. doi: 10.1111/j.1532-5415.1993.tb07299.x. [DOI] [PubMed] [Google Scholar]
- 67.Singh-Naz N, Brown M, Ganeshananthan M. Nosocomial adenovirus infection: molecular epidemiology of an outbreak. Pediatr Infect Dis J. 1993;12(11):922–925. [PubMed] [Google Scholar]
- 68.Lessa F C, Gould P L, Pascoe N. et al. Health care transmission of a newly emergent adenovirus serotype in health care personnel at a military hospital in Texas, 2007. J Infect Dis. 2009;200(11):1759–1765. doi: 10.1086/647987. [DOI] [PubMed] [Google Scholar]
- 69.Russell K L, Broderick M P, Franklin S E. et al. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis. 2006;194(7):877–885. doi: 10.1086/507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garnett C T, Erdman D, Xu W, Gooding L R. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76(21):10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bil-Lula I, Ussowicz M, Rybka B. et al. Hematuria due to adenoviral infection in bone marrow transplant recipients. Transplant Proc. 2010;42(9):3729–3734. doi: 10.1016/j.transproceed.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 72.Wadell G. Molecular epidemiology of human adenoviruses. Curr Top Microbiol Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- 73.Mattner F, Sykora K W, Meissner B, Heim A. An adenovirus type F41 outbreak in a pediatric bone marrow transplant unit: analysis of clinical impact and preventive strategies. Pediatr Infect Dis J. 2008;27(5):419–424. doi: 10.1097/INF.0b013e3181658c46. [DOI] [PubMed] [Google Scholar]
- 74.McNeill K M, Ridgely Benton F, Monteith S C, Tuchscherer M A, Gaydos J C. Epidemic spread of adenovirus type 4-associated acute respiratory disease between U.S. Army installations. Emerg Infect Dis. 2000;6(4):415–419. doi: 10.3201/eid0604.000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeon K, Kang C I, Yoon C H. et al. High isolation rate of adenovirus serotype 7 from South Korean military recruits with mild acute respiratory disease. Eur J Clin Microbiol Infect Dis. 2007;26(7):481–483. doi: 10.1007/s10096-007-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Padin D S, Faix D, Brodine S. et al. Retrospective analysis of demographic and clinical factors associated with etiology of febrile respiratory illness among US military basic trainees. BMC Infect Dis. 2014;14:576. doi: 10.1186/s12879-014-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez M P, Erdman D D, Torok T J, Freeman C J, Matyas B T. Outbreak of adenovirus 35 pneumonia among adult residents and staff of a chronic care psychiatric facility. J Infect Dis. 1997;176(3):760–763. doi: 10.1086/517295. [DOI] [PubMed] [Google Scholar]
- 78.Finn A, Anday E, Talbot G H. An epidemic of adenovirus 7a infection in a neonatal nursery: course, morbidity, and management. Infect Control Hosp Epidemiol. 1988;9(9):398–404. doi: 10.1086/645898. [DOI] [PubMed] [Google Scholar]
- 79.Klinger J R, Sanchez M P, Curtin L A, Durkin M, Matyas B. Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med. 1998;157(2):645–649. doi: 10.1164/ajrccm.157.2.9608057. [DOI] [PubMed] [Google Scholar]
- 80.Gerber S I, Erdman D D, Pur S L. et al. Outbreak of adenovirus genome type 7d2 infection in a pediatric chronic-care facility and tertiary-care hospital. Clin Infect Dis. 2001;32(5):694–700. doi: 10.1086/319210. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Z, Zhang Y, Xu S. et al. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol. 2009;47(3):697–703. doi: 10.1128/JCM.01769-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harris D J, Wulff H, Ray C G, Poland J D, Chin T D, Wenner H A. Viruses and disease. 3. An outbreak of adenovirus type 7A in a children's home. Am J Epidemiol. 1971;93(5):399–402. doi: 10.1093/oxfordjournals.aje.a121273. [DOI] [PubMed] [Google Scholar]
- 83.Chany C, Lepine P, Lelong M, Le T V, Satge P, Virat J. Severe and fatal pneumonia in infants and young children associated with adenovirus infections. Am J Hyg. 1958;67(3):367–378. doi: 10.1093/oxfordjournals.aje.a119941. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz H, Wigand R, Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983;117(4):455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- 85.Rubin B A. Clinical picture and epidemiology of adenovirus infections (a review) Acta Microbiol Hung. 1993;40(4):303–323. [PubMed] [Google Scholar]
- 86.Trei J S, Johns N M, Garner J L. et al. Spread of adenovirus to geographically dispersed military installations, May-October 2007. Emerg Infect Dis. 2010;16(5):769–775. doi: 10.3201/eid1605.091633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sauerbrei A, Sehr K, Brandstädt A, Heim A, Reimer K, Wutzler P. Sensitivity of human adenoviruses to different groups of chemical biocides. J Hosp Infect. 2004;57(1):59–66. doi: 10.1016/j.jhin.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 88.Hong J Y, Lee H J, Piedra P A. et al. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32(10):1423–1429. doi: 10.1086/320146. [DOI] [PubMed] [Google Scholar]
- 89.Lu Q B, Tong Y G, Wo Y. et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009-2012. Influenza Other Respi Viruses. 2014;8(3):302–308. doi: 10.1111/irv.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hakim F A, Tleyjeh I M. Severe adenovirus pneumonia in immunocompetent adults: a case report and review of the literature. Eur J Clin Microbiol Infect Dis. 2008;27(2):153–158. doi: 10.1007/s10096-007-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao B, Huang G H, Pu Z H. et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. 2014;145(1):79–86. doi: 10.1378/chest.13-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sly P D, Soto-Quiros M E, Landau L I, Hudson I, Newton-John H. Factors predisposing to abnormal pulmonary function after adenovirus type 7 pneumonia. Arch Dis Child. 1984;59(10):935–939. doi: 10.1136/adc.59.10.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cherry J. Philadelphia: Saunders; 2003. Adenoviruses; pp. 1843–1856. [Google Scholar]
- 94.Becroft D M. Bronchiolitis obliterans, bronchiectasis, and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol. 1971;24(1):72–82. doi: 10.1136/jcp.24.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalu S U, Loeffelholz M, Beck E. et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29(8):746–750. doi: 10.1097/INF.0b013e3181d743c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macek V, Sorli J, Kopriva S, Marin J. Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med. 1994;150(1):7–10. doi: 10.1164/ajrccm.150.1.8025775. [DOI] [PubMed] [Google Scholar]
- 97.Wurzel D F, Marchant J M, Yerkovich S T. et al. Prospective characterization of protracted bacterial bronchitis in children. Chest. 2014;145(6):1271–1278. doi: 10.1378/chest.13-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wurzel D F, Mackay I M, Marchant J M. et al. Adenovirus species C is associated with chronic suppurative lung diseases in children. Clin Infect Dis. 2014;59(1):34–40. doi: 10.1093/cid/ciu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wurzel D F, Marchant J M, Clark J E. et al. Respiratory virus detection in nasopharyngeal aspirate versus bronchoalveolar lavage is dependent on virus type in children with chronic respiratory symptoms. J Clin Virol. 2013;58(4):683–688. doi: 10.1016/j.jcv.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elliott W M, Hayashi S, Hogg J C. Immunodetection of adenoviral E1A proteins in human lung tissue. Am J Respir Cell Mol Biol. 1995;12(6):642–648. doi: 10.1165/ajrcmb.12.6.7766428. [DOI] [PubMed] [Google Scholar]
- 101.Matsuse T, Hayashi S, Kuwano K, Keunecke H, Jefferies W A, Hogg J C. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis. 1992;146(1):177–184. doi: 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 102.Percivalle E, Sarasini A, Torsellini M. et al. A comparison of methods for detecting adenovirus type 8 keratoconjunctivitis during a nosocomial outbreak in a Neonatal Intensive Care Unit. J Clin Virol. 2003;28(3):257–264. doi: 10.1016/s1386-6532(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 103.Hamada N, Gotoh K, Hara K. et al. Nosocomial outbreak of epidemic keratoconjunctivitis accompanying environmental contamination with adenoviruses. J Hosp Infect. 2008;68(3):262–268. doi: 10.1016/j.jhin.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Ishiko H, Shimada Y, Konno T. et al. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J Clin Microbiol. 2008;46(6):2002–2008. doi: 10.1128/JCM.01835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tabbara K F, Omar N, Hammouda E. et al. Molecular epidemiology of adenoviral keratoconjunctivitis in Saudi Arabia. Mol Vis. 2010;16:2132–2136. [PMC free article] [PubMed] [Google Scholar]
- 106.Aoki K, Tagawa Y. A twenty-one year surveillance of adenoviral conjunctivitis in Sapporo, Japan. Int Ophthalmol Clin. 2002;42(1):49–54. doi: 10.1097/00004397-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 107.Chang C H, Lin K H, Sheu M M, Huang W L, Wang H Z, Chen C W. The change of etiological agents and clinical signs of epidemic viral conjunctivitis over an 18-year period in southern Taiwan. Graefes Arch Clin Exp Ophthalmol. 2003;241(7):554–560. doi: 10.1007/s00417-003-0680-2. [DOI] [PubMed] [Google Scholar]
- 108.Matsui K, Saha S, Saitoh M. et al. Isolation and identification of adenovirus from conjunctival scrapings over a two-year period (between 2001 and 2003) in Yokohama, Japan. J Med Virol. 2007;79(2):200–205. doi: 10.1002/jmv.20779. [DOI] [PubMed] [Google Scholar]
- 109.Jin X H, Ishiko H, Nguyen T H. et al. Molecular epidemiology of adenoviral conjunctivitis in Hanoi, Vietnam. Am J Ophthalmol. 2006;142(6):1064–1066. doi: 10.1016/j.ajo.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 110.Ariga T, Shimada Y, Shiratori K. et al. Five new genome types of adenovirus type 37 caused epidemic keratoconjunctivitis in Sapporo, Japan, for more than 10 years. J Clin Microbiol. 2005;43(2):726–732. doi: 10.1128/JCM.43.2.726-732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee Y C, Chen N, Huang I T. et al. Human adenovirus type 8 epidemic keratoconjunctivitis with large corneal epithelial full-layer detachment: an endemic outbreak with uncommon manifestations. Clin Ophthalmol. 2015;9:953–957. doi: 10.2147/OPTH.S79697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Centers for Disease Control and Prevention (CDC) . Adenovirus-associated epidemic keratoconjunctivitis outbreaks—four states, 2008-2010. MMWR Morb Mortal Wkly Rep. 2013;62(32):637–641. [PMC free article] [PubMed] [Google Scholar]
- 113.Itakura S, Aoki K, Sawada H, Shinagawa M. Analysis with restriction endonucleases recognizing 4- or 5-base-pair sequences of human adenovirus type 3 isolated from ocular diseases in Sapporo, Japan. J Clin Microbiol. 1990;28(10):2365–2369. doi: 10.1128/jcm.28.10.2365-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ariga T, Shimada Y, Ohgami K. et al. New genome type of adenovirus serotype 4 caused nosocomial infections associated with epidemic conjunctivitis in Japan. J Clin Microbiol. 2004;42(8):3644–3648. doi: 10.1128/JCM.42.8.3644-3648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montessori V, Scharf S, Holland S, Werker D H, Roberts F J, Bryce E. Epidemic keratoconjunctivitis outbreak at a tertiary referral eye care clinic. Am J Infect Control. 1998;26(4):399–405. doi: 10.1016/s0196-6553(98)70035-5. [DOI] [PubMed] [Google Scholar]
- 116.Sendra-Gutiérrez J M, Martín-Rios D, Casas I, Sáez P, Tovar A, Moreno C. An outbreak of adenovirus type 8 keratoconjunctivitis in a nursing home in Madrid. Euro Surveill. 2004;9(3):27–30. doi: 10.2807/esm.09.03.00453-en. [DOI] [PubMed] [Google Scholar]
- 117.Saitoh-Inagawa W, Aoki K, Uchio E, Itoh N, Ohno S. Ten years' surveillance of viral conjunctivitis in Sapporo, Japan. Graefes Arch Clin Exp Ophthalmol. 1999;237(1):35–38. doi: 10.1007/s004170050191. [DOI] [PubMed] [Google Scholar]
- 118.Kuo I C, Espinosa C, Forman M, Pehar M, Maragakis L L, Valsamakis A. Detection and prevalence of adenoviral conjunctivitis among hospital employees using real-time polymerase chain reaction as an infection prevention tool. Infect Control Hosp Epidemiol. 2014;35(6):728–731. doi: 10.1086/676428. [DOI] [PubMed] [Google Scholar]
- 119.Kaneko H, Aoki K, Ishida S. et al. Recombination analysis of intermediate human adenovirus type 53 in Japan by complete genome sequence. J Gen Virol. 2011;92(Pt 6):1251–1259. doi: 10.1099/vir.0.030361-0. [DOI] [PubMed] [Google Scholar]
- 120.Huang G, Yao W, Yu W. et al. Outbreak of epidemic keratoconjunctivitis caused by human adenovirus type 56, China, 2012. PLoS ONE. 2014;9(10):e110781. doi: 10.1371/journal.pone.0110781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Enomoto M, Okafuji T, Okafuji T. et al. Isolation of an intertypic recombinant human adenovirus (candidate type 56) from the pharyngeal swab of a patient with pharyngoconjunctival fever. Jpn J Infect Dis. 2012;65(5):457–459. doi: 10.7883/yoken.65.457. [DOI] [PubMed] [Google Scholar]
- 122.Walsh M P, Chintakuntlawar A, Robinson C M. et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS ONE. 2009;4(6):e5635. doi: 10.1371/journal.pone.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaneko H, Suzutani T, Aoki K. et al. Epidemiological and virological features of epidemic keratoconjunctivitis due to new human adenovirus type 54 in Japan. Br J Ophthalmol. 2011;95(1):32–36. doi: 10.1136/bjo.2009.178772. [DOI] [PubMed] [Google Scholar]
- 124.Hiroi S, Morikawa S, Takahashi K, Komano J, Kase T. Molecular epidemiology of human adenoviruses d associated with epidemic keratoconjunctivitis in Osaka, Japan, 2001-2010. Jpn J Infect Dis. 2013;66(5):436–438. doi: 10.7883/yoken.66.436. [DOI] [PubMed] [Google Scholar]
- 125.Moyo S J, Hanevik K, Blomberg B. et al. Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; a case control study. BMC Infect Dis. 2014;14:666. doi: 10.1186/s12879-014-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kapelushnik J, Or R, Delukina M, Nagler A, Livni N, Engelhard D. Intravenous ribavirin therapy for adenovirus gastroenteritis after bone marrow transplantation. J Pediatr Gastroenterol Nutr. 1995;21(1):110–112. doi: 10.1097/00005176-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 127.Janoff E N, Orenstein J M, Manischewitz J F, Smith P D. Adenovirus colitis in the acquired immunodeficiency syndrome. Gastroenterology. 1991;100(4):976–979. doi: 10.1016/0016-5085(91)90272-m. [DOI] [PubMed] [Google Scholar]
- 128.Wang W H, Wang H L. Fulminant adenovirus hepatitis following bone marrow transplantation. A case report and brief review of the literature. Arch Pathol Lab Med. 2003;127(5):e246–e248. doi: 10.5858/2003-127-e246-FAHFBM. [DOI] [PubMed] [Google Scholar]
- 129.Arav-Boger R, Echavarria M, Forman M, Charache P, Persaud D. Clearance of adenoviral hepatitis with ribavirin therapy in a pediatric liver transplant recipient. Pediatr Infect Dis J. 2000;19(11):1097–1100. doi: 10.1097/00006454-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 130.Putra J, Suriawinata A A. Adenovirus hepatitis presenting as tumoral lesions in an immunocompromised patient. Ann Hepatol. 2014;13(6):827–829. [PubMed] [Google Scholar]
- 131.Yan Z, Nguyen S, Poles M, Melamed J, Scholes J V. Adenovirus colitis in human immunodeficiency virus infection: an underdiagnosed entity. Am J Surg Pathol. 1998;22(9):1101–1106. doi: 10.1097/00000478-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 132.Hedderwick S A, Greenson J K, McGaughy V R, Clark N M. Adenovirus cholecystitis in a patient with AIDS. Clin Infect Dis. 1998;26(4):997–999. doi: 10.1086/517647. [DOI] [PubMed] [Google Scholar]
- 133.Bateman C M, Kesson A M, Shaw P J. Pancreatitis and adenoviral infection in children after blood and marrow transplantation. Bone Marrow Transplant. 2006;38(12):807–811. doi: 10.1038/sj.bmt.1705526. [DOI] [PubMed] [Google Scholar]
- 134.Kir S, Aydin Y, Kocaman O. et al. Acute pancreatitis after severe opthalmic adenoviral infection. Acta Gastroenterol Belg. 2011;74(2):361–362. [PubMed] [Google Scholar]
- 135.Yokose N, Hirakawa T, Inokuchi K. Adenovirus-associated hemorrhagic cystitis in a patient with plasma cell myeloma treated with bortezomib. Leuk Res. 2009;33(8):e106. doi: 10.1016/j.leukres.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 136.Akiyama H, Kurosu T, Sakashita C. et al. Adenovirus is a key pathogen in hemorrhagic cystitis associated with bone marrow transplantation. Clin Infect Dis. 2001;32(9):1325–1330. doi: 10.1086/319992. [DOI] [PubMed] [Google Scholar]
- 137.Teramura T, Naya M, Yoshihara T, Kanoh G, Morimoto A, Imashuku S. Adenoviral infection in hematopoietic stem cell transplantation: early diagnosis with quantitative detection of the viral genome in serum and urine. Bone Marrow Transplant. 2004;33(1):87–92. doi: 10.1038/sj.bmt.1704320. [DOI] [PubMed] [Google Scholar]
- 138.Miyamura K, Hamaguchi M, Taji H. et al. Successful ribavirin therapy for severe adenovirus hemorrhagic cystitis after allogeneic marrow transplant from close HLA donors rather than distant donors. Bone Marrow Transplant. 2000;25(5):545–548. doi: 10.1038/sj.bmt.1702195. [DOI] [PubMed] [Google Scholar]
- 139.Fanourgiakis P, Georgala A, Vekemans M. et al. Intravesical instillation of cidofovir in the treatment of hemorrhagic cystitis caused by adenovirus type 11 in a bone marrow transplant recipient. Clin Infect Dis. 2005;40(1):199–201. doi: 10.1086/426594. [DOI] [PubMed] [Google Scholar]
- 140.Hofland C A, Eron L J, Washecka R M. Hemorrhagic adenovirus cystitis after renal transplantation. Transplant Proc. 2004;36(10):3025–3027. doi: 10.1016/j.transproceed.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira G F, Oliveira R A, Lucon M. et al. Hemorrhagic cystitis secondary to adenovirus or herpes simplex virus infection following renal transplantation: four case reports. Transplant Proc. 2009;41(10):4416–4419. doi: 10.1016/j.transproceed.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 142.Yagisawa T, Nakada T, Takahashi K, Toma H, Ota K, Yaguchi H. Acute hemorrhagic cystitis caused by adenovirus after kidney transplantation. Urol Int. 1995;54(3):142–146. doi: 10.1159/000282708. [DOI] [PubMed] [Google Scholar]
- 143.Keswani M, Moudgil A. Adenovirus-associated hemorrhagic cystitis in a pediatric renal transplant recipient. Pediatr Transplant. 2007;11(5):568–571. doi: 10.1111/j.1399-3046.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- 144.Koga S, Shindo K, Matsuya F, Hori T, Kanda S, Kanetake H. Acute hemorrhagic cystitis caused by adenovirus following renal transplantation: review of the literature. J Urol. 1993;149(4):838–839. doi: 10.1016/s0022-5347(17)36227-4. [DOI] [PubMed] [Google Scholar]
- 145.Lachiewicz A M, Cianciolo R, Miller M B, Derebail V K. Adenovirus causing fever, upper respiratory infection, and allograft nephritis complicated by persistent asymptomatic viremia. Transpl Infect Dis. 2014;16(4):648–652. doi: 10.1111/tid.12248. [DOI] [PubMed] [Google Scholar]
- 146.Sujeet K, Vasudev B, Desai P. et al. Acute kidney injury requiring dialysis secondary to adenovirus nephritis in renal transplant recipient. Transpl Infect Dis. 2011;13(2):174–177. doi: 10.1111/j.1399-3062.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 147.Bruno B, Zager R A, Boeckh M J. et al. Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation. 2004;77(7):1049–1057. doi: 10.1097/01.tp.0000122421.71556.71. [DOI] [PubMed] [Google Scholar]
- 148.Ito M, Hirabayashi N, Uno Y, Nakayama A, Asai J. Necrotizing tubulointerstitial nephritis associated with adenovirus infection. Hum Pathol. 1991;22(12):1225–1231. doi: 10.1016/0046-8177(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 149.Ardehali H, Volmar K, Roberts C, Forman M, Becker L C. Fatal disseminated adenoviral infection in a renal transplant patient. Transplantation. 2001;71(7):998–999. doi: 10.1097/00007890-200104150-00029. [DOI] [PubMed] [Google Scholar]
- 150.Echavarria M, Forman M, van Tol M J, Vossen J M, Charache P, Kroes A C. Prediction of severe disseminated adenovirus infection by serum PCR. Lancet. 2001;358(9279):384–385. doi: 10.1016/S0140-6736(01)05580-5. [DOI] [PubMed] [Google Scholar]
- 151.Mori K, Yoshihara T, Nishimura Y. et al. Acute renal failure due to adenovirus-associated obstructive uropathy and necrotizing tubulointerstitial nephritis in a bone marrow transplant recipient. Bone Marrow Transplant. 2003;31(12):1173–1176. doi: 10.1038/sj.bmt.1704077. [DOI] [PubMed] [Google Scholar]
- 152.Liddle O L, Samuel M I, Sudhanva M, Ellis J, Taylor C. Adenovirus urethritis and concurrent conjunctivitis: a case series and review of the literature. Sex Transm Infect. 2015;91(2):87–90. doi: 10.1136/sextrans-2014-051868. [DOI] [PubMed] [Google Scholar]
- 153.Sivaprakasam P, Carr T F, Coussons M. et al. Improved outcome from invasive adenovirus infection in pediatric patients after hemopoietic stem cell transplantation using intensive clinical surveillance and early intervention. J Pediatr Hematol Oncol. 2007;29(2):81–85. doi: 10.1097/MPH.0b013e318030875e. [DOI] [PubMed] [Google Scholar]
- 154.Robin M, Marque-Juillet S, Scieux C. et al. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007;92(9):1254–1257. doi: 10.3324/haematol.11279. [DOI] [PubMed] [Google Scholar]
- 155.Kroes A C, de Klerk E P, Lankester A C. et al. Sequential emergence of multiple adenovirus serotypes after pediatric stem cell transplantation. J Clin Virol. 2007;38(4):341–347. doi: 10.1016/j.jcv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 156.de Mezerville M H, Tellier R, Richardson S, Hébert D, Doyle J, Allen U. Adenoviral infections in pediatric transplant recipients: a hospital-based study. Pediatr Infect Dis J. 2006;25(9):815–818. doi: 10.1097/01.inf.0000233542.48267.fd. [DOI] [PubMed] [Google Scholar]
- 157.Venard V, Carret A, Corsaro D, Bordigoni P, Le Faou A. Genotyping of adenoviruses isolated in an outbreak in a bone marrow transplant unit shows that diverse strains are involved. J Hosp Infect. 2000;44(1):71–74. doi: 10.1053/jhin.1999.0656. [DOI] [PubMed] [Google Scholar]
- 158.Hoffman J A, Shah A J, Ross L A, Kapoor N. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(7):388–394. doi: 10.1053/bbmt.2001.v7.pm11529489. [DOI] [PubMed] [Google Scholar]
- 159.Kelsey D S. Adenovirus meningoencephalitis. Pediatrics. 1978;61(2):291–293. [PubMed] [Google Scholar]
- 160.Ladisch S, Lovejoy F H, Hierholzer J C. et al. Extrapulmonary manifestations of adenovirus type 7 pneumonia simulating Reye syndrome and the possible role of an adenovirus toxin. J Pediatr. 1979;95(3):348–355. doi: 10.1016/s0022-3476(79)80505-3. [DOI] [PubMed] [Google Scholar]
- 161.Gray G C, McCarthy T, Lebeck M G. et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin Infect Dis. 2007;45(9):1120–1131. doi: 10.1086/522188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Y C, Huang S L, Chen S P. et al. Adenovirus infection associated with central nervous system dysfunction in children. J Clin Virol. 2013;57(4):300–304. doi: 10.1016/j.jcv.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 163.Dubberke E R, Tu B, Rivet D J. et al. Acute meningoencephalitis caused by adenovirus serotype 26. J Neurovirol. 2006;12(3):235–240. doi: 10.1080/13550280600846633. [DOI] [PubMed] [Google Scholar]
- 164.Reyes-Andrade J, Sánchez-Céspedes J, Olbrich P. et al. Meningoencephalitis due to adenovirus in a healthy infant mimicking severe bacterial sepsis. Pediatr Infect Dis J. 2014;33(4):416–419. doi: 10.1097/INF.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 165.Frange P, Peffault de Latour R, Arnaud C. et al. Adenoviral infection presenting as an isolated central nervous system disease without detectable viremia in two children after stem cell transplantation. J Clin Microbiol. 2011;49(6):2361–2364. doi: 10.1128/JCM.00080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bowles N E, Ni J, Kearney D L. et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 167.Valdés O, Acosta B, Piñón A. et al. First report on fatal myocarditis associated with adenovirus infection in Cuba. J Med Virol. 2008;80(10):1756–1761. doi: 10.1002/jmv.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Melón S, Méndez S, Iglesias B. et al. Involvement of adenovirus in clinical mononucleosis-like syndromes in young children. Eur J Clin Microbiol Infect Dis. 2005;24(5):314–318. doi: 10.1007/s10096-005-1333-7. [DOI] [PubMed] [Google Scholar]
- 169.Couroucli X I, Welty S E, Ramsay P L. et al. Detection of microorganisms in the tracheal aspirates of preterm infants by polymerase chain reaction: association of adenovirus infection with bronchopulmonary dysplasia. Pediatr Res. 2000;47(2):225–232. doi: 10.1203/00006450-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 170.Guarner J, de Leon-Bojorge B, Lopez-Corella E. et al. Intestinal intussusception associated with adenovirus infection in Mexican children. Am J Clin Pathol. 2003;120(6):845–850. doi: 10.1309/LBRN-GF9M-JW2M-HT97. [DOI] [PubMed] [Google Scholar]
- 171.Bajanowski T, Wiegand P, Cecchi R. et al. Detection and significance of adenoviruses in cases of sudden infant death. Virchows Arch. 1996;428(2):113–118. doi: 10.1007/BF00193939. [DOI] [PubMed] [Google Scholar]
- 172.Rodríguez-Martínez C E, Rodríguez D A, Nino G. Respiratory syncytial virus, adenoviruses, and mixed acute lower respiratory infections in children in a developing country. J Med Virol. 2015;87(5):774–781. doi: 10.1002/jmv.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Russell K L, Baker C I, Hansen C. et al. Lack of effectiveness of the 23-valent polysaccharide pneumococcal vaccine in reducing all-cause pneumonias among healthy young military recruits: a randomized, double-blind, placebo-controlled trial. Vaccine. 2015;33(9):1182–1187. doi: 10.1016/j.vaccine.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 174.Top F H Jr. Control of adenovirus acute respiratory disease in U.S. Army trainees. Yale J Biol Med. 1975;48(3):185–195. [PMC free article] [PubMed] [Google Scholar]
- 175.Radin J M, Hawksworth A W, Blair P J. et al. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin Infect Dis. 2014;59(7):962–968. doi: 10.1093/cid/ciu507. [DOI] [PubMed] [Google Scholar]
- 176.Barraza E M, Ludwig S L, Gaydos J C, Brundage J F. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: report of an outbreak during a lapse in vaccination. J Infect Dis. 1999;179(6):1531–1533. doi: 10.1086/314772. [DOI] [PubMed] [Google Scholar]
- 177.Hendrix R M, Lindner J L, Benton F R. et al. Large, persistent epidemic of adenovirus type 4-associated acute respiratory disease in U.S. army trainees. Emerg Infect Dis. 1999;5(6):798–801. doi: 10.3201/eid0506.990609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gray G C, Goswami P R, Malasig M D. et al. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. Clin Infect Dis. 2000;31(3):663–670. doi: 10.1086/313999. [DOI] [PubMed] [Google Scholar]
- 179.Kuschner R A, Russell K L, Abuja M. et al. A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine. 2013;31(28):2963–2971. doi: 10.1016/j.vaccine.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 180.Leen A M, Rooney C M. Adenovirus as an emerging pathogen in immunocompromised patients. Br J Haematol. 2005;128(2):135–144. doi: 10.1111/j.1365-2141.2004.05218.x. [DOI] [PubMed] [Google Scholar]
- 181.Baldwin A, Kingman H, Darville M. et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant. 2000;26(12):1333–1338. doi: 10.1038/sj.bmt.1702716. [DOI] [PubMed] [Google Scholar]
- 182.La Rosa A M, Champlin R E, Mirza N. et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32(6):871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 183.George D, El-Mallawany N K, Jin Z. et al. Adenovirus infection in paediatric allogeneic stem cell transplantation recipients is a major independent factor for significantly increasing the risk of treatment related mortality. Br J Haematol. 2012;156(1):99–108. doi: 10.1111/j.1365-2141.2010.08468.x. [DOI] [PubMed] [Google Scholar]
- 184.Fowler C J, Dunlap J, Troyer D, Stenzel P, Epner E, Maziarz R T. Life-threatening adenovirus infections in the setting of the immunocompromised allogeneic stem cell transplant patients. Adv Hematol. 2010;2010:601548. doi: 10.1155/2010/601548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abinun M, Flood T J, Cant A J. et al. Autologous T cell depleted haematopoietic stem cell transplantation in children with severe juvenile idiopathic arthritis in the UK (2000-2007) Mol Immunol. 2009;47(1):46–51. doi: 10.1016/j.molimm.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 186.Kohno K, Nagafuji K, Tsukamoto H. et al. Infectious complications in patients receiving autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Transpl Infect Dis. 2009;11(4):318–323. doi: 10.1111/j.1399-3062.2009.00401.x. [DOI] [PubMed] [Google Scholar]
- 187.Mori Y, Miyamoto T, Kamezaki K. et al. Low incidence of adenovirus hemorrhagic cystitis following autologous hematopoietic stem cell transplantation in the rituximab era. Am J Hematol. 2012;87(8):828–830. doi: 10.1002/ajh.23247. [DOI] [PubMed] [Google Scholar]
- 188.Yusuf U, Hale G A, Carr J. et al. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81(10):1398–1404. doi: 10.1097/01.tp.0000209195.95115.8e. [DOI] [PubMed] [Google Scholar]
- 189.Leruez-Ville M, Minard V, Lacaille F. et al. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis. 2004;38(1):45–52. doi: 10.1086/380450. [DOI] [PubMed] [Google Scholar]
- 190.Runde V, Ross S, Trenschel R. et al. Adenoviral infection after allogeneic stem cell transplantation (SCT): report on 130 patients from a single SCT unit involved in a prospective multi center surveillance study. Bone Marrow Transplant. 2001;28(1):51–57. doi: 10.1038/sj.bmt.1703083. [DOI] [PubMed] [Google Scholar]
- 191.Lee Y J, Chung D, Xiao K. et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant. 2013;19(3):387–392. doi: 10.1016/j.bbmt.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Muller W J, Levin M J, Shin Y K. et al. Clinical and in vitro evaluation of cidofovir for treatment of adenovirus infection in pediatric hematopoietic stem cell transplant recipients. Clin Infect Dis. 2005;41(12):1812–1816. doi: 10.1086/498151. [DOI] [PubMed] [Google Scholar]
- 193.Sive J I, Thomson K J, Morris E C, Ward K N, Peggs K S. Adenoviremia has limited clinical impact in the majority of patients following alemtuzumab-based allogeneic stem cell transplantation in adults. Clin Infect Dis. 2012;55(10):1362–1370. doi: 10.1093/cid/cis689. [DOI] [PubMed] [Google Scholar]
- 194.Lankester A C, van Tol M J, Claas E C, Vossen J M, Kroes A C. Quantification of adenovirus DNA in plasma for management of infection in stem cell graft recipients. Clin Infect Dis. 2002;34(6):864–867. doi: 10.1086/339073. [DOI] [PubMed] [Google Scholar]
- 195.Schilham M W, Claas E C, van Zaane W. et al. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis. 2002;35(5):526–532. doi: 10.1086/341770. [DOI] [PubMed] [Google Scholar]
- 196.Humar A, Kumar D, Mazzulli T. et al. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005;5(10):2555–2559. doi: 10.1111/j.1600-6143.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 197.Hierholzer J C. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5(3):262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Michaels M G, Green M, Wald E R, Starzl T E. Adenovirus infection in pediatric liver transplant recipients. J Infect Dis. 1992;165(1):170–174. doi: 10.1093/infdis/165.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McGrath D, Falagas M E, Freeman R. et al. Adenovirus infection in adult orthotopic liver transplant recipients: incidence and clinical significance. J Infect Dis. 1998;177(2):459–462. doi: 10.1086/517375. [DOI] [PubMed] [Google Scholar]
- 200.Bridges N D, Spray T L, Collins M H, Bowles N E, Towbin J A. Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg. 1998;116(4):617–623. doi: 10.1016/S0022-5223(98)70168-0. [DOI] [PubMed] [Google Scholar]
- 201.Florescu M C, Miles C D, Florescu D F. What do we know about adenovirus in renal transplantation? Nephrol Dial Transplant. 2013;28(8):2003–2010. doi: 10.1093/ndt/gft036. [DOI] [PubMed] [Google Scholar]
- 202.Watcharananan S P, Avery R, Ingsathit A. et al. Adenovirus disease after kidney transplantation: course of infection and outcome in relation to blood viral load and immune recovery. Am J Transplant. 2011;11(6):1308–1314. doi: 10.1111/j.1600-6143.2011.03479.x. [DOI] [PubMed] [Google Scholar]
- 203.Florescu D F, Kwon J Y, Dumitru I. Adenovirus infections in heart transplantation. Cardiol Rev. 2013;21(4):203–206. doi: 10.1097/CRD.0b013e31828da5b7. [DOI] [PubMed] [Google Scholar]
- 204.Bruminhent J, Athas D M, Hess B D, Flomenberg P. Disseminated adenovirus disease in heart transplant recipient presenting with conjunctivitis. Transpl Infect Dis. 2015;17(1):125–128. doi: 10.1111/tid.12342. [DOI] [PubMed] [Google Scholar]
- 205.McLaughlin G E, Delis S, Kashimawo L. et al. Adenovirus infection in pediatric liver and intestinal transplant recipients: utility of DNA detection by PCR. Am J Transplant. 2003;3(2):224–228. doi: 10.1034/j.1600-6143.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 206.Florescu D F, Islam M K, Mercer D F. et al. Adenovirus infections in pediatric small bowel transplant recipients. Transplantation. 2010;90(2):198–204. doi: 10.1097/TP.0b013e3181e0de97. [DOI] [PubMed] [Google Scholar]
- 207.Ohori N P, Michaels M G, Jaffe R, Williams P, Yousem S A. Adenovirus pneumonia in lung transplant recipients. Hum Pathol. 1995;26(10):1073–1079. doi: 10.1016/0046-8177(95)90268-6. [DOI] [PubMed] [Google Scholar]
- 208.Humar A, Doucette K, Kumar D. et al. Assessment of adenovirus infection in adult lung transplant recipients using molecular surveillance. J Heart Lung Transplant. 2006;25(12):1441–1446. doi: 10.1016/j.healun.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Doan M L, Mallory G B, Kaplan S L. et al. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant. 2007;26(9):883–889. doi: 10.1016/j.healun.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 210.Florescu D F Hoffman J A; AST Infectious Diseases Community of Practice. Adenovirus in solid organ transplantation Am J Transplant 201313404206–211. [DOI] [PubMed] [Google Scholar]
- 211.Moulik M, Breinholt J P, Dreyer W J. et al. Viral endomyocardial infection is an independent predictor and potentially treatable risk factor for graft loss and coronary vasculopathy in pediatric cardiac transplant recipients. J Am Coll Cardiol. 2010;56(7):582–592. doi: 10.1016/j.jacc.2010.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shirali G S, Ni J, Chinnock R E. et al. Association of viral genome with graft loss in children after cardiac transplantation. N Engl J Med. 2001;344(20):1498–1503. doi: 10.1056/NEJM200105173442002. [DOI] [PubMed] [Google Scholar]
- 213.Schowengerdt K O, Ni J, Denfield S W. et al. Diagnosis, surveillance, and epidemiologic evaluation of viral infections in pediatric cardiac transplant recipients with the use of the polymerase chain reaction. J Heart Lung Transplant. 1996;15(2):111–123. [PubMed] [Google Scholar]
- 214.Simsir A, Greenebaum E, Nuovo G, Schulman L L. Late fatal adenovirus pneumonitis in a lung transplant recipient. Transplantation. 1998;65(4):592–594. doi: 10.1097/00007890-199802270-00027. [DOI] [PubMed] [Google Scholar]
- 215.Ferdman R M, Ross L, Inderlied C, Church J A. Adenovirus viremia in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1997;16(4):413–415. doi: 10.1097/00006454-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 216.Khoo S H, Bailey A S, de Jong J C, Mandal B K. Adenovirus infections in human immunodeficiency virus-positive patients: clinical features and molecular epidemiology. J Infect Dis. 1995;172(3):629–637. doi: 10.1093/infdis/172.3.629. [DOI] [PubMed] [Google Scholar]
- 217.Kolawole O M, Oladosu T O, Abdulkarim A A, Okoh A I. Prevalence of adenovirus respiratory tract and hiv co-infections in patients attending the University of Ilorin, teaching hospital, Ilorin, Nigeria. BMC Res Notes. 2014;7:870. doi: 10.1186/1756-0500-7-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.de Jong P J, Valderrama G, Spigland I, Horwitz M S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet. 1983;1(8337):1293–1296. doi: 10.1016/s0140-6736(83)92411-x. [DOI] [PubMed] [Google Scholar]
- 219.Winkelstein J A, Marino M C, Lederman H M. et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85(4):193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 220.Baum S. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. Adenovirus; pp. 1835–1841. [Google Scholar]
- 221.Centers for Disease Control and Prevention (CDC) . Acute respiratory disease associated with adenovirus serotype 14—four states, 2006-2007. MMWR Morb Mortal Wkly Rep. 2007;56(45):1181–1184. [PubMed] [Google Scholar]
- 222.Esposito D H, Gardner T J, Schneider E. et al. Outbreak of pneumonia associated with emergent human adenovirus serotype 14—Southeast Alaska, 2008. J Infect Dis. 2010;202(2):214–222. doi: 10.1086/653498. [DOI] [PubMed] [Google Scholar]
- 223.Chmielewicz B, Benzler J, Pauli G, Krause G, Bergmann F, Schweiger B. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J Med Virol. 2005;77(2):232–237. doi: 10.1002/jmv.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kajon A E, Mistchenko A S, Videla C, Hortal M, Wadell G, Avendaño L F. Molecular epidemiology of adenovirus acute lower respiratory infections of children in the south cone of South America (1991-1994) J Med Virol. 1996;48(2):151–156. doi: 10.1002/(SICI)1096-9071(199602)48:2<151::AID-JMV6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 225.Ison M G Green M; AST Infectious Diseases Community of Practice. Adenovirus in solid organ transplant recipients Am J Transplant 20099404S161–S165. [DOI] [PubMed] [Google Scholar]
- 226.Elnifro E M, Cooper R J, Klapper P E, Bailey A S, Tullo A B. Diagnosis of viral and chlamydial keratoconjunctivitis: which laboratory test? Br J Ophthalmol. 1999;83(5):622–627. doi: 10.1136/bjo.83.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Uhnoo I, Wadell G, Svensson L, Johansson M E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wold W, Horwitz M. Philadelphia, PA: Lippincott, Williams & Wilkins, Inc.; 2007. Adenoviruses; pp. 2395–2436. [Google Scholar]
- 229.Suparno C, Milligan D W, Moss P A, Mautner V. Adenovirus infections in stem cell transplant recipients: recent developments in understanding of pathogenesis, diagnosis and management. Leuk Lymphoma. 2004;45(5):873–885. doi: 10.1080/10428190310001628176. [DOI] [PubMed] [Google Scholar]
- 230.van Kraaij M G, van Elden L J, van Loon A M. et al. Frequent detection of respiratory viruses in adult recipients of stem cell transplants with the use of real-time polymerase chain reaction, compared with viral culture. Clin Infect Dis. 2005;40(5):662–669. doi: 10.1086/427801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wallot M A, Dohna-Schwake C, Auth M. et al. Disseminated adenovirus infection with respiratory failure in pediatric liver transplant recipients: impact of intravenous cidofovir and inhaled nitric oxide. Pediatr Transplant. 2006;10(1):121–127. doi: 10.1111/j.1399-3046.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 232.Landry M L, Lebeck M G, Capuano A W, McCarthy T, Gray G C. Adenovirus type 3 outbreak in connecticut associated with a novel variant. J Med Virol. 2009;81(8):1380–1384. doi: 10.1002/jmv.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Adhikary A K, Inada T, Banik U, Numaga J, Okabe N. Identification of subgenus C adenoviruses by fiber-based multiplex PCR. J Clin Microbiol. 2004;42(2):670–673. doi: 10.1128/JCM.42.2.670-673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kajon A E, Erdman D D. Assessment of genetic variability among subspecies b1 human adenoviruses for molecular epidemiology studies. Methods Mol Med. 2007;131:335–355. doi: 10.1007/978-1-59745-277-9_23. [DOI] [PubMed] [Google Scholar]
- 235.Xu W, Erdman D D. Type-specific identification of human adenovirus 3, 7, and 21 by a multiplex PCR assay. J Med Virol. 2001;64(4):537–542. doi: 10.1002/jmv.1083. [DOI] [PubMed] [Google Scholar]
- 236.Li Q G, Wadell G. Analysis of 15 different genome types of adenovirus type 7 isolated on five continents. J Virol. 1986;60(1):331–335. doi: 10.1128/jvi.60.1.331-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Robinson C M, Zhou X, Rajaiya J. et al. Predicting the next eye pathogen: analysis of a novel adenovirus. MBio. 2013;4(2):e00595–e12. doi: 10.1128/mBio.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Robinson C M, Seto D, Jones M S, Dyer D W, Chodosh J. Molecular evolution of human species D adenoviruses. Infect Genet Evol. 2011;11(6):1208–1217. doi: 10.1016/j.meegid.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Robinson C M, Singh G, Henquell C. et al. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology. 2011;409(2):141–147. doi: 10.1016/j.virol.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang Q, Seto D, Cao B, Zhao S, Wan C. Genome sequence of human adenovirus type 55, a re-emergent acute respiratory disease pathogen in China. J Virol. 2012;86(22):12441–12442. doi: 10.1128/JVI.02225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Seto D Chodosh J Brister J R Jones M S; Members of the Adenovirus Research Community. Using the whole-genome sequence to characterize and name human adenoviruses J Virol 201185115701–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yamadera S, Yamashita K, Akatsuka M, Kato N, Inouye S. Trend of adenovirus type 7 infection, an emerging disease in Japan. A report of the National Epidemiological Surveillance of Infectious Agents in Japan. Jpn J Med Sci Biol. 1998;51(1):43–51. doi: 10.7883/yoken1952.51.43. [DOI] [PubMed] [Google Scholar]
- 243.Azar R, Varsano N, Mileguir F, Mendelson E. Molecular epidemiology of adenovirus type 7 in Israel: identification of two new genome types, Ad7k and Ad7d2. J Med Virol. 1998;54(4):291–299. doi: 10.1002/(sici)1096-9071(199804)54:4<291::aid-jmv9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 244.Wadell G, Cooney M K, da Costa Linhares A. et al. Molecular epidemiology of adenoviruses: global distribution of adenovirus 7 genome types. J Clin Microbiol. 1985;21(3):403–408. doi: 10.1128/jcm.21.3.403-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Metzgar D, Osuna M, Yingst S. et al. PCR analysis of egyptian respiratory adenovirus isolates, including identification of species, serotypes, and coinfections. J Clin Microbiol. 2005;43(11):5743–5752. doi: 10.1128/JCM.43.11.5743-5752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kajon A E, Wadell G. Molecular epidemiology of adenoviruses associated with acute lower respiratory disease of children in Buenos Aires, Argentina (1984-1988) J Med Virol. 1992;36(4):292–297. doi: 10.1002/jmv.1890360411. [DOI] [PubMed] [Google Scholar]
- 247.Piedra P A, Poveda G A, Ramsey B, McCoy K, Hiatt P W. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics. 1998;101(6):1013–1019. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- 248.Cooper R J, Hallett R, Tullo A B, Klapper P E. The epidemiology of adenovirus infections in Greater Manchester, UK 1982-96. Epidemiol Infect. 2000;125(2):333–345. doi: 10.1017/s0950268899004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kim Y J, Hong J Y, Lee H J. et al. Genome type analysis of adenovirus types 3 and 7 isolated during successive outbreaks of lower respiratory tract infections in children. J Clin Microbiol. 2003;41(10):4594–4599. doi: 10.1128/JCM.41.10.4594-4599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Murtagh P, Cerqueiro C, Halac A, Avila M, Kajon A. Adenovirus type 7h respiratory infections: a report of 29 cases of acute lower respiratory disease. Acta Paediatr. 1993;82(6–7):557–561. doi: 10.1111/j.1651-2227.1993.tb12753.x. [DOI] [PubMed] [Google Scholar]
- 251.Li Q G, Wadell G. Comparison of 17 genome types of adenovirus type 3 identified among strains recovered from six continents. J Clin Microbiol. 1988;26(5):1009–1015. doi: 10.1128/jcm.26.5.1009-1015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Adrian T, Best B, Hierholzer J C, Wigand R. Molecular epidemiology and restriction site mapping of adenovirus type 3 genome types. J Clin Microbiol. 1989;27(6):1329–1334. doi: 10.1128/jcm.27.6.1329-1334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cassir N, Hraiech S, Nougairede A, Zandotti C, Fournier P E, Papazian L. Outbreak of adenovirus type 1 severe pneumonia in a French intensive care unit, September-October 2012. Euro Surveill. 2014;19(39):20914. doi: 10.2807/1560-7917.es2014.19.39.20914. [DOI] [PubMed] [Google Scholar]
- 254.Rebelo-de-Andrade H, Pereira C, Gíria M. et al. Outbreak of acute respiratory infection among infants in Lisbon, Portugal, caused by human adenovirus serotype 3 and a new 7/3 recombinant strain. J Clin Microbiol. 2010;48(4):1391–1396. doi: 10.1128/JCM.02019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kandel R, Srinivasan A, D'Agata E M, Lu X, Erdman D, Jhung M. Outbreak of adenovirus type 4 infection in a long-term care facility for the elderly. Infect Control Hosp Epidemiol. 2010;31(7):755–757. doi: 10.1086/653612. [DOI] [PubMed] [Google Scholar]
- 256.Top F H Jr, Buescher E L, Bancroft W H, Russell P K. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J Infect Dis. 1971;124(2):155–160. doi: 10.1093/infdis/124.2.155. [DOI] [PubMed] [Google Scholar]
- 257.Top F H Jr, Grossman R A, Bartelloni P J. et al. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis. 1971;124(2):148–154. doi: 10.1093/infdis/124.2.148. [DOI] [PubMed] [Google Scholar]
- 258.Choi E H, Kim H S, Eun B W. et al. Adenovirus type 7 peptide diversity during outbreak, Korea, 1995-2000. Emerg Infect Dis. 2005;11(5):649–654. doi: 10.3201/eid1105.041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Noda M, Yoshida T, Sakaguchi T, Ikeda Y, Yamaoka K, Ogino T. Molecular and epidemiological analyses of human adenovirus type 7 strains isolated from the 1995 nationwide outbreak in Japan. J Clin Microbiol. 2002;40(1):140–145. doi: 10.1128/JCM.40.1.140-145.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cho C T, Hiatt W O, Behbehani A M. Pneumonia and massive pleural effusion associated with adenovirus type 7. Am J Dis Child. 1973;126(1):92–94. doi: 10.1001/archpedi.1973.02110190080017. [DOI] [PubMed] [Google Scholar]
- 261.Yamamoto D, Okamoto M, Lupisan S. et al. Impact of human adenovirus serotype 7 in hospitalized children with severe fatal pneumonia in the Philippines. Jpn J Infect Dis. 2014;67(2):105–110. doi: 10.7883/yoken.67.105. [DOI] [PubMed] [Google Scholar]
- 262.Zhao S, Wan C, Ke C. et al. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in Southern China after a twenty-one year absence. Sci Rep. 2014;4:7365. doi: 10.1038/srep07365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Brown R S, Nogrady M B, Spence L, Wiglesworth F W. An outbreak of adenovirus type 7 infection in children in Montreal. Can Med Assoc J. 1973;108(4):434–439. [PMC free article] [PubMed] [Google Scholar]
- 264.Wadell G, Varsányi T M, Lord A, Sutton R N. Epidemic outbreaks of adenovirus 7 with special reference to the pathogenicity of adenovirus genome type 7b. Am J Epidemiol. 1980;112(5):619–628. doi: 10.1093/oxfordjournals.aje.a113034. [DOI] [PubMed] [Google Scholar]
- 265.Cui X, Wen L, Wu Z. et al. Human adenovirus type 7 infection associated with severe and fatal acute lower respiratory illness and nosocomial transmission. J Clin Microbiol. 2015;53(2):746–749. doi: 10.1128/JCM.02517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Centers for Disease Control (CDC) . Adenovirus type 7 outbreak in a pediatric chronic-care facility—Pennsylvania, 1982. MMWR Morb Mortal Wkly Rep. 1983;32(19):258–260. [PubMed] [Google Scholar]
- 267.Kajon A, Wadell G. Genome analysis of South American adenovirus strains of serotype 7 collected over a 7-year period. J Clin Microbiol. 1994;32(9):2321–2323. doi: 10.1128/jcm.32.9.2321-2323.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.de Silva L M, Colditz P, Wadell G. Adenovirus type 7 infections in children in New South Wales, Australia. J Med Virol. 1989;29(1):28–32. doi: 10.1002/jmv.1890290106. [DOI] [PubMed] [Google Scholar]
- 269.Straube R C, Thompson M A, Van Dyke R B. et al. Adenovirus type 7b in a children's hospital. J Infect Dis. 1983;147(5):814–819. doi: 10.1093/infdis/147.5.814. [DOI] [PubMed] [Google Scholar]
- 270.Sakata H, Taketazu G, Nagaya K. et al. Outbreak of severe infection due to adenovirus type 7 in a paediatric ward in Japan. J Hosp Infect. 1998;39(3):207–211. doi: 10.1016/s0195-6701(98)90259-6. [DOI] [PubMed] [Google Scholar]
- 271.Tang L, Wang L, Tan X, Xu W. Adenovirus serotype 7 associated with a severe lower respiratory tract disease outbreak in infants in Shaanxi Province, China. Virol J. 2011;8:23. doi: 10.1186/1743-422X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ersoy Y, Otlu B, Türkçüoğlu P, Yetkin F, Aker S, Kuzucu C. Outbreak of adenovirus serotype 8 conjunctivitis in preterm infants in a neonatal intensive care unit. J Hosp Infect. 2012;80(2):144–149. doi: 10.1016/j.jhin.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 273.Adhikary A K, Banik U. Human adenovirus type 8: the major agent of epidemic keratoconjunctivitis (EKC) J Clin Virol. 2014;61(4):477–486. doi: 10.1016/j.jcv.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 274.Metzgar D, Osuna M, Kajon A E, Hawksworth A W, Irvine M, Russell K L. Abrupt emergence of diverse species B adenoviruses at US military recruit training centers. J Infect Dis. 2007;196(10):1465–1473. doi: 10.1086/522970. [DOI] [PubMed] [Google Scholar]
- 275.Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41(3):696–701. doi: 10.1053/ajkd.2003.50133. [DOI] [PubMed] [Google Scholar]
- 276.Kendall E J, Riddle R W, Tuck H A, Rodan K S, Andrews B E, McDonald J C. Pharyngo-conjunctival fever; school outbreaks in England during the summer of 1955 associated with adenovirus types 3, 7, and 14. BMJ. 1957;2(5037):131–136. doi: 10.1136/bmj.2.5037.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tate J E, Bunning M L, Lott L. et al. Outbreak of severe respiratory disease associated with emergent human adenovirus serotype 14 at a US air force training facility in 2007. J Infect Dis. 2009;199(10):1419–1426. doi: 10.1086/598520. [DOI] [PubMed] [Google Scholar]
- 278.Lewis P F, Schmidt M A, Lu X. et al. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis. 2009;199(10):1427–1434. doi: 10.1086/598521. [DOI] [PubMed] [Google Scholar]
- 279.O'Flanagan D, O'Donnell J, Domegan L. et al. First reported cases of human adenovirus serotype 14p1 infection, Ireland, October 2009 to July 2010. Euro Surveill. 2011;16(8):1–5. [PubMed] [Google Scholar]
- 280.Van Der Veen J, Dijkman J H. Association of type 21 adenovirus with acute respiratory illness in military recruits. Am J Hyg. 1962;76:149–159. doi: 10.1093/oxfordjournals.aje.a120270. [DOI] [PubMed] [Google Scholar]
- 281.van der Avoort H G, Adrian T, Wigand R, Wermenbol A G, Zomerdijk T P, de Jong J C. Molecular epidemiology of adenovirus type 21 in the Netherlands and the Federal Republic of Germany from 1960 to 1985. J Clin Microbiol. 1986;24(6):1084–1088. doi: 10.1128/jcm.24.6.1084-1088.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Larsen R A, Jacobson J T, Jacobson J A, Strikas R A, Hierholzer J C. Hospital-associated epidemic of pharyngitis and conjunctivitis caused by adenovirus (21/H21 + 35) J Infect Dis. 1986;154(4):706–709. doi: 10.1093/infdis/154.4.706. [DOI] [PubMed] [Google Scholar]
- 283.Shult P A, Polyak F, Dick E C, Warshauer D M, King L A, Mandel A D. Adenovirus 21 infection in an isolated antarctic station: transmission of the virus and susceptibility of the population. Am J Epidemiol. 1991;133(6):599–607. doi: 10.1093/oxfordjournals.aje.a115932. [DOI] [PubMed] [Google Scholar]
- 284.Ooi M H, Wong S C, Clear D. et al. Adenovirus type 21-associated acute flaccid paralysis during an outbreak of hand-foot-and-mouth disease in Sarawak, Malaysia. Clin Infect Dis. 2003;36(5):550–559. doi: 10.1086/367648. [DOI] [PubMed] [Google Scholar]
- 285.Cardosa M J, Krishnan S, Tio P H, Perera D, Wong S C. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet. 1999;354(9183):987–991. doi: 10.1016/S0140-6736(98)11032-2. [DOI] [PubMed] [Google Scholar]
- 286.Seidemann K, Heim A, Pfister E D. et al. Monitoring of adenovirus infection in pediatric transplant recipients by quantitative PCR: report of six cases and review of the literature. Am J Transplant. 2004;4(12):2102–2108. doi: 10.1111/j.1600-6143.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 287.Kampmann B, Cubitt D, Walls T. et al. Improved outcome for children with disseminated adenoviral infection following allogeneic stem cell transplantation. Br J Haematol. 2005;130(4):595–603. doi: 10.1111/j.1365-2141.2005.05649.x. [DOI] [PubMed] [Google Scholar]
- 288.Leruez-Ville M, Chardin-Ouachée M, Neven B. et al. Description of an adenovirus A31 outbreak in a paediatric haematology unit. Bone Marrow Transplant. 2006;38(1):23–28. doi: 10.1038/sj.bmt.1705389. [DOI] [PubMed] [Google Scholar]
- 289.Slatter M A, Read S, Taylor C E. et al. Adenovirus type F subtype 41 causing disseminated disease following bone marrow transplantation for immunodeficiency. J Clin Microbiol. 2005;43(3):1462–1464. doi: 10.1128/JCM.43.3.1462-1464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Li X, Kong M, Su X. et al. An outbreak of acute respiratory disease in China caused by human adenovirus type B55 in a physical training facility. Int J Infect Dis. 2014;28:117–122. doi: 10.1016/j.ijid.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sun B, He H, Wang Z. et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. 2014;18(4):456. doi: 10.1186/s13054-014-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kajon A E, de Jong J C, Dickson L M. et al. Molecular and serological characterization of species B2 adenovirus strains isolated from children hospitalized with acute respiratory disease in Buenos Aires, Argentina. J Clin Virol. 2013;58(1):4–10. doi: 10.1016/j.jcv.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 293.Hierholzer J C, Pumarola A. Antigenic characterization of intermediate adenovirus 14-11 strains associated with upper respiratory illness in a military camp. Infect Immun. 1976;13(2):354–359. doi: 10.1128/iai.13.2.354-359.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li Q G, Hambraeus J, Wadell G. Genetic relationship between thirteen genome types of adenovirus 11, 34, and 35 with different tropisms. Intervirology. 1991;32(6):338–350. doi: 10.1159/000150218. [DOI] [PubMed] [Google Scholar]
- 295.Erard V, Huang M L, Ferrenberg J. et al. Quantitative real-time polymerase chain reaction for detection of adenovirus after T cell-replete hematopoietic cell transplantation: viral load as a marker for invasive disease. Clin Infect Dis. 2007;45(8):958–965. doi: 10.1086/521851. [DOI] [PubMed] [Google Scholar]
- 296.Lankester A C, Heemskerk B, Claas E C. et al. Effect of ribavirin on the plasma viral DNA load in patients with disseminating adenovirus infection. Clin Infect Dis. 2004;38(11):1521–1525. doi: 10.1086/420817. [DOI] [PubMed] [Google Scholar]
- 297.Lion T, Kosulin K, Landlinger C. et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24(4):706–714. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- 298.Sarantis H, Johnson G, Brown M, Petric M, Tellier R. Comprehensive detection and serotyping of human adenoviruses by PCR and sequencing. J Clin Microbiol. 2004;42(9):3963–3969. doi: 10.1128/JCM.42.9.3963-3969.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kajon A E, Dickson L M, Murtagh P, Viale D, Carballal G, Echavarria M. Molecular characterization of an adenovirus 3-16 intertypic recombinant isolated in Argentina from an infant hospitalized with acute respiratory infection. J Clin Microbiol. 2010;48(4):1494–1496. doi: 10.1128/JCM.02289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kajon A E, Lamson D, Shudt M. et al. Identification of a novel intertypic recombinant species D human adenovirus in a pediatric stem cell transplant recipient. J Clin Virol. 2014;61(4):496–502. doi: 10.1016/j.jcv.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Naesens L, Lenaerts L, Andrei G. et al. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob Agents Chemother. 2005;49(3):1010–1016. doi: 10.1128/AAC.49.3.1010-1016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Morfin F, Dupuis-Girod S, Mundweiler S. et al. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir Ther. 2005;10(2):225–229. [PubMed] [Google Scholar]
- 303.Gavin P J, Katz B Z. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics. 2002;110(1 Pt 1):e9. doi: 10.1542/peds.110.1.e9. [DOI] [PubMed] [Google Scholar]
- 304.Bhadri V A, Lee-Horn L, Shaw P J. Safety and tolerability of cidofovir in high-risk pediatric patients. Transpl Infect Dis. 2009;11(4):373–379. doi: 10.1111/j.1399-3062.2009.00391.x. [DOI] [PubMed] [Google Scholar]
- 305.Hartline C B, Gustin K M, Wan W B. et al. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis. 2005;191(3):396–399. doi: 10.1086/426831. [DOI] [PubMed] [Google Scholar]
- 306.Toth K, Spencer J F, Dhar D. et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci U S A. 2008;105(20):7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tollefson A E, Spencer J F, Ying B, Buller R M, Wold W S, Toth K. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res. 2014;112:38–46. doi: 10.1016/j.antiviral.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 308.Florescu D F, Pergam S A, Neely M N. et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18(5):731–738. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Paolino K, Sande J, Perez E. et al. Eradication of disseminated adenovirus infection in a pediatric hematopoietic stem cell transplantation recipient using the novel antiviral agent CMX001. J Clin Virol. 2011;50(2):167–170. doi: 10.1016/j.jcv.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 310.Marty F M, Winston D J, Rowley S D. et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 311.Ljungman P, Ribaud P, Eyrich M. et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31(6):481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 312.Legrand F, Berrebi D, Houhou N. et al. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 2001;27(6):621–626. doi: 10.1038/sj.bmt.1702820. [DOI] [PubMed] [Google Scholar]
- 313.Mynarek M, Ganzenmueller T, Mueller-Heine A. et al. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: results of a routine monitoring program. Biol Blood Marrow Transplant. 2014;20(2):250–256. doi: 10.1016/j.bbmt.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 314.Engelmann G, Heim A, Greil J. et al. Adenovirus infection and treatment with cidofovir in children after liver transplantation. Pediatr Transplant. 2009;13(4):421–428. doi: 10.1111/j.1399-3046.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 315.Refaat M, McNamara D, Teuteberg J. et al. Successful cidofovir treatment in an adult heart transplant recipient with severe adenovirus pneumonia. J Heart Lung Transplant. 2008;27(6):699–700. doi: 10.1016/j.healun.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 316.Saquib R, Melton L B, Chandrakantan A. et al. Disseminated adenovirus infection in renal transplant recipients: the role of cidofovir and intravenous immunoglobulin. Transpl Infect Dis. 2010;12(1):77–83. doi: 10.1111/j.1399-3062.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 317.Anderson E J, Guzman-Cottrill J A, Kletzel M. et al. High-risk adenovirus-infected pediatric allogeneic hematopoietic progenitor cell transplant recipients and preemptive cidofovir therapy. Pediatr Transplant. 2008;12(2):219–227. doi: 10.1111/j.1399-3046.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 318.Nagafuji K, Aoki K, Henzan H. et al. Cidofovir for treating adenoviral hemorrhagic cystitis in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2004;34(10):909–914. doi: 10.1038/sj.bmt.1704682. [DOI] [PubMed] [Google Scholar]
- 319.Chakrabarti S, Mautner V, Osman H. et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100(5):1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 320.Heemskerk B, Lankester A C, van Vreeswijk T. et al. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J Infect Dis. 2005;191(4):520–530. doi: 10.1086/427513. [DOI] [PubMed] [Google Scholar]
- 321.van Tol M J, Claas E C, Heemskerk B. et al. Adenovirus infection in children after allogeneic stem cell transplantation: diagnosis, treatment and immunity. Bone Marrow Transplant. 2005;35 01:S73–S76. doi: 10.1038/sj.bmt.1704852. [DOI] [PubMed] [Google Scholar]
- 322.Papadopoulou A, Gerdemann U, Katari U L. et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6(242):242ra83. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gerdemann U, Katari U L, Papadopoulou A. et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21(11):2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Feucht J, Opherk K, Lang P. et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125(12):1986–1994. doi: 10.1182/blood-2014-06-573725. [DOI] [PubMed] [Google Scholar]
- 325.Binn L N, Sanchez J L, Gaydos J C. Emergence of adenovirus type 14 in US military recruits—a new challenge. J Infect Dis. 2007;196(10):1436–1437. doi: 10.1086/522969. [DOI] [PubMed] [Google Scholar]


