Abstract
Human rhinovirus (HRV) and coronavirus (HCoV) infections are associated with both upper respiratory tract illness (“the common cold”) and lower respiratory tract illness (pneumonia). New species of HRVs and HCoVs have been diagnosed in the past decade. More sensitive diagnostic tests such as reverse transcription-polymerase chain reaction have expanded our understanding of the role these viruses play in both immunocompetent and immunosuppressed hosts. Recent identification of severe acute respiratory syndrome and Middle East respiratory syndrome viruses causing serious respiratory illnesses has led to renewed efforts for vaccine development. The role these viruses play in patients with chronic lung disease such as asthma makes the search for antiviral agents of increased importance.
Keywords: human rhinovirus, human coronavirus, polymerase chain reaction
More than 50% of all common colds are caused by human rhinoviruses (HRVs) and coronaviruses (HCoVs).1 2 3 The common cold includes rhinitis and pharyngitis, as well as sneezing, hoarseness, and cough.4 5 The illness is usually self-limited in healthy people, but can be associated with complications in individuals who suffer from heart or lung disease, or who are immunosuppressed.6 7 8 9 10 11 As a frequent cause of the common cold, these viruses are responsible for millions of lost work days, school absenteeism, and physician visits. In infants and young children, these viruses are associated with many cases of acute otitis media (AOM) and rhinosinusitis.12 Newer more sensitive diagnostic tests have increased the percentage of respiratory illnesses attributable to these viruses.13 14 15 Most HRV infections are symptomatic, but asymptomatic virus shedding has been reported with increasing frequency.
In the past 10 years, several new HRVs and HCoVs have been identified.16 17 18 In addition, recent studies have provided a better understanding of the pathogenesis of these viral infections which could lead to more specific treatments. This review will update recent advances in our knowledge of these two virus groups and their importance in respiratory viral infections.
Rhinoviruses
Virology
HRVs are positive-sense, single-stranded ribonucleic acid (RNA) viruses with icosahedral symmetry. The capsid is composed of four proteins: VP1, VP2, VP3, and VP4.4 19 VP4 is on the inside of the virus and anchors the RNA core to the viral capsid. The complete sequencing of all known HRV genomes has been reported.20
More than 150 serotypes of HRVs have been identified. Most of the HRV serotypes of the HRV-A and HRV-B families use intercellular adhesion molecule-1 (ICAM-1; major group) as the cell receptor for the virus entry. A few HRVs used heparin sulfate proteoglycan as an additional receptor and approximately 10 serotypes use low-density lipoprotein as the cell receptor (minor group). In 2002, a new clade of HRVs was reported and labeled HRV-C.19 Although HRV-C strains have been characterized by genome sequencing, the receptor used to infect epithelial cells is currently unidentified.
During replication, the RNA genome serves as an mRNA, which encodes the capsid or structural proteins, as well as the nonstructural proteins.4 After cell entry, the viral genome is translated into a polyprotein, which undergoes proteolytic cleavage to produce the structural and nonstructural gene products. Serotypes of HRVs are based on amino acid differences in the four capsid proteins.
Enteroviruses (EVs) are not typically thought of as causing acute respiratory tract infections. However, EV68 has properties of both EVs and HRVs, and has been independently identified as HRV serotype 87.21 EV68 and EV104 have been associated with respiratory illness in multiple countries.22 23 24 25 26
Pathogenesis
HRV infections initially involve the upper respiratory tract. After deposition of HRV in the eye or nose, the virus attaches to host cell epithelium. Infection of nasal epithelial cells results in increased neutrophils detectable in the nasal mucosa and secretions with release of inflammatory mediators. Although HRVs replicate best at 33°C, they have been found in lower respiratory tract cells where the temperature is 37°C.
HRVS do not produce cytopathologic changes; however, HRVs appear to disrupt tight junctions between cells, leading to increased vascular leakage and mucus secretion. Using primary nasal epithelial cells, HRV was found to alter the function and the expression of cystic fibrosis transmembrane conductance regulator (CFTR), α-epithelial sodium channel (α-ENaC), β-epithelial sodium channel (β-ENaC), and γ-epithelial sodium channel (γ-ENaC).27 These findings may help explain the mechanism by which altered mucociliary clearance is associated with HRV infections.
Most of the symptoms following HRV infection can be ascribed to the host response to the virus, including those mediated by the innate, mucosal, and cellular immune systems. Following epithelial cell attachment and internalization, HRV infection results in cytokine expression, including type I and type III interferons (IFNs), interleukin (IL)-6, IL-11, IL-12, IL-15, and IL-1B.28 29 30 31 32 Neutrophil recruitment results from release of growth factors and chemokines.
VP-I binds to ICAM-1 and is the major target for the memory immune response, residing in IgG1 subclass and IgA class antibodies. The majority of VP-1-specific antibodies are directed against an N-terminal 20-mer peptide (Pla), which is located inside the capsid. The antibody response to HRV in humans appears to be misdirected against a nonneutralizing epitope that is exposed during uncoating.
In a group of children with asthma and upper respiratory infections (URIs), type III IFN-λ levels were significantly higher in wheezing children infected with HRV compared with non-wheezing children.33 Although type III IFN-λ levels were lower at baseline in children with asthma compared with healthy children, they were found to be hyperexpressed during asthma exacerbation. In addition, a study reported that IFN-λ has antiviral and anti-inflammatory properties in primary epithelial cells from asthmatic patients exposed to rhinovirus.34
In a recently published mouse model of HRV infection, adult mice had an enhanced type 2 immune response and attenuated type 1 response compared with neonatal mice.35 The authors reported long-term mucosal metaplasia and airway responsiveness in HRV-infected neonatal mice. In addition, they found increased expression of epithelial IL-25 with neonatal rhinovirus infection. IL-25 appears to mediate metaplasia and airway hyper-responsiveness in the HRV-infected neonatal mice. Induction of type 1 cytokines, IFN-γ, IL-12p40, and tumor necrosis factor-α was diminished in neonates compared with adult mice. HRV infection elicits an increased ILC2 response in neonatal mice. These observations may help our understanding of the intersection of early HRV infections and subsequent development of asthma.
Epidemiology
HRVs cause respiratory illnesses throughout the world, in all age groups, and throughout the year, although most prevalent in the fall and spring in temperate climates.36 In one study, HRVs accounted for approximately 50% of common colds.37 HRVs commonly infect children in early childhood and into adulthood. School-aged children frequently introduce HRV infections into the home. Secondary attack rates range from 25 to 70%. Day-care centers and schools are also important locations for the spread of HRV. HRV transmission can occur by close contact, autoinoculation, fomites, or aerosols.
Clinical outcomes appear to be similar between the HRV species. The recently identified HRV-C infections can have symptoms of the common cold, pharyngitis, croup, AOM, bronchiolitis, and/or pneumonia.38 39 40 These infections have been reported in healthy children and adults, as well as in those with asthma, immunocompromised conditions, CF, or multiple sclerosis.41 42 43 44 45
HRV-Cs, more than HRV-As and -Bs, are major causes of wheezing in infants and of asthma exacerbations in older children.46 Of all viruses detected from middle ear fluids in children with otitis media, HRV-Cs accounted for half of the documented infections.47 Although reported infections have come mainly from respiratory tract specimens, HRV-Cs have been rarely reported in blood and pericardium.48 49
With more sensitive polymerase chain reaction (PCR) methods for HRV detection, several reports of long periods (>2–3 weeks) of HRV positivity have been published.50 51 Where strain typing has been used, however, HRV shedding normally stops within 11 to 21 days. Therefore, persistence may represent serial or overlapping infections by multiple untyped strains.52 In immunocompromised children, HRV-C strains were detected threefold longer (53 vs. 16 days) than in immunocompetent children.53
Recent studies have documented HRV species in all months of the year in tropical, subtropical, and semi-arid regions.54 55 Many HRV-C strains have been found to circulate during a single year and may be detected in subsequent years.56
Diagnosis
Standard tissue culture methods for isolation are useful for detecting HRV infection but are not very sensitive. With the development of PCR techniques, the ability to detect respiratory viruses has increased significantly. Detection of HRVs in respiratory specimens was enhanced by reverse transcription-PCR (RT-PCR), involving the use of hybridization probes or double-stranded DNA binding dye. Several studies have found an increased sensitivity of RT-PCR compared with viral culture techniques.57 58 59 60 61
Antibody assays are reported for HRVs, but are not readily available or helpful clinically. Since there is no common antigen for HRVs, serotype-specific neutralizing antibody assays are necessary to detect rises in serum antibodies following acute infections, but the large number of HRV serotypes makes this approach impractical.
Clinical Features
The incubation period for the common cold is 12 to 72 hours. Rhinorrhea and sneezing plus nasal congestion are usually the initial symptoms.62 63 Sore throat is common and may be an early symptom. Fever is unusual. Headache and malaise are often mild. Resolution of symptoms occurs in almost all cases within 4 to 9 days.
With the use of PCR techniques, HRVs have been reported commonly in asymptomatic children.3 This may be a result of prolonged shedding from a previous respiratory illness, a mild illness that went unrecognized, or a new virus infection during the incubation period. Asymptomatic HRV shedding is not as common in adults as it appears to be in children. In addition, coinfections with other respiratory viruses during respiratory illnesses are well described.64 65 66 67 68 69 70 71
With the global spread of influenza A (H1N1) in 2009, a series of surveillance protocols were activated to monitor and characterize the pandemic. Many countries have reported on their findings in patients with influenza-like illnesses (ILI) who had respiratory specimens tested for a wide range of respiratory viruses. Most of the reports used PCR techniques in children or adults (Table 1).70 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 The percentage of specimens that were positive with HRV ranged from 6.3 to 40.6%. In all the studies, the influenza illnesses could not be distinguished from HRV-related illnesses by clinical characteristics. During influenza outbreaks, other respiratory viruses, and especially HRV, continue to cause significant cases of URIs and lower respiratory infections (LRIs).
Table1. HRV isolates from patients with influenza-like illness.
| Reference | Location | Specimen | Age group | Positive for HRV (%) |
|---|---|---|---|---|
| Hombrouck et al72 | Belgium | NP | Children | 13 |
| Schnepf et al73 | France | NS | Children and adults | 20 |
| Memish et al74 | Saudi Arabia | NP/TS | Adults (health care workers) | 21 |
| Pascalis et al75 | Reunion Island | NS | Children and adults | 13.4 |
| Chang et al76 | Texas | NP | Children | 29.8 |
| Thiberville et al77 | Vietnam | NS | Children and adults | 20.3 |
| Yang et al78 | China | NP/TS | Adults | 6.3 |
| Nisii et al79 | Italy | NP | Adults | 10 |
| Kraft et al80 | United States/CDC | ? | Immunocompromised adults | 12.6 |
| Delangue et al81 | Bolivia | NP | Children and adults | 8 |
| Dia et al82 | Senegal | NP | Adults older than 50 years | 17.2 |
| Ju et al83 | China | NP/TS | Children and adults | 7.5 |
| Marcone et al84 | Argentina | NP | Children | 40.6 |
| Pariani et al85 | Italy | NS/BAL | Children and adults | 9 |
| Gilca et al70 | Canada | NS | Adults | 7 |
| Zimmerman et al86 | United States | NS | Children and adults | 15.4 |
Abbreviations: HRV, human rhinovirus; NP, nasopharyngeal swab; NS, nasal swab; TS, throat swab.
Asthma and HRV Infections
Asthma exacerbations in children and adults are frequently associated with respiratory virus infections, especially HRVs.87 88 89 90 HRV infections lead to more severe and longer-lasting lower respiratory tract symptoms.90
Lower airway dysfunction following HRV infection may result from direct infection of the lower airway or by stimulating inflammatory, immunologic, or neurogenic mechanisms in the upper airway, thereby impacting the lower airways. HRV has been detected in the columnar and basal cell layers of the lower airways.91 Using in situ hybridization, the replicative strand of HRV in the lower airways has been detected.92
Experimental HRV infections in asthmatic subjects have demonstrated airway narrowing, markers of eosinophil activation, bronchial infiltration with eosinophils, CD4 cells and CD8 cells, activation of prostaglandin and leukotriene pathways, and induction of nitric oxide.93 94 95 Innate immune responses were found to be defective in bronchial epithelial cells obtained from asthmatic subjects. Impaired Th1 responses to HRV were found in peripheral blood mononuclear cells, as reflected by significantly lower levels of IFN-α and IL-12, and higher levels of IL-10 from asthmatic patients compared with normal healthy volunteers.96
Several studies have found deficient induction of IFN-λ by HRV in bronchial epithelial cells from asthmatic patients.97 Induction of IFN-λ1 and IFN-λ2/3 mRNAs was significantly reduced in asthmatic compared with nonasthmatic subjects. Bronchoalveolar cells in asthma patients were deficient in IFN-λ after HRV was added. These studies support the view that innate immune responses in asthmatic subjects have deficiencies in two IFN families, in several lung cell types, and in response to HRV infection.
HRV infections are a major cause of wheezing episodes in infants and children.98 99 100 Wheezing episodes in infancy that are virus induced are often associated with asthma development in children. HRV infections that resulted in hospitalization during infancy were recently implicated as early predictors of subsequent development of asthma.101 102 Almost 90% of wheezing children in year 3 of the study had asthma diagnosed by 6 years of age. Outpatient HRV wheezing illnesses during infancy were also found to be predictors of wheezing through 3 years of age.103 104 105 106 107 The possible relationship between HRV wheezing illness and genetic risk of childhood-onset asthma was studied in a group of prospectively studied children.108 The participants were children who had been studied since birth and cultured for HRV. In children who had HRV wheezing illnesses, there was significant association with 17q21 gene locus.
COPD and HRV Infection
Multiple longitudinal studies have documented the importance of respiratory viral infections in acute exacerbations of chronic obstructive pulmonary disease (COPD).109 110 111 112 113 Other studies, using PCR techniques for respiratory virus detection, have found that more than 40% of COPD exacerbations are associated with respiratory viruses, especially HRVs. Inflammatory mediators, especially IL-8, have been found in increased levels of respiratory secretions obtained from stable COPD patients.
Studies have demonstrated an increase in Staphylococcus aureus and Streptococcus pneumonia adherence to respiratory epithelial cells infected with HRV. In an in vitro study using primary differentiated human cell culture lines, a strain of nontypeable Haemophilus influenza was found to potentiate airway epithelial cell responses to HRV by increasing ICAM-1 and Toll-like receptor-3 expression.114 However, it is unclear whether the interaction of respiratory virus with bacterial pathogens is a common cause of exacerbations of COPD or whether respiratory viruses, such as HRV, cause these pulmonary complications.
AOM and HRV Infections
Viruses, especially HRVs, result in an inflammatory reaction that results in mucociliary damage, impaired middle ear function, and increased mucus in the Eustachian tube. This leads to superinfection of the middle ear by bacteria and fluid accumulation (effusion). Recent studies detected HRVs in 40% of children with otitis media with effusion.115 116 117 118 HRVs were cultured in 24% of nasopharyngeal specimens. In a prospective study of 121 otitis-prone children, nasopharyngeal swabs were assayed by PCR for respiratory viruses and by culture for bacterial pathogens. HRVs were found at baseline in 30% of specimens. Positive PCR tests for HRV correlated with culturing Moraxella catarrhalis and S. pneumonia but not nontypeable H. influenzae. HRV and bacterial pathogens were found in otitis-prone children even in the absence of clinical symptoms. Most new otitis media episodes are coincident with a HRV URL.
In a recently published study in South African children with AOM, 74.2% of cases had respiratory viruses detected from middle ear fluid specimens.119 HRVs were detected in 37.7% of children with AOM. Respiratory viruses were recovered in 72% of episodes which had negative bacterial cultures. HIV status did not alter the spectrum of respiratory viruses recovered.
Rhinosinusitis and HRV Infections
Patients with the common cold syndrome have sinus abnormalities detectable by computed tomography.120 121 Abnormalities are most frequently detected in the maxillary and ethmoid sinuses, and resolve without antibiotics in 80% of patients followed over several weeks. Less than 20% of cases of viral rhinosinusitis are complicated by bacterial infection.122 In a study of 20 adults with acute rhinosinusitis, 15% had virus cultures positive for HRV, but 50% were positive using RT-PCR on maxillary sinus aspirates or nasal swabs.123 Intranasal pressure increases following nose blowing, sneezing, and coughing, and is high enough to propel virus-infected nasal reactions into the sinuses.124
Community-Acquired Pneumonia and Bronchiolitis in HRV Infections
Multiple studies using PCR assays have shown that HRVs do cause community-acquired pneumonia (CAP) in both children and adults.125 126 127 128 Studies in children have reported a range of 0 to 52% positive specimens for HRV (Table 2).129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 Bronchiolitis in children has been commonly reported in infants and young children. The most commonly reported virus recovered in acute cases has been RSV. As with CAP series, mixed infections with a second respiratory virus were common. In studies testing for respiratory viruses in adults with CAP, HRV was detected in 4 to 40% of cases (Table 3).149 150 151 152 153 154 155 As many cases of CAP had mixed infections, it is difficult to be certain if the HRV contributed to CAP.
Table 2. HRV isolates in childhood community-acquired pneumonia and/or bronchiolitis.
| Reference | Country | Design | Clinical | Specimen | % HRV ⊕ |
|---|---|---|---|---|---|
| Choi et al129 | Korea | Retrospective | Pn | BAL | 23.8 |
| Launes et al130 | Spain | Prospective | Pn | NPA | 52 |
| García-García et al131 | Spain | Prospective | Pn | NPA | 19 |
| Miller et al132 | Argentina | Prospective | Br | NS | 40 |
| Esposito et al133 | Italy | Prospective | Pn | NS | 29 |
| Homaira et al134 | Bangladesh | Prospective | Pn | NW | 12 |
| Guerrier et al135 | Cambodia | Prospective | Pn/Br | NPS | 34 |
| Cho et al136 | Korea | Prospective | Pn | NS | 18.5 |
| Suzuki et al137 | Philippines | Prospective | Pn | NPS | 30.5 |
| Uršič et al138 | Slovenia | Prospective | Pn/Br | NPS/TS | 33.1 |
| Pretorius et al139 | South Africa | Prospective | SARI | NPS | 25 |
| Lu et al140 | China | Prospective | Pn | NPA | 30.9 |
| Chidlow et al141 | New Guinea | Prospective | Pn | NS | 63 |
| Ghani et al142 | South Africa | Retrospective | Pn/Br | NPS/TS/BAL | 39 |
| Chen et al143 | Taiwan | Prospective | Br | NPS | 12.4 |
| Antunes et al144 | Portugal | Prospective | Br | ? | 0 |
| Mansbach et al145 | United States | Prospective | Br | NPA | 25.6 |
| Zeng et al146 | China | Prospective | Pn/Br | NPA | 23.2 |
| Ouédraogo et al147 | Burkina Faso | Prospective | Pn/Br | NPA | 40 |
| Gooskens et al148 | The Netherlands | Retrospective | LRTI | NPA/TS/S | 30 |
Abbreviations: BAL, bronchoalveolar lavage; Br, bronchiolitis; HRV, human rhinovirus; NPA, nasopharyngeal aspirate; NS, nasal swab; NW, nasal wash; Pn, pneumonia; TS, throat swab.
Table 3. HRV-associated CAP in adults.
| Reference | Country | Design | Clinical | % All virus + % HRV |
|---|---|---|---|---|
| Qu et al149 | China | Prospective | CAP | 15.2 |
| Seo et al150 | Korea | Prospective | LRTI | 12.0 |
| Walker and Ison151 | United States | Retrospective | CAP | 40 |
| Drieghe et al152 | Belgium | Prospective | LRTI | 5.2 |
| Fica et al153 | Chile | Prospective | CAP | 23.7 |
| Zhan et al154 | China | Prospective | CAP | 4.0 |
| Jain et al155 | United States | Prospective | CAP | 30.0 |
Abbreviations: CAP, community-acquired pneumonia; HRV, human rhinovirus; LRTI, lower respiratory tract infection.
Cystic Fibrosis and HIV Infections
A few studies have examined the role of respiratory viral infections in CF patients.156 Picornavirus was detected in more than 40% of URIs in children with underlying CF.157 There was no difference in pulmonary function in those children with proven HRV infection versus other respiratory viruses. Smyth et al followed up 108 patients with CF for 1 year, and detected HRV in 16% of exacerbations.158 Those patients with proven HRV infection did not show deterioration in clinical activity, but did receive more days of intravenous antibiotics. Goffard et al reported on 64 samples from 46 adult patients with CF.159 HRVs were most frequently identified in CF patients with clinical exacerbations and identified in 14%. Ramirez et al found similar HRV detection in CF patients with exacerbations and measured increased chemokine expression in sputum samples of HRV-positive patients.160
HRV Infections in Immunocompromised Hosts
Respiratory virus infections are common causes of acute respiratory illness in patients after solid-organ transplantation or following bone marrow transplantation.161 162 163 164 165 166 In these immunocompromised patients, HRV was the number one detected respiratory virus by PCR assays. Ison et al found an 83% (5/6) fatality rate in hematopoietic stem cell transplantation patients with bronchoalveolar lavage (BAL)-positive samples for HRV.162 In a study of 215 patients with underlying HCT, 30% had infections at 100 days posttransplant.166 The incidence for HRV was 22.3%. Median duration of virus shedding was 3 weeks. HRV infection was associated with URI symptoms.
In a prospective study of patients with malignancies and neutropenia, a virus was detected in 35% of patients.167 HRVs were the most common virus detected by quantitative PCR in nasopharyngeal aspirates. In a prospective, multicenter study in children with cancer and fever and neutropenia, 57% had a virus detected by PCR-DNA. A third of the patients had mixed HRV/bacterial infections.168 HRVs were the second most detected virus; RSV was most frequently identified. Significant morbidity and mortality in posttransplant patients have been reported following HRV infections.169 In bone marrow transplant recipients with fever and URI symptoms, HRV was the most commonly detected respiratory virus.170
Nosocomial HRV Infections
Hospital outbreaks of HRV infections have been reported in neonatal intensive care units (ICUs) and long-term care facilities.171 172 173 174 175 176 177 178 179 In one study, neonates acquired HRV infection during their hospital stay.179 In long-term care facilities where HRV outbreaks have occurred, there were potential deaths associated with the infection. Guidelines for hospital isolation recommend droplet precautions for patients with known HRV infection.180
Treatment
There are no approved antiviral medications for HRV respiratory tract infections. Anticholinergic medications can be used for rhinorrhea. Nasal congestion can be alleviated by nasal and systemic decongestants. Several studies have suggested that heated, humidified steam may reduce nasal congestion in common colds, but the data are not conclusive.181 Cough is a common accompanying problem in respiratory viral infections and can be suppressed with nonprescription cough suppressants. Other symptoms such as sore throat, myalgia, fever, or headache can be controlled with nonsteroidal anti-inflammatory drugs. Antibiotics are inappropriate for treating these viral infections.
Compounds targeting cell susceptibility, virus attachment, receptor blockage, virus uncoating, RNA replication, and viral protein synthesis have been evaluated. Although several agents have demonstrated both in vitro and in vivo success, none have received U.S. Food and Drug Administration approval due to poor bioavailability, poor side-effect profile, or limited potency.182 Viral capsid-binding compounds, such as pleconaril, block virus uncoating in vitro.183 Clinical trials demonstrated significant reduction in duration of respiratory symptoms in individuals receiving pleconaril, but the drug has not been approved.
Alternative medications, such as Echinacea angustifolia or zinc lozenges, have been tested in several volunteer trials but are not currently thought to be clinically effective.184 185 186 187 All of these studies suffer from inadequate control groups or incomplete virologic evaluation.
Quinolones have been used to treat exacerbation of COPD because of their broad antibacterial spectrum and anti-inflammatory properties. Recently, levofloxacin has been found to inhibit HRV infection in primary cell cultures employing human tracheal epithelial cells.188 Levofloxacin pretreatment decreased mRNA levels of ICAM-1. Macrolides have also been reported to reduce ICAM-1 expression and HRV-induced cytokines in vitro and in vivo.189
A recent randomized controlled study evaluated the clinical benefit of oral prednisolone for treating a first episode of HRV-associated wheezing in young children younger than 23 months.190 No long-term benefits were demonstrated in those children receiving oral prednisolone with their first HRV-associated wheezing episode. Short-term benefits were found in those receiving the oral prednisolone. This included less cough, rhinitis, and night-time respiratory symptoms. In a subset of children who had high viral loads (>7,000 copies/mL), a long-term benefit of prednisolone therapy was detected. Future studies will have to confirm these findings before a general recommendation can be made for corticosteroid treatment in asthma-prone children with HRV-associated wheezing episodes.
Prevention
HRV can be recovered from the hands of approximately 40% of adults with colds. Hand-to-hand transmission of HRV has led to the evaluation of disinfectants that will eliminate virus on human skin. Trials to reduce hand transmission have reported differing results.191 192 A recently published study in Bangladesh demonstrated that nasal swab samples and paired hand rinse samples had HRV detected by rRT-PCR in 21 and 29% of samples, respectively.193
A study using 2% of aqueous iodine decreased transmission in family members who were exposed to HRV-infected individuals. An evaluation of virucidal hand treatments confirmed the prevention of HRV infections by organic acids but not ethanol.192 Hand washing with soap and water effectively cleans HRV-contaminated hands better than single treatment with ethanol hand rub.192
Vaccines have not been thought to be useful against HRVs because of the serotype-specific neutralizing antibodies produced following infection and because of the numerous serotypes.194 195 196 197 However, recent studies in a mouse model have reported development of cross-serotype reactive antibodies to VP1, suggesting a basis for successful antibody-mediated vaccine development. Future studies will need to confirm and expand these preliminary observations.
Coronaviruses
Virology
Coronaviruses are positive, single-stranded RNA viruses that replicate in the cytoplasm and bud into cytoplasmic vesicles from the endoplasmic reticulum. Coronaviruses are divided into three groups: alpha, beta, and gamma. CD13 (human aminopeptidase N) is the cellular receptor for HCoV-229E (Table 4).198 199 200 Recent studies have shown that the newly reported HCoV-NL63 does not use CD13 as the receptor cell entry. HCoV-NL63, along with severe acute respiratory syndrome (SARS)-CoV, uses angiotensin-converting enzyme 2 (ACE2) as the entry receptor.201 ACE2 is found on the ciliated nasal and tracheobronchial epithelial cells. The receptor for HCoV-OC43 is not known. Carcinoembryonic antigen (CEA) is the receptor for mouse hepatitis virus.202 Phylogenetic relationships between coronaviruses have been based on deduced amino acid sequences of the coronavirus replicase, ORF1b gene.
Table 4. Coronavirus receptors.
| Virus | Group | Receptor |
|---|---|---|
| Human coronavirus 229E (HCoV-229E) | Alpha | APN |
| Bat coronaviruses (BCoVs-multiple species) | Alpha | Unknown |
| Human coronavirus NL63 (HCoV-NL63) | Alpha | ACE2 |
| Severe acute respiratory syndrome coronavirus (SARS-CoV) | Beta | ACE2 |
| Bat SARS-related coronavirus (Bat-SCoV) | Beta | ACE2? |
| Middle East respiratory syndrome coronavirus (MERS-CoV) | Beta | DPP4 |
| Human coronavirus OC43 (HCoV-OC43) | Beta | Unknown |
Abbreviations: ACE2, angiotensin-converting enzyme 2; APN, aminopeptidase N; DPP4, dipeptidyl peptidase 4.
Middle East respiratory syndrome (MERS)-CoV belongs to the genus Betacoronavirus (BCoV) in the family Coronaviridae.17 It is the first lineage C βCoV and the sixth coronavirus known to infect humans. MERS-CoV is an enveloped, positive-sense, single-stranded RNA virus. The replication cycle is similar to other CoVs. The host cell receptor for the MERS-CoV S protein is dipeptidyl peptidase-4 (DPP4) or CD26. This receptor is expressed on endothelial and epithelial cells of the kidney, lung, small intestine, liver, as well as on immune cells. This ability to infect many different organ cells may explain the extrapulmonary clinical characteristics in patients. After uncoating, there is translation of ORF/a/b, followed by transcription and genome replication. Following translation on the rough ER, budding and assembly of virions occur in the cytoplasm with exocytosis and virion release. ACE2 is a SARS-CoV receptor. Upon SARS-CoV infection, ACE2 expression in lungs is markedly downregulated and therefore helps explain SARS pathogenesis and progression to acute respiratory distress syndrome (Table 5).
Table 5. SARS and MERS coronavirus infections: virology, epidemiology, and diagnostic tests.
| SARS | MERS | |
|---|---|---|
| Virology | ||
| Virus | SARS-CoV | MERS-CoV |
| Phylogeny | BßCoV | CßCoV |
| Host receptor | ACE2 | DPP4 (CD26) |
| Cell entry pathway | Endosomal fusion | Cell membrane fusion |
| Epidemiology | ||
| Origin | China | Middle East |
| Natural reservoir | Bats | Bats |
| Intermediate host | Civets, raccoon dogs | Camels |
| Seasonality | Winter | ? |
| Mode of transmission | Person to person, droplet, contact, airborne | Animal to human, droplet, contact, airborne (?) |
| Incubation period | 2–14 d (up to 21 d) | 2–15 d |
| Diagnosis | ||
| 1° Specimens | Lower respiratory tract | Lower respiratory tract |
| RT-PCR test | + | + |
Abbreviations: CoV, coronavirus; MERS, Middle East respiratory syndrome; RT-PCR, reverse transcription-polymerase chain reaction; SARS, severe acute respiratory syndrome.
Pathogenesis
Coronaviruses attach to cellular receptors by the spike proteins on their surface.203 204 Internalization into host cells occurs by direct fusion with the plasma membrane or by endocytosis. Posttranslational proteolytic processes are important regulatory mechanisms. Polyproteins are cleaved by viral proteases, facilitating assembly of subunit protein complexes that are responsible for replication and transcription.205 There is little information on the host response to coronavirus replication. Humoral immune responses are detectable following natural infection, but the role of cell-mediated immunity is largely unknown.206 207
There are several in vitro and in vivo differences between SARS-CoV and MERS-CoV infections. There are radiographic differences, as well as differences in cytopathic effect. The host response to infection appears to be different. For example, there are observed differences in sensitivity to type I IFN in vitro. MERS-CoV is more sensitive to type I IFN compared with SARS-CoV.208 SARS pathogenesis is controlled in part by innate immune signaling. SARS-CoV encodes proteins that modulate innate immune signaling by antagonism of IFN induction and by avoiding IFN-stimulated gene effector functions (Table 6).209
Table 6. Clinical findings, treatment, and prevention of SARS and MERS coronavirus infections.
| SARS | MERS | |
|---|---|---|
| Clinical findings | ||
| Presenting syndrome | CAP or HAP | CAP or HAP |
| Extrapulmonary manifestations | Diarrhea | Acute renal failure, diarrhea |
| X-ray findings | Ground-glass opacities, ARDS | Focal to diffuse and/or consolidation, ARDS |
| Case-fatality rate | ∼10 | >35 |
| Treatment and prevention | ||
| Antivirals used | Interferon and ribavirin | Ribavirin and IFN-α2b |
| Passive immunization | Convalescent plasma therapy | Adaptive transfer of serum |
| Vaccine development | Yes—early phases | Yes—early phases |
Abbreviations: ARDS, acute respiratory distress syndrome; CAP, community-acquired pneumonia; HAP, healthcare assisted pneumonia; IFN, interferon; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome.
Epidemiology
Coronaviruses were detected as agents of respiratory infections approximately 40 years ago.210 211 212 They were later identified as coronaviruses, labeled “OC43” and “229E,” and accepted as a new genus in 1975. In epidemiologic studies in adults, coronaviruses were estimated to cause 15% of adult common colds. Coronaviruses were found to cause epidemics every 2 to 3 years with reinfections being common. All ages are susceptible. From epidemiologic studies, coronaviruses were found to be associated with respiratory illnesses, usually in the upper respiratory tract, but occasionally causing pneumonia. In temperate climates, HCoV-OC43 and HCoV-229E are transmitted primarily during the winter. They have been linked to asthma and COPD exacerbations in children and adults.125 213
In 2004 and 2005, three closely related coronavirus species were reported.214 215 216 217 218 219 NL63 was isolated from a 7-month-old girl with coryza, conjunctivitis, fever, and bronchiolitis. From epidemiologic studies, patients with HCoV-NL63 have ranged in age from 1 month to 100 years, with the highest infection rate occurring before age 5 years. Using molecular probes that targeted conserved regions of the coronavirus genome, a related coronavirus (HCoV-NH) was found in 79 of 895 young children tested by RT-PCR on respiratory specimens.
In a prospective study in Hong Kong, coronaviruses were detected in 2.1% of patients admitted to the hospital with signs and symptoms of acute respiratory illness.220 Of the 87 infected patients, 13 were positive for HCoV-HKU1, 17 were positive for HCoV-NL63, 53 were positive for HCoV-OC43, and 4 were positive for 229E. HCoV-HKU1 and HCoV-OC43 peak in the winter months. Upper respiratory tract illness was the most common presentation for HCoV-HKU1 infections. HCoV-NL63 infections occurred in early summer and fall but not in the winter.
Using newer molecular assays, the group at Vanderbilt reassessed the role of the newly described coronaviruses in a large cohort of healthy children who had been followed up prospectively for 20 years.221 Of the LRI samples available for screening, 8.4% had positive results for HCo-V and all were younger than 2 years. AOM was found in half of the HCo-V-infected children, but none of the children were hospitalized. Of the URI samples tested, 4.7% had detectable HCo-V RNA. Fifty-one percent of these positive children were diagnosed with AOM. The burden of URI attributable to HCo-V had significant year-to-year variation.
A recent study has provided evidence of genetic variability in OC43 strains.222 The complete nucleotide sequence of two contemporary OC43 strains compared with the prototype strain (ATCC VR 759) demonstrated important amino acid substitutions in the potential cleavage site sequence of the spike protein.
In 2002, the first cases of SARS were reported in Guangdong Province of China.223 Over the next few months, 29 countries reported cases in more than 8,000 patients with approximately 10% fatalities. A patient who acquired SARS in Guangdong traveled to Hong Kong and is thought to be the index case for over half of the cases. It was different from known human and animal coronaviruses by DNA sequencing. This new coronavirus was cultured from Himalayan palm civets, but it is now thought that bats are the primary reservoir (Fig. 1). SARS infected more than 8,000 people resulting in more than 700 deaths.
Fig. 1.
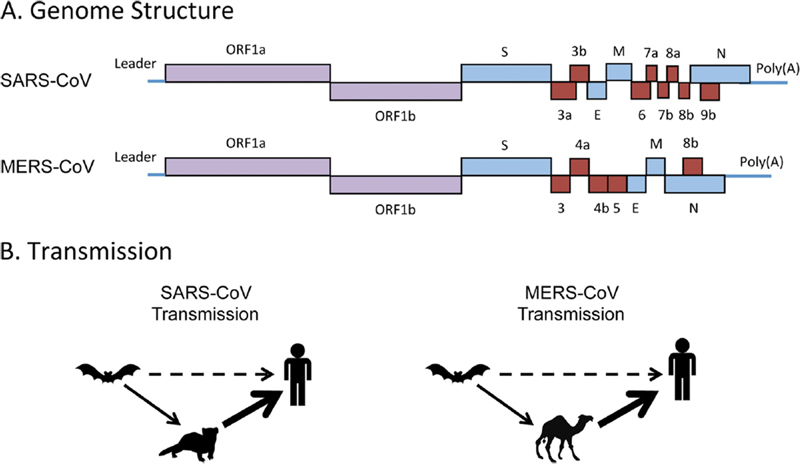
(A) Genome structures of SARS-CoV and MERS-CoV. The single-stranded, positive-sense coronavirus genomes encode the structural proteins (blue), membrane (M), spike (S), envelope (E), and nucleocapsid (N), two replicase polyproteins (purple), ORF1a and ORF1b, and unique accessory proteins (red) that perform important functions in coronavirus replication and pathogenesis, such as blocking the innate immune signaling pathway. (B) Transmission routes. SARS-CoV transmission is thought to be from bats harboring SARS-like viruses to palm civet cats, which infected humans. SARS-CoV could also have been transmitted from bats to humans directly. MERS-CoV is thought to be transmitted from camels to humans, with the possibility that at some point bats infected camels. The dashed line identifies a low-level transmission event, the thin solid line identified a potential transmission event, and the thick solid line identifies a probable transmission event. CoV, coronavirus; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome. (Reproduced from Coleman CM, Frieman MB. Coronaviruses: important emerging human pathogens. J Virol 2014;88:5209. doi: 10.1128/JVI.03488-13 with permission of the American Society for Microbiology.)
MERS epidemiology originally implicated the dromedary camel as the source of human cases (Fig. 1).224 225 Of the first 500 confirmed cases, 62% required hospitalization. Comorbid medical conditions were common and males were more likely to be identified as infected. The median age was 56 years. Secondary cases were commonly identified in younger health care workers. The incubation period was 5.2 days (range: 1.9–14.7 days). Mortality rates were reported to be approximately 35%.226 227
In May 2014, two cases of MERS-CoV infection in the United States were reported by the Centers for Disease Control and Prevention (CDC).228 229 Both patients had acquired their infection while working in health care in Saudi Arabia. No contacts of these patients developed infections with MERS-CoV.
Diagnosis
Coronaviruses require special cell lines or organ culture for detection by cultivation methods.230 These cell or organ culture techniques are labor intensive, time consuming, and relatively insensitive. Coronaviruses have also been detected by RT-PCR with greater sensitivity than standard culture techniques.231
SARS-CoV was initially detected by RT-PCR and culture. Viral RNA is found in high levels in the feces of SARS patients. To confirm SARS-CoV infection, the WHO criteria require detection of viral RNA by PCR, increase in antibody titers in body fluids, or isolation of SARS-CoV from clinical isolates.232 Laboratory diagnostic methods for SARS-CoV include (1) viral RNA detection using RT-PCR, (2) immunofluoroscent antibody (IFA), and (3) ELISA. Virus isolation is less sensitive than these other methods and requires a biosafety level 3 (BSL-3) facility.
Antibody assays are reported for coronaviruses, but are not readily available or helpful clinically. Complement-fixing and ELISA antibody assays for coronaviruses 229E and OC43 have been published, but are not available in clinical laboratories.233 234 Therefore, serologic tests for antibody-specific responses are mainly reserved for research or epidemiologic studies.
Serological assays are a complement to nucleic acid detection assays for SARS-CoV and MERS-CoV.235 236 237 They have been helpful in identifying the source of the infection, in transmission pattern analysis, patient contact studies, and diagnosing asymptomatic cases. Even with the number of assays available after the SARS and MERS outbreak, differentiating HCoVs by serologic means remains a problem. Viral neutralization tests remain the most specific assay available, but are limited to only a few laboratories.
Animal virologic data from surveillance studies support the theory that MERS originated from bats in Africa and crossed species barriers to infect camels many years ago. Infected camels were then transported to the Middle East where they transmitted MERS-CoV to humans. A recent report showed through complete genome sequencing that a virus isolated from a dromedary camel was identical to a human strain isolated from sick humans who had developed MERS following close contact with sick camels.238 The mode of transmission from camels to humans is currently unknown, although droplet transmission seems likely.
Laboratory confirmation of MERS required nucleic acid amplification assays. The WHO criteria for confirmed cases includes (1) + RT-PCR for two different specific targets or (2) +RT-PCR from one specific target plus an additional different RT-PCR product sequenced and confirmed as MERS-CoV. A BSL3 is required for viral culture and neutralizing antibody detection assays.
Clinical Features
In a prospective study of respiratory viral infections among hospitalized patients, 5.7% had coronaviruses identified.220 The 47 coronavirus infections represent 10.5% of all the respiratory viral infections. In 14 patients, coronaviruses were associated with another respiratory virus. Lower respiratory tract infections (bronchitis, bronchiolitis, and pneumonia) were far more common than upper respiratory tract (rhinitis, pharyngitis, and laryngitis) infections, 75 versus 25%, respectively. Over half of the infections were due to OC43-like strains. Approximately 20% were due to 229E-like strains and approximately 20% were due to NL63-strains. Coronavirus infections in the first year of life were associated predominantly with OC43-like strains.
HCoV was identified in 5.4% of specimens from 279 hospitalized adult patients with lower respiratory tract infections.239 240 The most frequently identified isolates were HCoV-OC43 in 12, followed by HCoV-229E in 7, HCoV-NL63 in 6, and HCoV-HKU1 in 4 specimens. Many patients had high-risk underlying conditions. In several recent studies evaluating multiplex PCR assays, HCoVs were detected in 3 to 8% of hospitalized children younger than 5 years with acute respiratory illnesses.241 242 243
Patients with SARS present with fever, cough, and/or myalgias. Dyspnea and hypoxemia were noted during the second week of illness. Rapidly progressive respiratory failure then occurred in a subset of patients. Patients appeared to be contagious after lower respiratory tract signs were observed.244 245
Nonspecific presenting symptoms are common in MERS-CoV-infected patients. Fever, chills, and sore throat are frequently reported.246 Respiratory tract symptoms are also quite common. Within a few days of symptoms, respiratory failure can develop. Chest radiographic findings progress from a unilateral focal lesion to extensive multifocal or bilateral involvement, but are not specific to MERS-CoV infections.247 248 The most common CT findings are bilateral, often basilar, airspace involvement. Cavitation has not been reported. Late CT findings in recovered patients may include fibrotic changes and organizing pneumonia.
Acute renal failure has often been observed during the second week of illness with MERS virus. This was also seen in a small percentage of SARS patients. Dialysis was often required. Other nonrespiratory tract involvement has been reported to include diarrhea (7–25% of patients), nausea, vomiting, pericarditis, and arrhythmias.249
Complications of MERS include superinfection or coinfection, septic shock, and delirium. ICU care is often needed within 5 days of admission. The case-fatality rates have ranged from 25 to 76%. As more cases were identified, there have been more asymptomatic or mild cases identified. These have predominantly been in young, healthy women without significant comorbidities.
Treatment
There are no published randomized controlled trials of specific antiviral agents in the treatment of SARS or MERS. Supportive therapy is currently the only recommended plan. When renal failure occurs, renal dialysis is necessary. Mechanical ventilation in an ICU is often required. Several candidate antiviral agents have been identified and include IFNs, ribavirin, and cyclophilin inhibitors.250 251 252 253 254 255 256 257 Both in vitro cell culture experiments and animal studies have shown antiviral activity of these agents against MERS-CoV. However, very few human cases who received a combination of IFN-α2b, ribavirin, and corticosteroids survived.
Infection Control
Prompt isolation of suspected SARS or MERS cases and contact tracing of case contacts are the keys to preventing nosocomial transmission.258 259 260 Standard, contact, and droplet precautions should be used in all cases. Airborne precautions should be employed for aerosol-generating procedures, bronchoscopy, tracheostomy, and airway insertion.
Vaccine design for SARS-CoV and MERS-CoV has focused on the development of chimeric spike glycoproteins containing neutralizing epitopes from multiple strains within or across subgroups.261 262 263 264 265 266 267 Inclusion of nucleocapsid protein in chimeric vaccines could broaden the protective response. The S protein is the major determinant of protective immunity. Antibodies against S protein appeared to protect from SARS-CoV challenges in animal studies. N protein-specific immune response provides little protection and only cross-react within, but not between, subgroups. Thus, there are no approved vaccines for MERS or any of the CoVs. The use of convalescent-phase plasma or immune globulin with high titers of neutralizing antibody has not been evaluated in randomized controlled trials.268
Conclusion
HRVs and HCoVs cause significant morbidity in immunocompetent people and in patients with underlying chronic or immunosuppressed medical conditions. Newer diagnostic tests have expanded our understanding of these respiratory viruses in clinical infections. These sensitive diagnostic tests have been used to detect new HRVs, such as HRV-C. Recent studies on the pathogenesis of HRVs and the host response to this group of viruses have provided insights into potential targets for therapeutic interventions. The recent outbreaks of SARS and MERS pose special problems in treatment and infection control.269 270 271 Vaccine development for HCoVs and/or coronaviruses appears to be many years away.
References
- 1.Greenberg S B. Update on rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2011;32(4):433–446. doi: 10.1055/s-0031-1283283. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg S B. Boca Raton: CRC Press; 2014. Human rhinovirus infections; pp. 353–376. [Google Scholar]
- 3.Cecere T E, Todd S M, Richmond O B. Boca Raton: CRC Press; 2014. Human coronavirus respiratory infections; pp. 547–558. [Google Scholar]
- 4.Jacobs S E, Lamson D M, St George K, Walsh T J. Human rhinoviruses. Clin Microbiol Rev. 2013;26(1):135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy J L, Turner R B, Braciale T, Heymann P W, Borish L. Pathogenesis of rhinovirus infection. Curr Opin Virol. 2012;2(3):287–293. doi: 10.1016/j.coviro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg S B, Allen M, Wilson J, Atmar R L. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(1):167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 7.El-Sahly H M, Atmar R L, Glezen W P, Greenberg S B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31(1):96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedzicha J A. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 9.Johnston S L, Pattemore P K, Sanderson G. et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiatt P W, Grace S C, Kozinetz C A. et al. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103(3):619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 11.Chonmaitree T, Howie V M, Truant A L. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics. 1986;77(5):698–702. [PubMed] [Google Scholar]
- 12.Pitkäranta A, Virolainen A, Jero J, Arruda E, Hayden F G. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102(2, Pt 1):291–295. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 13.Wolfromm A, Porcher R, Legoff J. et al. Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long-term incidence and outcome. Biol Blood Marrow Transplant. 2014;20(8):1238–1241. doi: 10.1016/j.bbmt.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brebion A, Mirand A, Regagnon C. et al. Evaluation of real-time RT-PCR Rhino&EV/Cc r-gene(®) (bioMérieux) kit versions 1 and 2 for rhinovirus detection. J Clin Virol. 2015;62:110–113. doi: 10.1016/j.jcv.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Grijalva C G, Griffin M R, Edwards K M. et al. Concordance between RT-PCR-based detection of respiratory viruses from nasal swabs collected for viral testing and nasopharyngeal swabs collected for bacterial testing. J Clin Virol. 2014;60(3):309–312. doi: 10.1016/j.jcv.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To K F, Lo A WI. Boca Raton: CRC Press; 2014. SARS-CoV infections in humans; pp. 559–581. [Google Scholar]
- 17.Chan J F, Lau S K, To K K, Cheng V C, Woo P C, Yuen K Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(2):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W M, Kiesner C, Pappas T. et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroda M, Niwa S, Sekizuka T. et al. Molecular evolution of the VP1, VP2, and VP3 genes in human rhinovirus species C. Sci Rep. 2015;5:8185. doi: 10.1038/srep08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmenberg A C, Spiro D, Kuzmickas R. et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324(5923):55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tapparel C, Junier T, Gerlach D. et al. New respiratory enterovirus and recombinant rhinoviruses among circulating picornaviruses. Emerg Infect Dis. 2009;15(5):719–726. doi: 10.3201/eid1505.081286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blomqvist S, Savolainen C, Råman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol. 2002;40(11):4218–4223. doi: 10.1128/JCM.40.11.4218-4223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa S, Hirano R, Okamoto-Nakagawa R, Ichiyama T, Shirabe K. Enterovirus 68 infection in children with asthma attacks: virus-induced asthma in Japanese children. Allergy. 2011;66(12):1618–1620. doi: 10.1111/j.1398-9995.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacques J, Moret H, Minette D. et al. Epidemiological, molecular, and clinical features of enterovirus respiratory infections in French children between 1999 and 2005. J Clin Microbiol. 2008;46(1):206–213. doi: 10.1128/JCM.01414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piralla A, Lilleri D, Sarasini A. et al. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008-2009. Diagn Microbiol Infect Dis. 2012;73(2):162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Rahamat-Langendoen J, Riezebos-Brilman A, Borger R. et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol. 2011;52(2):103–106. doi: 10.1016/j.jcv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Kim J H, Kwon H J, Jang Y J. Effects of rhinovirus infection on the expression and function of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel in human nasal mucosa. Ann Allergy Asthma Immunol. 2012;108(3):182–187. doi: 10.1016/j.anai.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Jayaraman A, Jackson D J, Message S D. et al. IL-15 complexes induce NK- and T-cell responses independent of type I IFN signaling during rhinovirus infection. Mucosal Immunol. 2014;7(5):1151–1164. doi: 10.1038/mi.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaheer R S, Wiehler S, Hudy M H. et al. Human rhinovirus-induced ISG15 selectively modulates epithelial antiviral immunity. Mucosal Immunol. 2014;7(5):1127–1138. doi: 10.1038/mi.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cakebread J A, Haitchi H M, Xu Y, Holgate S T, Roberts G, Davies D E. Rhinovirus-16 induced release of IP-10 and IL-8 is augmented by Th2 cytokines in a pediatric bronchial epithelial cell model. PLoS ONE. 2014;9(4):e94010. doi: 10.1371/journal.pone.0094010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagome K, Bochkov Y A, Ashraf S. et al. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134(2):332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulraiz F, Bellinghausen C, Dentener M A. et al. Efficacy of IFN-λ1 to protect human airway epithelial cells against human rhinovirus 1B infection. PLoS ONE. 2014;9(4):e95134. doi: 10.1371/journal.pone.0095134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller E K, Hernandez J Z, Wimmenauer V. et al. A mechanistic role for type III IFN-λ1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185(5):508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cakebread J A, Xu Y, Grainge C. et al. Exogenous IFN-β has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127(5):1148–1.154E12. doi: 10.1016/j.jaci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Hong J Y, Bentley J K, Chung Y. et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134(2):429–439. doi: 10.1016/j.jaci.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arruda E, Pitkäranta A, Witek T J Jr, Doyle C A, Hayden F G. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35(11):2864–2868. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mäkelä M J, Puhakka T, Ruuskanen O. et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36(2):539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McErlean P, Shackelton L A, Andrews E. et al. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS ONE. 2008;3(4):e1847. doi: 10.1371/journal.pone.0001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller E K, Khuri-Bulos N, Williams J V. et al. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol. 2009;46(1):85–89. doi: 10.1016/j.jcv.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renwick N, Schweiger B, Kapoor V. et al. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J Infect Dis. 2007;196(12):1754–1760. doi: 10.1086/524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savolainen-Kopra C, Blomqvist S, Kilpi T, Roivainen M, Hovi T. Novel species of human rhinoviruses in acute otitis media. Pediatr Infect Dis J. 2009;28(1):59–61. doi: 10.1097/INF.0b013e318182c90a. [DOI] [PubMed] [Google Scholar]
- 42.Urquhart G E, Grist N R. Virological studies of sudden, unexplained infant deaths in Glasgow 1967-70. J Clin Pathol. 1972;25(5):443–446. doi: 10.1136/jcp.25.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canducci F, Debiaggi M, Ceresola E R. et al. Infection and coinfection of human rhinovirus C in stem cell transplant recipients. Clin Dev Immunol. 2013;2013:236081. doi: 10.1155/2013/236081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khetsuriani N, Lu X, Teague W G, Kazerouni N, Anderson L J, Erdman D D. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14(11):1793–1796. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kneider M, Bergström T, Gustafsson C. et al. Sequence analysis of human rhinovirus aspirated from the nasopharynx of patients with relapsing-remitting MS. Mult Scler. 2009;15(4):437–442. doi: 10.1177/1352458508100038. [DOI] [PubMed] [Google Scholar]
- 46.Miller E K, Edwards K M, Weinberg G A. et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123(1):98–1040. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alper C M, Winther B, Mandel E M, Hendley J O, Doyle W J. Rate of concurrent otitis media in upper respiratory tract infections with specific viruses. Arch Otolaryngol Head Neck Surg. 2009;135(1):17–21. doi: 10.1001/archotol.135.1.17. [DOI] [PubMed] [Google Scholar]
- 48.Xatzipsalti M, Kyrana S, Tsolia M. et al. Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med. 2005;172(8):1037–1040. doi: 10.1164/rccm.200502-315OC. [DOI] [PubMed] [Google Scholar]
- 49.Tapparel C, L'Huillier A G, Rougemont A L, Beghetti M, Barazzone-Argiroffo C, Kaiser L. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol. 2009;45(2):157–160. doi: 10.1016/j.jcv.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72(4):695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 51.Winther B, Hayden F G, Hendley J O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78(5):644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 52.Dick E C, Blumer C R, Evans A S. Epidemiology of infections with rhinovirus types 43 and 55 in a group of university of Wisconsin student families. Am J Epidemiol. 1967;86(2):386–400. doi: 10.1093/oxfordjournals.aje.a120749. [DOI] [PubMed] [Google Scholar]
- 53.Piralla A, Rovida F, Campanini G. et al. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol. 2009;45(4):311–317. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 54.Savolainen C, Mulders M N, Hovi T. Phylogenetic analysis of rhinovirus isolates collected during successive epidemic seasons. Virus Res. 2002;85(1):41–46. doi: 10.1016/s0168-1702(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 55.Linder J E Kraft D C Mohamed Y et al. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades J Allergy Clin Immunol 2013131169–770., 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackay I M, Lambert S B, Faux C E. et al. Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period. J Infect Dis. 2013;207(9):1433–1441. doi: 10.1093/infdis/jis476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meerhoff T J, Houben M L, Coenjaerts F E. et al. Detection of multiple respiratory pathogens during primary respiratory infection: nasal swab versus nasopharyngeal aspirate using real-time polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2010;29(4):365–371. doi: 10.1007/s10096-009-0865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babady N E, Mead P, Stiles J. et al. Comparison of the Luminex xTAG RVP Fast assay and the Idaho Technology FilmArray RP assay for detection of respiratory viruses in pediatric patients at a cancer hospital. J Clin Microbiol. 2012;50(7):2282–2288. doi: 10.1128/JCM.06186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammond S P, Gagne L S, Stock S R. et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J Clin Microbiol. 2012;50(10):3216–3221. doi: 10.1128/JCM.00538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rand K H, Rampersaud H, Houck H J. Comparison of two multiplex methods for detection of respiratory viruses: FilmArray RP and xTAG RVP. J Clin Microbiol. 2011;49(7):2449–2453. doi: 10.1128/JCM.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loeffelholz M J, Pong D L, Pyles R B. et al. Comparison of the FilmArray Respiratory Panel and Prodesse real-time PCR assays for detection of respiratory pathogens. J Clin Microbiol. 2011;49(12):4083–4088. doi: 10.1128/JCM.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gwaltney J M Jr, Hendley J O, Patrie J T. Symptom severity patterns in experimental common colds and their usefulness in timing onset of illness in natural colds. Clin Infect Dis. 2003;36(6):714–723. doi: 10.1086/367844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris J M II, Gwaltney J M Jr. Incubation periods of experimental rhinovirus infection and illness. Clin Infect Dis. 1996;23(6):1287–1290. doi: 10.1093/clinids/23.6.1287. [DOI] [PubMed] [Google Scholar]
- 64.Drews A L, Atmar R L, Glezen W P, Baxter B D, Piedra P A, Greenberg S B. Dual respiratory virus infections. Clin Infect Dis. 1997;25(6):1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rehder K J, Wilson E A, Zimmerman K O, Cunningham C K, Turner D A. Detection of multiple respiratory viruses associated with mortality and severity of illness in children. Pediatr Crit Care Med. 2015;16(7):e201–e206. doi: 10.1097/PCC.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damasio G A, Pereira L A, Moreira S D, Duarte dos Santos C N, Dalla-Costa L M, Raboni S M. Does virus-bacteria coinfection increase the clinical severity of acute respiratory infection? J Med Virol. 2015;87(9):1456–1461. doi: 10.1002/jmv.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong-Chew R M, Espinoza M A, Taboada B. et al. Prevalence of respiratory virus in symptomatic children in private physician office settings in five communities of the state of Veracruz, Mexico. BMC Res Notes. 2015;8:261. doi: 10.1186/s13104-015-1239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei L, Liu W, Zhang X A. et al. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illnesses, Chongqing, 2009-2013. Medicine (Baltimore) 2015;94(16):e742. doi: 10.1097/MD.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morikawa S, Hiroi S, Kase T. Detection of respiratory viruses in gargle specimens of healthy children. J Clin Virol. 2015;64:59–63. doi: 10.1016/j.jcv.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilca R, Amini R, Douville-Fradet M. et al. Other respiratory viruses are important contributors to adult respiratory hospitalizations and mortality even during peak weeks of the influenza season. Open Forum Infect Dis. 2014;1(2):ofu086. doi: 10.1093/ofid/ofu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asner S A, Rose W, Petrich A, Richardson S, Tran D J. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin Microbiol Infect. 2015;21(3):2640–2.64E8. doi: 10.1016/j.cmi.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hombrouck A, Sabbe M, Van Casteren V. et al. Viral aetiology of influenza-like illness in Belgium during the influenza A(H1N1)2009 pandemic. Eur J Clin Microbiol Infect Dis. 2012;31(6):999–1007. doi: 10.1007/s10096-011-1398-4. [DOI] [PubMed] [Google Scholar]
- 73.Schnepf N, Resche-Rigon M, Chaillon A. et al. High burden of non-influenza viruses in influenza-like illness in the early weeks of H1N1v epidemic in France. PLoS ONE. 2011;6(8):e23514. doi: 10.1371/journal.pone.0023514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Memish Z A, Assiri A M, Alshehri M, Hussain R, Alomar I. The prevalance of respiratory viruses among healthcare workers serving pilgrims in Makkah during the 2009 influenza A (H1N1) pandemic. Travel Med Infect Dis. 2012;10(1):18–24. doi: 10.1016/j.tmaid.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pascalis H, Temmam S, Turpin M. et al. Intense co-circulation of non-influenza respiratory viruses during the first wave of pandemic influenza pH1N1/2009: a cohort study in Reunion Island. PLoS ONE. 2012;7(9):e44755. doi: 10.1371/journal.pone.0044755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang M L, Jordan-Villegas A, Evans A. et al. Respiratory viruses identified in an urban children's hospital emergency department during the 2009 influenza A(H1N1) pandemic. Pediatr Emerg Care. 2012;28(10):990–997. doi: 10.1097/PEC.0b013e31826ca980. [DOI] [PubMed] [Google Scholar]
- 77.Thiberville S D, Ninove L, Vu Hai V. et al. The viral etiology of an influenza-like illness during the 2009 pandemic. J Med Virol. 2012;84(7):1071–1079. doi: 10.1002/jmv.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Wang Z, Ren L. et al. Influenza A/H1N1 2009 pandemic and respiratory virus infections, Beijing, 2009-2010. PLoS ONE. 2012;7(9):e45807. doi: 10.1371/journal.pone.0045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nisii C, Meschi S, Selleri M. et al. Frequency of detection of upper respiratory tract viruses in patients tested for pandemic H1N1/09 viral infection. J Clin Microbiol. 2010;48(9):3383–3385. doi: 10.1128/JCM.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kraft C S, Jacob J T, Sears M H, Burd E M, Caliendo A M, Lyon G M. Severity of human rhinovirus infection in immunocompromised adults is similar to that of 2009 H1N1 influenza. J Clin Microbiol. 2012;50(3):1061–1063. doi: 10.1128/JCM.06579-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delangue J, Roca Sanchez Y, Piorkowski G. et al. Viral aetiology influenza like illnesses in Santa Cruz, Bolivia (2010-2012) Virol J. 2014;11:35–45. doi: 10.1186/1743-422X-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dia N, Richard V, Kiori D. et al. Respiratory viruses associated with patients older than 50 years presenting with ILI in Senegal, 2009 to 2011. BMC Infect Dis. 2014;14:189–194. doi: 10.1186/1471-2334-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ju X, Fang Q, Zhang J, Xu A, Liang L, Ke C. Viral etiology of influenza-like illnesses in Huizhou, China, from 2011 to 2013. Arch Virol. 2014;159(8):2003–2010. doi: 10.1007/s00705-014-2035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marcone D N, Culasso A, Carballal G, Campos R, Echavarría M. Genetic diversity and clinical impact of human rhinoviruses in hospitalized and outpatient children with acute respiratory infection, Argentina. J Clin Virol. 2014;61(4):558–564. doi: 10.1016/j.jcv.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pariani E, Martinelli M, Canuti M. et al. Influenza and other respiratory viruses involved in severe acute respiratory disease in northern Italy during the pandemic and postpandemic period (2009-2011) Biomed Res Int. 2014;2014:241298. doi: 10.1155/2014/241298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmerman R K, Rinaldo C R, Nowalk M P. et al. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011-12 influenza season. Influenza Other Respi Viruses. 2014;8(4):397–405. doi: 10.1111/irv.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atmar R L, Guy E, Guntupalli K K. et al. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158(22):2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 88.Nicholson K G, Kent J, Ireland D C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307(6910):982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiang Z, Gonzalez R, Xie Z. et al. Human rhinovirus group C infection in children with lower respiratory tract infection. Emerg Infect Dis. 2008;14(10):1665–1667. doi: 10.3201/eid1410.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grünberg K, Timmers M C, de Klerk E P, Dick E C, Sterk P J. Experimental rhinovirus 16 infection causes variable airway obstruction in subjects with atopic asthma. Am J Respir Crit Care Med. 1999;160(4):1375–1380. doi: 10.1164/ajrccm.160.4.9810083. [DOI] [PubMed] [Google Scholar]
- 91.Papadopoulos N G, Bates P J, Bardin P G. et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181(6):1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 92.Gern J E, Galagan D M, Jarjour N N, Dick E C, Busse W W. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155(3):1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Z, Tang W, Gwaltney J M Jr, Wu Y, Elias J A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol. 1997;273(4, Pt 1):L814–L824. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]
- 94.Gern J E, Vrtis R, Grindle K A, Swenson C, Busse W W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162(6):2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 95.Zhu Z, Tang W, Ray A. et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest. 1996;97(2):421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wark P A, Johnston S L, Bucchieri F. et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forbes R L, Gibson P G, Murphy V E, Wark P A. Impaired type I and III interferon response to rhinovirus infection during pregnancy and asthma. Thorax. 2012;67(3):209–214. doi: 10.1136/thoraxjnl-2011-200708. [DOI] [PubMed] [Google Scholar]
- 98.Contoli M, Message S D, Laza-Stanca V. et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 99.Durrani S R, Montville D J, Pratt A S. et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130(2):489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heymann P W, Kennedy J L. Rhinovirus-induced asthma exacerbations during childhood: the importance of understanding the atopic status of the host. J Allergy Clin Immunol. 2012;130(6):1315–1316. doi: 10.1016/j.jaci.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beigelman A, Bacharier L B. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol. 2013;13(2):211–216. doi: 10.1097/ACI.0b013e32835eb6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piotrowska Z, Vázquez M, Shapiro E D. et al. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J. 2009;28(1):25–29. doi: 10.1097/INF.0b013e3181861da0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung J Y, Han T H, Kim S W, Kim C K, Hwang E S. Detection of viruses identified recently in children with acute wheezing. J Med Virol. 2007;79(8):1238–1243. doi: 10.1002/jmv.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson D J, Gangnon R E, Evans M D. et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemanske R F Jr, Jackson D J, Gangnon R E. et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 106.Kim W K, Gern J E. Updates in the relationship between human rhinovirus and asthma. Allergy Asthma Immunol Res. 2012;4(3):116–121. doi: 10.4168/aair.2012.4.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jackson D J, Evans M D, Gangnon R E. et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185(3):281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calışkan M, Bochkov Y A, Kreiner-Møller E. et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seemungal T, Harper-Owen R, Bhowmik A. et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 110.Rohde G, Wiethege A, Borg I. et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ko F W, Ip M, Chan P K. et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132(3):900–908. doi: 10.1378/chest.07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perotin J M, Dury S, Renois F. et al. Detection of multiple viral and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a pilot prospective study. J Med Virol. 2013;85(5):866–873. doi: 10.1002/jmv.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kherad O, Kaiser L, Bridevaux P O. et al. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138(4):896–904. doi: 10.1378/chest.09-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sajjan U S, Jia Y, Newcomb D C. et al. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J. 2006;20(12):2121–2123. doi: 10.1096/fj.06-5806fje. [DOI] [PubMed] [Google Scholar]
- 115.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003;16(2):230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bulut Y, Güven M, Otlu B. et al. Acute otitis media and respiratory viruses. Eur J Pediatr. 2007;166(3):223–228. doi: 10.1007/s00431-006-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McBride T P, Doyle W J, Hayden F G, Gwaltney J M Jr. Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg. 1989;115(9):1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- 118.Chantzi F M, Papadopoulos N G, Bairamis T. et al. Human rhinoviruses in otitis media with effusion. Pediatr Allergy Immunol. 2006;17(7):514–518. doi: 10.1111/j.1399-3038.2006.00448.x. [DOI] [PubMed] [Google Scholar]
- 119.Madhi S A, Govender N, Dayal K. et al. Bacterial and respiratory viral interactions in the etiology of acute otitis media in HIV-infected and HIV-uninfected South African children. Pediatr Infect Dis J. 2015;34(7):753–760. doi: 10.1097/INF.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gwaltney J M Jr, Phillips C D, Miller R D, Riker D K. Computed tomographic study of the common cold. N Engl J Med. 1994;330(1):25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 121.Elkhatieb A, Hipskind G, Woerner D, Hayden F G. Middle ear abnormalities during natural rhinovirus colds in adults. J Infect Dis. 1993;168(3):618–621. doi: 10.1093/infdis/168.3.618. [DOI] [PubMed] [Google Scholar]
- 122.Pitkäranta A, Roivainen M, Blomgren K. et al. Presence of viral and bacterial pathogens in the nasopharynx of otitis-prone children. A prospective study. Int J Pediatr Otorhinolaryngol. 2006;70(4):647–654. doi: 10.1016/j.ijporl.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 123.Pitkäranta A, Arruda E, Malmberg H, Hayden F G. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35(7):1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gwaltney J M Jr, Hendley J O, Phillips C D, Bass C R, Mygind N, Winther B. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30(2):387–391. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 125.Falsey A R, Walsh E E, Hayden F G. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185(9):1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Franz A, Adams O, Willems R. et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48(4):239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marcos M A, Esperatti M, Torres A. Viral pneumonia. Curr Opin Infect Dis. 2009;22(2):143–147. doi: 10.1097/QCO.0b013e328328cf65. [DOI] [PubMed] [Google Scholar]
- 128.Nascimento-Carvalho C M, Ribeiro C T, Cardoso M R. et al. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. Pediatr Infect Dis J. 2008;27(10):939–941. doi: 10.1097/INF.0b013e3181723751. [DOI] [PubMed] [Google Scholar]
- 129.Choi S H, Hong S B, Ko G B. et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 130.Launes C, de-Sevilla M F, Selva L, Garcia-Garcia J J, Pallares R, Muñoz-Almagro C. Viral coinfection in children less than five years old with invasive pneumococcal disease. Pediatr Infect Dis J. 2012;31(6):650–653. doi: 10.1097/INF.0b013e31824f25b0. [DOI] [PubMed] [Google Scholar]
- 131.García-García M L, Calvo C, Pozo F, Villadangos P A, Pérez-Breña P, Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(8):808–813. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 132.Miller E K, Bugna J, Libster R. et al. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics. 2012;129(1):e60–e67. doi: 10.1542/peds.2011-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Esposito S, Daleno C, Tagliabue C. et al. Impact of rhinoviruses on pediatric community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2012;31(7):1637–1645. doi: 10.1007/s10096-011-1487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Homaira N, Luby S P, Petri W A. et al. Incidence of respiratory virus-associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009-2011. PLoS ONE. 2012;7(2):e32056. doi: 10.1371/journal.pone.0032056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guerrier G, Goyet S, Chheng E T. et al. Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr Infect Dis J. 2013;32(1):e8–e13. doi: 10.1097/INF.0b013e31826fd40d. [DOI] [PubMed] [Google Scholar]
- 136.Cho H J, Shim S Y, Son D W, Sun Y H, Tchah H, Jeon I S. Respiratory viruses in neonates hospitalized with acute lower respiratory tract infections. Pediatr Int. 2013;55(1):49–53. doi: 10.1111/j.1442-200X.2012.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suzuki A, Lupisan S, Furuse Y. et al. Respiratory viruses from hospitalized children with severe pneumonia in the Philippines. BMC Infect Dis. 2012;12:267. doi: 10.1186/1471-2334-12-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Uršič T, Jevšnik M, Zigon N. et al. Human bocavirus and other respiratory viral infections in a 2-year cohort of hospitalized children. J Med Virol. 2012;84(1):99–108. doi: 10.1002/jmv.22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pretorius M A, Madhi S A, Cohen C. et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness—South Africa, 2009-2010. J Infect Dis. 2012;206 01:S159–S165. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- 140.Lu G, Li J, Xie Z. et al. Human metapneumovirus associated with community-acquired pneumonia in children in Beijing, China. J Med Virol. 2013;85(1):138–143. doi: 10.1002/jmv.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chidlow G R, Laing I A, Harnett G B. et al. Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J Clin Virol. 2012;54(3):235–239. doi: 10.1016/j.jcv.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghani A S, Morrow B M, Hardie D R, Argent A C. An investigation into the prevalence and outcome of patients admitted to a pediatric intensive care unit with viral respiratory tract infections in Cape Town, South Africa. Pediatr Crit Care Med. 2012;13(5):e275–e281. doi: 10.1097/PCC.0b013e3182417848. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y W, Huang Y C, Ho T H, Huang C G, Tsao K C, Lin T Y. Viral etiology of bronchiolitis among pediatric inpatients in northern Taiwan with emphasis on newly identified respiratory viruses. J Microbiol Immunol Infect. 2014;47(2):116–121. doi: 10.1016/j.jmii.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Antunes H, Rodrigues H, Silva N. et al. Etiology of bronchiolitis in a hospitalized pediatric population: prospective multicenter study. J Clin Virol. 2010;48(2):134–136. doi: 10.1016/j.jcv.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mansbach J M, Piedra P A, Teach S J. et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng S Z, Xiao N G, Xie Z P. et al. Prevalence of human rhinovirus in children admitted to hospital with acute lower respiratory tract infections in Changsha, China. J Med Virol. 2014;86(11):1983–1989. doi: 10.1002/jmv.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ouédraogo S, Traoré B, Nene Bi Z A. et al. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso) PLoS ONE. 2014;9(10):e110435. doi: 10.1371/journal.pone.0110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gooskens J, van der Ploeg V, Sukhai R N, Vossen A C, Claas E C, Kroes A C. Clinical evaluation of viral acute respiratory tract infections in children presenting to the emergency department of a tertiary referral hospital in the Netherlands. BMC Pediatr. 2014;14:297–305. doi: 10.1186/s12887-014-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qu J X, Gu L, Pu Z H. et al. Viral etiology of community-acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis. 2015;15:89–96. doi: 10.1186/s12879-015-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seo Y B, Song J Y, Choi M J. et al. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother. 2014;46(2):67–76. doi: 10.3947/ic.2014.46.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Walker E, Ison M G. Respiratory viral infections among hospitalized adults: experience of a single tertiary healthcare hospital. Influenza Other Respi Viruses. 2014;8(3):282–292. doi: 10.1111/irv.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drieghe S, Ryckaert I, Beuselinck K, Lagrou K, Padalko E. Epidemiology of respiratory viruses in bronchoalveolar lavage samples in a tertiary hospital. J Clin Virol. 2014;59(3):208–211. doi: 10.1016/j.jcv.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fica A, Dabanch J, Andrade W. et al. Clinical relevance of rhinovirus infections among adult hospitalized patients. Braz J Infect Dis. 2015;19(2):118–124. doi: 10.1016/j.bjid.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhan Y, Yang Z, Chen R, Wang Y, Guan W, Zhao S. Respiratory virus is a real pathogen in immunocompetent community-acquired pneumonia: comparing to influenza like illness and volunteer controls. BMC Pulm Med. 2014;14:144–152. doi: 10.1186/1471-2466-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jain S, Self W H, Wunderink R G. et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shah A, Connelly M, Whitaker P. et al. Pathogenicity of individual rhinovirus species during exacerbations of cystic fibrosis. Eur Respir J. 2015;45(6):1748–1751. doi: 10.1183/09031936.00229114. [DOI] [PubMed] [Google Scholar]
- 157.Collinson J, Nicholson K G, Cancio E. et al. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51(11):1115–1122. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smyth A R, Smyth R L, Tong C Y, Hart C A, Heaf D P. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child. 1995;73(2):117–120. doi: 10.1136/adc.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goffard A, Lambert V, Salleron J. et al. Virus and cystic fibrosis: rhinoviruses are associated with exacerbations in adult patients. J Clin Virol. 2014;60(2):147–153. doi: 10.1016/j.jcv.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ramirez I A Caverly L J Kalikin L M et al. Differential responses to rhinovirus- and influenza-associated pulmonary exacerbations in patients with cystic fibrosis Ann Am Thorac Soc 2014114554–561. (Erratun: Caverly, Lindsay L [corrected to Caverly, Lindsay J]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Garbino J, Gerbase M W, Wunderli W. et al. Respiratory viruses and severe lower respiratory tract complications in hospitalized patients. Chest. 2004;125(3):1033–1039. doi: 10.1378/chest.125.3.1033. [DOI] [PubMed] [Google Scholar]
- 162.Ison M G, Hayden F G, Kaiser L, Corey L, Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36(9):1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hassan I A, Chopra R, Swindell R, Mutton K J. Respiratory viral infections after bone marrow/peripheral stem-cell transplantation: the Christie hospital experience. Bone Marrow Transplant. 2003;32(1):73–77. doi: 10.1038/sj.bmt.1704048. [DOI] [PubMed] [Google Scholar]
- 164.Ljungman P, Ward K N, Crooks B N. et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28(5):479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 165.Ghosh S, Champlin R, Couch R. et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29(3):528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 166.Milano F, Campbell A P, Guthrie K A. et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115(10):2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ohrmalm L, Wong M, Aust C. et al. Viral findings in adult hematological patients with neutropenia. PLoS ONE. 2012;7(5):e36543. doi: 10.1371/journal.pone.0036543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Torres J P, Labraña Y, Ibañez C. et al. Frequency and clinical outcome of respiratory viral infections and mixed viral-bacterial infections in children with cancer, fever and neutropenia. Pediatr Infect Dis J. 2012;31(9):889–893. doi: 10.1097/INF.0b013e31825c4b7e. [DOI] [PubMed] [Google Scholar]
- 169.Seo S, Renaud C, Kuypers J M. et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood. 2015;125(24):3789–3797. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Campbell A P, Guthrie K A, Englund J A. et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis. 2015;61(2):192–202. doi: 10.1093/cid/civ272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Longtin J, Winter A L, Heng D. et al. Severe human rhinovirus outbreak associated with fatalities in a long-term care facility in Ontario, Canada. J Am Geriatr Soc. 2010;58(10):2036–2038. doi: 10.1111/j.1532-5415.2010.03091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Valenti W M, Clarke T A, Hall C B, Menegus M A, Shapiro D L. Concurrent outbreaks of rhinovirus and respiratory syncytial virus in an intensive care nursery: epidemiology and associated risk factors. J Pediatr. 1982;100(5):722–726. doi: 10.1016/s0022-3476(82)80571-4. [DOI] [PubMed] [Google Scholar]
- 173.Reid A B, Anderson T L, Cooley L, Williamson J, Mcgregor A R. An outbreak of human rhinovirus species C infections in a neonatal intensive care unit. Pediatr Infect Dis J. 2011;30(12):1096–1095. doi: 10.1097/INF.0b013e31822938d7. [DOI] [PubMed] [Google Scholar]
- 174.van Piggelen R O, van Loon A M, Krediet T G, Verboon-Maciolek M A. Human rhinovirus causes severe infection in preterm infants. Pediatr Infect Dis J. 2010;29(4):364–365. doi: 10.1097/INF.0b013e3181c6e60f. [DOI] [PubMed] [Google Scholar]
- 175.Longtin J, Marchand-Austin A, Winter A L. et al. Rhinovirus outbreaks in long-term care facilities, Ontario, Canada. Emerg Infect Dis. 2010;16(9):1463–1465. doi: 10.3201/eid1609.100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wald T G, Shult P, Krause P, Miller B A, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med. 1995;123(8):588–593. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 177.Schoop R, Klein P, Suter A, Johnston S L. Echinacea in the prevention of induced rhinovirus colds: a meta-analysis. Clin Ther. 2006;28(2):174–183. doi: 10.1016/j.clinthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 178.Louie J K, Yagi S, Nelson F A. et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41(2):262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hicks L A, Shepard C W, Britz P H. et al. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J Am Geriatr Soc. 2006;54(2):284–289. doi: 10.1111/j.1532-5415.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 180.Siegel J D Rhinehart E Jackson M Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings Am J Infect Control 2007351002S65–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Singh M. Heated, humidified air for the common cold. Cochrane Database Syst Rev. 2004;(2):CD001728. doi: 10.1002/14651858.CD001728.pub2. [DOI] [PubMed] [Google Scholar]
- 182.Rotbart H A. Antiviral therapy for enteroviruses and rhinoviruses. Antivir Chem Chemother. 2000;11(4):261–271. doi: 10.1177/095632020001100402. [DOI] [PubMed] [Google Scholar]
- 183.Hayden F G, Herrington D T, Coats T L. et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36(12):1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koenighofer M, Lion T, Bodenteich A. et al. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Respir Med. 2014;9(1):57. doi: 10.1186/2049-6958-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sperber S J, Shah L P, Gilbert R D, Ritchey T W, Monto A S. Echinacea purpurea for prevention of experimental rhinovirus colds. Clin Infect Dis. 2004;38(10):1367–1371. doi: 10.1086/386324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Turner R B. Ineffectiveness of intranasal zinc gluconate for prevention of experimental rhinovirus colds. Clin Infect Dis. 2001;33(11):1865–1870. doi: 10.1086/324347. [DOI] [PubMed] [Google Scholar]
- 187.Kurugöl Z, Akilli M, Bayram N, Koturoglu G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr. 2006;95(10):1175–1181. doi: 10.1080/08035250600603024. [DOI] [PubMed] [Google Scholar]
- 188.Yamaya M, Nishimura H, Hatachi Y. et al. Levofloxacin inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Antimicrob Agents Chemother. 2012;56(8):4052–4061. doi: 10.1128/AAC.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Min J Y, Jang Y J. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. doi: 10.1155/2012/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jartti T, Nieminen R, Vuorinen T. et al. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135(3):691–8.0E9. doi: 10.1016/j.jaci.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Turner R B, Hendley J O. Virucidal hand treatments for prevention of rhinovirus infection. J Antimicrob Chemother. 2005;56(5):805–807. doi: 10.1093/jac/dki329. [DOI] [PubMed] [Google Scholar]
- 192.Savolainen-Kopra C, Korpela T, Simonen-Tikka M L. et al. Single treatment with ethanol hand rub is ineffective against human rhinovirus—hand washing with soap and water removes the virus efficiently. J Med Virol. 2012;84(3):543–547. doi: 10.1002/jmv.23222. [DOI] [PubMed] [Google Scholar]
- 193.Luby S P, Lu X, Cromeans T, Sharker M A, Kadir M A, Erdman D D. Hand contamination with human rhinovirus in Bangladesh. J Med Virol. 2014;86(12):2177–2180. doi: 10.1002/jmv.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Blanco J CG, Core S, Pletneva L M, March T H, Boukhvalova M S, Kajon A E. Prophylactic antibody treatment and intramuscular immunization reduce infectious human rhinovirus 16 load in the lower respiratory tract of challenged cotton rats. Trials Vaccinol. 2014;3:52–60. doi: 10.1016/j.trivac.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Glanville N, Johnston S L. Challenges in developing a cross-serotype rhinovirus vaccine. Curr Opin Virol. 2015;11:83–88. doi: 10.1016/j.coviro.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 196.McLean G R. Developing a vaccine for human rhinoviruses. J Vaccines Immun. 2014;2(3):16–20. doi: 10.14312/2053-1273.2014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McLean G R, Walton R P, Shetty S. et al. Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1. Antiviral Res. 2012;95(3):193–201. doi: 10.1016/j.antiviral.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 198.Yeager C L, Ashmun R A, Williams R K. et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reguera J, Mudgal G, Santiago C, Casasnovas J M. A structural view of coronavirus-receptor interactions. Virus Res. 2014;194:3–15. doi: 10.1016/j.virusres.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol. 2015;89(4):1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A. 2005;102(22):7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Williams R K, Jiang G S, Holmes K V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang X, Dong W, Milewska A. et al. HCoV-HKU1 spike protein uses O-acetylated sialic acid as an attachment receptor determinant and employs HE protein as a receptor-destroying enzyme. J Virol. 2015;89(14):7202–7213. doi: 10.1128/JVI.00854-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gralinski L E, Baric R S. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235(2):185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schäfer A, Baric R S, Ferris M T. Systems approaches to Coronavirus pathogenesis. Curr Opin Virol. 2014;6:61–69. doi: 10.1016/j.coviro.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Coleman C M, Liu Y V, Mu H. et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tang X C, Agnihothram S S, Jiao Y. et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A. 2014;111(19):E2018–E2026. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van den Brand J M, Smits S L, Haagmans B L. Pathogenesis of Middle East respiratory syndrome coronavirus. J Pathol. 2015;235(2):175–184. doi: 10.1002/path.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Totura A L, Baric R S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schmidt O W, Kenny G E. Immunogenicity and antigenicity of human coronaviruses 229E and OC43. Infect Immun. 1981;32(3):1000–1006. doi: 10.1128/iai.32.3.1000-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McIntosh K, Dees J H, Becker W B, Kapikian A Z, Chanock R M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967;57(4):933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McIntosh K Ellis E F Hoffman L S Lybass T G Eller J J Fulginiti V A Association of viral and bacterial respiratory infection with exacerbations of wheezing in young asthmatic children Chest 197363(Suppl):43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Falsey A R, McCann R M, Hall W J. et al. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc. 1997;45(6):706–711. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dijkman R, Jebbink M F, El Idrissi N B. et al. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46(7):2368–2373. doi: 10.1128/JCM.00533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Drosten C, Günther S, Preiser W. et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 216.Vabret A, Mourez T, Dina J. et al. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11(8):1225–1229. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Arden K E, Nissen M D, Sloots T P, Mackay I M. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75(3):455–462. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van der Hoek L, Pyrc K, Jebbink M F. et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Esper F, Weibel C, Ferguson D, Landry M L, Kahn J S. Coronavirus HKU1 infection in the United States. Emerg Infect Dis. 2006;12(5):775–779. doi: 10.3201/eid1205.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Esper F, Weibel C, Ferguson D, Landry M L, Kahn J S. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191(4):492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Talbot H K, Shepherd B E, Crowe J E Jr. et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. 2009;28(8):682–687. doi: 10.1097/INF.0b013e31819d0d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vijgen L, Keyaerts E, Lemey P. et al. Circulation of genetically distinct contemporary human coronavirus OC43 strains. Virology. 2005;337(1):85–92. doi: 10.1016/j.virol.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cheng V C, Lau S K, Woo P C, Yuen K Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Memish Z A, Cotten M, Meyer B. et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Saad M, Omrani A S, Baig K. et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Assiri A, McGeer A, Perl T M. et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Memish Z A, Cotten M, Watson S J. et al. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. doi: 10.1016/j.ijid.2014.03.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Centers for Disease Control and Prevention CDC announces second imported case of Middle East Respiratory Syndrome (MERS) in the United States [press release]. Available at: http://www.cdc.gov/media/releases/2014/p0512-US-MERS.html. Published May 12, 2014
- 229.Centers for Disease Control and Prevention CDC telebriefing: updates on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) investigation in the United States Available at: http://www.cdc.gov/media/releases/2014/t0517-mers.html. Published May 17, 2014. Updated May 19, 2014
- 230.Atmar R, Greenberg S B. New York: Informa Healthcare; 2010. Respiratory viral infections; pp. 246–271. [Google Scholar]
- 231.Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J Virol Methods. 2001;97(1–2):59–66. doi: 10.1016/S0166-0934(01)00343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cao Z, Liu L, Du L. et al. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol J. 2010;7:299. doi: 10.1186/1743-422X-7-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Adachi D, Johnson G, Draker R. et al. Comprehensive detection and identification of human coronaviruses, including the SARS-associated coronavirus, with a single RT-PCR assay. J Virol Methods. 2004;122(1):29–36. doi: 10.1016/j.jviromet.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Walsh E E, Shin J H, Falsey A R. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis. 2013;208(10):1634–1642. doi: 10.1093/infdis/jit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Reusken C, Mou H, Godeke G J. et al. Specific serology for emerging human coronaviruses by protein microarray. Euro Surveill. 2013;18(14):20441. doi: 10.2807/1560-7917.es2013.18.14.20441. [DOI] [PubMed] [Google Scholar]
- 236.Che X Y, Qiu L W, Pan Y X. et al. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004;42(6):2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhao G, Du L, Ma C. et al. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol J. 2013;10:266. doi: 10.1186/1743-422X-10-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Azhar E I, El-Kafrawy S A, Farraj S A. et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 239.Gerna G, Campanini G, Rovida F. et al. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol. 2006;78(7):938–949. doi: 10.1002/jmv.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gerna G, Percivalle E, Sarasini A. et al. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J Clin Virol. 2007;38(3):244–250. doi: 10.1016/j.jcv.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis. J Med Virol. 2005;75(3):463–465. doi: 10.1002/jmv.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vabret A, Dina J, Gouarin S. et al. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J Paediatr Child Health. 2008;44(4):176–181. doi: 10.1111/j.1440-1754.2007.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wu P S, Chang L Y, Berkhout B. et al. Clinical manifestations of human coronavirus NL63 infection in children in Taiwan. Eur J Pediatr. 2008;167(1):75–80. doi: 10.1007/s00431-007-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Peiris J S, Yuen K Y, Osterhaus A D, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 245.Ksiazek T G, Erdman D, Goldsmith C S. et al. SARS Working Group . A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 246.Milne-Price S, Miazgowicz K L, Munster V J. The emergence of the Middle East respiratory syndrome coronavirus. Pathog Dis. 2014;71(2):121–136. doi: 10.1111/2049-632X.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ajlan A M, Ahyad R A, Jamjoom L G, Alharthy A, Madani T A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203(4):782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 248.Gogna A, Tay K H, Tan B S. Severe acute respiratory syndrome: 11 years later—a radiology perspective. AJR Am J Roentgenol. 2014;203(4):746–748. doi: 10.2214/AJR.14.13062. [DOI] [PubMed] [Google Scholar]
- 249.Cunha C B, Opal S M. Middle East respiratory syndrome (MERS): a new zoonotic viral pneumonia. Virulence. 2014;5(6):650–654. doi: 10.4161/viru.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wong S S, Yuen K Y. The management of coronavirus infections with particular reference to SARS. J Antimicrob Chemother. 2008;62(3):437–441. doi: 10.1093/jac/dkn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Frausto S D, Lee E, Tang H. Cyclophilins as modulators of viral replication. Viruses. 2013;5(7):1684–1701. doi: 10.3390/v5071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Falzarano D, de Wit E, Martellaro C, Callison J, Munster V J, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt T S, Al Mutairy E. Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. 2015;20(1):87–91. doi: 10.3851/IMP2792. [DOI] [PubMed] [Google Scholar]
- 254.de Wilde A H, Jochmans D, Posthuma C C. et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kindrachuk J, Ork B, Hart B J. et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chu C M, Cheng V C, Hung I F. et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vincent M J, Bergeron E, Benjannet S. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Al-Tawfiq J A, Memish Z A. Middle East respiratory syndrome coronavirus: epidemiology and disease control measures. Infect Drug Resist. 2014;7:281–287. doi: 10.2147/IDR.S51283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Graham R L, Donaldson E F, Baric R S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Maltezou H C, Tsiodras S. Middle East respiratory syndrome coronavirus: implications for health care facilities. Am J Infect Control. 2014;42(12):1261–1265. doi: 10.1016/j.ajic.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mou H, Raj V S, van Kuppeveld F J, Rottier P J, Haagmans B L, Bosch B J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87(16):9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59(1–3):118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ma C, Wang L, Tao X. et al. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments—the importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32(46):6170–6176. doi: 10.1016/j.vaccine.2014.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ying T, Du L, Ju T W. et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J Virol. 2014;88(14):7796–7805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ma C, Li Y, Wang L. et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32(18):2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang N, Tang J, Lu L, Jiang S, Du L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015;202:151–159. doi: 10.1016/j.virusres.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ohnuma K, Haagmans B L, Hatano R. et al. Inhibition of Middle East respiratory syndrome coronavirus infection by anti-CD26 monoclonal antibody. J Virol. 2013;87(24):13892–13899. doi: 10.1128/JVI.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mair-Jenkins J, Saavedra-Campos M, Baillie J K. et al. Convalescent Plasma Study Group . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cheng V C, Chan J F, To K K, Yuen K Y. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100(2):407–419. doi: 10.1016/j.antiviral.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Agnihothram S, Gopal R, Yount B L Jr. et al. Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J Infect Dis. 2014;209(7):995–1006. doi: 10.1093/infdis/jit609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sampathkumar P. Middle East respiratory syndrome: what clinicians need to know. Mayo Clin Proc. 2014;89(8):1153–1158. doi: 10.1016/j.mayocp.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


