Abstract
Introduction:
Androgen deprivation therapy (ADT) is the mainstay for advanced, hormone-sensitive prostate cancer, and options include surgical castration, luteinizing hormone-releasing hormone (LHRH) agonist, and more recently, gonadotropin releasing hormone (GnRH) antagonist therapy. Our understanding of the mechanisms and adverse effects of ADT has increased substantially, including the class-specific adverse effects of ADT.
Areas Covered:
This review will summarize the pharmacodynamic and pharmacokinetic properties of the GnRH antagonist degarelix and its role in the management of advanced prostate cancer, the clinical evidence supporting its regulatory approval, as well as potential benefits and disadvantages over traditional LHRH agonist therapy.
Expert Opinion:
Degarelix represents a newer class of ADT that results in a rapid and reliable decline in serum testosterone, a quality that makes it particularly advantageous in men presenting with symptomatic, hormone-sensitive prostate cancer. Due to differences in mechanism of action, there is observational data suggesting a potential cardiovascular and even oncologic benefit over traditional LHRH agonist therapy. Further research is ongoing to more clearly define this potential benefit.
Keywords: degarelix, androgen deprivation therapy, LHRH agonist, GnRH antagonist, prostate cancer
1.0. Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer-related death in the United States. [1] The link between prostate cancer and androgens has been known since the 1940s when it was discovered that lower serum testosterone levels by orchiectomy or estrogen injection could improve the symptoms of men with metastatic prostate cancer. [2] Since then, various forms of androgen deprivation therapy (ADT) have become the mainstay in the management of advanced prostate cancer. This strategy remains an important tool in the management of this disease in a number of clinical settings including treatment of metastatic disease, adjuvant treatment to radiation therapy, and biochemical failure following definitive local therapy. Despite the identification of androgen ablation in the treatment of prostate cancer there remains continued debate as to the timing, utility and preferential treatment options for ADT. This article reviews the pharmacodynamic and pharmacokinetic properties of gonadotropin-releasing hormone (GnRH) antagonist degarelix, including its role in the management of prostate cancer and its perceived benefits and disadvantages as compared to traditional luteinizing hormone-releasing hormone (LHRH) agonists.
2.0. Mechanism of Action of ADT
Since Huggins and Hodges were able to identify prostate cancer as a hormonally-sensitive disease, ADT has been a standard treatment at different points within the disease process. [2,3] The adequacy of ADT is routinely assessed based on achieving castrate levels of serum testosterone. The production of testosterone begins with the hypothalamic-pituitary axis when LHRH (also known as GnRH) is released by the hypothalamus and stimulates production of luteinizing hormone (LH) from the anterior pituitary. In response to LH, testosterone is produced by Leydig cells in the testes. It is important to note that the adrenal gland also produces a small amount of testosterone. To be termed correctly, ADT is a ligand-reduction therapy, with the receptor for testosterone being the androgen receptor (AR). [4] In the inactive form, AR is bound by heat-shock proteins in the cell cytoplasm. When bound by androgens, the AR localizes to the nucleus and allows for target gene transcription. [5] These genes are specific to a number of processes including the development of male sexual characteristics, the maturation of the prostate and the production of proteins such as PSA.
ADT therefore attempts to disrupt this pathway either at the pre-receptor, receptor or post-receptor levels. [4,6] ADT can be achieved with various modalities. The simplest form is via surgical castration (i.e. bilateral orchiectomy), which removes all Leydig cells, however leaves adrenal production of testosterone intact. Medical castration has become the mainstay for ADT due to the potential reversibility, avoidance of surgical castration and its associated psychosocial implications and decreased toxicity profile when compared to the historic use of diethylstilbestrol. [3] The focus of this review will be on medical castration treatments aimed at the pre-receptor targets.
The first of these pre-receptor treatments was the discovery of the LHRH analog as a treatment for advanced and metastatic prostate cancer and is based on the idea of a negative feedback loop. [7] The administration of this treatment, after an initial surge in LH release (and thereby testosterone levels), results in the desensitization and inhibition through negative feedback loops resulting in plummeting LH levels by 4 weeks. [8] As the hypothalamus does not stimulate LH production this results in a decrease in gonadal testosterone production. The concern remained regarding the initial LH and testosterone surge possibly resulting in a clinical flare phenomenon, to be described in the following section.
Given the clinical concern of testosterone surge and the delay to castration, development of LHRH receptor antagonists was the next logical progression. [9] These agents bind competitively to the LHRH receptors in the pituitary, thereby preventing production of LH and downstream the production of testosterone. It should be noted that a difference in the concentration of FSH differs between LHRH agonists and LHRH receptor antagonists. The agonists over time have only partial suppression of FSH, whereas the LHRH receptor antagonists maintain suppression of FSH. [10,11] Without the agonist-activity the hormonal surge, as seen with the LHRH agonists, does not occur. These mechanisms of action are extremely important to understanding the clinical utility of different treatments within ADT.
2.1. Flare Phenomenon
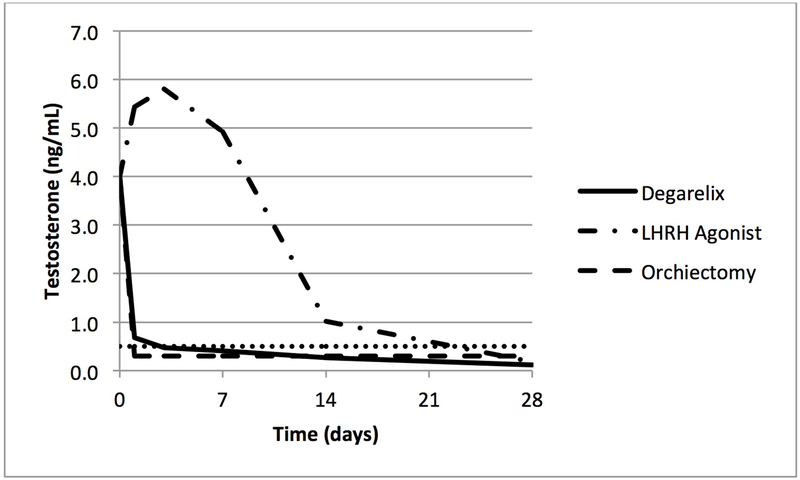
The initial response from a LHRH agonist is a transient increase in the LH and testosterone levels (Fig 1). This increase can last up to 20 days and be associated with a 10-fold rise in LH. This has been shown to accompany a clinical response known as a clinical flare, which is a result of the increased testosterone on the hormonally-drive prostate cancer. [12,13] Depending on the sites of metastasis, the flare symptoms can include bone pain, bladder outlet obstruction, ureteral obstruction, spinal cord compression and cardiovascular effects. [12] Clinical flare phenomenon has been a known problem for patients with metastatic disease with a wide range of reported incidence of 4-63%. [13] Studies looking at the concomitant use of antiandrogens with LHRH agonists had demonstrated an improvement in clinical flare symptoms. [14] To combat the hormonal surge and clinical flare, it has been shown that the use of an antiandrogen prior to LHRH agonist administration can further decrease the risk of flare. [15–17] Although there is no consensus as to the routine administration of antiandrogen within guidelines [3], those most at risk of clinical flare (symptomatic advanced prostate cancer, spinal cord metastasis, obstructive voiding symptoms) are typically given a blockade with antiandrogen for at least one week. [15–18]
Figure 1.
Median testosterone levels during the first 28 days of treatment with degarelix, leuprolide or orchiectomy in men with prostate cancer. [20,78]
3.0. Overview of the Market
Discovery of synthetic LHRH analogues introduced a novel treatment strategy from prior management with surgical castration or estrogen administration. [3] The initial treatments developed were LHRH receptor agonists with the first analog developed being Leuprolide, which obtained FDA-approval in 1985 and has remained the traditional standard for ADT. [4,6] A second analog was developed called goserelin, with FDA-approval in 1989. Two more LHRH analogs, triptorelin and histrelin, were approved in 2000 and 2004 respectively. [6] While there have been no trials comparing these agents head-to-head, a meta-analysis of 24 trials of monotherapy found no differences in androgen suppression between the different LHRH agonists. [19]
As described previously the testosterone surge after administration of LHRH agonists led to the development of LHRH receptor antagonists. The first FDA-approved agent was abarelix in 2003. [20,21] Unfortunately due to significant adverse allergic reactions this has since been removed from the market in the United States. [22] The only available LHRH receptor antagonist currently available is degarelix with a low risk of histamine release and garnered FDA-approval in 2008. [23,24] While there are no newly approved agents within the LHRH receptor antagonist classification or in the pre-receptor category, CYP17 inhibitors and AR antagonists are gaining much recognition and will start to garner new therapeutic utility in different stages of advanced prostate cancer.
4.0. Chemistry and Pharmacodynamics
Degarelix is a decapeptide with the sequence Ac-D-2-Nal-D-4Cpa-D-3Pal-Ser-4Aph(L-Hor)-D-4Aph(Cbm)-Leu-Ilys-Pro-D-Ala-NH2. When compared to prior LHRH receptor antagonists, degarelix was highly water soluble with a comparatively low propensity to form gels and ability to diffuse at high concentrations in a manner similar to that of slow release formulations of peptides. Given the potentially life-threatening allergic reaction side effects of prior generations of LHRH receptor antagonists, degarelix was found to be significantly less potent at releasing histamine from mast cells. [25,26]
Degarelix is a 3rd-generation LHRH receptor antagonist that competitively and reversibly binds LHRH receptors in the pituitary gland resulting in immediate suppression of LH and FSH release, thereby resulting in a very rapid decrease in testosterone secretion by testicular Leydig cells. [27] In general, ADT results in a 90-95% reduction in circulating levels of testosterone [8] and the within 8-24 hours after initial dose of a LHRH receptor antagonist, LH concentrations are reduced by 51-84%. [28] By 3 days after administration over 96% of patients will reach castrate levels of testosterone.
4.1. Pharmacokinetics and Metabolism
Degarelix is reconstituted in a mannitol solution and given as a subcutaneous injection. Once injected it forms a gel-like depot at the injection site when in contact with body fluids. This results in a sustained release of the agent in a prolonged duration of action. It is released into the systemic circulation in a biphasic manner characterized by an initial fast phase, then undergoes a maturation stage and is limited by its diffusion out of the depot resulting in a later slow phase. [29]
In the Phase III trial (CS21) the pharmacokinetics of degarelix 240mg was evaluated. With the initial dose the AUC0-28 days was 635ng/day per mL and Cmax was 66.0ng/mL and occurred at tmax = 40h. Degarelix is eliminated biphasically with a median terminal half-life of 43 days for initial dose and 28 days for the maintenance dose. [10,26,27,30]
Degarelix undergoes degradation by the hepato-biliary system but is fully excreted by both hepatic and renal mechanisms. [31] Degarelix has not been evaluated in those with renal impairment. Prescribing information at this time states it does not require dose adjustment in those with mild renal impairment but should be use with caution in those with moderate or severe impairment. [32] The same is true for those with hepatic impairment; those with mild or moderate impairment do not require dose adjustment, whereas caution should be take for those with severe hepatic impairment. There is no adjustment for age or weight and drug interactions are unlikely with degarelix as it is a poor substrate of the CYP450 system. [30,33]
5.0. Potential Oncologic Advantages Over LHRH Agonists
There are several hypotheses that purport to explain the supposed advantages of degarelix over LHRH agonist therapy. The first is the elimination of testosterone surge, which is seen with LHRH agonist therapy but not with degarelix. This eliminates the potential dangers of clinical flare phenomenon. The second advantage is time to castration. The reduction in circulating testosterone levels reach castrate levels within 3 days of administrating of degarelix but requires 28 days of treatment with Leuprolide. This delay can be critical when treating aggressive prostate cancer at high risk for rapid progression.
There is also evidence of maintenance repeat injections of LHRH agonists resulting in testosterone mircosurges. Testosterone levels were shown to increase to above castration levels temporarily in about a quarter of patients after goserelin injections. [34,35] The clinical implications of these microsurges have not been established.
Lastly, FSH may be an independent growth factor due to the levels of FSH receptors correlating with prostate volume and pathologic staging of the prostate. It is possible that agonists binding higher levels of FSH receptors may contribute to prostate cancer, and treatment with degarelix has been shown to suppress not only testosterone production but FSH as well. A recent experiment in a gonadotropin-suppressed nude mouse model xenograft with PC-3 human prostate cancer demonstrated that FSH supplementation resulted in tumor growth, while treatment with degarelix resulted in suppression of this growth. [36]
5.1. Potential Oncologic Disadvantages
There are a few theoretical disadvantages with degarelix that should be noted. The first disadvantage is the requirement of monthly depot injections in contrast to 3-6 month depot injections for the LHRH agonists. While this is a consideration for some patients, it should not limit the utility or use of degarelix. A second recent development was a published phase II study that randomized patients to neoadjuvant degarelix, degarelix plus antiandrogen, or LHRH agonist plus bicalutamide for 3 months prior to prostatectomy. Unexpectedly, the authors found a higher level of dihydrotestosterone (DHT) and normal levels of testosterone precursors in patients treated with degarelix alone. They hypothesized that rapid suppression of gonadotropins might lead to activation of alternative androgen production pathways or possible off-target effects of antiandrogen therapy. Although this study is limited by a small patient sample size, the results warrant further investigation. [37]
6.0. Clinical Efficacy
Metrics for systemic therapy in prostate cancer are different than those used in chemotherapy for other malignancies, where objective responses are typically gauged by radiographic criteria or survival outcomes. While such outcomes are the most relevant in any malignancy, including prostate cancer, the disease course for metastatic prostate cancer is long. Therefore, the surrogate markers of testosterone suppression and PSA reduction are often utilized. Indeed, the Southwest Oncology Group (SWOG) trial 9346 in patients with hormone-sensitive prostate cancer and SWOG trial 9916 in patients with castration-resistant prostate cancer demonstrated that PSA progression predicts overall survival and this endpoint may be a surrogate endpoint in similar such trials. [38]
After demonstrating efficacy in the pre-clinical setting and in a randomized trial of healthy, eugonadal young men, [39] degarelix was evaluated in two phase II, dose-escalation studies. [23,40] Both studies were open-label, randomized trials in patients of all stages of prostate cancer in whom ADT was indicated. In the North American study, patients were given a starting dose of 200mg degarelix and followed by 1 year of monthly injections of 60 or 80mg. [23] In the European study, patients were given starting dose 200 or 240mg of degarelix followed by 1 year of monthly injections of 80, 120, or 160mg. [40] Of the formulations, the 240mg dose was associated with the highest percentage of patients achieving a castrate-level of serum testosterone within 3 days and there did not appear to be a dose-dependent relationship with adverse events noted in the various strengths of maintenance doses. [23,40]
The pivotal study (CS21) of degarelix that led to approval of the drug was a phase III, randomized, open-label trial comparing degarelix vs LHRH agonists in a non-inferiority design in which patients were randomized into three arms: degarelix 240mg starting dose with either 80mg or 160mg monthly maintenance or a standard dose of leuprolide 7.5mg IM monthly. [24] Patients enrolled in the trial for any indication of ADT (excluding neoadjuvant therapy) including biochemical failure after treatment with curative intent and hormone-sensitive metastatic disease. Patients were followed on their assigned treatment regimens for 1 year and the study was analyzed for the primary endpoint of castration with testosterone level of ≤ 0.5 ng/mL between 28-364 days and secondary outcomes included the speed at which castration occurred, PSA failure following castration, adverse events, and quality of life analysis. Of the 610 patients included in the analysis, there was no differences between either degarelix dosing arms or the leuprolide arm in terms of the primary outcome, with all arms achieving castration level of testosterone at 28-364 days in >96% of patients. However, treatment with degarelix did result in a more rapid suppression of testosterone compared to leuprolide with >95% of patients achieving castration levels by day 3 compared to a median testosterone increase of 65% by day 3 in the leuprolide arm. [24] Although this trial utilized two doses of degarelix maintenance, it should be noted that the 240mg/80mg dose is the approved regimen. Secondary outcomes including PSA levels and progression-free survival were the subject of a more in-depth analysis. [41] The incidence of PSA progression, defined as two consecutive increases in PSA of 50% compared to nadir and ≥ 5 ng/mL on two consecutive measurements at least two weeks apart or death, were significantly lower in the degarelix 240/80mg arm (7.7%), compared to the degarelix 240/160mg (12.9%), and leuprolide arms (12.9%). When stratified by baseline prostate cancer stage, there was no difference in PSA progression in patients with localized or locally advanced disease. However, in patients with metastatic disease the PSA progression incidence was 36.2% and 21.6% for the leuprolide and degarelix 240/80mg respectively, although this difference did not reach statistical significance (p=0.156), nor was the study powered for sub-group analysis. When stratifying PSA progression by baseline PSA level, patients with a PSA > 20 ng/mL at the onset of ADT had a significantly lower risk of PSA progression with degarelix vs. leuprolide (p=0.04). After adjustment for baseline disease stage and PSA, the hazard ratio of PSA progression or death was 0.664 (95% CI 0.385-1.146). It is difficult to interpret why PSA progression was lowest in the degarelix 240/80mg arm while the risk of PSA progression between the higher dose of degarelix 240/160mg was similar to the leuprolide arm, but this may be due to different concentrations of maintenance doses (160mg was 40mg/mL, 80mg was 20mg/mL) or differences in baseline characteristics (a higher proportion of patients in the degarelix 240/160mg arm had baseline PSA > 50 ng/mL). [41] An exploratory analysis of serum alkaline phosphatase (S-ALP) from the CS21 trial concluded that degarelix was associated with a greater reduction in S-ALP level in patients with metastatic disease or those with a PSA ≥ 50 ng/mL and that degarelix was able to maintain S-ALP suppression throughout the study period. In contrast, treatment with leuprolide was associated with a gradual rise in S-ALP levels as the study continued. As S-ALP is a marker of bone turnover, the implication of this analysis is that degarelix may be superior to LHRH agonist therapy for skeletal metastasis control; however this hypothesis remains to be tested. [42]
Following conclusion of this trial, some of the patients voluntarily continued in a 5-year open-label extension phase of the trial (CS21A). Patients receiving degarelix were continued on their previous dose while those patients who initially received leuprolide were randomized to either 80mg or 160mg of degarelix, ultimately with all converting to 80mg of degarelix following regulatory approval of this maintenance dose. While the trial has been completed, the only published report is an interim analysis at a median follow-up of 27.5 months. In patients who continued on degarelix, there was no difference in the PSA progression-free survival hazard rate in the extension phase. The patients who crossed-over from leuprolide to degarelix saw the PSA progression-free survival hazard rate decrease by more than half, with 0.20 events per year with leuprolide in the first year and 0.08 events per year following cross-over to degarelix (p=0.003). [43]
An industry sponsored, pooled-analysis of 1,925 patients from five prospective randomized trials concluded that compared to LHRH agonists, degarelix was associated with an improved PSA progression-free survival, longer overall survival (HR 0.47), decreased joint, musculoskeletal, and urinary tract events. [44] Yet this should be interpreted with caution, as these trials were performed in very heterogeneous patient populations, with follow-up periods as low as 3 months and the events reported in the pooled analysis were not the primary endpoints of the original studies. [45–48] As an example, Gleason scoring was excluded from the multivariate analysis due to lack of central review and study heterogeneity. [44]
7.0. Other Indications
7.1. Lower Urinary Tract Symptom Reduction
Beyond standard prostate cancer control, degarelix has also been evaluated in a number of other clinical settings including reduction of prostate volume for lower urinary tract symptoms relief. Anderson et al. reported on a small number of patients (n=40) who were randomized to degarelix vs. LHRH agonist plus antiandrogen for 3 months and found a significantly larger reduction in International Prostate Symptom Score (IPSS) and prostate volume in patients treated with degarelix. [45] This advantage of more rapid prostate shrinkage was not confirmed in a larger trial of neoadjuvant degarelix vs. LHRH agonist plus antiandrogen prior to radiation therapy and a separate trial of ADT for primary treatment of prostate cancer in patients not eligible for definitive therapy. [46,47]
7.2. Intermittent ADT
Guidelines suggest that intermittent ADT should be considered in certain patients, as it is associated with lower cost, improved sexual function, and possible improvement in quality of life indicators during off-treatment cycles, while maintaining oncologic efficacy. [49–51] Degarelix has been studied in this setting in an open-label, single-arm study reporting improved sexual function and fewer adverse events during off-treatment periods, while maintaining PSA suppression and allowing for testosterone recovery in line with previous studies of intermittent ADT. [52]
7.3. Second-Line ADT
Despite the initial benefits of hormonal therapy in men with prostate cancer, most patients will eventually progress to castration-resistant disease. Treatment options in this setting have expanded recently and include sipuleucel-T, abiraterone, enzalutamide, taxane-based chemotherapy, and bone protective agents. [53] Degarelix has been evaluated in a few exploratory studies in the second-line setting for men with failure after an LHRH agonist. A 1-year, open-label phase II trial reported on 37 patients with castrate-resistant prostate cancer. At 3 months, the response rate was 16.7%-33.3% and probability of finishing 1 year of treatment without PSA progression was 8—9%. [54] Given the growing array of more effective options for castrate-resistant prostate cancer, it is unlikely that the modest results with second-line degarelix will result in significant adoption in approach.
7.4. ADT + Radiation
Numerous trials have demonstrated improved oncologic outcomes through the combination of ADT with definitive radiotherapy for intermediate and high-risk prostate cancer. [55] Given the rapid onset of castration with degarelix, there is interest in the use of GnRH antagonist treatment in this setting in order to minimize the duration of ADT and the associated side effects. [50] A trial is currently underway comparing GnRH antagonist as neoadjuvant therapy for prostatic brachytherapy [56] and a pilot study for combined degarelix and external beam radiation therapy has recently completed, though the results are still pending. [57]
8.0. Safety and Tolerability
As with all forms of ADT, degarelix is associated with a significant amount of adverse effects (AEs) including ‘hot flashes’, prolongation of the QT interval, development of gynecomastia, sexual dysfunction, bone loss and skeletal morbidity, anemia, psychological and cognitive effects, metabolic alterations (weight gain, muscle loss, insulin resistance, increased triglycerides), and cardiovascular morbidity. [58,59] The most notable AE specific to degarelix are injection-site reactions that include pain (31%), erythema (21%), swelling (8%), and nodules (7%). The risk of injection-site reaction was 31% after the first injection, though this risk was diminished (2.5%) for subsequent maintenance doses and only 2% of patients discontinued degarelix due to these reactions. Less common treatment related AEs include pyrexia, chills, and elevated alanine aminotransferase and elevated aspartate aminotransferase. [60] Previous iterations of GnRH antagonists were associated with significant histamine release, however the 3rd-generation degarelix is associated with minimal histamine release and there have been very few reported incidence of anaphylaxis in post-marketing surveillance. [61,30]
8.1. Cardiovascular Effects of GnRH Antagonists
One of the most concerning, and controversial, AEs related to ADT are the purported cardiovascular comorbidities. [59] Although the true impact of ADT on cardiovascular risk is still being actively debated, with differing conclusions in the literature, this risk is reported by the FDA for LHRH agonist therapy. [62,63] Retrospective analysis from pooled data of phase III clinical trials suggests a lower risk of major cardiovascular events (HR 0.44) with degarelix in patients with a history of cardiovascular disease. [64] Such post hoc analysis is fraught as the trials were not designed for this endpoint, but prompted the recently announced PRONOUNCE trial, a phase III study of degarelix vs. leuprolide to compare the incidence of major cardiovascular events in patients with cardiovascular disease at one year. [65] There are several biologic mechanisms that may explain the results. A mouse model of low-density lipoprotein receptor knockout mice demonstrated that those mice treated with GnRH antagonists develop less adiposity, characteristics of metabolic syndrome, and atherosclerosis compared with mice that had undergone orchiectomy or LHRH agonist therapy. [66] Most acute cardiovascular events (e.g. myocardial infarction, stroke) are caused by rupture of atherosclerotic plaque resulting in an occlusive thrombus or emboli. [67] Factors involved with atherosclerotic plaque rupture are the study of complex analysis, but simplistically involve a core of lipid and necrosis covered by a thin layer of smooth muscle cell and connective tissue, which can be degraded by infiltrating macrophages. [68,69] A mouse model of ApoE−/− fed a high-fat diet to induce carotid artery atherosclerosis noted that 4 weeks of LHRH agonist therapy was associated with an increase in the plaque necrosis and macrophage infiltration, theoretically making such plaques more susceptible to rupture, while treatment with degarelix was not associated with this histologic change. [70] Another possible biologic explanation may lie in the presence of FSH receptors within the endothelial surface of blood vessels. FSH receptors that would be stimulated by LHRH agonist therapy but would presumably be less stimulated through GnRH antagonist therapy which also suppresses FSH. [71] Additionally, T-cells have demonstrated to express GnRH receptors, which can cause a pro-inflammatory T-helper type 1 milieu resulting in macrophage activation that may destabilize atherosclerotic plaques. [72–74]
9.0. Regulatory Affairs and Cost
Degarelix was approved for the treatment of advanced, hormone-sensitive prostate cancer by the United States Food and Drug Administration in 2008 and by the European Medicine Agency in 2009. The recommended dosage is 240mg SQ administered in two 120mg injections followed by monthly 80mg maintenance dosing. [30] Cost-effectiveness analysis is complicated by assumptions of effectiveness and estimates of outcomes and costs, which may vary dramatically by setting. Conclusions about the cost effectiveness of degarelix are contradictory based on available literature. [75–77]
10.0. Conclusion
Despite numerous advances in therapies for advanced prostate cancer, ADT remains the mainstay of therapy. While orchiectomy and LHRH agonist treatment have been the most commonly used form of ADT, the GnRH antagonist degarelix has shown to be at least equally effective from an oncologic standpoint and through differences in its mechanism of action and pharmacodynamic profile may have possible advantages over the traditional forms of ADT – in particular for patients with preexisting cardiovascular disease, although this remains a subject of debate. Degarelix treatment reliably and rapidly results in castration-levels of testosterone, and this quality may be particularly attractive in patients who are presenting with symptomatic metastases. Disadvantages include a higher incidence of injection-site reactions (although this lessens with subsequent treatments), lack of a longer acting depot, and questions about cost-effectiveness. Nonetheless, the observed differences in cardiovascular events are an attractive aspect of the drug and warrant the ongoing prospective evaluation.
11.0. Expert Opinion
Degarelix is a GnRH antagonist that results in a more rapid decrease in testosterone and is not associated with the flare phenomenon noted in LHRH agonists. Although data from the CS21 trial and extension study of that trial suggest a possible benefit in PSA progression in patients with more advanced disease and higher baseline PSA levels, conclusions are limited due to inadequate power. Additionally there is potential benefit to degarelix over LHRH agonist therapy in terms of cardiovascular morbidity, although this data is not yet clear. The most obvious clinical benefit of degarelix over LHRH agonists is in patients presenting with symptomatic metastatic hormone-naïve disease, where the rapid achievement of castration level of serum testosterone and avoidance of flare is particularly attractive. Although the concepts of microsurges in testosterone with LHRH agonist therapy and FSH suppression with degarelix are interesting, the clinical effects are not proven. The benefits of degarelix versus traditional LHRH agonist therapy beyond this relatively narrow indication are not clearly defined, as there is no evidence that suggests a more rapid decline in testosterone is associated with a more lasting oncologic benefit. Further research is warranted to define the benefits of GnRH antagonist degarelix over LHRH agonists.
Drug Summary Box.
Box 1. Comparison summary of LHRH agonists and degarelix30
| Degarelix | LHRH agonist |
|---|---|
| Rapid testosterone suppression (~3days) | Prolonged suppression of testosterone (10-20days) |
| No initial testosterone flare | Initial testosterone flare |
| No microsurges of testosterone | Microsurges of testosterone with each injection |
| Complete FSH suppression | Partial FSH suppression |
| Monthly maintenance injection | 3-6 month maintenance injection schedule |
Acknowledgments
Funding
This paper was not funded
Footnotes
Declaration of interest
S L Woldu is supported by an NIH T32 Ruth L. Kirschstein Institutional National Research Award. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer Journal for Clinicians. 2017;67:7–30 [DOI] [PubMed] [Google Scholar]
- [2].Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J Urol 1941;168:9–12 [DOI] [PubMed] [Google Scholar]
- [3].National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer, version 2.2016. 2016. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [Last accessed 21 Feb 2016]
- [4].Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Onc 2011;29(27):3651–58 [DOI] [PubMed] [Google Scholar]
- [5].Black BE, Paschal BM. Intranuclear organization and function of the androgen receptor. Trends in Endocrinology and Metabolism 2004;15(9):411–17 [DOI] [PubMed] [Google Scholar]
- [6].Wadosky KM, Koochekpour S. Therapeutic rationales, progresses, failures, and future directions for advanced prostate cancer. International Journal of Biological Science 2016;12(4):409–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schally AV, Coy DH. Stimulatory and inhibitory analogs of luteinizing hormone releasing hormone (LHRH). Adv Exp Med Biol 1977;87:99–121 [DOI] [PubMed] [Google Scholar]
- [8].Eisenberger MA, O’Dwyer PJ, Friedman MA. Gonadotropin Hormone-releasing hromone analogues: a new therapeutic approach for prostatic carcinoma. J Clin Onc 1986;4(3):414–24 [DOI] [PubMed] [Google Scholar]
- [9].Cook T, Sheridan WP. Development of GnRH antagonists for prostate cancer: new approaches to treatment. The Oncologist 2000;5(2):162–8 [DOI] [PubMed] [Google Scholar]
- [10].Bhasin S, Berman N, Swerdloff RS. Follicle-stimulating hromone (FSH) escape during chronic gonadotropin-releasing hormone (GnRH) agonist and testosterone treatments. Journal of Andrology 1994;15:386–91 [PubMed] [Google Scholar]
- [11].Garnick MD, Campion M. Abarelix depot, a GnRH antagonist, v LHRH super-agonists in prostate cancer: differential effects on follicle-stimulating hormone. Abarelix Deport Study group. Mol Urol 2000;4:275–7 [PubMed] [Google Scholar]
- [12].Bubley GJ. Is the flare phenomenon clincally significant?. Urology 2001;58:5–9 [DOI] [PubMed] [Google Scholar]
- [13].Van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers?. Urology 2008;71:1001–6 [DOI] [PubMed] [Google Scholar]
- [14].Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Eng J Med 1989;321:419–24 [DOI] [PubMed] [Google Scholar]
- [15].Labrie F, Dupont A, Belanger A, Lachance R. Flutamide eliminates the risk of disease flare in prostatic cancer patients treated with a luteinizing hormone-releasing hormone agonist. J Urol 1987;138(4):804–6. [DOI] [PubMed] [Google Scholar]
- [16].Shulze H, Senge T. Influence of different types of antiandrogens on luteinizing hormone-releasing hormone analogue-induced testosterone surge in patients with metastatic carcinoma of the prostate. J Urol 1990;144:934–41. [DOI] [PubMed] [Google Scholar]
- [17].Kuhn J, Billebaud T, Navratil H, et al. Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide). N Eng J Med 1989;321(7):413–8. [DOI] [PubMed] [Google Scholar]
- [18].Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol 2001;3(suppl 3):S10–14. [PMC free article] [PubMed] [Google Scholar]
- [19].Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Annals Internal Medicine 2000;132:566–77 [DOI] [PubMed] [Google Scholar]
- [20].Tomera K, Gleason D, Gittelman M, et al. The gonadotropin-releasing hormone antagonist abarelix deport versus luteinizing hormone releasing hormone agonists leuprolide or goserlin: initial results of endocrinological and biochemical efficacies in patients with prostate cancer. J Urol 2001;165(5):1585–9 [PubMed] [Google Scholar]
- [21].Trachtenberg J, Gittleman M, Steidle C, et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol 2002;167(4):1670–4 [DOI] [PubMed] [Google Scholar]
- [22].Koch M, Steidle C, Brosman S, et al. An open-label study of abarelix in men with symptomatic prostate cancer at risk of treatment with LHRH agonists. Urology 2003;62(5):877–82 [DOI] [PubMed] [Google Scholar]
- [23].Gittelman M, Pommerville PJ, Persson BE, et al. A 1-year, open label, randomized phase II dose finding study of Degarelix for the treeatment of prostate cancer in North America. J Urol 2008;180:1986–92. [DOI] [PubMed] [Google Scholar]
- [24].Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label parallel-group phase III study in patients with prostate cancer. Brithish Journal of Urology International 2008;102:1531–8 [DOI] [PubMed] [Google Scholar]; ** Phase III, randomized trial comparing degarelix vs LHRH agonist, which led to its approval
- [25].Jiang G, Stalewski J, Galyean R, et al. GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanies at positions 5 and 6. J Med Chem 2001;44(3):4553–67 [DOI] [PubMed] [Google Scholar]
- [26].Klotz L. Pharmacokinetic and pharmacodynami profile for degarelix for prostate cancer. Expert Opinion on Drug Metabolism and Toxicology 2015;11(11):1795–1802 [DOI] [PubMed] [Google Scholar]
- [27].Boccon-Gibod L, van der Meulen E, Persson BE. An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer. Ther Adv Urol 2011;3(3):127–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weckermann D, Harzmann R. Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. Eur Uro 2004;46:279–84 [DOI] [PubMed] [Google Scholar]
- [29].Tornoe CW, Agerso H, Nielsen HA, et al. Population pharmacokinetic modeling of a subcutaneous depot for GnRH antagonist Degarelix. Pharmaceutical Research 2004;21(4):574–84 [DOI] [PubMed] [Google Scholar]
- [30].Firmagon (degarelix for inection) US prescribing information. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022201s003lbl.pdf [Last accessed 21 February 2017]; *Prescribing information for degarelix
- [31].Sonesson A, Koechling W, Stalewski J, et al. Metabolite Profiles of Degareix, a new gonadtropin-releasing hormone receptor antagonist, in rat, dog and mokey. Drug Metabolism and Disposition 2011;39(10):1895–1903 [DOI] [PubMed] [Google Scholar]
- [32].Sonesson A, Rasmussen BB. In vitro and in vivo human metabolism of degarelix, a gonadotropin-releasing hromone receptor blocker. Drug metabolism and disposition 2013;41(7):1339–46 [DOI] [PubMed] [Google Scholar]
- [33].Shaw GL, Whitaker H, Corcoran M, et al. The Early Effects of Rapid Androgen Deprivation on Human Prostate Cancer. Eur Urol 2016;70:214–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shore ND, Abrahamsson PA, Andersone J, et al. New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists. Prostate Cancer and Prostatic Diseases 2013;16:7–15 [DOI] [PubMed] [Google Scholar]
- [35].Zinner NR, Bidair M, Centeno A, et al. Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6mg and 10.8mg: results of a randomized open-label trial. Urology 2004;64:1177–81 [DOI] [PubMed] [Google Scholar]
- [36].Oduwole O, Rawson P, Rahman N, et al. FSH supplementation increases the growth of PC-3 human prostate cancer cell xenograft in gonadotropin-suppressed nude mice. 18th European Congress of Endocrinology, May 2016 Munich, Germany: European Society of Endocrinology. Endocrine Abstracts 2016;4:GP117 [Google Scholar]
- [37].Sayyid RK, Evans A, Hersey K, et al. A Phase II, Randomized, Open-Label Study of Neoadjuvant Degarelix versus LHRH Agonist in Prostate Cancer Patients Prior to Radical Prostatectomy. Clin Cancer Res 2016: published online 2 February 2017, doi: 10.1158/1078-0432.CCR-16-1790 [DOI] [PubMed] [Google Scholar]
- [38].Hussain M, Goldman B, Tangen C, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Onc 2009;27:2450–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Swensson USH, Senderovitz T, Karlsson MO. Population PK/PD modeling of testosterone (T), LH and dihydrotestosterone (DHT) response to single SC degarelix in male volunteers. American Society for Clinical Pharmacology and Therapeutics 2003;73(2):87 [Google Scholar]
- [40].Van Poppel H, Tombal B, de la Rosette JJ, Persson BE, Jensen JK, Kold Olesen T. Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker--results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur Urol 2008;54:805–13 [DOI] [PubMed] [Google Scholar]
- [41].Tombal B, Miller K, Boccon-Gibod L, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics.Eur Urol 2010;57:836–42 [DOI] [PubMed] [Google Scholar]
- [42].Schroder FH, Tombal B, Miller K, et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12-month, comparative, phase III study. BJU International 2010;106:182–7 [DOI] [PubMed] [Google Scholar]
- [43].Crawford ED, Tombal B, Miller K, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol 2011;186:889–97 [DOI] [PubMed] [Google Scholar]; *5-year extension of the CS21A Phase III trial demonstrating continued efficacy of degarelix.
- [44].Klotz L, Miller K, Crawford ED, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol 2014;66:1101–8 [DOI] [PubMed] [Google Scholar]
- [45].Anderson J, Al-Ali G, Wirth M, et al. Degarelix versus goserelin (+ antiandrogen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study (NCT00831233). Urologia internationalis 2013;90:321–8 [DOI] [PubMed] [Google Scholar]
- [46].Mason M, Maldonado Pijoan X, Steidle C, et al. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clinical Oncology (Royal College of Radiologists (Great Britain)) 2013;25:190–6 [DOI] [PubMed] [Google Scholar]
- [47].Axcrona K, Aaltomaa S, da Silva CM, et al. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU International 2012;110:1721–8 [DOI] [PubMed] [Google Scholar]
- [48].Tombal B, Tammela TLJ, Wolff JM, et al. Efficacy and safety of a 3-monthly depot of degarelix compared with goserelin in prostate cancer. Eur Urol Supp 2012;11(5):109 [Google Scholar]
- [49].Schulman C, Cornel E, Matveev V, et al. Intermittent Versus Continuous Androgen Deprivation Therapy in Patients with Relapsing or Locally Advanced Prostate Cancer: A Phase 3b Randomised Study (ICELAND). Eur Urol 2016;69:720–7 [DOI] [PubMed] [Google Scholar]
- [50].Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Eng J Med 2012;367:895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014;65:467–79 [DOI] [PubMed] [Google Scholar]
- [52].Boccon-Gibod L, Albers P, Morote J, et al. Degarelix as an intermittent androgen deprivation therapy for one or more treatment cycles in patients with prostate cancer. Eur Urol 2014;66:655–63 [DOI] [PubMed] [Google Scholar]
- [53].Lowrance WT, Roth BJ, Kirkby E, et al. Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2015. J Urol 2016;195:1444–52 [DOI] [PubMed] [Google Scholar]
- [54].Miller K, Simson G, Goble S, Persson BE. Efficacy of degarelix in prostate cancer patients following failure on luteinizing hormone-releasing hormone agonist treatment: results from an open-label, multicentre, uncontrolled, phase II trial (CS27). Therapeutic advances in urology 2015;7:105–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and Goserelin. N Eng J Med 1997;337:295–300 [DOI] [PubMed] [Google Scholar]
- [56].Miki K, Sasaki H, Kido M, et al. A comparative study on the efficacies of gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist in neoadjuvant androgen deprivation therapy combined with transperineal prostate brachytherapy for localized prostate cancer. BMC Cancer 2016;16:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Degarelix Acetate Before and During Radiation Therapy in Treating Patients With Prostate Cancer (NCT01731912). Available at: https://clinicaltrials.gov/ct2/show/NCT01731912 [Last accessed 21 February 2017]
- [58].Sountoulides P, Rountos T. Adverse effects of androgen deprivation therapy for prostate cancer: prevention and management. ISRN Urology 2013;2013(240108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–36 [DOI] [PubMed] [Google Scholar]
- [60].Crawford ED, Shore ND, Moul JW, et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology. 2014;83:1122–8 [DOI] [PubMed] [Google Scholar]
- [61].Koechling W, Hjortkjaer R, Tanko LB. Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. British journal of clinical pharmacology 2010;70:580–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 2011;306(21):2359–66. [DOI] [PubMed] [Google Scholar]
- [63].Bosco C, Bosnyak Z, Malmberg A, et al. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol 2015;68(3):386–96. [DOI] [PubMed] [Google Scholar]
- [64].Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol 2014;65:565–73 [DOI] [PubMed] [Google Scholar]
- [65].A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease (PRONOUNCE). Available from: https://clinicaltrials.gov/ct2/show/NCT02663908 [last accessed 17 February 2017]
- [66].Hopmans SN, Duivenvoorden WC, Werstuck GH, et al. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Uro Onc 2014;32:1126–34 [DOI] [PubMed] [Google Scholar]
- [67].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Eng J Med 2005;352:1685–95 [DOI] [PubMed] [Google Scholar]
- [68].Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005;111:3481–8 [DOI] [PubMed] [Google Scholar]
- [69].Shah PK. Role of inflammation and metalloproteinases in plaque disruption and thrombosis. Vascular medicine 1998;3:199–206 [DOI] [PubMed] [Google Scholar]
- [70].Knutsson A, Hsiung S, Celik S, et al. Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(−/−) mice. Scientific Reports 2016;6:26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Eng J Med 2010;363:1621–30 [DOI] [PubMed] [Google Scholar]
- [72].Chen HF, Jeung EB, Stephenson M, Leung PC. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrine and Metabolism 1999;84:743–50 [DOI] [PubMed] [Google Scholar]
- [73].Tanriverdi F, Gonzalez-Martinez D, Hu Y, et al. GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clinical and experimental immunology 2005;142:103–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dixit VD, Yang H, Udhayakumar V, Sridaran R. Gonadotropin-releasing hormone alters the T helper cytokine balance in the pregnant rat. Biology of reproduction 2003;68:2215–21 [DOI] [PubMed] [Google Scholar]
- [75].Lu L, Peters J, Roome C, Stein K. Cost-effectiveness analysis of degarelix for advanced hormone-dependent prostate cancer. BJU International 2012;109:1183–92 [DOI] [PubMed] [Google Scholar]
- [76].Lee D, Porter J, Gladwell D, Brereton N, Nielsen SK. A cost-utility analysis of degarelix in the treatment of advanced hormone-dependent prostate cancer in the United Kingdom. Journal of medical economics 2014;17:233–47 [DOI] [PubMed] [Google Scholar]
- [77].Hatoum HT, Crawford ED, Nielsen SK, et al. Cost-effectiveness analysis comparing degarelix with leuprolide in hormonal therapy for patients with locally advanced prostate cancer. Expert review of pharmacoeconomics & outcomes research 2013;13:261–70 [DOI] [PubMed] [Google Scholar]
- [78].Lin BJT, Chen K, Chen M, et al. The time for serum testosterone to reach castrate level after bilateral orchiectomy or oral estrogen in the management of metastatic prostatic cancer. Urology 1994;43(6):834–7 [DOI] [PubMed] [Google Scholar]