Abstract
Background
Stimulant use and sexual behaviors have been linked in behavioral and epidemiological studies. Although methamphetamine-related neurofunctional differences have been investigated, few studies have examined neural responses to drug and sexual cues with respect to shorter or longer term methamphetamine abstinence in individuals with methamphetamine dependence.
Methods
Forty-nine men with shorter term methamphetamine abstinence, 50 men with longer term methamphetamine abstinence, and 47 non–drug-using healthy comparison men completed a functional magnetic resonance imaging cue-reactivity task consisting of methamphetamine, sexual, and neutral visual cues.
Results
Region-of-interest analyses revealed greater methamphetamine cue–related activation in shorter term methamphetamine abstinence and longer term methamphetamine abstinence individuals relative to healthy comparison men in the ventromedial prefrontal cortex. A significant interaction of group and condition in the anterior insula was found. Relative to healthy comparison participants, both shorter term methamphetamine abstinence and longer term methamphetamine abstinence groups displayed greater sexual cue–related anterior insula activation relative to methamphetamine cues and neutral cues, but there were no differences between shorter term methamphetamine abstinence and longer term methamphetamine abstinence groups in anterior insula responses. Subsequent whole-brain analyses indicated a group-by-condition interaction with longer term methamphetamine abstinence participants showing greater sexual-related activation in the left superior frontal cortex relative to healthy comparison men. Shorter term methamphetamine abstinence participants showed greater superior frontal cortex activation to sexual relative to neutral cues, and longer term methamphetamine abstinence participants showed greater superior frontal cortex activation to sexual relative to neutral and methamphetamine cues.
Conclusions
The findings suggest that abstinence from methamphetamine may alter how individuals respond to drug and sexual cues and thus may influence drug use and sexual behaviors. Given the use of methamphetamine for sexual purposes and responses to natural vs drug rewards for addiction recovery, the findings may have particular clinical relevance.
Keywords: methamphetamine dependence, ventral striatum, dorsal striatum, ventral medial prefrontal cortex, anterior insula, superior frontal cortex
Significance Statement.
Methamphetamine use and sexual behaviors are related, but neural responses to drug and sexual cues with respect to shorter or longer term methamphetamine abstinence in individuals with methamphetamine dependence are unclear. Relatively greater methamphetamine cue–related activation in the ventromedial prefrontal cortex was found in men with shorter and longer term methamphetamine abstinence compared with healthy comparison men. Greater sexual cue–related anterior insula activation relative to methamphetamine cues and neutral cues was found in men with shorter and longer term methamphetamine abstinence compared with healthy comparison men. Relatively greater sexual cue–related activation in the left superior frontal cortex was also found in men with longer term methamphetamine abstinence compared with healthy comparison men. Abstinence from methamphetamine may alter how individuals respond to drug and sexual cues and may thus influence drug use and sexual behaviors.
Introduction
Methamphetamine is a psychostimulant that is widely abused and linked to serious public health problems globally (World Drug Report, 2016). Methamphetamine use has been associated with sexual activity, as methamphetamine may enhance sexual self-confidence (Semple et al., 2004), diminish sexual inhibitions (Frohmader et al., 2010a), and increase sexual desire, arousal, and pleasure and delay orgasm both in humans (Frosch et al., 1996; Molitor et al., 1999) and animal models (Frohmader et al., 2011). Some individuals who use methamphetamine have reported that sexual enhancement was the primary motivation for their drug use (Semple et al., 2002; Bosma-Bleeker and Blaauw, 2018). Animal studies have found that methamphetamine activates the same cells in reward systems as sexual behavior, which in turn suggests that methamphetamine may influence compulsive sexual behaviors (Frohmader et al., 2010b).
Multiple addiction studies have focused on dopamine-related reward systems involving the ventral striatum (Balodis et al., 2016). The ventral striatum may be activated by methamphetamine or cocaine administration in humans and animals (Porrino et al., 2004; Völlm et al., 2004). The ventral striatum receives projections of midbrain dopaminergic neurons and has been implicated in reward-based learning (Daniel and Pollmann, 2014) and drug-associated conditioned reinforcement learning (Everitt et al., 1999); thus, it may mediate the rewarding effects of drugs. However, the transition from voluntary drug-taking to compulsive drug-seeking may be the result of dynamic shifts from ventral to dorsal striatal involvement (Everitt and Robbins, 2013). For example, chronic stimulant use may shift activation from the ventral to dorsal striatum during drug seeking (See et al., 2007). As such, different stages of addiction and abstinence may be linked to differences in striatal correlates of reward processing.
However, dopamine-related processes in the striatum may be insufficient to account fully for addiction processes, and other brain regions implicated in reward processing and behavioral control, including the prefrontal cortex (PFC), are important to consider (Goldstein and Volkow, 2002). Within the PFC, the ventromedial PFC (vmPFC) may contribute importantly to reward processing and the development of addictions, as this region engages in emotional processes, stress reactivity, and attentional processes underlying goal-directed behaviors and decision-making (Hitchcott et al., 2007). Damage to the vmPFC has been associated with blunted emotional reactivity, poorly modulated emotional reactions, disadvantageous social decision-making, impaired goal-directed behaviors, and lack of insight (Barrash et al., 2000). Individuals with substance dependence show similar behaviors to patients with bilateral vmPFC lesions (Bechara et al., 2001), which suggests vmPFC dysfunction in substance dependence.
The anterior insula is another crucial region to consider, as it is implicated in both drug and non-drug craving (Garavan, 2010). As the anterior insula has been implicated in motivation, executive functioning, interoceptive awareness, and decision-making processes, abnormalities in anterior insula function may promote addictive behaviors (Naqvi et al., 2014). Moreover, the relationship between cue-elicited anterior insula activation and treatment outcome has been shown in prior fMRI studies. Janes et al. found that cue-elicited bilateral anterior insula activation may reflect potentiated relapse vulnerability in treated, abstinent smokers, and suggest that insula activation may help identify individuals vulnerable to engaging in addictive disorders (Janes et al., 2010).
According to the impaired response inhibition and salience attribution (iRISA) model of addiction (Goldstein and Volkow, 2011), disrupted PFC function could lead to increased salience of drugs and drug-related cues, decreased sensitivity to non-drug cues, and fewer tendencies to inhibit maladaptive behaviors. Enhanced PFC drug-related cue activation has been reported after short-term abstinence (up to a mean of 8 days) in studies of tobacco smoking (Janes et al., 2009). However, it was proposed in the iRISA model that in early abstinence, the sensitivity of the PFC to non-drug–related rewards may be markedly attenuated, leading to impaired inhibitory control in individuals with drug use disorders and promoting relapse (Goldstein and Volkow, 2011). As a result, drug seeking and drug taking may become main motivational drives relative to others during early abstinence (Volkow et al., 2003). However, studies investigating the impact of abstinence on PFC cue reactivity on exposure to drug, drug-related, or non-drug–related cues are limited. The evidence that methamphetamine may increase sexual desire and heighten sexual rewards (Rawson et al., 2002; Frohmader et al., 2010a) may be contrary to the iRISA model. Moreover, although sexual pleasure and orgasm may be significantly impaired after chronic drug use and abstinence, sexual desire and arousal may not be similarly impacted (Vallejo‐Medina and Sierra, 2013). Thus, investigating how individuals with methamphetamine dependence respond to sexual cues at various stages of abstinence is important from theoretical and clinical perspectives.
Changes in brain systems associated with chronic methamphetamine use may be difficult to reverse merely through drug termination (Volkow et al., 2016). Vulnerabilities to relapse may persist in relationship to drug cues or drug-use motivations (e.g., to enhance sexual pleasure and performance) after years of abstinence (Kalivas and Volkow, 2005). Furthermore, self-reported impulsivity may increase when abstaining from methamphetamine, potentially promoting relapse (Jones et al., 2016). While some drug-related brain alterations may be reversed or partially recovered with increasing abstinence (e.g., longer term abstinence [12–17 months] vs shorter term abstinence [<6 months] (Volkow et al., 2001; Wang et al., 2004), understanding neural responses at varying stages of abstinence may provide insight into potential risks for relapse or engagement in other risky behaviors (Volkow et al., 2001). However, human studies investigating functional responses to cues relating to drug or sexual cues at various stages of abstinence in chronic methamphetamine dependence are lacking.
In this investigation, we employed a functional magnetic resonance imaging (fMRI) cue-reactivity task involving methamphetamine, sexual, and neutral cues to examine neural differences between 2 groups of men with varying degrees of methamphetamine abstinence (shorter term methamphetamine abstinence [STMA] and longer term methamphetamine abstinence [LTMA]) and relative to a group of healthy comparison (HC) men. We investigated 3 primary hypotheses. First, consistent with addiction relapse theories, we hypothesized that when exposed to methamphetamine cues, both STMA and LTMA groups would exhibit increased activation in the ventral striatum, dorsal striatum, vmPFC, and anterior insula compared with HC participants. Second, consistent with evidence of increased sexual motivations associated with methamphetamine use, we hypothesized that both STMA and LTMA groups relative to HC participants would exhibit relatively increased activation in the ventral striatum, dorsal striatum, vmPFC, and anterior insula when exposed to sexual cues. Third, if protracted abstinence may be associated with recovery of functioning in these regions, we hypothesized that there was the possibility that the brain activation in the ventral striatum, dorsal striatum, vmPFC, and anterior insula in the LTMA groups would be approximating more close than the brain activation in HC participants when exposed to both methamphetamine and sexual cues and would exhibit relatively decreased activation compared with the STMA group. Subsequent whole-brain analyses were performed to investigate potential functional alterations between groups beyond regions of interest. Finally, as engagement in both stimulant use and risky sexual behaviors has been associated with impulsivity (Leeman and Potenza, 2012; Leeman et al., 2019), we explored relationships between regional activations and baseline methamphetamine use and impulsivity in STMA and LTMA participants.
Methods
Participants
Participants were 146 men aged 19–45 years recruited from Hunan Province, China. Individuals with a period of methamphetamine use more than 18 months before entering treatment and meeting the diagnosis criteria of methamphetamine dependence as determined by the Chinese version of the Structured Clinical Interview for Diagnosis and Statistical Manual of Mental Disorders were divided into 2 subsamples. The LTMA (n = 50) sample was a convenience sample that was recruited from individuals scheduled for release from the Pingtang Isolated Compulsory Drug Rehabilitation Center, and the STMA (n = 49) sample was recruited from the Kangda Voluntary Drug Rehabilitation Center. The HC group (n = 47) was recruited from the local community.
Individuals were excluded for meeting criteria for Structured Clinical Interview for Diagnosis and Statistical Manual of Mental Disorders dependence on a substance other than nicotine (or for the methamphetamine groups, methamphetamine); the presence or history of a psychiatric disorder or general medical illness (e.g., cardiovascular disease, high blood pressure); a history of brain injury or loss consciousness of 10 minutes or more; left-handedness; and any contraindications of MRI scanning. All participants reported not having consumed alcohol or any other psychoactive substances for at least 48 hours before scanning, with corroborating information obtained from the doctors caring for the LTMA and STMA participants (as they were inpatients).
The final participant sample of 146 individuals represents a total enrollment of 162 males, 155 of whom completed fMRI procedures, and the exclusion of 9 individuals for excessive head motion. A portion of the LTMA (n = 28) and HC (n = 27) was included in a previous report (Huang et al., 2018). All participants completed the Barratt Impulsiveness Scale (Chinese version; Patton et al., 1995; Li et al., 2011). This study was approved by the ethical review board of the Second Xiangya Hospital of Central South University, which evaluated the study specifically related to participation of incarcerated individuals and conditions for use of incarcerated individuals in research were met. Participants from the compulsory and voluntary drug rehabilitation centers could decline involvement in the study if they had any concerns, and all participants provided voluntary written informed consent.
Cue-Reactivity Task
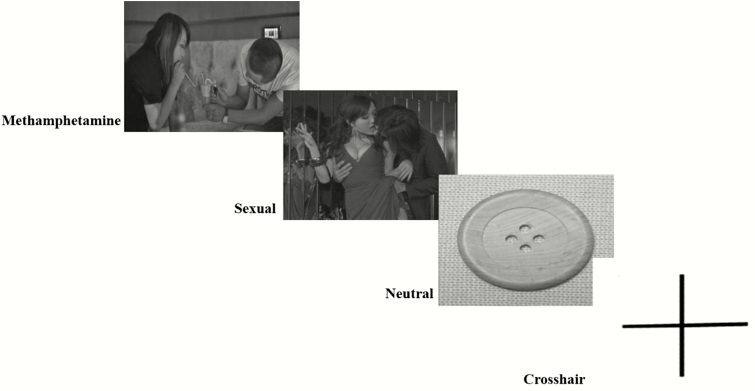
The details for image selection and task design have been described previously (Huang et al., 2018). Briefly, the 450-second cue-reactivity task consisted of 6 epochs, each containing three 20-second blocks (methamphetamine, sexual, and neutral images; Figure 1) and one 15-second rest (crosshair). The methamphetamine cue-related images were the images of methamphetamine itself, people who were smoking methamphetamine, or the instruments they used to smoke methamphetamine. Each block contained 5 unique images presented for 3 seconds with a 1-second inter-stimulus interval. Thus, a total 30 images of each cue condition (i.e., methamphetamine, sexual, and neutral) were presented during the fMRI scan. The order of the images and the blocks within epochs were all presented pseudo-randomly to control for order effects across participants (George et al., 2001; Myrick et al., 2004).
Figure 1.
Images of representative methamphetamine, sexual, and neutral cues, and the crosshair of the cue-reactivity task.
fMRI Acquisition and Analysis
Images were obtained using a 3.0-T Siemens scanner (Allegra; Siemens Medical System, Erlangen, Germany) equipped with a standard 20-channel head coil. Blood oxygen-level–dependent data were acquired with an echo-planar imaging sequence: repetition time (TR) = 2000 milliseconds, echo time (TE) = 30 milliseconds, flip = 80°, field of view = 220 × 220 mm, voxel size = 3.4 × 3.4 × 4.0 mm, slice thickness = 4 mm, gap = 1 mm, number of slices = 36 (Huang et al., 2018). The first 5 volumes (10 seconds) were removed from analyses to enable the signal to achieve steady-state equilibrium. Image analysis was performed using the Statistical Parametric Mapping 12 software package (SPM12, http://www.fil.ion.ucl.ac.uk/spm/). Functional images were realigned to the first volume of each session individually, normalized spatially into Montreal Neurological Institute template standardized space (resampling into 3 × 3 × 3 mm3 voxels), and smoothed with an 8-mm full-width-at-half-maximum Gaussian filter. Participants (n = 9) were excluded from further analyses due to head motion in excess of 3 mm and/or 3°.
A general linear model was used to conduct a functional data analysis. First-level models included each series of five 3-second pictures (1-second inter-stimulus interval) modeled as 20-second blocks by condition (methamphetamine, sexual, and neutral) and the 6 motion parameters as regressors. Given that prior findings showed no lateralized effects in cue reactivity in these regions (Völlm et al., 2004; Huang et al., 2018), bilateral region-of-interest (ROI) was used in ROI analyses. Bilateral ROIs were created using labeled regions of the Neuromorphometrics atlas (www.neuromorphometrics.com) for the ventral striatum using the accumbens area, for the dorsal striatum using the putamen and the caudate nucleus, for the vmPFC using the medial frontal cortex, and for the anterior insula using the anterior insula (supplemental Figure 1). For whole-brain analyses, a cluster-level familywise-error correction threshold of P < .05 was applied to the voxel-level uncorrected threshold of P < .01. Individual average parameter estimates within ROIs and whole brain–identified clusters were extracted for statistical analyses.
Statistical Analysis
Group-level ROI and whole-brain analyses were performed using 3 × 3 mixed-effects ANOVA with group (STMA, LTMA, HC) as a between-subjects factor, condition (methamphetamine, sexual, and neutral) as a within-subjects factor, and subject as a random-effects factor (McFarquhar, 2018). ROI analyses and post-hoc testing of whole-brain findings were conducted with R (R Development Core Team, 2018). Post-hoc t tests were applied at a regional level to further investigate the nature of findings in regions demonstrating a main effect of group or an interaction of group by condition. One-way ANOVAs were performed to examine group differences within condition, and paired-samples t tests were performed in each group to assess condition effects. Subsequent analyses including age, education, tobacco use, and alcohol use as covariates were performed. Relationships between brain activation and baseline methamphetamine use (i.e., duration [months] of methamphetamine use and dosage [grams] of methamphetamine use) and impulsivity scores were examined using Pearson correlations with a significance threshold of P < .05. A Bonferroni-correction for multiple comparisons threshold of P < (.05/4 = .0125) was applied.
Results
Participant Characteristics
Participant characteristics were summarized (Table 1). All STMA and LTMA participants used methamphetamine by smoking the drug. STMA were younger than HC participants (P = .006). STMA participants had the highest education (P < .001), and HC participants reported more education than did LTMA participants (P = .027). The duration of methamphetamine abstinence ranged from 1 to 3 months for the STMA group and from 16 to 40 months for the LTMA group. The 3 groups did not differ in proportions reporting smoking, but HC participants were the least likely to report drinking alcohol among the 3 groups (P = .001).
Table 1.
Participant characteristics
| Variable | HC | STMA | LTMA | F/χ 2 | df | P |
|---|---|---|---|---|---|---|
| n | 47 | 49 | 50 | – | – | – |
| Age, y (SD) | 34.19 (7.39) | 30.06 (5.51) | 32.54 (6.33) | 5.02 | 2, 143 | .008 |
| Education, y (SD) | 10.06 (2.33) | 11.90 (2.86) | 8.72 (2.25) | 20.22 | 2, 143 | <.001 |
| Duration of MA abstinence, mo (SD) | – | 1.65 (0.84) | 19.58 (3.89) | −31.88 | 1, 97 | <.001 |
| Age of first MA use, y (SD) | – | 24.59 (5.25) | 25.44 (6.64) | −0.70 | 1, 97 | .483 |
| Duration of MA use, mo (SD) | – | 66.12 (39.73) | 61.64 (32.60) | 0.61 | 1, 97 | .541 |
| Tobacco use, N (%) | 41 (87.23) | 42 (85.71) | 49 (98.00) | 5.12 | 2 | .077 |
| Alcohol use, N (%) | 7 (14.89) | 24 (48.98) | 22 (44.00) | 13.92 | 2 | .001 |
| Attentionala, (SD) | 38.00 (12.89) | 42.30 (16.49) | 51.45 (18.59) | 8.76 | 2143 | <.001 |
| Motora, (SD) | 29.09 (15.72) | 39.59 (16.51) | 41.95 (18.18) | 7.91 | 2143 | .001 |
| Nonplanninga, (SD) | 35.63 (13.76) | 49.80 (17.87) | 63.80 (16.68) | 36.50 | 2143 | <.001 |
| Impulsivitya, mean (SD) | 34.24 (8.96) | 43.86 (14.14) | 52.40 (14.68) | 23.94 | 2143 | <.001 |
Abbreviations: HC, healthy comparison; LTMA, longer term methamphetamine abstinence; STMA, shorter-term methamphetamine abstinence; F, F-distribution; df, degrees of freedom.
aSubscale and total scores reflect validated transformation of Chinese version of Barratt Impulsiveness Scale-11 onto a 100-point scale (Li et al., 2011).
Results
Methamphetamine Cue–Related Activation
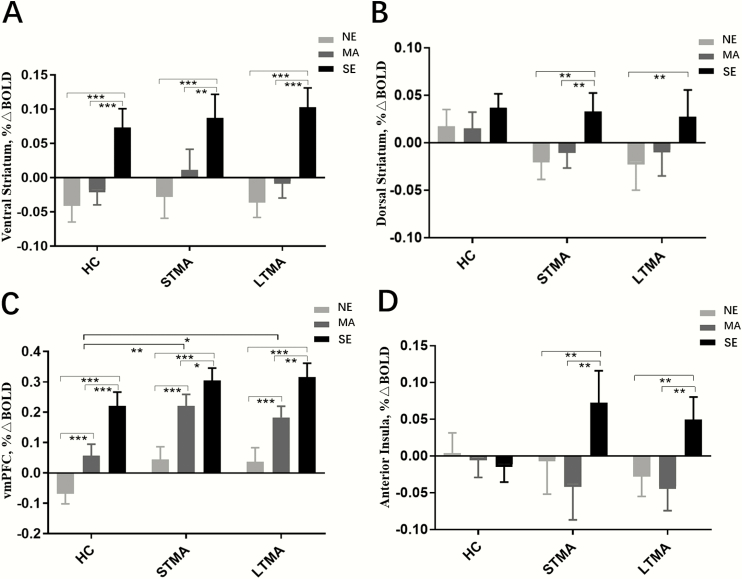
There were no group differences or within-subject effects or group-by-condition interaction of methamphetamine cues relative to neutral cues in the ventral striatum, dorsal striatum, and anterior insula (Figure 2A, B, D). ROI analyses revealed a group difference in methamphetamine cue–related activation in the vmPFC (Figure 2C). The STMA relative to the HC group showed greater methamphetamine cue–related vmPFC activation (P = .003). The LTMA relative to the HC group showed greater methamphetamine cue–related vmPFC activation (P = .020), though this did not survive multiple-comparisons correction. The scatterplot and linear relationships between methamphetamine abstinence duration and methamphetamine cue–related vmPFC activations are shown in supplemental Figure 2 for STMA and LTMA separately. Within each group, methamphetamine cue–related vmPFC activation was greater than neutral cue–related activation (P < .001). The group difference of methamphetamine cue–related vmPFC activation survived inclusion of covariates for tobacco use, alcohol use, age, and education (P = .024). Age and education were mean-centered within groups to adjust for group differences, and there was a negative main effect of age on vmPFC activation in response to methamphetamine cues (beta = −0.244, P = .004), and no effects of tobacco use, alcohol use, or education were found (P > .1). No group-by-condition interaction of methamphetamine cues relative to neutral cues in the vmPFC was found. Whole-brain analyses did not identify additional regions with significant methamphetamine cue–related group or condition effects on brain activation.
Figure 2.
Average regional blood oxygen-level–dependent signal differences between and within groups in the ventral striatum (A), dorsal striatum (B), ventromedial prefrontal cortex (vmPFC) (C), and anterior insula (D). Error bars indicate standard error. HC, healthy comparison; LTMA, longer-term methamphetamine abstinence participants; MA, methamphetamine cue; NE, neutral cue; SE, sexual cue; STMA, shorter term methamphetamine abstinence participants. *P < .05, **P < .01, ***P < .001.
Sexual Cue–Related Activation
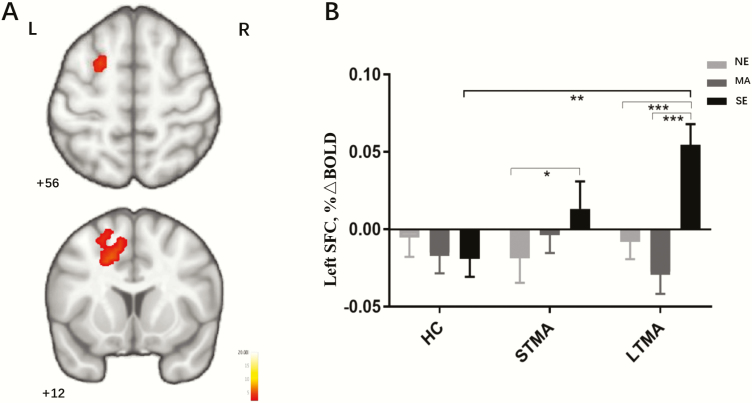
There were no group differences or group-by-condition interactions in sexual cue–related activation in the ventral striatum, dorsal striatum, or vmPFC. Within groups, sexual cue–related positive activation was greater than neutral and/or methamphetamine cue–related activation in the ventral striatum, dorsal striatum, and vmPFC (Figure 2A–C). ROI analyses revealed a significant interaction of group and condition in the anterior insula (Figure 2D). Relative to HC participants, both STMA and LTMA groups displayed greater sexual cue–related anterior insula activation relative to methamphetamine cues (STMA vs HC: P = .004; LTMA vs HC: P = .016) and neutral cues (STMA vs HC: P = .009; LTMA vs HC: P = .010), and there were no differences between the STMA and LTMA groups in anterior insula responses (P > .5). Whole-brain analysis identified a significant interaction of group and condition on activation in the left superior frontal cortex (SFC) (peak F = 7.14; peak x, y, z coordinates = −27, −22, 32; Brodmann area 6; cluster size 305, pFWE = 0.042) (Figure 3) that was associated with sexual cues. STMA participants showed greater sexual cue–related SFC activation relative to neutral cue–related negative activity (P < .05). LTMA participants showed greater sexual cue–related SFC activation relative to neutral and methamphetamine cue–related deactivation (P < .001). Post-hoc pairwise comparisons revealed LTMA sexual cue–related SFC activation was greater than that in HC participants (P < .001) (Figure 3B). The scatterplot and linear relationships between methamphetamine abstinence duration and sexual cue–related anterior insula activations, methamphetamine abstinence duration, and sexual cue–related SFC activations are shown in supplemental Figure 3 and supplemental Figure 4 for STMA and LTMA separately. The significant interaction of group by condition in the anterior insula survived inclusion of covariates for tobacco use, alcohol use, age, and education (P = .007), the group difference of sexual cue-related SFC activation also survived inclusion of covariates for tobacco use, alcohol use, age, and education (P = .004). Age and education were mean-centered within groups to adjust for group differences, and there were no effects of tobacco use, alcohol use, age, or education on group by condition interaction of anterior insula, or SFC in response to sexual cues (P > .05).
Figure 3.
Interaction of group and condition in the left SFC (A). Average regional blood oxygen-level–dependent signal differences between and within groups in the left SFC are shown in (B). Error bars indicate standard error. HC, healthy controls; LTMA, longer term methamphetamine abstinence participants; MA, methamphetamine cue; NE, neutral cue; SE, sexual cue; STMA, shorter term methamphetamine abstinence participants. *P < .05, **P < .01, ***P < .001.
Relationships Between Methamphetamine Use, Impulsivity, and Regional Brain Activations
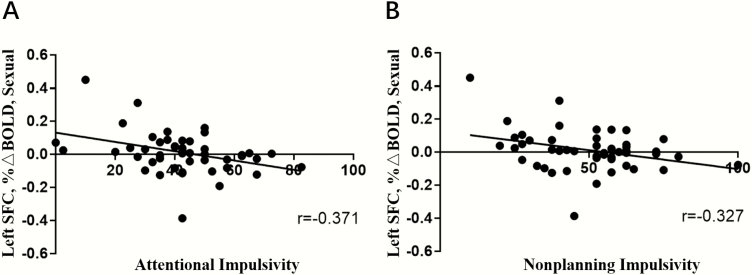
LTMA participants reported greater impulsivity relative to HC and STMA participants (P = .001), and STMA participants reported greater impulsivity relative to HC participants (P < .001). In STMA participants, attentional impulsivity negatively correlated with sexual cue–related SFC activation (r = −0.371, P = .009) (Figure 4A) and methamphetamine cue–related SFC activation (r = −0.340, P = .017) (supplemental Figure 5a), nonplanning impulsivity negatively correlated with sexual cue–related SFC activation (r = −0.327, P = .022) (Figure 4B) and methamphetamine cue–related SFC activation (r = −0.308, P = .031) (supplemental Figure 5b), though these findings did not survive Bonferroni correction. There were no other relationships between activation in ROI- or whole brain–identified clusters and impulsivity or baseline methamphetamine use.
Figure 4.
Scatterplot demonstrating in the shorter term methamphetamine abstinence (STMA) group a negative correlation between attentional impulsivity and activation in the left superior frontal cortex (SFC) relative to sexual cues (A), a negative correlation between nonplanning impulsivity and activation in the left SFC relative to sexual cues (B).
All significant findings persisted in subsequent analyses excluding 1 LTMA participant with 40 months of abstinence.
Discussion
The present study used a cue-reactivity task involving neutral, methamphetamine, and sexual cues to investigate neurofunctional changes associated with shorter and longer term methamphetamine abstinence. To our knowledge, this is the first study to compare neural responses with methamphetamine and sexual cues after abstinence of varying durations in a large sample with methamphetamine dependence. When exposed to methamphetamine cues, both STMA and LTMA groups relative to HCs exhibited increased activation in the vmPFC but not the ventral or dorsal striatum or anterior insula. Group by condition interaction showed that compared with HCs, sexual cue–related anterior insula activations were greater relative to methamphetamine cues in STMA and LTMA groups, but no anterior insula response differences between STMA and LTMA groups were found. LTMA participants exhibited increased activation relative to HCs in the left SFC when exposed to sexual cues; there were no differences in cue-induced brain activation between the STMA and LTMA groups. However, there was a pattern that the sexual cue–related brain activations in the STMA group appeared intermediate in magnitude between the HC and LTMA groups in the ventral striatum, more so in the SFC.
In this study, when exposed to methamphetamine cues, both STMA and LTMA relative to HC participants exhibited increased activation in the vmPFC, but not the ventral or dorsal striatum or anterior insula, which is partially consistent with our first hypothesis. Previous research has demonstrated an important role of the vmPFC in encoding expected value representations, including greater responses when experiencing greater rewards (Blair et al., 2006; Gläscher et al., 2009). In patients addicted to methamphetamine, activation of the vmPFC in response to methamphetamine cues may reflect a dysregulation of this region, demonstrated behaviorally as a preference for smaller immediate over larger delayed rewards (Paulus et al., 2002) or for the drug itself given a role for the vmPFC in stimulant craving (Wexler et al., 2001). The vmPFC has also been implicated in the processing and tracking of rewards (Knutson et al., 2003). As such, the STMA and LTMA groups may be demonstrating greater salience at a neural level to drug-related cues after shorter and longer term abstinence, which is consistent with the iRISA model (Goldstein and Volkow, 2011). The findings also suggest a biological mechanism for recovery in methamphetamine dependence (Kalivas and Volkow, 2005) and a need for interventions beyond abstinence (e.g., cognitive behavioral therapies that involve learning new skills and approaches; Carroll and Kiluk, 2017).
Mixed findings have been reported regarding the ventral striatum, the dorsal striatum, and the processing of drug rewards in drug-abusing individuals, with some studies showing hypoactivity during drug cue exposure (Vollstädt‐ Klein et al., 2010), others showing hyperactivity (Prisciandaro et al., 2014). One possible explanation for seemingly conflicting results may relate to different stages of addiction and abstinence. The ventral striatum has been implicated in mediating reinforcing effects of stimulant drugs and neural mechanisms of voluntary drug taking (Wise, 2004); however, the dorsal striatum has been implicated in compulsive drug seeking (Everitt and Robbins, 2013). How different stages of abstinence may be linked to differences in striatal correlates of reward processing and how such neural correlates of reward processing may in turn impact abstinence warrant further investigation.
Anterior insula activations in response to sexual cues were greater relative to methamphetamine cues and neutral cues in the STMA and LTMA groups compared with HCs in our study, which is consistent with our second hypothesis. Insula activation in response to sexual stimuli has been reported both in non-drug–using and drug-using individuals (Garavan et al., 2000; Safron et al., 2007). Our study also found a relationship between anterior insula activation and sexual stimuli. Although the anterior insula may be involved in drug craving and drug seeking under specific conditions, our findings suggest that the anterior insula may also contribute importantly to interoception for specifically survival-related functions, such as sex, hunger, and thirst (Garavan, 2010; Naqvi et al., 2014), but this warrants further investigation.
Contrary to hypotheses, there were no group differences in activation in the ventral striatum, dorsal striatum, and vmPFC when exposed to sexual cues. However, within groups, sexual cue–related activation was greater than neutral and/or methamphetamine cue–related activation in the ventral striatum, dorsal striatum, and vmPFC. The greater activation to sexual cues in these 3 regions suggests that chronic methamphetamine use may not be associated with blunted sexual reward processing, which is in seeming contrast to the iRISA and reward deficiency models of addiction (Goldstein and Volkow, 2011; Volkow et al., 2016) but is consistent with animal research indicating that chronic methamphetamine exposure impairs sexual performance but not sexual conditioning (Bolin and Akins, 2012). However, more research is needed to determine if these sexual cue–related neural responses are a consequence of methamphetamine abstinence (i.e., sexual-reward sensitivity has replaced methamphetamine reward sensitivity after abstaining from methamphetamine) or if these alterations are also present in individuals currently abusing methamphetamine.
A whole-brain group-by-condition interaction and post-hoc contrasts indicated that LTMA relative to HC participants showed greater activation of the left SFC in response to sexual cues. The SFC has been implicated in attentional control (Hopfinger et al., 2000; Fan et al., 2005) and in memory and decision-making processes relevant to goal-directed behaviors (Rushworth et al., 2004; Rose et al., 2011). As such, the relatively increased SFC activation may reflect increased attention or attentional control in LTMA in response to the sexual stimuli, although this possibility remains speculative. Given that methamphetamine dependence has been associated with cognitive deficits relating to attention (Salo et al., 2009; Potvin et al., 2018) and attentional deficits have been linked to sexual risk-taking (Isaksson et al., 2018), it will be important to examine further how such features may relate to sexual behaviors among individuals with LTMA.
Within groups, the greater activation in response to sexual cues in the ventral striatum, dorsal striatum, vmPFC, anterior insula, and left SFC in both STMA and LTMA groups may provide insight into the use of methamphetamine for sexual purposes in individuals with methamphetamine dependence. Methamphetamine use can increase sexual libido, pleasure, and stamina while increasing risk-taking behavior, such as unprotected sexual intercourse and sex with multiple partners (Piyaraj et al., 2018). Understanding how methamphetamine use, and extended abstinence from methamphetamine, may alter responses to sexual cues may provide insight into this population and inform interventions.
There was a significant negative correlation between attentional impulsivity and left SFC activation to sexual cues in the STMA group, but not among LTMA participants. The findings suggest a complex relationship between SFC activation to methamphetamine and sexual cues, impulsivity, and duration of methamphetamine abstinence among individuals with methamphetamine dependence. Dysfunction of the PFC has been implicated in poor inhibitory control in addictions (Goldstein and Volkow, 2011), and individuals with SFC damage show poor inhibitory control (Floden and Stuss, 2006). Speculatively, after longer term abstinence, brain function may have partially recovered from impacts of methamphetamine, but shorter term abstinence may not be sufficient for such effects. Previous studies in attention-deficit/hyperactivity disorder (ADHD) (Schneider et al., 2010) and healthy individuals (Horn et al., 2003) have also reported negative correlations between impulsivity and neural activations, which may suggest a “trait-like” characteristic across different groups. However, impulsivity as assessed here may also reflect a self-perception of one’s tendencies that may be sensitive to stage of abstinence from methamphetamine. As such, given stimulant use in ADHD groups (Wilens et al., 2007; Mitchell et al., 2016), further research is needed to examine whether the current findings may have implications for the understanding of the neurobiology and clinical features of ADHD, especially with respect to stimulant use and sexual behaviors.
The current findings suggest that brain function may not fully change simply through drug abstinence (Volkow et al., 2016). Some behavioral and medication interventions in treating drug addictions have focused on reducing cue-elicited craving (Taylor et al., 2009; Vollstädt-Klein et al., 2011; Park et al., 2015); however, interventions are not uniformly successful (Conklin and Tiffany, 2002). A recent study suggests that training in selective attentional processing of natural rewards over drug rewards may help alter reward processing networks to be more responsive to natural rewards and reduce drug-related motivations and relapse (Garland et al., 2014). However, the current findings suggest that individuals in remission from methamphetamine dependence may exhibit heightened responses to sexual cues, and the extent to which such training may influence responses to sexual rewards warrants consideration and research.
In the present study, there were no differences in cue-induced brain activation between the STMA and LTMA groups when exposed to both methamphetamine and sexual cues. However, the magnitudes of responses suggested that the brain activation to sexual cues in the STMA group was intermediate (albeit not in a statistically significant fashion) between the HC and LTMA groups in the ventral striatum and the SFC, but not in the dorsal striatum, vmPFC, or anterior insula. The ventral striatum has been found to activate in response to methamphetamine in drug-naïve humans (Völlm et al., 2004) and has shown greater activation in individuals with current methamphetamine dependence compared with those without when performing the Balloon Analogue Risk Task (Kohno et al., 2014). However, the ventral striatum was not implicated in methamphetamine cue responsiveness across groups, although responses to sexual cues were linked to ventral striatal activation. The extent to which these findings may relate to functional recovery from drug dependence following extended abstinence (i.e., up to 40 months) warrants investigation in longitudinal studies (Balodis et al., 2016). Protracted abstinence from methamphetamine may reverse some drug-induced alterations in brain function (Volkow et al., 2001; Wang et al., 2004), and this may explain some findings in the LTMA group relative to the STMA group, although this too warrants additional investigation.
Several limitations of the present study should be noted. First, this was a cross-sectional study. Preexisting differences in neural functioning in the LTMA and STMA groups may have been present; hence, future longitudinal studies are indicated. Second, STMA participants were not well matched on age and education to the LTMA or HC participants, as STMA participants tended to be younger and have more education. HC participants were not well matched on alcohol use to the STMA or LTMA participants, as HC participants were the least likely to report drinking alcohol among the 3 groups. However, when including age, education, and alcohol use as covariates, the main findings persisted. Third, STMA and LTMA participants were recruited from voluntary and compulsory drug rehabilitation centers, respectively. As such, differences in motivations for treatment and the abstention from sexual activities may have influenced neural responses to methamphetamine and sexual cues. Hence, future studies should gather information on motivations for treatment and other domains that may differ in voluntary vs compulsory treatment programs. Fourth, due to a limited number of methamphetamine-dependent females completing study procedures (n = 3), the current analyses were limited to males, and it will be important to examine neurofunctioning in women with methamphetamine dependence in future studies given gender-related differences noted in studies of stimulant dependence (Potenza et al., 2012; Kober et al., 2016). Fifth, we did not evaluate participants’ craving or other subjective responses to the sexual and methamphetamine cues, such as their use of methamphetamine for sexual purposes. This may have been relevant to the current study in that SFC activation has been related to cravings for drug and responses to drug-related cues (McClernon et al., 2005; Rose et al., 2011). Sixth, we did not test the lateralized effects in the ROI analyses; further study could consider exploring each region for effects of laterality. Seventh, the whole-brain analysis was performed without covariates to adjust for group differences in age, education, and alcohol use. It is possible that analysis incorporating these covariates may have resulted in an altered pattern of regional group differences. Another limitation was the absence of the use of urine screening tests to confirm absence of psychoactive drug use in all participants.
Duration of abstinence may impact responses to sexual cues more so than to methamphetamine cues in individuals with methamphetamine dependence. Greater vmPFC activation to methamphetamine cues was observed in both the STMA and LTMA groups relative to HC participants, but our approach identified no differences in methamphetamine cue–related activation between STMA and LTMA groups. Greater anterior insula activations in response to sexual cues relative to methamphetamine cues and neutral cues in STMA and LTMA groups compared with HCs were observed. Greater SFC activation to sexual cues was observed in LTMA but not STMA participants compared with HCs. Activation in the ventral striatum, dorsal striatum, vmPFC, anterior insula, and SFC in response to sexual cues was greater than responses to neutral and/or methamphetamine cues both in STMA and LTMA participants. These findings may have clinical relevance given the use of methamphetamine for sexual purposes and the relevance of neural and behavioral responses to natural vs drug rewards for addiction recovery. Future studies investigating individuals at multiple stages of addiction and abstinence could help clarify functional alterations that may represent preexisting features, consequences of chronic methamphetamine exposure, and recovery from methamphetamine dependence.
Supplementary Material
Acknowledgments
Shubao Chen, Shucai Huang, Xuyi Wang, Tieqiao Liu, Wei Hao, and Hongxian Chen conceptualized and designed the research; Shubao Chen and Shucai Huang collected most of the data, Cheng Yang and Weifu Cai helped with data collection; Shubao Chen and Patrick D. Worhunsky performed image data processing and analyses; Xuyi Wang, Tieqiao Liu, Patrick D. Worhunsky, and Marc N. Potenza assisted with interpretation of findings; Shubao Chen drafted the manuscript; Marc N. Potenza and Patrick D. Worhunsky worked with Shubao Chen on editing the manuscript. All authors approved the final version of the manuscript submitted for publication.
This work was supported by National Key R and D Program of China (no. 2017YFC1310400), National Natural Science Foundation of China (no. 81571307, no. 81371465 and no. 81671324), Fundamental Research Funds for Central Universities of the Central South University (no. 2016zzts148), National Basic Research Program of China (973 Program) (no. 2015CB553504), National Key R and D Program of China (no. 2016YFC0800908-Z02), and the National Institutes of Health (K01 DA042998, R01 DA039136).
Statement of Interest
The authors declare no conflicts of interest with the content of the manuscript. Dr Potenza has consulted for Rivermend Health, Opiant/Lightlake Therapeutics, Addiction Policy Forum, Jazz Pharmaceuticals, and Game Day Data, LLC; has received research support from the Mohegan Sun Casino and the National Center for Responsible Gaming; and has consulted for and/or advised gambling and legal entities on issues related to impulse-control/addictive disorders.
References
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, Potenza MN (2016) Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology 41:2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW (2000) Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol 18:355–381. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE (2001) Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39:376–389. [DOI] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair JR (2006) Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci 26:11379–11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin BL, Akins CK (2012) Chronic pre-exposure to methamphetamine following 31 days of withdrawal impairs sexual performance but not sexual conditioning in male Japanese quail. Behav Processes 91:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma-Bleeker MH, Blaauw E (2018) Substance use disorders and sexual behavior; the effects of alcohol and drugs on patients’ sexual thoughts, feelings and behavior. Addict Behav 87:231–237. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD (2017) Cognitive behavioral interventions for alcohol and drug use disorders: through the stage model and back again. Psychol Addict Behav 31:847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167. [DOI] [PubMed] [Google Scholar]
- Daniel R, Pollmann S (2014) A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem 114:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW (1999) Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci 877:412–438. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2013) From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37:1946–1954. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. Neuroimage 26:471–479. [DOI] [PubMed] [Google Scholar]
- Floden D, Stuss DT (2006) Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci 18:1843–1849. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, Pitchers KK, Balfour ME, Coolen LM (2010a) Mixing pleasures: review of the effects of drugs on sex behavior in humans and animal models. Horm Behav 58:149–162. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, Wiskerke J, Wise RA, Lehman MN, Coolen LM (2010b) Methamphetamine acts on subpopulations of neurons regulating sexual behavior in male rats. Neuroscience 166:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohmader KS, Lehman MN, Laviolette SR, Coolen LM (2011) Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci 31:16473–16482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch D, Shoptaw S, Huber A, Rawson RA, Ling W (1996) Sexual HIV risk among gay and bisexual male methamphetamine abusers. J Subst Abuse Treat 13:483–486. [DOI] [PubMed] [Google Scholar]
- Garavan H. (2010) Insula and drug cravings. Brain Struct Funct 214:593–601. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. [DOI] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO (2014) Effects of mindfulness-oriented recovery enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 231:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ (2001) Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 58:345–352. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Hampton AN, O’Doherty JP (2009) Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb Cortex 19:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcott PK, Quinn JJ, Taylor JR (2007) Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex 17:2820–2827. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000) The neural mechanisms of top-down attentional control. Nat Neurosci 3:284–291. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW (2003) Response inhibition and impulsivity: an fMRI study. Neuropsychologia 41:1959–1966. [DOI] [PubMed] [Google Scholar]
- Huang S, Zhang Z, Dai Y, Zhang C, Yang C, Fan L, Liu J, Hao W, Chen H (2018) Craving responses to methamphetamine and sexual visual cues in individuals with methamphetamine use disorder after long-term drug rehabilitation. Front Psychiatry 9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson J, Stickley A, Koposov R, Ruchkin V (2018) The danger of being inattentive - ADHD symptoms and risky sexual behaviour in Russian adolescents. Eur Psychiatry 47:42–48. [DOI] [PubMed] [Google Scholar]
- Janes AC, Frederick Bd, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ (2009) Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol 17:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ (2010) Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HW, Dean AC, Price KA, London ED (2016) Increased self-reported impulsivity in methamphetamine users maintaining drug abstinence. Am J Drug Alcohol Abuse 42:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D (2003) A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18:263–272. [DOI] [PubMed] [Google Scholar]
- Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN (2016) Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology 41:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED (2014) Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry 71:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN (2012) Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (Berl) 219:469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Rowland BHP, Gebru NM, Potenza MN (2019) Relationships among impulsive, addictive and sexual tendencies and behaviours: a systematic review of experimental and prospective studies in humans. Philos Trans R Soc Lond B Biol Sci 374:20180129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Phillips MR, Xu D, Zhang Y, Yang S, Tong Y, Wang Z, Niu Y (2011) Reliability and validity of an adapted Chinese version of Barratt Impulsiveness Scale. Chin Ment Health J 25:610–615. [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE (2005) Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology 30:1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarquhar M. (2018) Modelling group-level repeated measures of neuroimaging data using the univariate general linear model. Front Neurosci 13:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, Sweitzer MM, Tunno AM, Kollins SH, McClernon FJ (2016) “I use weed for my ADHD”: a qualitative analysis of online forum discussions on cannabis use and ADHD. Plos One 11:e0156614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor F, Ruiz JD, Flynn N, Mikanda JN, Sun RK, Anderson R (1999) Methamphetamine use and sexual and injection risk behaviors among out-of-treatment injection drug users. Am J Drug Alcohol Abuse 25:475–493. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS (2004) Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 29:393–402. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A (2014) The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci 1316:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CB, Park SM, Gwak AR, Sohn BK, Lee JY, Jung HY, Choi SW, Kim DJ, Choi JS (2015) The effect of repeated exposure to virtual gambling cues on the urge to gamble. Addict Behav 41:61–64. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA (2002) Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology 26:53–63. [DOI] [PubMed] [Google Scholar]
- Piyaraj P, van Griensven F, Holtz TH, Mock PA, Varangrat A, Wimonsate W, Thienkrua W, Tongtoyai J, McNamara A, Chonwattana W, Nelson KE (2018) The finding of casual sex partners on the internet, methamphetamine use for sexual pleasure, and incidence of HIV infection among men who have sex with men in Bangkok, Thailand: an observational cohort study. Lancet HIV 5:e379–e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004) Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24:3554–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012) Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Pelletier J, Grot S, Hébert C, Barr AM, Lecomte T (2018) Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict Behav 80:154–160. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J, Brady KT (2014) The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction 109:2062–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Rawson RA, Washton A, Domier CP, Reiber C (2002) Drugs and sexual effects: role of drug type and gender. J Subst Abuse Treat 22:103–108. [DOI] [PubMed] [Google Scholar]
- Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD (2011) Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry 70:794–799. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM (2004) Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Safron A, Barch B, Bailey JM, Gitelman DR, Parrish TB, Reber PJ (2007) Neural correlates of sexual arousal in homosexual and heterosexual men. Behav Neurosci 121:237–248. [DOI] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C (2009) Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry 65:706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MF, Krick CM, Retz W, Hengesch G, Retz-Junginger P, Reith W, Rösler M (2010) Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults - a functional magnetic resonance imaging (fMRI) study. Psychiatry Res 183:75–84. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW (2007) The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 194:321–331. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I (2002) Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat 22:149–156. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Grant I, Patterson TL (2004) Female methamphetamine users: social characteristics and sexual risk behavior. Women Health 40:35–50. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009) Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56(Suppl 1):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo‐Medina P, Sierra JC (2013) Effect of drug use and influence of abstinence on sexual functioning in a Spanish male drug‐dependent sample: a multisite study. J Sex Med 10:333–341. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J (2001) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111:1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic advances from the brain disease model of addiction. N Engl J Med 374:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM (2004) Methamphetamine activates reward circuitry in drug naïve human subjects. Neuropsychopharmacology 29:1715–1722. [DOI] [PubMed] [Google Scholar]
- Vollstädt‐Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K (2010) Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105:1741–1749. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, von der Goltz C, Hermann D, Mann K, Kiefer F (2011) Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry 69:1060–1066. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS (2004) Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 161:242–248. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC (2001) Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158:86–95. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adamson J, Sgambati S, Whitley J, Santry A, Monuteaux MC, Biederman J (2007) Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. Am J Addict 16(Suppl 1):14–21; quiz 22. [DOI] [PubMed] [Google Scholar]
- Wise RA. (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. [DOI] [PubMed] [Google Scholar]
- World Drug Report (2016) In, Sales No. E. 16XI.7. Edition. United Nations Office on Drugs and Crime. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.