Abstract
Following cutaneous injury, keratinocytes secrete paracrine factors that regulate wound cell functions; dysregulation of this signaling can lead to wound pathologies. Previously, we established that keratinocyte integrin α3β1 promotes wound angiogenesis through paracrine stimulation of endothelial cells. We hypothesize here that α3β1-dependent paracrine signaling from keratinocytes regulates the differentiation state of myofibroblasts. We report that epidermal α3 knockout mice exhibit more wound myofibroblasts and fewer cyclooxygenase 2 (Cox-2) positive dermal cells than controls. We also find that conditioned medium (CM) from α3-expressing mouse keratinocytes (MKα3+), but not from α3-null MK cells (MKα3-), induces expression of Cox-2 in fibroblasts in a time and dose-dependent manner, and that this induction is mediated by interleukin-1α (IL-1α). Compared to MKα3- cells, MKα3+ cells secrete more IL-1α and less interleukin 1 receptor antagonist (IL-1RA), a natural IL-1 receptor antagonist. Treatment with an IL-1α neutralizing antibody, recombinant IL-1RA, or IL-1 receptor (IL-1R1)-targeting siRNA suppresses MKα3+ CM-dependent induction of Cox-2 expression in fibroblasts. Finally, active recombinant IL-1α is sufficient to induce Cox-2 in fibroblasts and to inhibit TGF-β-induced α-SMA expression. Our findings support a role for keratinocyte integrin α3β1 in controlling the secretion of IL-1α, a paracrine factor that regulates the wound myofibroblast phenotype.
Keywords: epidermal integrin α3β1, IL-1α, IL-1RA, IL-1R1, fibroblast differentiation, myofibroblast, Cox-2, PGE2, α-SMA, TGF-β, wound healing, paracrine signaling, keratinocyte, extracellular matrix
INTRODUCTION
Growth and repair processes, both normal and pathological, require bidirectional interactions between cells and the extracellular matrix (ECM) (Bissell et al., 1982; Bornstein and Sage, 2002; Hynes, 2009; Hynes and Naba, 2012). When this “dynamic reciprocity” is disrupted pathogenic processes can ensue, including tumors and chronic wounds (Alexander and Cukierman, 2016; Schultz et al., 2011). It is crucial that different wound cells interact collaboratively in a temporally and tightly regulated manner to ensure a proper healing outcome (DiPersio et al., 2016; Gurtner et al., 2008). Yet, the roles of specific integrin-mediated paracrine signals in coordinating this interplay are not understood. Our recent work has focused on the function of keratinocyte integrin-ECM interactions in governing wound processes (DiPersio et al., 2016; Longmate and DiPersio, 2017).
Dramatic changes in the ECM occur following injury. Prior to wounding, dermal fibroblasts reside in a collagen-rich ECM and are relatively quiescent. However, following injury these quiescent fibroblasts experience a shift to a predominantly fibrin-/fibronectin-rich provisional matrix (Tomasek et al., 2002; Van De Water et al., 2013; Vedrenne et al., 2012). In addition, alterations in the mechanical microenvironment contribute to the differentiation of fibroblasts into myofibroblasts, the cell type that deposits, rigidifies, and contracts wound ECM (Hinz, 2010; Van De Water et al., 2013). The persistence of α-SMA-expressing myofibroblasts is characteristic of organ fibrosis, pathogenic scarring and tumor stroma (Hinz et al., 2012; Tomasek et al., 2002; Van De Water et al., 2013). Conversely, a lack of myofibroblasts is characteristic of chronic wounds such as diabetic ulcers, and a reduced number of myofibroblasts and wound contraction is closely associated with reduced epidermal barrier function, persistent bacterial infection and inflammation (Brem and Tomic-Canic, 2007; Mustoe, 2004).
Integrins are major surface receptors on basal keratinocytes that mediate epidermal adhesion to basement membrane in normal skin, and they control key keratinocyte functions following injury, such as proliferation, migration, and paracrine stimulation of other wound cells (DiPersio et al., 2016; Grose et al., 2002; Margadant et al., 2010; Watt, 2002). Integrin α3β1, a laminin-binding integrin, is essential for maintaining basement membrane integrity and regulating secretion of soluble factors that stimulate wound angiogenesis (Longmate et al., 2014; Mitchell et al., 2009). Paracrine signals from epidermal keratinocytes have also been shown to regulate fibroblast functions, including wound contraction, and the loss of such intercellular crosstalk is associated with pathologies such as hypertrophic scarring and organ fibrosis (Ghahary and Ghaffari, 2007; Werner et al., 2007). Indeed, studies using co-culture models demonstrated that keratinocytes secrete factors (e.g., growth factors, cytokines, ECM components, MMPs) that have both positive and negative effects on fibroblast functions (Nowinski et al., 2004; Werner et al., 2007). Keratinocyte-derived TGF-β promotes α-SMA expression while secretion of IL-1α by keratinocytes at early time points in co-culture temporally inhibits fibroblast α-SMA expression (Shephard et al., 2004). In response to IL-1α, fibroblasts upregulate Cox-2 expression and prostaglandin E2 (PGE2) production (Di Mari et al., 2003; Ogata et al., 2007) and lung fibroblast-derived PGE2 can inhibit TGF-β-mediated induction of α-SMA expression in an autocrine manner (Garrison et al., 2013; Penke et al., 2014). Interestingly, integrin α3β1 has been demonstrated to regulate the response of Caco-2 cells to Interleukin 1 (Li et al., 2004; Lubin et al., 2003; Rafferty et al., 2010; Stulic et al., 2007). However, the role of epidermal integrins, particularly α3β1, in regulating keratinocyte-derived paracrine signals that modulate fibroblast gene expression and differentiation is unknown.
In the current study, we investigated a role for epidermal integrin α3β1 in regulating paracrine signaling from keratinocytes that modulates the differentiation state of myofibroblasts. We identify a role for keratinocyte integrin α3β1 in promoting the secretion of IL-1α and reducing the secretion of IL-1RA, thereby inducing Cox-2 expression in fibroblasts and inhibiting their differentiation to myofibroblasts during wound healing. Our work elucidates an important role for keratinocyte integrins – α3β1 specifically – in regulating the differentiation state of wound cells.
RESULTS
Genetic deletion of α3 in the epidermis leads to reduced Cox-2 expression and enhanced α-SMA expression in dermal cells of cutaneous wounds, in vivo
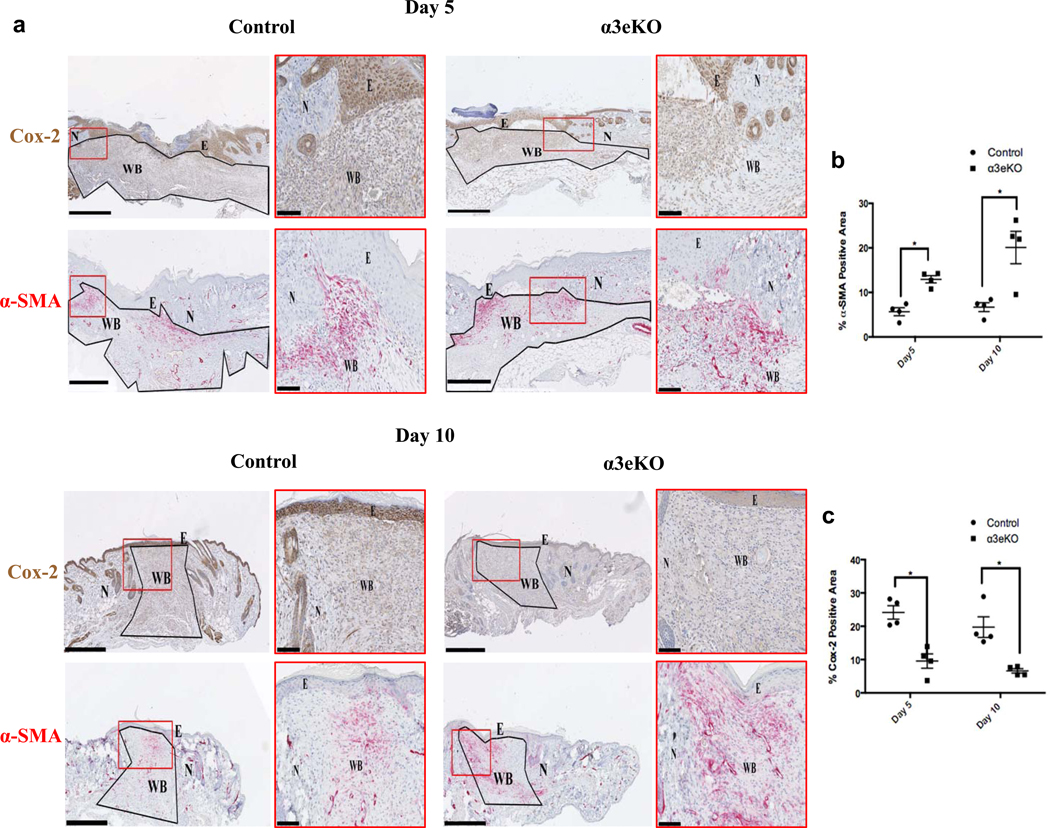
To assess the influence of epidermal α3β1 on wound healing, we deleted α3 from the epidermis in mice homozygous for floxed Itga3 alleles (Itga3flx/flx) that also express a Cre recombinase transgene under the control of epidermal-specific keratin 14 promoter (K14-Cre Itga3flx/flx, hereafter referred to as α3eKO). Control Itga3flx/flx mice lacked K14-Cre (Mitchell et al., 2009). Successful K14-driven epidermal specific deletion of α3 was confirmed by immunostaining for α3 and K14 (Fig. S1e). Building on our recent work with these mice, we sought to determine if α3β1-dependent signals from epidermal keratinocytes control wound myofibroblast differentiation. One potential fibroblast control point is Cox-2. Fibroblast Cox-2 is induced during wound healing, and has been shown to inhibit lung fibroblast differentiation (Futagami et al., 2002). To determine whether such induction of Cox-2 in fibroblasts is dependent on α3β1 expression in keratinocytes, excisional wounds from both control mice and α3eKO mice were stained for Cox-2. We found that fibroblast Cox-2 expression was induced in the wound bed but this induction was blunted in wounds from α3eKO mice relative to controls (Fig.1a, 1c). Moreover, wounds of α3eKO mice contained more α-SMA positive myofibroblasts than control wounds (Fig. 1a, 1b), suggesting a suppressive effect of epidermal α3β1 on myofibroblast differentiation. Thus, α3-dependent upregulation of dermal Cox-2 was inversely related to the amount of α-SMA staining (Fig. 1). Appropriate controls were performed to confirm the specificity of α-SMA and Cox-2 antibodies (Fig. S1a–d). Together, these data suggest a role for epidermal integrin α3β1 in regulating fibroblast differentiation during cutaneous wound healing via induction of dermal Cox-2 expression.
Figure 1. Genetic deletion of α3 in the epidermis leads to reduced Cox-2 expression and enhanced α-SMA expression in dermal cells of cutaneous wounds, in vivo.
(a) Mouse wound sections (5 and 10 days post wounding) from control and α3eKO mice were immunostained for Cox-2 (brown) or α-SMA (red). E=epidermis; N=normal skin; WB=wound bed; scale bar=500μm. Wound beds were outlined (black) and defined as the ROI. Insets (red boxes) illustrate staining and wound features (scale bar=100μm). Stains were separated by a color-deconvolution algorithm, and percentages of α-SMA-positive staining (b) or Cox-2-positive staining (c) within each ROI were quantitated (ImageJ). 2-way ANOVA followed by Bonferroni post-hoc analysis, *p > 0.05, n= 4 mice per time point / genotype.
Epidermal integrin α3β1 promotes fibroblast Cox-2 expression, in vitro
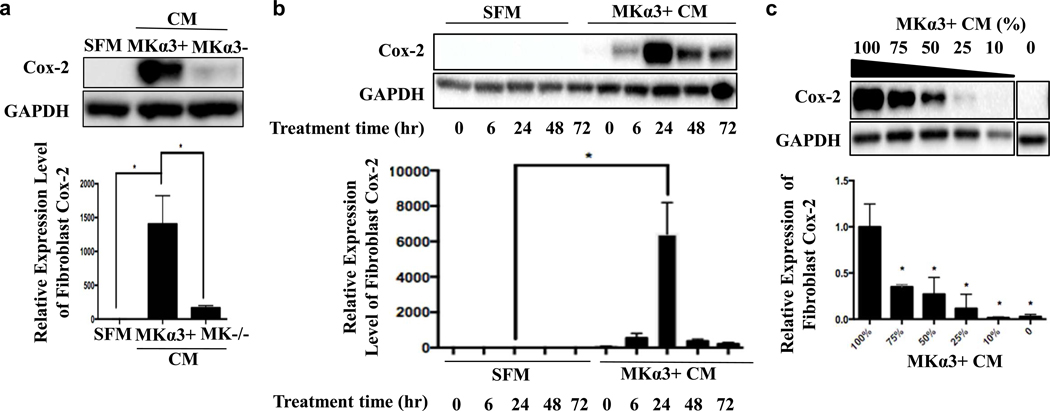
Previously, we developed mouse keratinocyte (MK) cell lines that either lack α3β1 (i.e., MKα3-, derived from α3-null mouse) or have α3 expression restored through a stable transfection with human α3 cDNA (i.e., MKα3+) (Mitchell et al., 2009). Conditioned medium (CM) was prepared from MKα3- and MKα3+ cells and used to culture (24 hrs) low passage primary normal human dermal fibroblasts (NHDF). We observed that CM from MKα3+ cells robustly induced fibroblast Cox-2 protein compared to CM from MKα3- cells, indicating that MK cells secrete soluble factor(s) in an α3β1-dependent manner that induce fibroblast Cox-2 expression in a time and dose-dependent manner (Fig. 2). This α3β1-mediated Cox-2 induction was not restricted to NHDFs but also occurred in IMR-90 human lung fibroblasts, human foreskin fibroblasts, and primary mouse embryonic fibroblasts (Fig. S2). Importantly, we also found that levels of PGE2 secreted into CM did not differ between MK cells that express or lack α3β1 (not shown), indicating that the α3β1-dependent fibroblast response (Fig 1, 2) was not a direct consequence of MK cell-derived PGE2.
Figure 2. MKα3+ CM induces expression of fibroblast Cox-2 in a dose and time-dependent manner.
(a) Fibroblast Cox-2 protein expression was measured in response to conditioned media (CM) from mouse keratinocytes (MK) that express (MKα3+) or lack α3β1 (MKα3-) or unconditioned serum free medium (SFM). n=3, 1-way ANOVA, *p<0.05; error bars are +/− SEM. (b) Primary dermal fibroblasts were treated with SFM or MKα3+ CM for various time points, and fibroblast Cox-2 expression was measured by western blot. n=3, 2-way ANOVA, * p<0.0001, error bars are +/− SEM. (c) Protein expression of fibroblast Cox-2 was measured in response to decreasing concentration of MKα3+ CM, or to SFM (0% non-adjacent lanes from the same blot). n=3, 1-way ANOVA, * p<0.05; error bars are +/− SEM.
Cox-2 inhibits TGF-β-induced fibroblast differentiation by upregulating secreted levels of PGE2 in primary normal human dermal fibroblasts
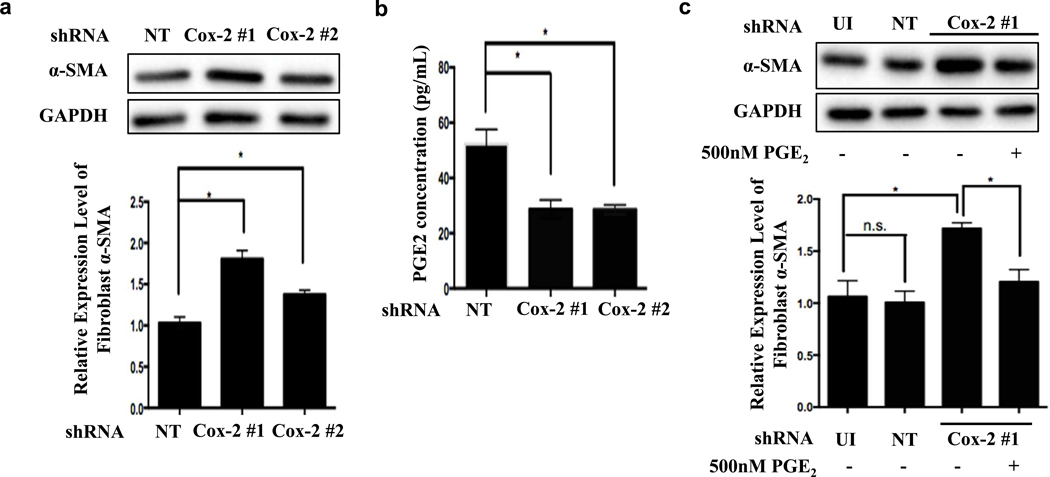
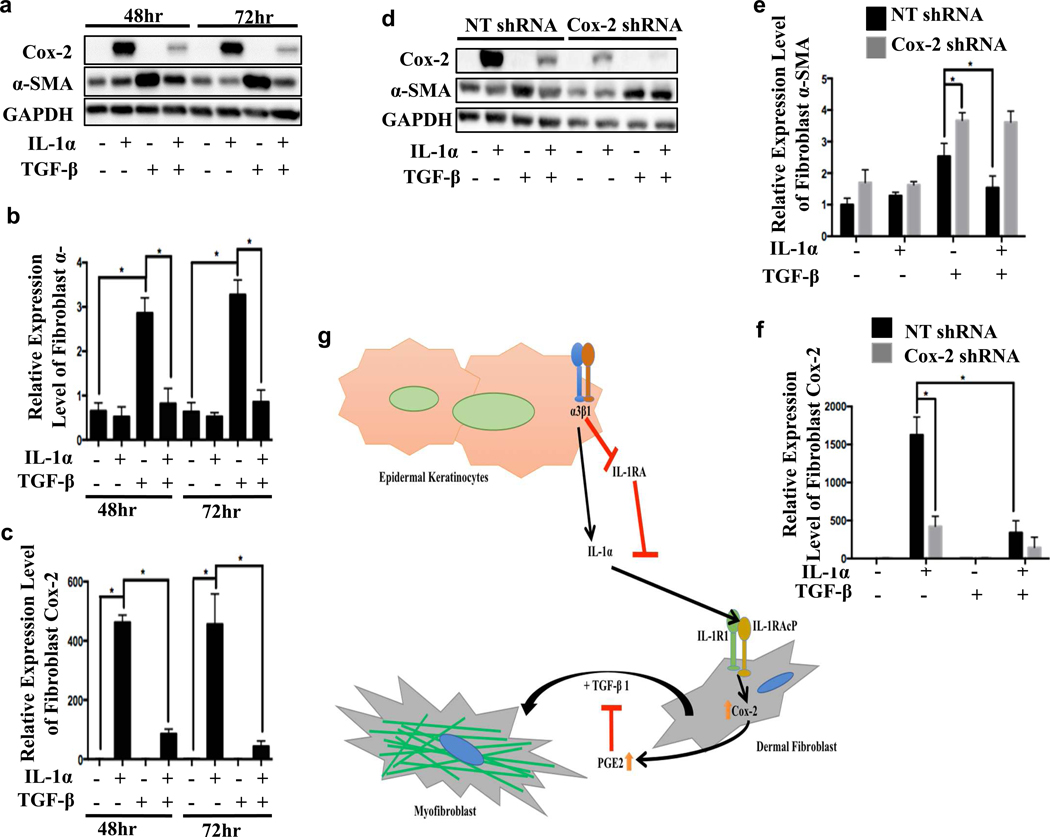
To assess the functional significance of Cox-2 induction in fibroblasts, we infected NHDFs with lentiviruses expressing either non-targeting (control) shRNA or Cox-2 targeting shRNAs to generate stable control and Cox-2 knockdown cell lines. Each of two Cox-2 shRNAs efficiently inhibited Cox-2 protein expression (Fig. S3). Furthermore, both Cox-2 targeting shRNAs led to a 2-fold reduction in secreted PGE2 compared to control NHDFs, and also increased TGF-β-mediated α-SMA induction, consistent with an inhibitory function for Cox-2 / PGE2 (Fig. 3a, 3b and Fig. S4). Moreover, addition of PGE2 to NHDFs with Cox-2 knockdown returned TGF-β-dependent α-SMA expression to control levels (Fig. 3c). Addition of exogenous PGE2 to control NHDFs strongly inhibited TGF-β-induced stress fiber formation, α-SMA induction, and nuclear localization of myocardin related transcription factor A (MRTF-A, Fig. S4). These results demonstrated that Cox-2/PGE2 signaling is a negative regulator of primary NHDF differentiation.
Figure 3. Cox-2 knockdown in fibroblasts decreases PGE2 secretion and promotes TGF-β-mediated induction of α-SMA expression.
(a-c) NHDFs were infected with lentivirus expressing control non-targeting shRNA (NT), Cox-2 targeting shRNAs or uninfected (UI) and recovered for 48 hrs. NHDFs were serum starved overnight followed by 2 ng/mL of TGF-β for 3 days; (a) α-SMA expression was measured by western blot (normalized to GAPDH). n=3, 1-way ANOVA, *p<0.05; error bars are +/− SEM; (b) secreted PGE2 concentration was measured by ELISA; n=3, 1-way ANOVA, *p<0.05; error bars are +/− SEM. (c) NHDFs incubated with TGF-β with or without 500 nM PGE2 for 3 days, and α-SMA expression was measured by western blot (normalized to GAPDH). n=3, 2-way ANOVA analysis, * p<0.05; error bars are +/− SEM.
Keratinocyte integrin α3β1 regulates the expression and secretion of IL-1α and IL-1RA
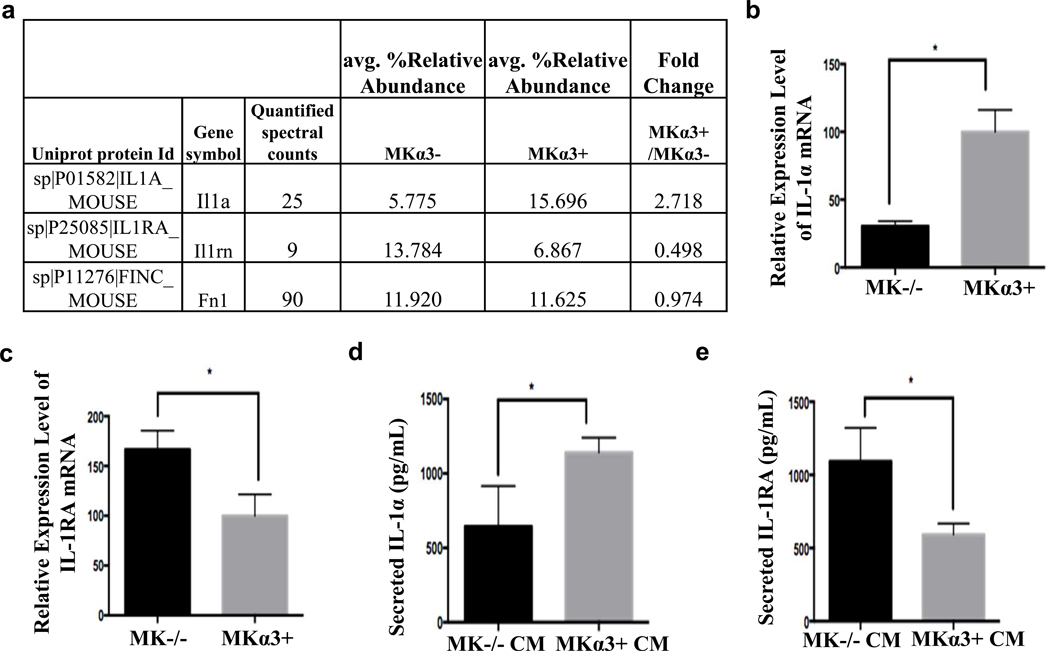
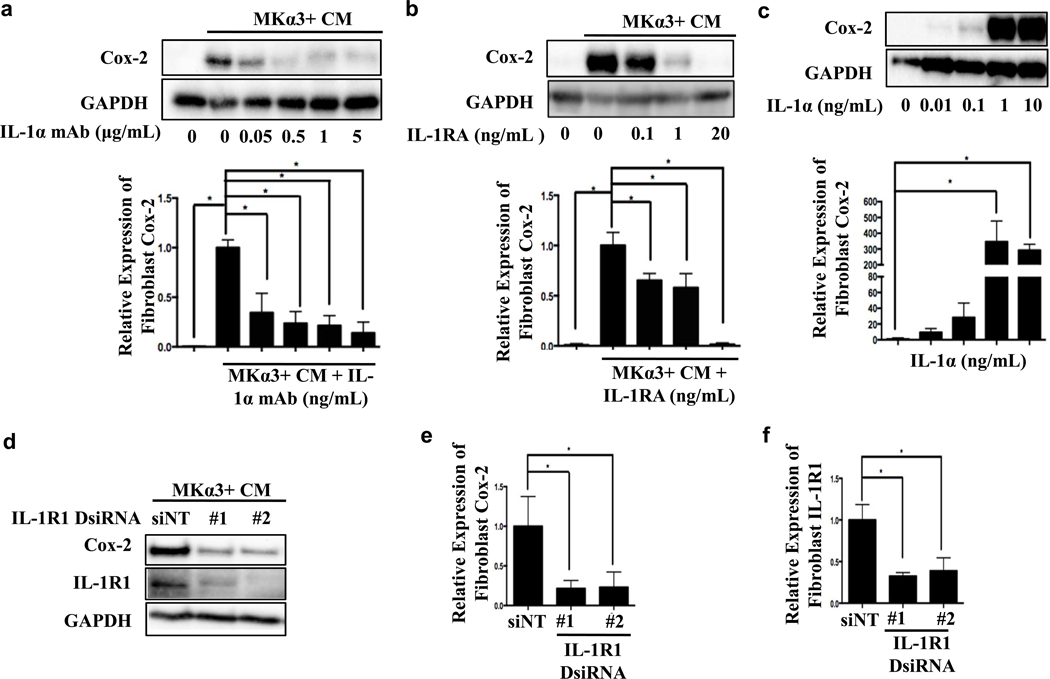
Previously, we determined that genetic depletion of α3β1 in MK cells reduced the secretion of pro-angiogenic factors, such as MMP-9 and MRP3 and we recently performed proteomics on CM from MKα3+ and MKα3- cells (Longmate et al., 2018). Deletion of α3 from keratinocytes reduced the secretion of a number of proteins, including the cytokine, IL-1α and increased secretion of IL-1RA, a natural inhibitor of IL-1α function (Fig. 4a). These findings were confirmed by RT-qPCR and ELISA using MK mRNA or MK CM, respectively (Fig. 4b–e). Collectively, these data show that keratinocyte α3β1 induced the expression and secretion of IL-1α and reduced the expression and secretion of IL-1RA (Fig. 4).
Figure 4. Epidermal integrin α3β1 regulates expression and secretion of IL-1α and IL-1RA.
(a) Relative amounts of secreted IL-1α (gene symbol: Il1a) and IL-1RA (Il1rn) as determined by mass spectrometry from conditioned media derived from MKα- and MK α3+ cells. (b+c) RNA was isolated from MK cells that express or lack α3, and RT-qPCR was used to determine mRNA expression levels of (b) IL-1α and (c) IL-1RA (normalized to housekeeping genes). N=3, 1-way ANOVA, *p<0.05; error bars are + SEM. (d+e) MK cells were conditioned in SFM for 2 days, and secretion of IL-1α (d), or IL-1RA (e) was measured by ELISA. N=3, 1-way ANOVA, *p<0.05; error bars are + SEM.
IL-1α mediates α3β1-dependent, paracrine induction of fibroblast Cox-2 by MK cells.
To test a role for IL-1α in MK-conditioned medium, we pre-incubated MKα3+ CM with either an IL-1α neutralizing monoclonal antibody (IL-1α mAb) or recombinant IL-1RA protein, and then incubated (24 hrs) NHDFs either with these pretreated CM, MKα3+ CM (positive control), or with SFM (negative control). As before, MKα3+ CM alone induced Cox-2 in NHDFs (Fig. 2a, 5a & 5b) but this induction was dramatically reduced by pretreating CM with IL-1α mAb (Fig. 5a) or with active IL-1RA (Fig. 5b & S5b). Although MK cells secrete low levels of interleukin 1 beta (IL-1β) this is not regulated in an α3β1-dependent manner and pretreatment of CM with IL-1β blocking antibody (IL-1β mAb) did not reduce the MKα3+ CM-mediated induction of fibroblast Cox-2 (Fig. S6). These data demonstrate that IL-1α, but not IL-1β, mediates α3β1-dependent induction of fibroblast Cox-2 expression. To determine if IL-1α is sufficient to induce Cox-2 in NHDFs, we treated cells with increasing doses of active recombinant IL-1α protein for 24 hours and measured Cox-2 expression. Active recombinant IL-1α was sufficient to induce fibroblast Cox-2 expression in a dose-dependent manner and this recombinant IL-1α was functionally active as shown by its ability to phosphorylate interleukin receptor associated protein 4 (IRAK4) (Fig 5c, FigS5a). Next, we determined whether IL-1 receptor type 1 (IL-1R1) on fibroblasts is necessary to mediate the IL-1α-dependent induction of Cox-2. Both IL-1R1 siRNAs significantly attenuated α3β1-mediated induction of Cox-2 expression relative to non-targeting siRNA (Fig. 5d–f). Together, these data identify the IL-1α/IL-1RA/IL-1R1 pathway as a major signaling axis that mediates α3β1-dependent, paracrine induction of fibroblast Cox-2 expression.
Figure 5. IL-1α signaling mediates induction of fibroblast Cox-2 by MKα3+ CM.
(a & b) NHDFs were treated with MKα3+ CM with or without increasing concentration of IL-1α blocking antibody (IL-1α mAb) (a) or recombinant IL-1RA (b) for 24 hours. Fibroblast Cox-2 and GAPDH were measured by western blot. 2-way ANOVA, n=3, *p<0.05; error bars are + SEM. (c) NHDFs were treated with increasing doses of recombinant human IL-1α for 24 hours and western blotted for Cox-2 and GAPDH. 1-way ANOVA, n=3, *p<0.05; error bars are + SEM. (d-f) NHDFs were transfected with non-targeting (siNT) or IL-1R1 DsiRNAs followed by MKα3+ CM treatment for 24 hours, and western blotted for Cox-2 and IL-1R1 (normalized to GAPDH). 1-way ANOVA, n=3, *p<0.05; error bars are + SEM.
IL-1α-mediated induction of Cox-2 inhibits TGF-β-induced α-SMA expression in NHDFs
To determine whether IL-1α is sufficient to inhibit TGF-β-induced α-SMA expression, NHDFs were treated with or without recombinant active IL-1α in the presence or absence of TGF-β for 2 days or 3 days. We observed a TGF-β-dependent increase in α-SMA expression as early as 24-hr, which was statistically significant at 48-hr and further increased at 72-hr post TGF-β stimulation (Fig. 6a, b). However, NHDFs that had been cultured with both IL-1α and TGF-β showed significantly reduced α-SMA induction by TGF-β at the 48-hr and 72-hr time points (Fig. 6a–c), and Cox-2 shRNA inhibited this IL-1α-dependent reduction (Fig. 6d–f). Our results reveal a mechanism in which keratinocyte integrin α3β1-dependent, balanced expression of IL-1α and its antagonist, IL-1RA, control the TGF-β-mediated fibroblast differentiation state during wound healing.
Figure 6. IL-1α-mediated induction of Cox-2 inhibits TGF-β-induced α-SMA expression in NHDFs.
Uninfected (a), non-targeting shRNA (NT) or Cox-2 targeting shRNA-infected NHDFs (d) were pretreated with IL-1α (1 ng/mL) for 6 hrs followed by serum free medium, IL-1α (1 ng/mL), TGF-β (2 ng/mL), or combination of IL-1α and TGF-β for 48 or 72 hours (replaced solution every 24 hours). NHDFs were lysed and blotted for Cox-2, α-SMA, and GAPDH; expression levels of α-SMA (b & e) and Cox-2 (c & f) quantitated as fold changes to control at time =0 (not shown). N=3, 2-way ANOVA followed by Turkey’s post-hoc analysis, *P<0.05; error bars are + SEM. (g) Model: keratinocytes secrete IL-1α and IL-1RA in an α3β1-dependent manner to antagonistically regulate fibroblast gene expression and differentiation.
DISCUSSION
Our previous work established an important role for the integrin α3β1 in the regulation of paracrine signaling from epidermal keratinocytes during wound healing (DiPersio et al., 2016; Longmate and Dipersio, 2014; Longmate et al., 2017; Longmate et al., 2018). Following injury, α3β1 controls the secretion by wound keratinocytes of factors that regulate both proximal (e.g., epidermal basement membrane assembly) and more distal (e.g., angiogenesis) tissue remodeling processes (DiPersio et al., 1997; Longmate et al., 2017; Mitchell et al., 2009). This role is consistent with a model of “dynamic reciprocity” in which an integrin is a central component of a mechanism that interrogates the adhesive state of wound keratinocytes, in turn dictating proximal ECM assembly and influencing distal wound processes (DiPersio et al., 2016; Schultz et al., 2011). Whether integrin-mediated paracrine signals from keratinocytes can also control functions of other stromal cells, such as inflammatory cells and fibroblasts/myofibroblasts, has not been explored previously. Here we report a role for epidermal α3β1 in regulating secretion of IL-1α and IL-1RA from keratinocytes to modulate fibroblast gene expression and differentiation.
Our work shows that expression and secretion of both IL-1α and IL-1RA are regulated by epidermal integrin α3β1. The balance between levels of IL-1α and IL-1RA must be maintained to ensure proper wound outcomes, and disruption of such a tightly controlled ratio results in pathological healing. For example, mice deficient in IL-1RA show an exaggerated and persistent inflammatory response (Hirsch et al., 1996; Horai et al., 1998). In addition, myofibroblasts isolated from patients with systemic sclerosis express higher levels of intracellular IL-1RA compared to normal skin fibroblasts, and overexpression of IL-1RA in normal fibroblasts promotes the myofibroblast phenotype, with higher levels of α-SMA and plasminogen activator inhibitor, and lower levels of collagenase and MMP-1 mRNA, implicating important roles for IL-1RA in the pathogenesis of fibrosis (Kanangat et al., 2006). Our findings provide a role for epidermal α3β1 in mediating these paracrine signals to control fibroblast gene expression and differentiation (Fig. 6g).
The Cox-2 / PGE2 signaling axis has been implicated in autocrine suppression of TGF-β-induced α-SMA expression in human lung fibroblasts (Garrison et al., 2013; Penke et al., 2014). Here we demonstrate that IL-1α from MKα3+ CM enhances Cox-2 / PGE2 signaling in NHDFs and Cox-2 shRNA treated NHDFs show lower PGE2 secretion and higher levels of TGF-β-induced α-SMA (Fig. 3 & Fig. 5a–c). In addition, we show that IL-1α alone is sufficient to inhibit TGF-β-induced α-SMA via an autoinhibitory Cox-2 mechanism (Fig. 6 a–f). Interestingly, co-incubating IL-1α with TGF-β attenuates IL-1α-mediated fibroblast Cox-2 induction, suggesting that Cox-2 induction by IL-1α is antagonized by TGF-β (Fig. 6 c & f). These observations are consistent with a model in which the balance of proinflammatory and TGF-β stimuli governs the phenotype of fibroblasts in tissue repair (Shephard et al., 2004). Our current studies identify an α3β1 / IL-1α / Cox-2 regulatory pathway that may be a potential therapeutic target to treat pathogenic scarring and organ fibrosis.
Our findings in α3-deficient MK cells indicate that other keratinocyte integrins do not robustly stimulate IL-1α secretion or fibroblast Cox-2 induction (Fig. 1, 2 & 4), suggesting that compensation by other integrins does not support the Cox-2 suppression that we observed in α3eKO wounds or in NHDFs treated with MKα3- CM (Fig. 1 & 2A). In addition, we demonstrate that such α3β1-dependent, IL-1α signaling from keratinocytes plays a critical role in maintaining a local source of PGE2 by fibroblasts. Many studies have shown that levels of eicosanoids and their effects are highly cell / tissue specific, and all eicosanoids including PGE2, function locally at the site of synthesis because they have very short half-lives (Dennis and Norris, 2015; Van der Walt, 1989). Our data demonstrate a mechanism in which keratinocyte integrin-dependent paracrine signaling to fibroblasts supports an autocrine loop to maintain a local source of PGE2 to ensure proper wound outcomes.
In summary, our data provide evidence demonstrating a critical role for α3β1-dependent IL-1α paracrine signaling from keratinocytes that modulates fibroblast gene expression and differentiation. Although integrins are attractive targets for therapeutic agents aimed at enhancing wound healing, direct targeting of epidermal α3β1 in chronic wounds might be problematic due to its previously established roles in basement membrane assembly, and pro- angiogenic functions (DiPersio et al., 1997; Mitchell et al., 2009; Reynolds et al., 2008). However, the current study provides a fresh approach for targeting specific paracrine signaling pathways that are downstream of epidermal α3β1, which may allow for the therapeutic modulation of specific gene groups such as IL-1α, IL-1RA, and / or IL-1R1 to ensure proper wound outcomes.
MATERIALS AND METHODS
Chemicals and reagents
Recombinant human IL-1α and IL-1β blocking antibody were purchased from ThermoFisher Scientific (Waltham); recombinant human IL-1RA was from MilliporeSigma (Burlington); IL-1α blocking antibody and recombinant mouse IL-1β were purchased from Abcam (United Kingdom); recombinant active human TGF-β was from R&D Systems (Minneapolis), and PGE2 was purchased from Cayman Chemical (Ann Arbor). RNAi and qPCR primer sequences are in Table S1.
In vivo wounding and histology of tissue sections
All animal experiments were carried out with the approval of the Albany Medical College IACUC. Mice homozygous for floxed α3 allele (Itga3flx/flx) express a Cre recombinase transgene under the control of epidermal-specific keratin 14 promoter (K14-Cre). Mice expressing Itga3flx/flx but lacking the K14-Cre transgene served as controls (Mitchell et al., 2009). Full-thickness wounds (4 mm) on the back of 16 mice (6–10 weeks old) from 3 different experiments were generated. Wounds (5 or 10 days post-wounding) were harvested from both control and α3eKO mice (n=4 mice per time point / genotype), fixed (4% paraformaldehyde) overnight, embedded in paraffin, and sectioned (5 microns). Immunostaining was performed using alkaline phosphatase directly conjugated to anti-α-SMA (MilliporeSigma, Burlington) or using unconjugated rabbit monoclonal antibody to Cox-2 followed by Horse Radish Peroxidase (HRP)-conjugated secondary reagents (Cell Signaling, Danvers) and DAB method (BD Pharmingen, San Diego). The field within the wound bed below the re-epithelialized epidermis (excluding normal adjacent tissue and subjacent fat and muscle) was imaged, outlined and defined as a ROI. Stains were separated by a color-deconvolution algorithm as previously described (Ruifrok and Johnston, 2001), and percentages of Cox-2 and α-SMA positive areas within each ROI were measured using area fraction plugin of ImageJ software (Schindelin et al., 2012).
Cell Culture
Mouse Keratinocytes (MK) were cultured at 33°C and 8% CO2 on collagen (30 μg/ml) coated tissue culture dishes in MK growth medium (DiPersio et al. 2000). MK cells were cultured (2 days) in SFM and CM was collected, spun down to remove cell debris, aliquoted and stored at - 20°C. Primary NHDFs were isolated from adult tissue using protocols approved by the Albany Medical Center IRB. NHDFs, neonatal foreskin fibroblasts, IMR-90 fibroblasts (ATCC, Lot 60286830, Manassas) were cultured (37°C and 10% CO2 on uncoated tissue culture dishes in 10% fetal bovine serum-containing medium (Varney et al., 2016). All cells used in these experiments were routinely tested for mycoplasma and found negative by two independent methods, fluorescence microscopy and a PCR-based test (InvivoGen, San Diego).
Western blot analysis
Protein lysates (Laemmli buffer) were separated by SDS-PAGE, transferred to nitrocellulose and incubated overnight with the following antibodies: Cox-2 (1:500 dilution), p-IRAK4 (1:500 dilution) and IRAK4 (1:1000 dilution) from Cell Signaling (Danvers); α-SMA (1:1000 dilution) from MilliporeSigma (Burlington), GAPDH (1:4000 dilution) and IL-1R1 (1:1000 dilution) from ThermoFisher Scientific (Waltham). Relative protein expression was normalized to that of an internal loading control (GAPDH) and expressed as a fold change relative to the average ratio in control lysates.
RNA interference
DsiRNAs (10 nM each) were reverse transfected (24 hrs) with RNAimax (ThermoFisher Scientific, Waltham), replated and recovered (24 hrs) in growth medium before washing and treatment in SFM with additions noted. Lentivirus was produced in HEK 293 FT cells as before (Varney et al., 2016). NHDFs were treated with virus for 24 hours in the presence of 6 μg/mL of polybrene. After removal of virus, cells were replated, drug selected and recovered for 48 hours prior to the start of the experiment.
Mass spectrometry analysis of secreted proteins from MK cells
Duplicate samples of CM were prepared (see above) and equal protein from each CM was analyzed by mass spectrometry (Thermo Fisher Center for Multiplexed Proteomics, Harvard Medical School, Boston, MA). Spectra were searched using method as previously described, and peptide spectral matches were filtered to a less than 1% false discovery rate using the target-decoy strategy combined with linear discriminant analysis (Longmate et al., 2018).
Quantitative RT-PCR
Total RNA was isolated (TRIzol Reagent, Life Technologies, Carlsbad), cDNA generated (iScript Reverse Transcription Supermix, Bio-Rad Laboratories, Hercules) and quantitative PCR reactions performed (iQ™ SYBR® Green Supermix, Bio-Rad Laboratories, Hercules) on a CFX96 C1000 Touch PCR machine (Bio-Rad Laboratories, Hercules). Conditions were as follows: IL-1α and IL-1RA (95°C, 3 minutes, 1 cycle; 95°C, 15 seconds; 60°C, 30 seconds; 72°C, 30 seconds; 40 amplification cycles); IL-1β and 3 housekeeping control genes - Polr2a, B2m, and Ppia (95°C, 3 minutes, 1 cycle; followed, 95°C, 15 seconds; 55°C, 30 second; 72°C, 30 seconds; 40 amplification cycles). Relative mRNA levels were calculated using the following formula: (2((−1)(avg. Ct gene of interest–geometric mean of Ct housekeeping genes)) )*100.
ELISA
ELISAs were performed according to manufacturer’s instructions to quantify cytokines in conditioned media. IL-1α, IL-1β and IL-1RA from ThermoFisher Scientific (Waltham), and PGE2 from Enzo Life Sciences (Farmingdale).
Immunofluorescence
Cells were fixed, permeabilized, blocked in 5% BSA and incubated with primary antibody overnight (1:1000 α-SMA, MilliporeSigma, Burlington; 1:500 MRTF-A, Bethyl Laboratories, Montgomery; 1:750 integrin α3, rabbit polyclonal antisera ((DiPersio et al., 1995); or 1:200 K14, Abcam, United Kingdom). Cells were washed before and after incubation with secondary antibodies, phalloidin and Hoechst 33342 from ThermoFisher Scientific (Waltham) for 1 h (Varney et al., 2016). Images were taken on a Roper/Photometrics Coolsnap ES camera and processed using Nikon Elements software.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Christina Nickerson (Albany Medical Center Histology Core) for assistance with tissue processing and sectioning, and Derek Power and Karl Anderson for technical assistance. We thank Drs. Jonathan Harton and Michelle Lennartz for reagents. Special thanks to Drs. Susan LaFlamme, Peter Vincent, James Drake, and Gabrielle Fredman for providing critical feedback. This research was supported by National Institute of Health grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to L. Van De Water and C.M. DiPersio (MPI, R01AR063778).
ABBREVIATIONS
- α-SMA
Alpha Smooth Muscle Actin
- CM
Conditioned Medium
- MKα3+ CM
Conditioned Medium from α3-expressing Mouse Keratinocytes
- Cox-2
Cyclooxygenase-2
- ECM
Extracellular Matrix
- IL-1α
Interleukin 1 alpha
- IL-1β
Interleukin 1 beta
- IL-1RA
Interleukin 1 Receptor Antagonist
- IL-1R1
Interleukin 1 Receptor type 1
- MK
Mouse Keratinocytes
- NHDFs
Primary Normal Human Dermal Fibroblasts
- PGE2
Prostaglandin E2
CRediT Statement:
| Rui Zheng | |
| Roles: | Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – Original Draft Preparation, Writing – Reviewing and Editing |
| Affiliation: | Departments of Surgery and
Regenerative and Cancer Cell Biology Albany Medical College, Albany, NY 12208 |
| Whitney Longmate | |
| Roles: | Resources, Methodology, Writing – Reviewing and Editing |
| Affiliation: | Departments of Surgery and
Regenerative and Cancer Cell Biology Albany Medical College, Albany, NY 12208 |
| Lori DeFreest | |
| Roles: | Investigation |
| Affiliation: | Department of Surgery Albany Medical College, Albany, NY 12208 |
| Scott Varney | |
| Roles: | Conceptualization, Methodology, Writing – Reviewing and Editing |
| Affiliation: | Departments of Surgery and
Regenerative and Cancer Cell Biology Albany Medical College, Albany, NY 12208 |
| Lei Wu | |
| Roles: | Methodology, Resources, Validation |
| Affiliation: | Departments of Surgery and
Regenerative and Cancer Cell Biology Albany Medical College, Albany, NY 12208 |
| C. Michael DiPersio | |
| Roles: | Conceptualization, Funding Acquisition, Writing – Reviewing and Editing |
| Affiliation: | Departments of Surgery and
Regenerative and Cancer Cell Biology Albany Medical College, Albany, NY 12208 |
| Livingston Van De Water | |
| Roles: | Conceptualization, Funding Acquisition, Project Administration, Mentorship, Writing – Original Draft Preparation, Writing – Reviewing and Editing |
| Affiliation: | Departments of Surgery and
Regenerative and Cancer Cell Biology Albany Medical College, Albany, NY 12208 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT:
Datasets related to this article can be found at [http://dx.doi.org/10.17632/zyhpsk9ryx.1#folder-d41ccca3-ac52-4be0-88f3-cdb1ea57c99e] an open-source online data repository hosted at Mendelev Data.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Alexander J, Cukierman E. Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr Opin Cell Biol. 2016;42:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99(1):31–68. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–16. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15(8):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mari JF, Mifflin RC, Adegboyega PA, Saada JI, Powell DW. IL-1alpha-induced COX-2 expression in human intestinal myofibroblasts is dependent on a PKCzeta-ROS pathway. Gastroenterology. 2003;124(7):1855–65. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137(3):729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shah S, Hynes RO. alpha 3A beta 1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J Cell Sci. 1995;108 ( Pt 6):2321–36. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Zheng R, Kenney J, Van De Water L. Integrin-mediated regulation of epidermal wound functions. Cell Tissue Res. 2016;365(3):467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagami A, Ishizaki M, Fukuda Y, Kawana S, Yamanaka N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab Invest. 2002;82(11):1503–13. [DOI] [PubMed] [Google Scholar]
- Garrison G, Huang SK, Okunishi K, Scott JP, Kumar Penke LR, Scruggs AM, et al. Reversal of myofibroblast differentiation by prostaglandin E(2). Am J Respir Cell Mol Biol. 2013;48(5):550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15 Suppl 1:S46–53. [DOI] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, et al. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129(9):2303–15. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. [DOI] [PubMed] [Google Scholar]
- Hinz B The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–55. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93(20):11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4(1):a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanangat S, Postlethwaite AE, Higgins GC, Hasty KA. Novel functions of intracellular IL-1ra in human dermal fibroblasts: implications in the pathogenesis of fibrosis. J Invest Dermatol. 2006;126(4):756–65. [DOI] [PubMed] [Google Scholar]
- Li G, Lubin FD, McGee DW. alpha3beta1 integrin induced suppression of the Caco-2 epithelial cell IL-1 signaling pathway leading to NF-(kappa)B activation. Cell Immunol. 2004;231(1–2):30–9. [DOI] [PubMed] [Google Scholar]
- Longmate W, DiPersio CM. Beyond adhesion: emerging roles for integrins in control of the tumor microenvironment. F1000Res. 2017;6:1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Dipersio CM. Integrin Regulation of Epidermal Functions in Wounds. Adv Wound Care (New Rochelle). 2014;3(3):229–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Lyons SP, Chittur SV, Pumiglia KM, Van De Water L, DiPersio CM. Suppression of integrin alpha3beta1 by alpha9beta1 in the epidermis controls the paracrine resolution of wound angiogenesis. J Cell Biol. 2017;216(5):1473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Lyons SP, DeFreest L, Van De Water L, DiPersio CM. Opposing Roles of Epidermal Integrins alpha3beta1 and alpha9beta1 in Regulation of mTLD/BMP-1-Mediated Laminin-gamma2 Processing during Wound Healing. J Invest Dermatol. 2018;138(2):444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Monichan R, Chu ML, Tsuda T, Mahoney MG, DiPersio CM. Reduced fibulin-2 contributes to loss of basement membrane integrity and skin blistering in mice lacking integrin alpha3beta1 in the epidermis. J Invest Dermatol. 2014;134(6):1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Segal M, McGee DW. Regulation of epithelial cell cytokine responses by the alpha3beta1 integrin. Immunology. 2003;108(2):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24(11):4133–52. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, et al. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122(Pt 11):1778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe T Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187(5A):65S–70S. [DOI] [PubMed] [Google Scholar]
- Nowinski D, Lysheden AS, Gardner H, Rubin K, Gerdin B, Ivarsson M. Analysis of gene expression in fibroblasts in response to keratinocyte-derived factors in vitro: potential implications for the wound healing process. J Invest Dermatol. 2004;122(1):216–21. [DOI] [PubMed] [Google Scholar]
- Ogata S, Kubota Y, Yamashiro T, Takeuchi H, Ninomiya T, Suyama Y, et al. Signaling pathways regulating IL-1alpha-induced COX-2 expression. J Dent Res. 2007;86(2):186–91. [DOI] [PubMed] [Google Scholar]
- Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits alpha-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem. 2014;289(24):17151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty BJ, Li G, McGee DW. Activation of the alpha3 integrin affects TRAF6 function in the IL-1 signaling pathway of CACO-2 epithelial cells. Immunol Invest. 2010;39(1):1–15. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, et al. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest. 2008;118(3):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23(4):291–9. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164(6):2055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulic M, Lubin FD, O’Donnell PM, Tammariello SP, McGee DW. Effect of the alpha3beta1 integrin on the IL-1 stimulated activation of c-Jun N-terminal kinase (JNK) in CACO-2 cells. Cytokine. 2007;37(2):163–70. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. [DOI] [PubMed] [Google Scholar]
- Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv Wound Care (New Rochelle). 2013;2(4):122–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Walt JG. Eicosanoids: a short review. J S Afr Vet Assoc. 1989;60(1):65–8. [PubMed] [Google Scholar]
- Varney SD, Betts CB, Zheng R, Wu L, Hinz B, Zhou J, et al. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J Cell Sci. 2016;129(4):774–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedrenne N, Coulomb B, Danigo A, Bonte F, Desmouliere A. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol Biol (Paris). 2012;60(1):20–7. [DOI] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21(15):3919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.