Abstract
Aim
Glutamate has been considered as neurotransmitter that is critical in triggering relapse to drugs of abuse, including ethanol and cocaine. Extracellular glutamate concentrations are tightly regulated by several mechanisms, including reuptake through glutamate transporters. Glutamate transporter type 1 (GLT-1) is responsible for clearing the majority of extracellular glutamate. The astrocytic cystine/glutamate antiporter (xCT) regulates also glutamate homeostasis. In this study, we investigated the effects of cocaine exposure and ampicillin/sulbactam (AMP/SUL), a β-lactam antibiotic known to upregulate GLT-1 and xCT, on relapse-like ethanol intake and the expression of astrocytic glutamate transporters in mesocorticolimbic brain regions.
Methods
Male alcohol-preferring (P) rats had free access to ethanol for 5 weeks. On Week 6, rats were exposed to either cocaine (20 mg/kg, i.p.) or saline for 12 consecutive days. Ethanol bottles were then removed for 7 days; during the last 5 days, either AMP/SUL (100 or 200 mg/kg, i.p.) or saline was administered to the P rats. Ethanol bottles were reintroduced, and ethanol intake was measured for 4 days.
Results
Cocaine exposure induced an alcohol deprivation effect (ADE), which was associated in part by a decrease in the expression of GLT-1 and xCT in the nucleus accumbens (NAc) core. AMP/SUL (100 mg/kg, i.p.) attenuated the ADE, while AMP/SUL (200 mg/kg, i.p.) reduced ethanol intake during 4 days of ethanol re-exposure and upregulated GLT-1 and xCT expression in the NAc core, NAc shell and dorsomedial prefrontal cortex (dmPFC).
Conclusion
This study suggests that these astrocytic glutamate transporters might be considered as potential targets for the treatment of polysubstance abuse.
INTRODUCTION
Relapse to ethanol is considered as a significant problem and can occur even after long periods of abstinence (Moos and Moos, 2006). The increase in voluntary ethanol intake after a short or a long abstinence period is a phenomenon called the alcohol deprivation effect (ADE). ADE is used as an animal model for ethanol craving (Heyser et al., 1998; Spanagel and Holter, 1999). Several pharmacological agents were investigated for their efficacy in preventing relapse-like consumption using ADE as an animal model (Spanagel and Zieglgansberger, 1997; Qrunfleh et al., 2013; Marti-Prats et al., 2015).
Studies have shown that different drugs of abuse can induce drug-seeking behavior and relapse to other drugs of abuse (Fox et al., 2005). Importantly, treatment outcomes of addicts with polysubstance abuse problems are often less successful than in addicts with dependence to only one of the drugs (Schmitz et al., 1997; Heil et al., 2001; Anderson et al., 2009). A preclinical study has shown that cocaine given to alcohol-preferring (P) rats 4 hours prior to reinstatement session significantly increased ethanol seeking as compared to control animal group (Hauser et al., 2014).
Findings revealed that synaptic changes within glutamatergic systems in the mesocorticolimbic circuit play a major role in the relapse-like to drugs of abuse in rodents [for review see (Tzschentke and Schmidt, 2003)]. The prefrontal cortex (PFC) and the nucleus accumbens (NAc) are two brain regions within the mesocorticolimbic circuit that have major roles in facilitating relapse to drugs of abuse (Childress et al., 1999; Goldstein and Volkow, 2002; McFarland et al., 2003). Importantly, glutamatergic projections from the PFC to the NAc have been suggested to be fundamentally important in drug dependence and relapse-like behaviors [for review see (Kalivas, 2009; Rao and Sari, 2012)]. Moreover, previous studies revealed that dorsomedial PFC (dmPFC) acts as a critical structure that engages, directs and activates drug-seeking behavior under a variety of environmental conditions (McLaughlin and See, 2003; Berglind et al., 2009). The NAc is a brain region that plays an important role in processing the motivational properties of drugs of abuse (Koob, 1996; Childress et al., 1999). The NAc is comprised of two structural and anatomical subregions (Heimer et al., 1997; Meredith, 1999). The core is responsible for mediating relapse-like drug-seeking behavior (Everitt and Robbins, 2005), while the NAc shell mediates the behaviorally energizing effects of conditioned and novel stimuli (Parkinson et al., 1999).
Extracellular glutamate is regulated by several glutamate transporters (Danbolt, 2001). Glutamate transporter type 1 (GLT-1) is the major glutamate transporter that regulates the majority of extracellular glutamate and is essential in clearing glutamate from the synaptic cleft (Danbolt, 2001). Nearly 80% of the total GLT-1 expression is mainly distributed in astrocytes, and around 5–10% of the total GLT-1 expression is found in neuronal terminals. Mice that lack GLT-1 experience spontaneous seizures linked to high mortality (Tanaka et al., 1997). Furthermore, mice with a partial absence of GLT-1 in the forebrain survive to adulthood and exhibit intermittent focal seizure (Sugimoto et al., 2018). The glutamate aspartate transporter (GLAST) is another astroglial glutamate transporter that is partially involved in regulating glutamate homeostasis in certain brain regions (Tzingounis and Wadiche, 2007). The cystine/glutamate antiporter (xCT) also has a key role in regulating glutamate homeostasis and was found to have functional role in glutamate modulation (Baker et al., 2003).
Several studies from ours and Kalivas’s laboratory showed the important role of upregulating GLT-1 and xCT for the attenuation of reinstatement to cocaine using self-administration of cocaine paradigm (Sari et al., 2009; Knackstedt et al., 2010). In addition, our laboratory has reported that chronic ethanol and cocaine exposure decreased the expression of GLT-1 in the NAc (Hammad et al., 2017b) and that ampicillin/sulbactam (AMP/SUL), a β-lactam antibiotic known to upregulate GLT-1 and xCT, attenuated cocaine-induced reinstatement (Hammad et al., 2017a). In this study, using an ADE model, we investigated for the first time the effect of cocaine on ethanol relapse-like behavior in male alcohol-preferring (P) rats during 4 days of ethanol re-exposure. We further investigated the effects of AMP/SUL on ethanol intake and preference during the 4 days of ethanol re-exposure as well as GLT-1, GLAST and xCT expression in the NAc core, NAc shell and dmPFC.
MATERIALS AND METHODS
Drugs
Cocaine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO). AMP/SUL (Fresenius Kabi USA, LLC) was purchased from the University of Toledo Medical Center Pharmacy. Saline solution (0.9% NaCl) was used to dissolve both drugs. Ethanol 95% (190 proof, Decan Labs) was diluted using deionized water for preparation of two concentrations of ethanol (15 and 30%, v/v).
Subjects
Selectively bred male alcohol-preferring (P) rats were received from Indiana University, School of Medicine (Indianapolis, IN, USA) at the age of 21–30 days and placed in the Department of Laboratory Animal Resources, University of Toledo, Health Science Campus. At the age of 75 days, rats were single-housed in plastic cages lined with corncob bedding, and rats were exposed to free access to food and water throughout the experiments and assigned to five control and experimental groups, as described below. The room temperature was kept at 21°C and 50% humidity with a 12-hour light/dark cycle. All animal procedures were conducted in compliance with, and approved by, the Institutional Animal Care and Use Committee of the University of Toledo in accordance with the guidelines of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
Behavioral drinking paradigms
The experimental design and timeline are illustrated in Fig. 1. At the age of 75 days, rats were assigned to five separate groups: (a) ethanol-naïve (water control) group had free access to food and water ad libitum and were treated with saline throughout the experiment; (b) ethanol-saline group had free access to food and home-cage three-bottle paradigm (water, 15% ethanol and 30% ethanol concurrently) throughout the initial 5 weeks, conditioning phase and re-exposure phase of the experiment, and rats were treated with saline throughout the experiment; (c) ethanol-cocaine-saline group had free access to food and home-cage three bottles (water, 15% ethanol and 30% ethanol concurrently) throughout the initial 5 weeks, conditioning phase and re-exposure phase of the experiment, and rats were exposed to 12 cocaine injections (20 mg/kg, i.p) during the conditioning phase and then treated with saline during the withdrawal period; (d) ethanol-cocaine-AMP/SUL 100 group had free access to food and home-cage three bottles (water and 15% ethanol and 30% ethanol concurrently) throughout the initial 5 weeks, conditioning phase and re-exposure phase of the experiment, and rats were exposed to 12 cocaine injections (20 mg/kg, i.p) and then treated with 5 AMP/SUL injections (100 mg/kg, i.p) during the withdrawal period; and (e) ethanol-cocaine-AMP/SUL 200 group had free access to food home-cage three bottles (water, 15% ethanol and 30% ethanol concurrently) throughout the initial 5 weeks, conditioning phase and re-exposure phase of the experiment, and rats were exposed to 12 cocaine injections (20 mg/kg, i.p) during the conditioning phase and then treated with 5 AMP/SUL injections (200 mg/kg, i.p.) during the withdrawal phase. Groups 2–5 were exposed to ethanol for 5 weeks prior to cocaine conditioning. After the third week of the drinking procedure, ethanol and water intakes were measured three times per week for 2 weeks and used as a baseline. Ethanol and water intake measurements were expressed as g/kg/day. In this study, we have excluded any animals that drank <4 g/kg/day of ethanol, in accordance with previous studies from our laboratory (Sari et al., 2009; Sari and Sreemantula, 2012). After 5 weeks of free-choice ethanol drinking, groups 3–5 were given cocaine (20 mg/kg, i.p) for 12 consecutive days, while groups 1–2 received saline injections. Ethanol bottles were then removed from the cage for 7 days and during the last 5 days of this period, rats in groups 4 and 5 were given AMP/SUL (100 and 200 mg/kg, i.p., respectively). Twenty-four hours after the last AMP/SUL i.p. (or saline) injection, ethanol bottles (15 and 30%) were reintroduced to home cage for 4 days. Ethanol and water intakes were measured daily, and the amount of intake was determined to the nearest 10th of gram by subtracting the obtained bottle weight from their previous day’s value with consideration of the density of ethanol. Weight results were presented as g/kg/day. Ethanol and water bottles were changed twice weekly throughout the experiment. Supplementary Table S1 summarizes animal groups and injections given throughout the experiment.
Fig. 1.
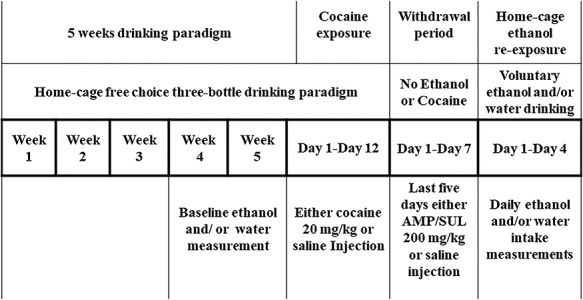
Experimental timeline for relapse-like intake.
Brain tissue harvesting
Rats were promptly euthanized using carbon dioxide after taking the last ethanol and water measurements and decapitated using a guillotine. Brains were extracted and immediately frozen on dry ice and stored at −80°C. Subsequently, brain regions (NAc core, NAc shell and dmPFC) were dissected using a micro-punch procedure in a cryostat apparatus as described previously (Rao et al., 2015a). The Rat Brain Stereotaxic Atlas was used to identify all brain regions (Paxinos and Watson, 2007).
Western blot protocol for detection of GLT-1, xCT and GLAST
Brain samples were lysed using regular lysis buffer as described in previous study from our laboratory (Sari et al., 2011). Equal amounts of extracted proteins were mixed with 5× Laemmli loading dye and then were separated in 10% polyacrylamide gels. Proteins were then transferred onto a PVDF membrane (Bio-Rad, Hercules, CA). Membranes were then blocked with 3% milk in TBST (50 mM Tris-HCl; 150 mM NaCl, pH 7.4; 0.1% Tween 20) for 30 minutes at room temperature. Membranes were then incubated overnight at 4°C with one of the following primary antibodies: guinea pig anti-GLT-1 (1:5000; Millipore, 60 kDa; ab1783), rabbit anti-xCT antibody (1:1000; Abcam; 57 kDa; ab37185) or rabbit anti-EAAT1 (GLAST) antibody (1:5000; Abcam, 64 kDa; ab416). Mouse anti-β-tubulin antibody was used as a loading control (1:5000; Cell Signaling Technology; 55 kDa; #2146). On the next day, membranes were washed with TBST five times and then blocked with 3% milk in TBST for 30 minutes. Membranes were further incubated with secondary antibody for 90 minutes at room temperature. Secondary antibodies were anti-mouse (1:5000; Cell Signaling Technology; #7076) and anti-rabbit (1:5000; Thermo Fisher Scientific; #A-21206). Membranes were then incubated with the SuperSignal West Pico Chemiluminescent substrate and further exposed to Kodak BioMax MR Film (Thermo Fisher Scientific Inc.), and films were developed on a Konica SRX-101A machine. An MCID system was used to quantify the bands, and the results were presented as a percentage of the ratio of tested protein/β-tubulin, relative to the ethanol-naïve (water) control group 1 (100% control value). Antibodies were validated by determining that the bands were produced at the expected molecular weight for the target protein. We fixed the protein expression level for GLT-1, xCT and GLAST of the ethanol-naïve (group 1) as 100% in each gel run as stated in previous studies from our laboratory and others (Li et al., 2003; Miller et al., 2008; Hakami et al., 2016).
Statistical analyses
Two-way mixed model ANOVA with repeated measures was used to analyze behavioral data, followed by Bonferroni post hoc means comparisons. Western blot data were analyzed using one-way ANOVA, followed by Newman–Keuls multiple post hoc means comparisons. All statistical analyses were based on a P < 0.05 level of significance.
RESULTS
Effect of cocaine and AMP/SUL treatment on daily ethanol intake, daily ethanol preference, daily water intake and body weight during relapse-like ethanol-drinking behavior
We measured daily ethanol consumption (g/kg/day), ethanol preference (%), water intake (g/kg/day) and body weight (g) during the 4 days of re-exposure to ethanol, after 12 days of i.p. cocaine or saline injections, and then 5 days of treatment (i.p.) with saline, AMP/SUL 100 mg/kg or AMP/SUL 200 mg/kg during the las 5 days of the 7 days of withdrawal (see Supplementary Table S1).
Daily ethanol intake
Two-way mixed model ANOVA with repeated measures revealed a significant main effect of Day [F (4, 100) = 7.218, P < 0.0001], a significant effect of Treatment [F (3, 25) = 49.25, P < 0.0001] and a significant Day × Treatment interaction [F (12, 100 = 19.84, P < 0.0001]. Bonferroni post hoc comparisons showed a significant increase in ethanol consumption during the 4 days of ethanol re-exposure in the ethanol-cocaine-saline group as compared to the ethanol-saline group (P < 0.05; Fig. 2A). Rats exposed to cocaine and treated with AMP/SUL (100 mg/kg, i.p.) showed no significant increase in ethanol consumption compared to the ethanol-saline group, while rats exposed to cocaine and treated with AMP/SUL (200 mg/kg, i.p.) revealed a decrease in ethanol intake during the 4 days of ethanol re-exposure compared to the ethanol-saline group (P < 0.0001; Fig. 2A).
Fig. 2.
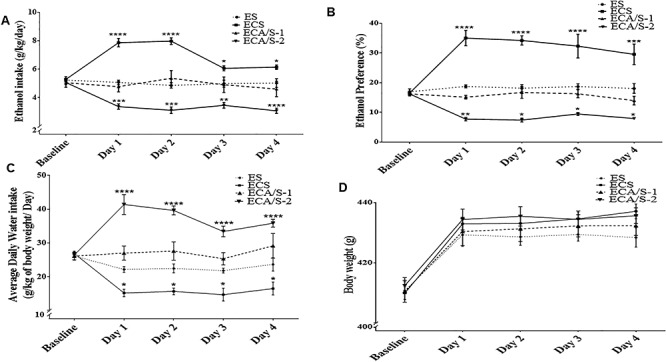
Average daily ethanol consumption (A), ethanol preference (B), water intake (C) and body weight (D) in male P rats during the baseline period (Week 4 and 5) and ethanol re-exposure (Day 1–Day 4) (mean ± SEM). Two-way mixed model ANOVA with repeated measures showed a significant increase in ethanol consumption and preference during the 4 days of ethanol re-exposure in the ethanol-cocaine-saline group as compared to the ethanol-saline group. Rats exposed to cocaine and treated with AMP/SUL (100 mg/kg, i.p.) showed no significant increase in ethanol consumption and preference compared to the ethanol-saline group, while rats exposed to cocaine and treated with AMP/SUL (200 mg/kg, i.p.) revealed a decrease in ethanol intake during the 4 days of ethanol re-exposure compared to the ethanol-saline group. A significant decrease in water intake was observed during the 4 days of ethanol re-exposure in the ethanol-cocaine-saline group as compared to the ethanol-saline group. Rats exposed to cocaine and treated with AMP/SUL (100 mg/kg, i.p.) showed no significant increase in water intake compared to the ethanol-saline group, while rats exposed to cocaine and treated with AMP/SUL (200 mg/kg, i.p.) revealed an increase in water intake during the 4 days of ethanol re-exposure compared to the ethanol-saline group. No significant effect of groups on body weight was revealed (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.00001), (n = 7–10 for each group). Abbreviations: WS, water-saline; ES, ethanol-saline; ECS, ethanol-cocaine-saline; ECA/S-1, ethanol-cocaine-AMP/SUL 100; and ECA/S-2, ethanol-cocaine-AMP/SUL 200.
Daily ethanol preference
Two-way mixed model ANOVA with repeated measures revealed no significant main effect of Days [F (4, 100) = 1.576, P = 0.1865], a significant effect of Treatment [F (3, 25) = 66.19, P < 0.0001] and a significant Day × Treatment interaction [F (12, 100 = 6.878, P < 0.0001]. Bonferroni post hoc comparisons showed a significant increase in ethanol preference during the 4 days of ethanol re-exposure in the ethanol-cocaine-saline group as compared to the ethanol-saline group (P < 0.0001; Fig. 2B). Rats exposed to cocaine and treated with AMP/SUL (100 mg/kg, i.p.) showed no significant increase in ethanol preference compared to the ethanol-saline group, while rats exposed to cocaine and treated with AMP/SUL (200 mg/kg, i.p.) revealed a decrease in preference during the 4 days of ethanol re-exposure compared to the ethanol-saline group (P < 0.0001; Fig. 2B).
Daily water intake
Two-way mixed model ANOVA with repeated measures revealed no significant main effect of Days [F (4, 100) = 2.339, P = 0.0603], a significant effect of Treatment [F (3, 25) = 57.94, P < 0.0001] and a significant Day × Treatment Interaction [F (12, 100 = 10.58, P < 0.0001]. Bonferroni post hoc comparisons showed a significant increase in water consumption during the 4 days of ethanol re-exposure in the ethanol- cocaine-AMP/SUL 200 group (P < 0.0001; Fig. 2B). Rats exposed to cocaine and treated with AMP/SUL (100 mg/kg, i.p.) showed no significant increase in water intake compared to the ethanol-saline group, while the ethanol-cocaine-saline-treated group revealed a decrease in water intake compared to the ethanol-saline group during the 4 days of ethanol re-exposure (P < 0.0001; Fig. 2B).
Body weight
Two-way ANOVA revealed a significant main effect of Day [F (4, 100) = 139.0, P < 0.0001], a non-significant effect of Treatment [F (3, 25) = 0.8266, P = 0.4917] and a non-significant Day × Treatment interaction [F (12, 100 = 0.9967, P = 0.4577]. Bonferroni post hoc comparisons showed no significant effect in body weight between all groups (Fig. 2D).
Effects of cocaine and AMP/SUL treatment on GLT-1, xCT and GLAST expression in the NAc core, NAc shell and dmPFC during relapse-like ethanol-drinking behavior
GLT-1 expression
One-way ANOVA revealed a significant main effect of treatment among the ethanol-naïve (water control), ethanol-saline, ethanol-cocaine-saline and ethanol-cocaine-AMP/SUL 100 and ethanol-cocaine-AMP/SUL 200 groups in the NAc core [F (4, 30) = 24.04, P < 0.0001; Fig. 3A; Supplementary Table S2A], NAc shell [F (4, 30) = 8.247, P = 0.0002; Fig. 4A; Supplementary Table S3A] and dmPFC [F (4, 30) = 4.931, P = 0.0043; Fig. 5A; Supplementary Table S4A]. Newman–Keuls multiple post hoc comparisons showed a significant decrease in the expression of GLT-1 in the ethanol-cocaine-saline-treated group compared to all other groups (P < 0.05; Fig. 3A) and a significant increase in the ethanol-cocaine-AMP/SUL 200-treated group compared to all other groups in the NAc core (P < 0.001; Fig. 3A). Newman–Keuls multiple post hoc comparisons showed a significant increase in the expression of GLT-1 in the ethanol-cocaine-AMP/SUL 200-treated group compared all other groups in the NAc shell (P < 0.01; Fig. 4A). In addition, Newman–Keuls multiple post hoc comparisons showed significant increase in the expression of GLT-1 in the ethanol- cocaine-AMP/SUL 200-treated group compared to all other groups in the dmPFC (P < 0.05; Fig. 5A).
Fig 3.
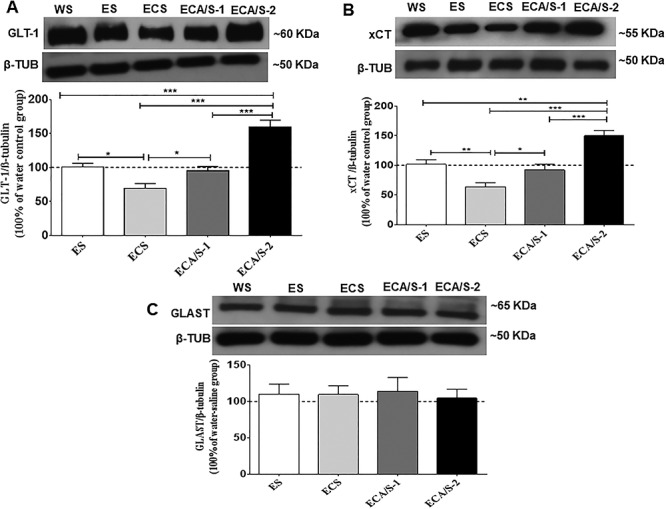
The effects of cocaine (20 mg/kg) and AMP/SUL (100 and 200 mg/kg) on GLT-1/β-tubulin (A), xCT/β-tubulin (B) and GLAST/β-tubulin (C) in the NAc core following 4-day ethanol re-exposure (mean ± SEM). One-way ANOVA revealed a significant reduction in GLT-1/β-tubulin and xCT/β-tubulin in the ethanol-cocaine-saline-treated group and an increase in GLT-1/β-tubulin and xCT/β-tubulin in the ethanol-cocaine–AMP/SUL (200 mg/kg) treated group. No significant difference was revealed in GLAST/β-tubulin between groups. (*P < 0.05, **P < 0.01 and ***P < 0.001), (n = 5–7 for each group). Abbreviations: WS, water-saline; ES, ethanol-saline; ECS, ethanol-cocaine-saline; ECA/S-1, ethanol-cocaine-AMP/SUL 100; and ECA/S-2, ethanol-cocaine-AMP/SUL 200.
Fig 4.
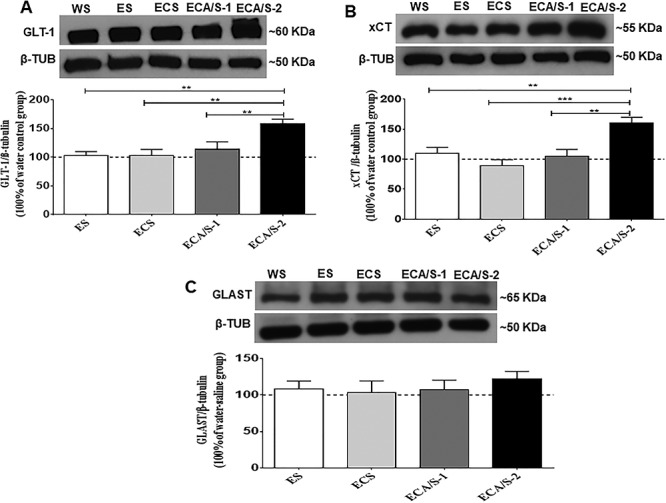
The effects of cocaine (20 mg/kg) and AMP/SUL (100 and 200 mg/kg) on GLT-1/β-tubulin (A), xCT/β-tubulin (B) and GLAST/β-tubulin (C) in the NAc shell following 4-day ethanol re-exposure (mean ± SEM). One-way ANOVA revealed an increase in GLT-1/β-tubulin and xCT/β-tubulin in the ethanol-cocaine-AMP/SUL (200 mg/kg) treated group. No significant difference was revealed in GLAST/β-tubulin between groups. (**P < 0.01 and ***P < 0.001), (n = 5–7 for each group). Abbreviations: WS, water-saline; ES, ethanol-saline; ECS, ethanol-cocaine-saline; ECA/S-1, ethanol-cocaine-AMP/SUL 100; and ECA/S-2, ethanol-cocaine-AMP/SUL 200.
Fig 5.
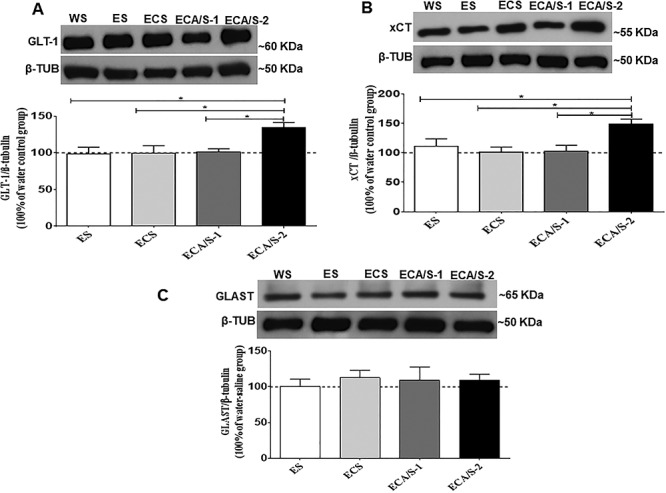
The effects of cocaine (20 mg/kg) and AMP/SUL (100 mg/kg and 200 mg/kg) on GLT-1/β-tubulin (A), xCT/β-tubulin (B) and GLAST/β-tubulin (C) in the dmPFC following 4-day ethanol re-exposure (mean ± SEM). One-way ANOVA revealed increases in GLT-1/β-tubulin and xCT/β-tubulin in the ethanol-cocaine-AMP/SUL (200 mg/kg) treated group. No significant difference was revealed in GLAST/β-tubulin between all groups. (*P < 0.05), (n = 5–7 for each group). Abbreviations: WS, water-saline; ES, ethanol-saline; EDS, ethanol-cocaine-saline; ECA/S-1, ethanol-cocaine-AMP/SUL 100; and ECA/S-2, ethanol-cocaine-AMP/SUL 200.
xCT expression
One-way ANOVA revealed a significant main effect among the ethanol-naïve (water control), ethanol-saline, ethanol-cocaine, ethanol-cocaine-AMP/SUL 100 and ethanol-cocaine-AMP/SUL 200 groups in the NAc core [F (4, 30) = 18.28, P < 0.0001; Fig. 3B; Supplementary Table S2B], NAc shell [F (4, 30) = 9.323, P < 0.0001; Fig. 4B; Supplementary Table S3B] and dmPFC [F (4, 30) = 5.812, P = 0.0018; Fig. 5B; Supplementary Table S4B]. Newman–Keuls multiple post hoc comparisons showed a significant decrease in the expression of xCT in the ethanol-cocaine-treated group compared to all other groups (P < 0.05; Fig. 3B) and a significant increase in xCT expression in the ethanol-cocaine-AMP/SUL 200-treated group compared to all other groups in the NAc core (P < 0.01; Fig. 3B). Newman–Keuls multiple post hoc comparisons showed a significant decrease in the expression of xCT in the ethanol-cocaine-treated group compared to all other groups (P < 0.05; Fig. 5B) and a significant increase in the ethanol-cocaine-AMP/SUL 200-treated group compared to all other groups in the NAc shell (P < 0.05; Fig. 4B). Newman–Keuls multiple post hoc comparisons showed also a significant increase in the expression of xCT in the ethanol-cocaine-AMP/SUL 200-treated group compared to all other groups in the dmPFC (P < 0.05; Fig. 5B).
GLAST expression
One-way ANOVA revealed no significant main effect of treatment among the ethanol-naïve (water control), ethanol-saline, ethanol-cocaine, ethanol-cocaine-AMP/SUL 100 and ethanol-cocaine-AMP/SUL 200 groups in the NAc core [F (4, 30) = 0.1764, P = 0.9485; Fig. 3C; Supplementary Table S2C], NAc shell [F (4, 30) = 0.6846, P = 0.6090; Fig. 4C; Supplementary Table S3C] or dmPFC [F (4, 30) = 0.3294, P = 0.8557; Fig. 5C; Supplementary Table S4C].
DISCUSSION
We report in this study that rats exposed to cocaine (20 mg/kg, i.p.) for 12 days showed an increase in relapse-like ethanol-drinking behavior compared to the control saline-treated rats. Previous studies have reported that P rats show a robust relapse-like drinking behavior following a single prolonged (2 weeks or more) ethanol deprivation when reaccess to ethanol (Rodd-Henricks et al., 2001; Sari et al., 2006). Our current ethanol relapse-like drinking findings for the ethanol-saline group did not show any ADE effect after 1 week of deprivation. The discrepancy of these results might be due to the length of ethanol deprivation (1 week versus 2 weeks). Importantly, studies have shown that cocaine exposure can trigger drug seeking and relapse for other drugs of abuse, indicating a common neurobiological pathway for seeking behavior between cocaine and other drugs of abuse. Indeed, cocaine exposure for 4-hours prior to an ethanol re-exposure session increased ethanol-seeking and relapse-like drinking behavior (Hauser et al., 2014). Similarly, a single non-contingent cocaine injection stimulated heroin self-administration following a 3-week extinction period (De Vries et al., 1998). In this study, a robust ADE was shown after 1 week of deprivation in the cocaine-saline group even after a single short deprivation period. These findings are in parallel to the human studies showing that cocaine increased ethanol relapse behavior (Fox et al., 2005). Similar ADE was demonstrated in both male and female rodents. A two-bottle option model of 24-hour exposure every other day, female and male mice exposed to 3-week sporadic access to ethanol drinking, developed excessive ethanol intake and then shown pronounced ADE after 1-week abstinence (Zhou et al., 2018). In this study, male P rats were used to determine the effects of cocaine exposure on a short deprivation period. Further studies are warranted to examine the effect of cocaine exposure on female P rats.
Treatment with AMP/SUL (100 mg/kg, i.p.) attenuated this increase, while treatment with AMP/SUL (200 mg/kg, i.p.) decreased ethanol drinking below the baseline levels of control rats that had not received cocaine. Water intake was reduced in rats exposed to cocaine. However, AMP/SUL (200 mg/kg, i.p.) increased water intake. The increase in water intake for AMP/SUL (200 mg/kg, i.p.) treated group is parallel to the decrease in ethanol intake and is speculated to be a compensatory mechanism to conserve the overall fluid balance during relapse to ethanol intake and β-lactam treatment (Bell et al., 2008; Qrunfleh et al., 2013).
Evidence has demonstrated the important role of the glutamatergic system in relapse to drugs of abuse, including ethanol and cocaine, and that modulating astroglial and neuronal proteins that control excitatory synaptic plasticity may have therapeutic effects in the treatment of drug dependence [for review see (Vengeliene et al., 2005b; Gass and Olive, 2008; Kalivas and Volkow, 2011)]. Acamprosate (N-methyl-d-aspartate, NMDA, receptor antagonist) attenuated relapse-like ethanol-drinking behavior (Spanagel et al., 1996; Holter et al., 1997; Heyser et al., 1998). In addition, other glutamatergic target drugs attenuate relapse-like ethanol-drinking behavior such as MPEP, a metabotropic glutamate receptor antagonist (Backstrom et al., 2004; Schroeder et al., 2005), and competitive and non-competitive NMDA antagonists (Holter et al., 2000; Vengeliene et al., 2005a). Importantly, restoring GLT-1 and xCT expression may prevent cocaine reinstatement, chronic ethanol intake and ADE (Kalivas, 2009; Sari et al., 2009, 2011; Knackstedt et al., 2010; Qrunfleh et al., 2013; Das et al., 2015). Previous studies from our laboratory showed that AMP/SUL treatment attenuated cocaine-induced reinstatement, reduced ethanol intake and attenuated dependence to cannabinoids, in part, through upregulation of GLT-1 and xCT expression (Hammad et al., 2017a; Hakami et al., 2019). In this study, we used AMP/SUL in order to attenuate ADE in P rats. It is important to note that cocaine exposure alone reduced GLT-1 expression in the NAc (Knackstedt et al., 2010). We suggest here that AMP/SUL may increase GLT-1 and xCT expression and decrease ethanol drinking regardless of prior cocaine or ethanol exposure. Importantly, there is possibility that the pharmacological effects of AMP/SUL on decreasing ethanol drinking may involve other mechanisms that are unrelated to glutamate transporters. Studies are warranted to determine other pharmacological effects of AMP/SUL on decreasing ethanol intake as well as cocaine-seeking behavior.
Astrocytic GLT-1 has been long suggested to play a key role in glutamate homeostasis. Thus, deletion of astroglial GLT-1 caused a reduction of 80% of GLT-1 protein as well as glutamate uptake; however, deletion of neuronal GLT-1 did not affect the expression of GLT-1 or glutamate uptake in mice (Petr et al., 2015). This latter study demonstrated that the deletion of astrocytic GLT-1 increased the mortality, decrease body weight and induced seizures. The importance of neuronal GLT-1 is warranted investigation regarding glutamate function in a disease model. Note that in our present study, western blot technique revealed the overall GLT-1 expression (neuronal and astrocytic). Studies are warranted to determine the effects of cocaine, ethanol and β-lactams on neuronal GLT-1 versus astrocytic GLT-1. These studies may involve electrophysiological, confocal and electron microscopic techniques. It is important to note that post-translational regulation of GLT-1 has a role in several neurological disorders (Peterson and Binder, 2019). Studies are warranted to determine the potential role of post-translational regulation of GLT-1 in neurological diseases and drug dependence animal models with the uses of AMP/SUL as a potential therapeutic drug. In this study, we further investigated GLAST as another astrocytic glutamate transporter that is co-expressed with GLT-1 throughout the brain (Berger and Hediger, 1998). We did not detect any significant changes in the level of GLAST with AMP/SUL treatment, which suggests the specific regulatory effect of AMP/SUL on GLT-1 and xCT expression.
Similarly, glutamate transmission between the NAc and the PFC is implicated in cocaine- seeking behavior (Baker et al., 2003; Berglind et al., 2009). Chronic cocaine exposure produced an increase in extracellular glutamate concentrations in the mesocorticolimbic circuits, which result in the development and expression of behavioral sensitization [for review see (Wolf, 1998; Vanderschuren and Kalivas, 2000)]. Similarly, chronic cocaine exposure reduced basal extracellular glutamate concentrations in the NAc (Hotsenpiller et al., 2001). It is noteworthy that reinstatement to cocaine is attenuated by a local injection of glutamate ionotropic receptor antagonists into the NAc core (Cornish and Kalivas, 2000; McFarland and Kalivas, 2001). Similarly, cue to cocaine-seeking behavior was blocked by a local injection of glutamate ionotropic receptor antagonists into the NAc core, but not NAc shell (Di Ciano and Everitt, 2001). Several studies showed a reduction in GLT-1 expression in the NAc core and NAc shell, but not dmPFC, following cocaine self-administration (Sondheimer and Knackstedt, 2011; Reissner et al., 2014, 2015). Studies from our laboratory and Kalivas’s laboratory demonstrated that deficits in GLT-1 expression is suggested to be associated with an elevation in extracellular glutamate concentrations, in part due to reduction in glutamate uptake in animal models of drugs of abuse such as ethanol, cocaine and nicotine (Knackstedt et al., 2009, 2010;Das et al., 2015). These studies showed that ceftriaxone, β-lactam antibiotic known to upregulate GLT-1 and xCT, reversed these effects. Based in these published data, we suggest here that deficits in the expression of GLT-1 and xCT are associated with elevation in extracellular glutamate concentrations in key reward brain regions, including NAc. Furthermore, previous study from our laboratory showed that intra-accumbal injection of GLT-1 blocker, dihydrokainic acid, reversed the effects of ceftriaxone in extracellular glutamate concentration in NAc in P rats exposed to ethanol (Das et al., 2015). We suggest here that blocking accumbal GLT-1 function would increase extracellular glutamate concentration and consequently lead to enhancement of ADE. Studies are warranted to evaluate this hypothesis.
Importantly, cocaine withdrawal decreased GLT-1 expression, and this reduction is strongly correlated with the length of access and withdrawal of the drug (Fischer et al., 2013). Indeed, the decrease was higher in the NAc core than in the NAc shell following longer periods of cocaine access (Fischer-Smith et al., 2012). Findings revealed that chronic cocaine administration decreased xCT expression (Knackstedt et al., 2010) and activity (Trantham-Davidson et al., 2012) in the NAc. Repeated cocaine exposure produced hyper-excitability in the mPFC (Nasif et al., 2005). Higher glutamate release, following chronic cocaine exposure, was found in the mPFC after 1 and 7 days of cocaine withdrawal (Williams and Steketee, 2004). However, less is known about the effect of ethanol and cocaine co-exposure on other mesocorticolimbic brain regions and the aspects of glutamatergic function, particularly the expression of astrocytic glutamate transporters. Studies are warranted to determine the link between GLT-1 and xCT expression and the relapse-like drinking behavior that might clarify the potential mechanism. In this study, we showed for the first time that GLT-1 and xCT expression were reduced in the NAc core in animals that developed an ADE-like effect after combined non-contingent cocaine and ethanol exposure. However, GLT-1 and xCT expression were not altered in the NAc core, NAc shell or dmPFC after ethanol exposure alone. These results are in agreement with a previous study conducted in our laboratory (Alhaddad et al., 2014). Importantly, the increase in ethanol intake was associated in part with a reduction in GLT-1 and xCT expression in the NAc core. This study also revealed that AMP/SUL (200 mg/kg, i.p.) treatment upregulated GLT-1 and xCT expression in the NAc core, NAc shell and dmPFC, which may be the mechanism underlying the decrease in relapse-like ethanol-drinking behavior.
In addition, studies from our laboratory have reported that β-lactam antibiotics increased GLT-1 and xCT expression, partly by increasing the expression of the nuclear factor kappa-B (NFκB) and phospho-AKT (Rao and Sari, 2012; Goodwani et al., 2015; Rao et al., 2015a, 2015b). In accordance, it has been clearly shown that ceftriaxone-induced upregulation of GLT-1 is mediated through NFkB signaling pathway (Lee et al., 2008). These data indicate that these signaling pathways could be a conceivable mechanism for β-lactam antibiotics. Future studies are warranted to demonstrate if these pathways are specific to the effect of β-lactam.
In conclusion, we found in this study that AMP/SUL (200 mg/kg, i.p.) attenuated ethanol relapse-like drinking behavior, which was accentuated by concurrent cocaine administration. Upregulations of GLT-1 and xCT expression have also been observed with AMP/SUL (200 mg/kg, i.p.) treatment in the major mesocorticolimbic brain regions. These findings emphasized that GLT-1 and xCT may be potential targets for the treatment of relapse-like drinking behavior in polysubstance abusers. In addition, AMP/SUL might be considered as a potential agent for the treatment of relapse-like drinking behavior. Further studies are warranted to demonstrate the effects of AMP/SUL alone on GLT-1 and xCT expression, as well as the effects of cocaine on ethanol intake regardless of prior ethanol exposure.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Award Number R01AA019458 (Y.S.) from the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. A.M.H. was supported by a scholarship from Al-Zaytoonah University, Amman, Jordan.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Alhaddad H, Das SC, Sari Y (2014) Effects of ceftriaxone on ethanol intake: A possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology (Berl) 231:4049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li S-H, et al. (2009) Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend 104:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, et al. (2004) mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29:921–8. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, et al. (2003) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–9. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Schultz JA, et al. (2008) Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol 42:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Hediger MA (1998) Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol 198:13–30. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW Jr, LaLumiere RT, et al. (2009) A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci 29:3715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, et al. (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:Rc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. (2001) Glutamate uptake. Prog Neurobiol 65:1–105. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, et al. (2015) Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 97:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, et al. (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10:3565–71. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ (2001) Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology 25:341–60. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci 8:1481–9. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV (2012) Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience 210:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV (2013) Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci 33:9319–27.23719800 [Google Scholar]
- Fox HC, Talih M, Malison R, et al. (2005) Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 30:880–91. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF (2008) Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75:218–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwani S, Rao PS, Bell RL, et al. (2015) Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res 1622:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Hammad AM, Sari Y (2016) Effects of amoxicillin and augmentin on cystine-glutamate exchanger and glutamate transporter 1 isoforms as well as ethanol intake in alcohol-preferring rats. Front Neurosci 10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Alshehri FS, Sari Y (2019) Beta-lactams modulate astroglial glutamate transporters and attenuate dependence to CP 55,940, a CB1 receptor agonist, in rat model. Behav Brain Res 359:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Alasmari F, Althobaiti YS, et al. (2017a) Modulatory effects of ampicillin/sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav Brain Res 332:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Althobaiti YS, Das SC, et al. (2017b) Effects of repeated cocaine exposure and withdrawal on voluntary ethanol drinking, and the expression of glial glutamate transporters in mesocorticolimbic system of P rats. Mol Cell Neurosci 82:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Wilden JA, Deehan GA Jr, et al. (2014) Cocaine influences alcohol-seeking behavior and relapse drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res 38:2678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Badger GJ, Higgins ST (2001) Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. J Stud Alcohol 62:14–22. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, et al. (1997) The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci 9:354–81. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, et al. (1998) Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology 18:125–33. [DOI] [PubMed] [Google Scholar]
- Holter SM, Landgraf R, Zieglgansberger W, et al. (1997) Time course of acamprosate action on operant ethanol self-administration after ethanol deprivation. Alcohol Clin Exp Res 21:862–8. [PubMed] [Google Scholar]
- Holter SM, Danysz W, Spanagel R (2000) Novel uncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonist MRZ 2/579 suppresses ethanol intake in long-term ethanol-experienced rats and generalizes to ethanol cue in drug discrimination procedure. J Pharmacol Exp Ther 292:545–52. [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME (2001) Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci 14:1843–55. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–72. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, et al. (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (1996) Drug addiction: the yin and yang of hedonic homeostasis. Neuron 16:893–6. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, et al. (2008) Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem 283:13116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olinger AB, Dassow MS, et al. (2003) Up-regulation of GABA(B) receptor mRNA and protein in the hippocampus of cocaine- and lidocaine-kindled rats. Neuroscience 118:451–62. [DOI] [PubMed] [Google Scholar]
- Marti-Prats L, Zornoza T, Lopez-Moreno JA, et al. (2015) Acetaldehyde sequestration by D-penicillamine prevents ethanol relapse-like drinking in rats: Evidence from an operant self-administration paradigm. Psychopharmacology (Berl) 232:3597–606. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 168:57–65. [DOI] [PubMed] [Google Scholar]
- Meredith GE. (1999) The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci 877:140–56. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, et al. (2008) Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience 153:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS (2006) Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 101:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ (2005) Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci 25:3674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, et al. (1999) Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci 19:2401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. Amsterdam; Boston: Academic Press/Elsevier. [Google Scholar]
- Peterson AR, Binder DK (2019) Post-translational regulation of GLT-1 in neurological diseases and its potential as an effective therapeutic target. Front Mol Neurosci 12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr GT, Sun Y, Frederick NM, et al. (2015) Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci 35:5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y (2013) Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol 27:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y (2012) Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem 19:5148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Goodwani S, Bell RL, et al. (2015a) Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience 295:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Saternos H, Goodwani S, et al. (2015b) Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 232:2333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, et al. (2014) Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology 39:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, et al. (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol 20:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, et al. (2001) Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res 25:1140–50. [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC (2006) Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res 30:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, et al. (2009) Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci 29:9239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, et al. (2011) Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol 46:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN (2012) Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience 227:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Bordnick PS, Kearney ML, et al. (1997) Treatment outcome of cocaine-alcohol dependent patients. Drug Alcohol Depend 47:55–61. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW (2005) The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 179:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA (2011) Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res 225:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, Allingham K, et al. (1996) Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 305:39–44. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W (1997) Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci 18:54–9. [PubMed] [Google Scholar]
- Spanagel R, Holter SM (1999) Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol 34:231–43. [DOI] [PubMed] [Google Scholar]
- Sugimoto J, Tanaka M, Sugiyama K, et al. (2018) Region-specific deletions of the glutamate transporter GLT1 differentially affect seizure activity and neurodegeneration in mice. Glia 66:777–88. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, et al. (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–702. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, et al. (2012) Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci 32:12406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI (2007) Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 8:935–47. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ (2003) Glutamatergic mechanisms in addiction. Mol Psychiatry 8:373–82. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, et al. (2005a) The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology 48:822–9. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Vollmayr B, Henn FA, et al. (2005b) Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology (Berl) 178:125–32. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD (2004) Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: a time course study. Eur J Neurosci 20:1639–46. [DOI] [PubMed] [Google Scholar]
- Wolf ME. (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Crowley R, Prisinzano T, et al. (2018) Effects of mesyl salvinorin B alone and in combination with naltrexone on alcohol deprivation effect in male and female mice. Neurosci Lett 673:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


