Abstract
Background
The genetic etiology of schizophrenia (SCZ) overlaps with that of other major psychiatric disorders in samples of European ancestry. The present study investigated transethnic polygenetic features shared between Japanese SCZ or their unaffected first-degree relatives and European patients with major psychiatric disorders by conducting polygenic risk score (PRS) analyses.
Methods
To calculate PRSs for 5 psychiatric disorders (SCZ, bipolar disorder [BIP], major depressive disorder, autism spectrum disorder, and attention-deficit/hyperactivity disorder) and PRSs differentiating SCZ from BIP, we utilized large-scale European genome-wide association study (GWAS) datasets as discovery samples. PRSs derived from these GWASs were calculated for 335 Japanese target participants [SCZ patients, FRs, and healthy controls (HCs)]. We took these PRSs based on GWASs of European psychiatric disorders and investigated their effect on risk in Japanese SCZ patients and unaffected first-degree relatives.
Results
The PRSs obtained from European SCZ and BIP patients were higher in Japanese SCZ patients than in HCs. Furthermore, PRSs differentiating SCZ patients from European BIP patients were higher in Japanese SCZ patients than in HCs. Interestingly, PRSs related to European autism spectrum disorder were lower in Japanese first-degree relatives than in HCs or SCZ patients. The PRSs of autism spectrum disorder were positively correlated with a young onset age of SCZ.
Conclusions
These findings suggest that polygenic factors related to European SCZ and BIP and the polygenic components differentiating SCZ from BIP can transethnically contribute to SCZ risk in Japanese people. Furthermore, we suggest that reduced levels of an ASD-related genetic factor in unaffected first-degree relatives may help protect against SCZ development.
Keywords: schizophrenia, bipolar disorder, autism spectrum disorder, first-degree relative, polygenic risk score, GWAS
Significance Statement.
Polygenic risk scores for European schizophrenia and bipolar disorder were higher in Japanese patients with schizophrenia than in healthy participants.
Polygenic risk scores differentiating schizophrenia from European bipolar disorder were higher in Japanese schizophrenia patients than in healthy participants.
Polygenic risk scores related to European autism spectrum disorder were lower in Japanese unaffected first-degree relatives of schizophrenia than in healthy participants or schizophrenia patients.
Higher polygenic risk scores for autism spectrum disorder were correlated with earlier age at onset in patients with schizophrenia.
Introduction
Schizophrenia (SCZ) is a common psychiatric disorder with a lifetime morbidity rate of 0.5% to 1.0%. SCZ is a highly heritable disorder, with an estimated heritability of approximately 80% (Sullivan et al., 2003), and the risk of onset of the disorder is increased approximately 10-fold in first-degree relatives (FRs) of SCZ patients (Cardno and Gottesman, 2000; Tsuang, 2000). Large-scale genome-wide association studies (GWASs) by the SCZ Working Group of the Psychiatric Genomics Consortium (PGC) have shown by polygenic risk score (PRS) analysis that the risk of SCZ is mediated by a polygenic component comprising the additive effects of a large number of common single-nucleotide polymorphisms (SNPs) with weak effects (Purcell et al., 2009; Ripke et al., 2014; Pardinas et al., 2018; Taniguchi et al., 2020). Based on the PRS for SCZ in large-scale European discovery GWAS samples, approximately 20% of the variance in SCZ susceptibility could be explained in independent European target samples (Ripke et al., 2014). The size of the discovery sample affects whether the PRS can predict the risk of the disorder in an independent target sample (Dudbridge, 2013; Ohi et al., 2016a). We have further shown that the PRS for SCZ based on the large-scale European discovery GWAS could transethnically explain susceptibility to SCZ in independent Japanese target samples (Ohi et al., 2016a).
The genetic etiology of SCZ overlaps with that of other major psychiatric disorders in samples of European ancestry. The Cross-Disorder Group of the PGC has examined shared polygenetic components among major psychiatric disorders (SCZ, bipolar disorder [BIP], major depressive disorder [MDD], autism spectrum disorder [ASD], and attention-deficit/hyperactivity disorder [ADHD]) in individuals of European descent and found shared common cross-disorder factors among SCZ, BIP, and MDD (Smoller et al., 2013). Of these major psychiatric disorders, common transethnic genetic factors for the risk of SCZ and BIP were revealed between individuals of European and non-European ancestry (Ohi et al., 2016a; Ikeda et al., 2018, 2019). On the other hand, these major psychiatric disorders are distinct diagnoses that have disorder-specific genetic factors. Recently, the BIP and SCZ Working Group of the PGC identified 2 genome-wide significant loci differentiating the 2 disorders in individuals of European descent (Ruderfer et al., 2018). To our knowledge, it is unknown whether the European PRSs differentiating SCZ from BIP can explain SCZ risk in an independent sample comprising individuals of non-European ancestry.
Unaffected FRs exhibit slight impairments in intermediate phenotypes, such as cognitive functions and brain structures/functions that are broadly impaired in patients with SCZ (Green, 2006; Toulopoulou et al., 2010; Hill et al., 2013; Ohi et al., 2017b, 2019a). Therefore, these intermediate phenotypes are useful tools for understanding the genetic mechanisms implicated in the pathophysiology of SCZ. Impairments in these intermediate phenotypes occur in a genetic continuum among SCZ patients, FRs, and healthy controls (HCs) (Ohi et al., 2017b, 2019a). Since unaffected FRs share approximately one-half of the genetic risk for SCZ, an unaffected FR would have one-half the PRS for SCZ. Among participants of European ancestry, PRSs for SCZ and BIP in unaffected FRs were intermediate between those of SCZ patients and HCs (Ranlund et al., 2018; Toulopoulou et al., 2019). Furthermore, childhood-onset SCZ patients had higher PRSs for both SCZ and ASD than their unaffected siblings (Ahn et al., 2016). By identifying a genetic factor specific to unaffected FRs of patients, we may gain an advantage in reducing the incidence of SCZ. However, it is unclear whether PRSs based on GWASs of other psychiatric disorders (MDD, ASD, and ADHD) in addition to SCZ and BIP are related to unaffected FRs of SCZ patients.
We hypothesized that genetic variants differentiating SCZ from BIP in Europeans as well as genetic variants related to psychiatric disorders in Europeans would overlap with genetic risk variants in Japanese SCZ patients and unaffected FRs, that is, individuals at high risk of developing SCZ. We investigated the effects of PRSs based on GWASs of European psychiatric disorders (SCZ, BIP, SCZ vs BIP, MDD, ASD, and ADHD) on risk in Japanese SCZ patients ([1] SCZ vs FRs vs HCs, [2] SCZ vs HCs ,and [3] SCZ vs FRs) or unaffected FRs ([4] FRs vs HCs) by PRS analyses. We used publicly available GWAS datasets as discovery samples to calculate PRSs for 5 psychiatric disorders [SCZ (Ripke et al., 2014), BIP (Stahl et al., 2019), MDD (Wray et al., 2018), ASD (Grove et al., 2019), and ADHD (Demontis et al., 2019)] and a PRS differentiating SCZ from BIP (Ruderfer et al., 2018). PRSs derived from these GWASs were calculated for Japanese target participants (131 patients with SCZ, 57 of their unaffected FRs, and 147 HCs).
Methods
Discovery Samples
Several publicly available GWAS datasets [SCZ (Ripke et al., 2014), BIP (Stahl et al., 2019), SCZ vs BIP (Ruderfer et al., 2018), MDD (Wray et al., 2018), ASD (Grove et al., 2019), and ADHD (Demontis et al., 2019)] through the PGC (https://www.med.unc.edu/pgc/results-and-downloads) were used as discovery samples to identify risk variants for each disorder and their P values and odds ratios (ORs). The sample information and the details regarding the sample collection, genotyping, processing, quality control (QC), and imputation procedures applied in each discovery cohort have been described previously (Ripke et al., 2014; Ruderfer et al., 2018; Wray et al., 2018; Demontis et al., 2019; Grove et al., 2019; Stahl et al., 2019) and briefly summarized in Supplementary Methods. We further provide information regarding the sample sizes (Table 1) and the number of SNPs used for PRS analyses at each liberal significance threshold (PT cutoff) (supplementary Table 1).
Table 1.
Sample sizes for discovery samples
| GWS loci | Cases (n) | Controlsa (n) | ||
|---|---|---|---|---|
| Discovery samples | ||||
| SCZ | Ripke et al. (2014) | 108 | 35 476 | 46 839 |
| BIP | Stahl et al. (2019) | 30 | 20 352 | 31 358 |
| SCZ vs BIP | Ruderfer et al. (2018) | 2 | 23 585b | 15 270c |
| MDD | Wray et al. (2018) | 44 | 59 851 | 113 154 |
| ASD | Grove et al. (2019) | 5 | 18 381 | 27 969 |
| ADHD | Demontis et al. (2019) | 12 | 20 183 | 35 191 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder ASD, autism spectrum disorder; BIP, bipolar disorder; GWS, genome-wide significant; MDD, major depressive disorder; SCZ, schizophrenia.
aControls or pseudocontrols from trio samples.
bSCZ.
cBIP.
Target Sample
The target sample (n = 335) comprised 131 patients with SCZ (50 males/81 females, mean age ± SD: 42.9 ± 13.1 years), 57 of their unaffected FRs (41 parents/12 siblings/4 offspring, 18 males/39 females, 59.7 ± 13.6 years), and 147 HCs (98 males/49 females, 37.2 ± 14.1 years). The study sample was recruited from the Schizophrenia Non-Affected Relative Project at Kanazawa Medical University. All participants were of Japanese descent and had no biological first- or second-degree relatives within their own diagnostic groups. Patients and their unaffected FRs were recruited from Kanazawa Medical University Hospital. Each patient was diagnosed by at least 2 trained psychiatrists on the basis of unstructured clinical interviews, medical records, and clinical conferences (Ohi et al., 2016b; 2017a, 2017b, 2019, 2019a, 2019b; Yasuyama et al., 2017). The patients were diagnosed according to the criteria in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. FRs were evaluated using the Structured Clinical Interview for DSM-IV-Non-Patient version to exclude individuals who had had current or past contact with psychiatric services or had received psychiatric medication. HCs were recruited through local advertisements and among hospital staff at Kanazawa Medical University and were also evaluated using the Structured Clinical Interview for DSM-IV-Non-Patient version to exclude individuals who had had current or past contact with psychiatric services, had received psychiatric medication, or had any family history of neuropsychiatric disease in their first- or second-degree relatives. Written informed consent was obtained from all participants after the procedures had been thoroughly explained. This study was performed in accordance with the World Medical Association’s Declaration of Helsinki and was approved by the Research Ethical Committee of Kanazawa Medical University.
Genotyping
Venous blood was collected from the target participants, and genomic DNA was extracted from whole blood according to standard procedures at Kanazawa Medical University. Genotyping was performed using the Infinium OmniExpressExome-8 v1.4 BeadChip with an iScan system (Illumina, San Diego, CA) according to the manufacturer’s protocol at Tokyo Metropolitan Institute of Medical Science. The genotype data were analyzed using GenomeStudio 2.0 with the Genotyping module v2.0 (Illumina) to evaluate the QC. In the data-cleaning process (Nishizawa et al., 2014), 1 sample with a poor genotype call rate of <0.95 (HC, n = 1) was excluded from further analyses. SNPs with a low genotype call frequency of <0.95 or a “Cluster sep” (an index of genotype cluster separation) of <0.1 were excluded from the further analyses. We then applied the following QC criteria to exclude samples: (1) sex chromosome anomalies (SCZ, n = 1) and sample relatedness in each diagnostic group (pi-hat > 0.2) (FR, n = 1) using PLINK v1.9. Finally, 332 individuals, comprising 130 SCZ patients, 56 FRs, and 146 HCs, were included in this study. The demographic information of the 3 diagnostic groups (SCZ, FR, and HC) is summarized in Table 2. Next, we excluded SNPs that (1) were duplicated or ambiguous, (2) were localized on the Y chromosome or mitochondria, (3) deviated from Hardy-Weinberg equilibrium (P < 1.0 × 10–5), or (4) had a low minor allele frequency < 0.001. After all of these exclusions, 627 789 SNPs were retained.
Table 2.
Demographic information for target sample
| HC | FR | SCZ | ||
|---|---|---|---|---|
| Variables | (n = 146) | (n = 56) | (n = 130) | P values (F or χ2) |
| Age (y) | 37.2 ± 14.1 | 56.8 ± 15.6 | 42.9 ± 13.1 | 2.49 × 10 –21 (55.0) |
| Gender (male/female) | 97/49 | 18/38 | 50/80 | 3.22 × 10 –7 (30.0) a |
| Education (y) | 16.1 ± 2.4 | 12.8 ± 2.1 | 12.6 ± 2.2 | 2.05 × 10 –32 (91.8) |
| Estimated premorbid IQ | 108.5 ± 7.7 | 99.7 ± 9.1 | 98.7 ± 10.4 | 5.38 × 10 –18 (45.0) |
| CPZ-eq. (mg/d) | 0 | 0 | 509.6 ± 512.7 | – |
| Age at onset (y) | – | – | 26.9 ± 10.6 | – |
| DOI (y) | – | – | 15.8 ± 11.3 | – |
| PANSS positive symptoms | – | – | 16.0 ± 6.2 | – |
| PANSS negative symptoms | – | – | 17.8 ± 6.8 | – |
Abbreviations: CPZ-eq., chlorpromazine equivalent of total antipsychotics; DOI, duration of illness; FR, first-degree relative; HC, healthy controls; PANSS, Positive and Negative Syndrome Scale; SCZ, schizophrenia. The mean ± SD and P values are shown. Complete demographic information was not obtained for all participants (estimated premorbid IQ in HCs, n = 145). Continuous variables were analyzed using parametric ANOVA. The difference in categorical variables was analyzed using Pearson’s χ2 test.
a χ 2 test.
To test for the existence of genetic structure in the data, we performed a principal component analysis, and the first 10 principal components were calculated using PLINK. Genotype information from the JPT (Japanese in Tokyo, Japan), CHB (Han Chinese in Beijing, China), CEU (Utah residents with ancestors from Northern and Western Europe), and YRI (Yoruba in Ibadan, Nigeria) samples in HapMap 3 (http://hapmap.ncbi.nlm.nih.gov/downloads/genotypes/2010-05_phaseIII/) was compared with our dataset to check for population stratification (supplementary Figure 1). We confirmed that the principal components in our target samples (SCZ, FRs, and HCs) were located on those in the JPT.
Imputation
Genotype imputation was performed using the pre-phasing/imputation stepwise approach implemented in IMPUTE2/SHAPEIT (chunk size of 2 Mb and default parameters). To incorporate pedigree information into the haplotype estimates, we utilized the software package duoHMM, implemented in SHAPEIT (O’Connell et al., 2014). Genotype imputation was performed using the 1000 Genomes Project Phase 3 dataset [https://mathgen.stats.ox.ac.uk/impute/1000GP_Phase3.html (Auton et al., 2015)] as a reference panel. X chromosome imputation was conducted for participants passing QC for the autosomal analysis. For PRS analysis, to obtain a highly informative SNP set, SNPs localized on the X chromosome and insertion-deletion polymorphisms were excluded, and SNPs with high imputation quality ( >0.9) were retained. Ultimately, 8 339 066 variants were considered for the PRS analysis.
Statistical Analyses
To remove SNPs that were in linkage disequilibrium in the target sample, the SNPs were pruned based on a pairwise r2 threshold of 0.25 and a window size of 200 SNPs using PLINK. After pruning, 1 354 311 independent SNPs remained. We then calculated PRSs constructed from alleles showing a nominal association with each disorder in discovery GWASs under the following PT cutoff values: PT ≤ .01, PT ≤ .05, PT ≤ .1, PT ≤ .2, PT ≤ .5, and PT ≤ 1. For each individual included in the target sample, a PRS was calculated by weighing the scores for “risk variants” by the logarithm of the OR observed in each discovery dataset. The score, consisting of the number of risk variants (0, 1, or 2) multiplied by the logarithm of the OR, was summed over all of the SNPs in the PT-SNP sets for each individual in the target sample. To examine the effects of the European PRSs based on each PT cutoff on the risk of Japanese SCZ or FR, we used R 3.6.1 (http://www.r-project.org/) to perform linear regression or logistic regression with 3 diagnostic statuses (SCZ vs FRs vs HCs) or 2 diagnostic statuses (SCZ vs HCs, SCZ vs FRs, and FRs vs HCs) as the dependent variables, respectively, and each PRS based on each European GWAS as the independent variables. We compared the differences in adjusted R2 for linear regression or Nagelkerke’s pseudo-R2 for logistic regression, which are a measure of the variance explained by the model. The PRSs at each PT cutoff were highly correlated with each other and were not independent. Therefore, the P values based on different PT cutoff values were not corrected. The significance level for PRS analyses was set at P < .05. A Bonferroni-corrected P value threshold of P < .01 (α = .05/5 disorders) was used to avoid type I error.
Results
Effects of PRSs for SCZ and BIP in Europeans on Risk Among Japanese SCZ Patients and FRs
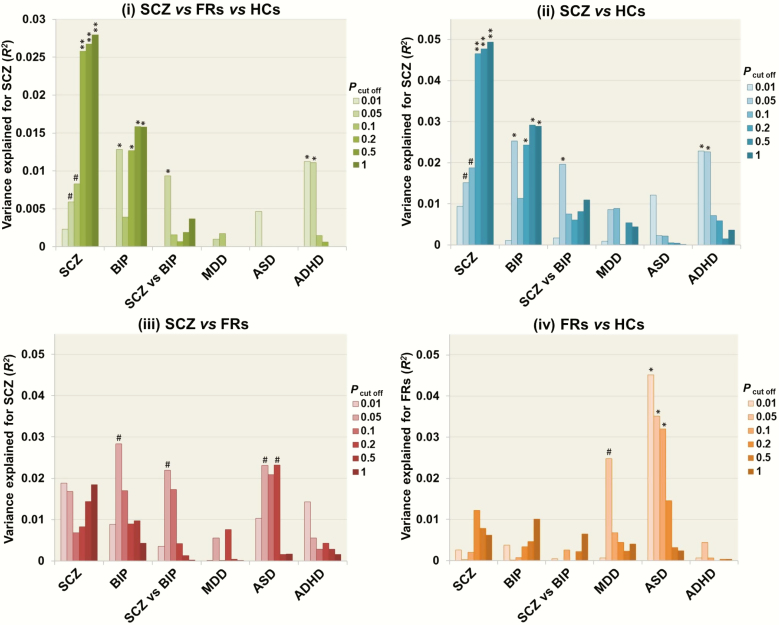
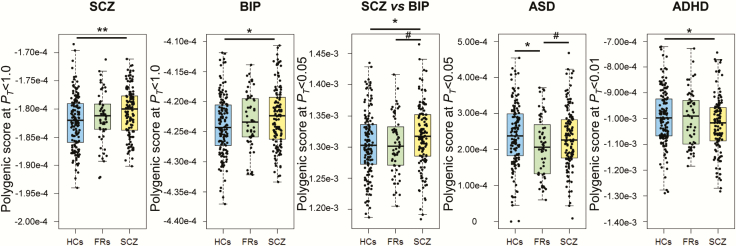
We first investigated the effects of PRSs based on European GWASs of SCZ and BIP (SCZ, BIP, and SCZ vs BIP) on risk levels in Japanese SCZ patients ([i1] SCZ vs FRs vs HCs, [2] SCZ vs HCs, and [3] SCZ vs FRs) or unaffected FRs ([4] FRs vs HCs) at different PT cutoff levels (Figure 1). The PRSs obtained from European SCZ samples were significantly higher in Japanese patients with SCZ than in HCs [Figures 1 and 2, (1) SCZ vs FRs vs HCs, a maximum at PT ≤ 1.0: adjusted R2 = 0.028, P = 1.30 × 10–3; (2) SCZ vs HCs, a maximum at PT ≤ 1.0: Nagelkerke’s R2 = 0.049, P = 1.66 × 10–3]. In addition, the PRSs related to European BIP were nominally higher in Japanese patients with SCZ than in HCs [Figures 1 and 2, (1) SCZ vs FRs vs HCs, a maximum at PT ≤ 0.5: adjusted R2 = 0.016, P = .012; (2) SCZ vs HCs, a maximum at PT ≤ .5: Nagelkerke’s R2 = 0.029, P = .015]. The PRSs differentiating SCZ from European BIP were marginally higher in Japanese patients with SCZ than in HCs [Figures 1 and 2, (1) SCZ vs FRs vs HCs, a maximum at PT ≤ .05: adjusted R2 = 0.010, P = .043; (2) SCZ vs HCs, a maximum at PT ≤ .05: Nagelkerke’s R2 = 0.020, P = .046]. In contrast, there were no significant differences in the PRSs obtained from European SCZ and BIP or the PRSs differentiating SCZ from BIP between (3) SCZ and FRs or (4) FRs and HCs (Figures 1 and 2, P > .05), although the PRSs obtained from European BIP and the PRSs differentiating SCZ from BIP were marginally higher in patients with SCZ than in FRs (a maximum at PT ≤ .05: P < .10).
Figure 1.
Effects of polygenic risk scores (PRSs) for European psychiatric disorders (SCZ, BIP, SCZ vs BIP, MDD, ASD, and ADHD) based on each threshold (PT cutoff) on the risk of Japanese SCZ ([1] SCZ vs FRs vs HCs, [2] SCZ vs HCs, or [3] SCZ vs FRs) or FRs ([4] FRs vs HCs). The y-axis shows adjusted R2 or Nagelkerke’s pseudo-R2, indicating the power of explanation of the model. SCZ, schizophrenia; FR, first-degree relative; HC, healthy control; BIP, bipolar disorder; MDD, major depressive disorder; ASD, autism spectrum disorder; ADHD, attention-deficit/hyperactivity disorder. **P < .01, *P < .05, #P < .1.
Figure 2.
Differences in PRSs based on GWASs (SCZ, BIP, SCZ vs BIP, ASD and ADHD) among HCs, FRs, and patients with SCZ. SCZ, schizophrenia; FR, first-degree relative; HC, healthy control; BIP, bipolar disorder; MDD, major depressive disorder; ASD, autism spectrum disorder; ADHD, attention-deficit/hyperactivity disorder; PRSs, polygenic risk scores; GWAS, genome-wide association study. **P < .01, *P < .05, #P < .1.
Effects of PRSs for MDD, ASD, and ADHD in Europeans on the Risk Levels of Japanese SCZ Patients or FRs
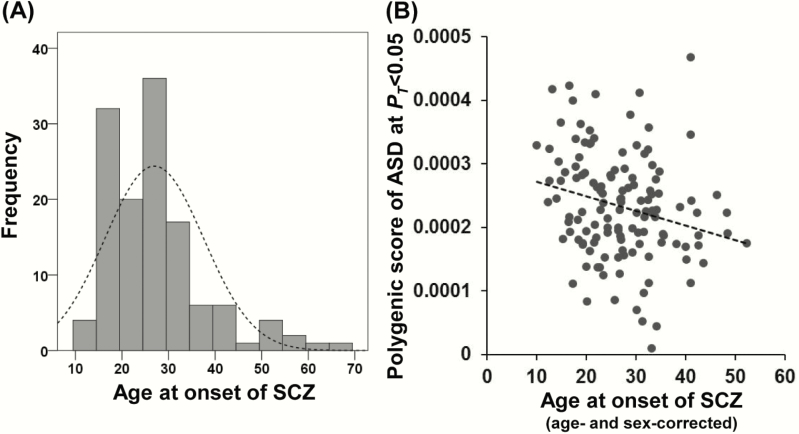
We next investigated the effects of PRSs based on European GWASs of other psychiatric disorders on the risk levels of Japanese SCZ patients or FRs at different PT levels (Figure 1). The PRSs obtained from European ADHD were marginally lower in Japanese patients with SCZ than in HCs [Figures 1 and 2, (1) SCZ vs FRs vs HCs, a maximum at PT ≤ .01: adjusted R2 = 0.011, P = .030; (2) SCZ vs HCs, a maximum at PT ≤ .01: Nagelkerke’s R2 = 0.023, P = .031], while there were no significant differences in the PRSs for European ADHD between (3) SCZ and FRs or (4) FRs and HCs (P > .05). Interestingly, the PRSs obtained from European ASD were marginally lower in Japanese FRs compared with HCs [Figures 1 and 2, (4) FRs vs HCs, a maximum at PT ≤ .01: Nagelkerke’s R2 = 0.045, P = .013] and patients with SCZ ([3] SCZ vs FRs, a maximum at PT ≤ 0.2: Nagelkerke’s R2 = 0.023, P = .084), while there were no significant differences in the PRSs for European ASD between (2) SCZ and HCs (P > .05). As childhood-onset patients with SCZ showed higher PRSs for both SCZ and ASD than their unaffected siblings (Ahn et al., 2016), we further investigated the correlation between age at onset and PRSs for both SCZ and ASD in our target SCZ samples (Figure 3). Lower age at onset of SCZ was significantly associated with higher PRSs for ASD (PT ≤ .05: beta = −0.20, P = 7.13 × 10–3) but not PRSs for SCZ (P > .05). There were no significant differences in the PRSs for MDD among patients with SCZ, FRs, and HCs (Figures 1 and 2, P > .05).
Figure 3.
Correlation between age at onset of SCZ and PRSs related to ASD in patients with SCZ. (A) A histogram of age at onset of SCZ is shown. (B) The PRSs for ASD were negatively correlated with the age at onset of SCZ. SCZ, schizophrenia; FR, first-degree relative; HC, healthy control; BIP, bipolar disorder; MDD, major depressive disorder; ASD, autism spectrum disorder; ADHD, attention-deficit/hyperactivity disorder; PRSs, polygenic risk scores; GWAS, genome-wide association study.
Discussion
The aim of the present study was to investigate whether European-derived PRSs for major psychiatric disorders (SCZ, BIP, MDD, ASD, and ADHD) based on large-scale GWASs from the PGC transethnically influence the risk level of Japanese SCZ patients or their unaffected FRs. As expected, the PRSs related to European SCZ and BIP were higher in Japanese patients with SCZ than in HCs. Furthermore, we report for the first time, to our knowledge, that PRSs differentiating SCZ from European BIP were higher in Japanese patients with SCZ than in HCs. Of the other 3 psychiatric disorders, the PRSs related to European ADHD were lower in Japanese patients with SCZ than in HCs. Intriguingly, PRSs related to European ASD were lower in Japanese FRs than in HCs or patients with SCZ. Considering that higher PRSs for ASD were correlated with earlier age at onset in patients with SCZ, a low genetic risk of ASD may contribute to protection against developing SCZ.
Although the sample sizes of our Japanese target cohort are relatively small, we preliminarily performed GWASs of (1) SCZ vs HCs, (2) SCZ vs FRs vs HCs, (3) FRs vs HCs, and (4) SCZ vs FRs in the target cohort (supplementary Methods). As expected, we did not find any genome-wide significant SNPs associated with SCZ or FRs in any GWASs (supplementary Figure 2, P > 5.0 × 10–8). Marginally associated SNPs (P < 1.0 × 10–5) in the GWAS of 3 diagnostic groups are summarized in supplementary Table 2. The strongest association with SCZ was observed at rs59454283, an intronic SNP in the endoplasmic reticulum membrane protein complex subunit 8 (EMC8) gene on 16q24.1 (minor allele frequency: SCZ = 0.19; FRs = 0.12; HCs = 0.05; t = 5.25, P = 2.82 × 10–7). All statistical t values in the GWAS of SCZ vs FRs vs HCs (range, t = −4.92 – 5.25) were higher than those in the GWAS of SCZ vs HCs (t = −4.55 – 4.68). The directions of all these associations between SCZ vs FRs vs HCs and SCZ vs HCs were identical (supplementary Table 2). Compared with the GWAS of SCZ vs HCs, the GWAS of SCZ vs FRs vs HCs including FRs identified an increased number of SNPs nominally associated with SCZ (supplementary Figure 3). The increased statistical power might be derived from the increased sample sizes of FRs. Therefore, we took 10 randomized samples (56 HCs and 146 FRs + HCs) from FRs and HCs and repeatedly performed GWASs of the SCZ vs randomized HCs vs FRs + HCs 10 times. The number of SNPs nominally associated with SCZ in both GWASs of SCZ vs FRs vs HCs and SCZ vs HCs was significantly higher than those in the GWASs using 10 randomizations (supplementary Figure 3, all P < .001). These findings suggest that GWASs utilizing FRs in addition to SCZ patients and HCs could be effective in detecting SNPs associated with SCZ despite small sample sizes.
Consistent with a previous study (Ohi et al., 2016a), the PRSs related to SCZ and BIP in Europeans predicted the risk of SCZ in Japanese participants regardless of the difference in sample ethnicity. We previously indicated that the PRS based on SCZ and BIP in Europeans explained 5.6% and 1.0% of the variance in Japanese participants’ SCZ and BIP status, respectively, in a distinct target sample (341 patients with SCZ and 588 HCs). In this study, the PRSs related to SCZ and BIP explained 4.9% and 2.9% of the variance in the SCZ and BIP status, respectively. The amounts of variance explained by the PRSs for SCZ were similar between studies, while the estimated variance explained by the PRSs for BIP was much stronger in this study than in the previous study. The difference in the variance explained by the PRSs for BIP is presumably due to the difference in sample sizes in discovery GWASs of BIP (Smoller et al., 2013; Stahl et al., 2019), as a larger scale GWAS could provide a more reliable catalog for the prediction of disease status.
The PRSs for SCZ and BIP in European FRs were intermediate between those of European SCZ patients and HCs (Ranlund et al., 2018; Toulopoulou et al., 2019). However, in our Japanese target sample, the PRSs obtained from European SCZ and BIP patients were not significantly different between SCZ and FRs or FRs and HCs, although Figure 2 shows that the PRSs in FRs were impaired to an intermediate degree, falling between those of SCZ patients and HCs. The likely cause is the difference in sample sizes rather than the difference in ethnicities between the target cohorts. In contrast, this study is the first, to our knowledge, to investigate a difference in PRSs differentiating SCZ from BIP among patients with SCZ, FRs, and HCs. The PRSs in patients with SCZ were much stronger than those in FRs and HCs, while the PRSs were similar between FRs and HCs, although the FR sample size was small. Genetic risk factors for developing SCZ but not BIP might specifically contribute to the pathogenesis of patients with SCZ compared with unaffected FRs.
A strong positive genetic correlation between the risks of SCZ and MDD and a weak positive genetic correlation between the risk of SCZ and risks of ASD and ADHD have been indicated in individuals with European ancestry by linkage disequilibrium score regression analysis, which can directly estimate the genetic SNP correlations from GWASs (Wen et al., 2019), but not by PRS analysis. Of 3 psychiatric disorders (MDD, ASD, and ADHD), the European PRSs related to ADHD in Japanese patients with SCZ were perversely lower than those in HCs. No associations between the European PRSs for MDD or ASD and risk of SCZ were observed between Japanese patients with SCZ and HCs. Because our target sample size may have been insufficient to identify such a nominal association between PRS related to these 3 psychiatric disorders and the risk of SCZ, further study using a larger target sample is needed.
Different types of genetic variations, including common genetic variants (Smoller et al., 2013; Ripke et al., 2014; Grove et al., 2019), rare genetic variants (de Sena Cortabitarte et al., 2017), and rare copy number variants (CNVs) (Moreno-De-Luca et al., 2010; Kushima et al., 2018), contribute to a common risk of both SCZ and ASD. Social communication difficulties are phenotypically shared with both SCZ and ASD (St Pourcain et al., 2018). Childhood-onset SCZ (COS) is a rare phenotype of SCZ involving a potentially higher occurrence of comorbid ASD and pathogenic CNVs compared with adult-onset SCZ (Forsyth and Asarnow, 2020). However, common genetic variants as well as CNVs contribute to the risk of COS (Ahn et al., 2016). The PRSs related to social communication difficulties were genetically overlapped in both SCZ and ASD, especially at early developmental stage (St Pourcain et al., 2018). In this study, lower age at onset of SCZ (range, 12–67 years, median, 26 years) was significantly correlated with higher PRSs for ASD. Although a few of our SCZ patients had childhood-onset psychosis (before age 13, n = 3), our finding would support evidence that COS patients had higher PRSs for ASD (Ahn et al., 2016) and there was shared genetic overlap in both SCZ and ASD at an early developmental stage (St Pourcain et al., 2018). Furthermore, the PRSs for ASD were lower in unaffected FRs than in HCs or patients with SCZ, suggesting that a low genetic risk of ASD in FRs may have a protective effect against developing SCZ, particularly early-onset SCZ. Further studies are warranted to investigate whether PRSs for SCZ vs ASD or PRSs for both SCZ and ASD based on European GWASs transethnically influence the risk of Japanese SCZ patients or their unaffected FRs.
There are some limitations to the interpretations of our findings. Compared with the sample sizes of SCZ patients and HCs, those of the FRs were relatively small, potentially resulting in false positive and negative findings. Considering the peak onset age of SCZ, unaffected parents of SCZ may have a lower risk of onset than siblings and offspring. As we did not have any Japanese BIP samples, we could not examine whether we classify Japanese SCZ and BIP patients using PRSs differentiating SCZ from European BIP. Adding the first 4 principal components (PCs) of the genotyping data as covariates to the control for any confounding effects due to population stratification did not change our findings. However, any admixture of Europeans in Japan or Japanese in Europe could be highly confounding. We could not completely exclude the admixture effect even if the PCs were treated as covariates. The estimated variances were assessed using adjusted R2 and Nagelkerke’s R2; however, we could not simply compare the adjusted R2 for linear regression and Nagelkerke’s pseudo-R2 for logistic regression.
In conclusion, we showed that the PRSs for SCZ and BIP based on large-scale European discovery GWASs and the PRS for differentiating SCZ from BIP could explain the risk of SCZ in independent Japanese target samples, suggesting the existence of transethnically shared genetic susceptibility contributing to SCZ. Furthermore, our analyses showed that FRs might be protected against developing SCZ if they lack ASD risk alleles. Further study using a larger FR sample is required.
Supplementary Material
Acknowledgments
We thank all individuals who participated in this study. This work was supported by Grants-in-Aid for Scientific Research (C) (19K08081) and Young Scientists (B) (16K19784) from the Japan Society for the Promotion of Science, a grant from the Uehara Memorial Foundation, a grant from the Takeda Science Foundation, a grant from the YOKOYAMA Foundation for Clinical Pharmacology (YRY-1807), a grant from the Smoking Research Foundation, and a Grant for Assist KAKEN (K2017-8, K2018-16, and K2018-17) and Promoted Research (S2017-3 and S2018-5) from Kanazawa Medical University.
Statement of Interest
None.
References
- Ahn K, An SS, Shugart YY, Rapoport JL (2016) Common polygenic variation and risk for childhood-onset schizophrenia. Mol Psychiatry 21:94–96. [DOI] [PubMed] [Google Scholar]
- Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II (2000) Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet 97:12–17. [PubMed] [Google Scholar]
- Demontis D, et al. ; ADHD Working Group of the Psychiatric Genomics Consortium (PGC); Early Lifecourse & Genetic Epidemiology (EAGLE) Consortium; 23andMe Research Team (2019) Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 51:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sena Cortabitarte A, Degenhardt F, Strohmaier J, Lang M, Weiss B, Roeth R, Giegling I, Heilmann-Heimbach S, Hofmann A, Rujescu D, Fischer C, Rietschel M, Nöthen MM, Rappold GA, Berkel S (2017) Investigation of SHANK3 in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 174:390–398. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. (2013) Power and predictive accuracy of polygenic risk scores. Plos Genet 9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JK, Asarnow RF (2020) Genetics of childhood-onset schizophrenia 2019 update. Child Adolesc Psychiatr Clin N Am 29:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67(Suppl 9):3–8; discussion 36–42. [PubMed] [Google Scholar]
- Grove J, et al. ; Autism Spectrum Disorder Working Group of the Psychiatric Genomics Consortium; BUPGEN; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; 23andMe Research Team (2019) Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 51:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA (2013) Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry 170:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, et al. (2019) Genome-wide association study detected novel susceptibility genes for schizophrenia and shared trans-populations/diseases genetic effect. Schizophr Bull 45:824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Saito T, Kondo K, Iwata N (2018) Genome-wide association studies of bipolar disorder: a systematic review of recent findings and their clinical implications. Psychiatry Clin Neurosci 72:52–63. [DOI] [PubMed] [Google Scholar]
- Kushima I, et al. (2018) Comparative analyses of copy-number variation in autism spectrum disorder and schizophrenia reveal etiological overlap and biological insights. Cell Rep 24:2838–2856. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca D, et al. (2010) Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet 87:618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa D, et al. (2014) Genome-wide association study identifies a potent locus associated with human opioid sensitivity. Mol Psychiatry 19:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell J, et al. (2014) A general approach for haplotype phasing across the full spectrum of relatedness. Plos Genet 10:e1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Kataoka Y, Shimada T, Kuwata A, Okubo H, Kimura K, Yasuyama T, Uehara T, Kawasaki Y (2019) Meta-analysis of physical activity and effects of social function and quality of life on the physical activity in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 269:517–527. [DOI] [PubMed] [Google Scholar]
- Ohi K, Kikuchi M, Ikeda M, Yamamori H, Yasuda Y, Fujimoto M, Fujino H, Miura K, Fukunaga M, Nakaya A, Iwata N, Hashimoto R (2016a) Polygenetic components for schizophrenia, bipolar disorder and rheumatoid arthritis predict risk of schizophrenia. Schizophr Res 175:226–229. [DOI] [PubMed] [Google Scholar]
- Ohi K, Matsuda Y, Shimada T, Yasuyama T, Oshima K, Sawai K, Kihara H, Nitta Y, Okubo H, Uehara T, Kawasaki Y (2016b) Structural alterations of the superior temporal gyrus in schizophrenia: detailed subregional differences. Eur Psychiatry 35:25–31. [DOI] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kihara H, Yasuyama T, Sawai K, Matsuda Y, Oshima K, Okubo H, Nitta Y, Uehara T, Kawasaki Y (2017a) Impact of familial loading on prefrontal activation in major psychiatric disorders: a near-infrared spectroscopy (NIRS) study. Sci Rep 7:44268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Nemoto K, Kataoka Y, Yasuyama T, Kimura K, Okubo H, Uehara T, Kawasaki Y (2017b) Cognitive clustering in schizophrenia patients, their first-degree relatives and healthy subjects is associated with anterior cingulate cortex volume. Neuroimage Clin 16:248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kataoka Y, Koide Y, Yasuyama T, Uehara T, Okubo H, Kawasaki Y (2019a) Intelligence decline between present and premorbid IQ in schizophrenia: schizophrenia non-affected relative project (SNARP). Eur Neuropsychopharmacol 29:653–661. [DOI] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kuwata A, Kataoka Y, Okubo H, Kimura K, Yasuyama T, Uehara T, Kawasaki Y (2019b) Smoking rates and number of cigarettes smoked per day in schizophrenia: a large cohort meta-analysis in a Japanese population. Int J Neuropsychopharmacol 22:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardiñas AF, et al. ; GERAD1 Consortium; CRESTAR Consortium (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 50:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P(2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranlund S, et al. ; GROUP; PEIC; WTCCC2 (2018) A polygenic risk score analysis of psychosis endophenotypes across brain functional, structural, and cognitive domains. Am J Med Genet B Neuropsychiatr Genet 177:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JT, Farh KH, Holmans PA, Lee P, Bulik-Sullivan B, Collier DA, Huang H(2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderfer D, et al. (2018) Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173:1705–1715; e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Ripke S, Lee PH, Neale B, Nurnberger JI, Santangelo S, Sullivan PF, Perlis RH, Purcell SM, Fanous A, Neale MC(2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, et al. ; eQTLGen Consortium; BIOS Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium (2019) Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet 51:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pourcain B, et al. ; iPSYCH-SSI-Broad Autism Group (2018) ASD and schizophrenia show distinct developmental profiles in common genetic overlap with population-based social communication difficulties. Mol Psychiatry 23:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC (2003) Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60:1187–1192. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Ninomiya K, Kushima I, Saito T, Shimasaki A, Sakusabe T, Momozawa Y, Kubo M, Kamatani Y, Ozaki N, Ikeda M, Iwata N(2020). Polygenic risk scores in schizophrenia with clinically significant copy number variants. Psychiatry Clin Neurosci 74:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulopoulou T, Goldberg TE, Mesa IR, Picchioni M, Rijsdijk F, Stahl D, Cherny SS, Sham P, Faraone SV, Tsuang M, Weinberger DR, Seidman LJ, Murray RM (2010) Impaired intellect and memory: a missing link between genetic risk and schizophrenia? Arch Gen Psychiatry 67:905–913. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Zhang X, Cherny S, Dickinson D, Berman KF, Straub RE, Sham P, Weinberger DR (2019) Polygenic risk score increases schizophrenia liability through cognition-relevant pathways. Brain 142:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M. (2000) Schizophrenia: genes and environment. Biol Psychiatry 47:210–220. [DOI] [PubMed] [Google Scholar]
- Wen Y, Zhang F, Ma X, Fan Q, Wang W, Xu J, Zhu F, Hao J, He A, Liu L, Liang X, Du Y, Li P, Wu C, Wang S, Wang X, Ning Y, Guo X (2019) eQTLs weighted genetic correlation analysis detected brain region differences in genetic correlations for complex psychiatric disorders. Schizophr Bull 45:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2018) Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama T, Ohi K, Shimada T, Uehara T, Kawasaki Y (2017) Differences in social functioning among patients with major psychiatric disorders: interpersonal communication is impaired in patients with schizophrenia and correlates with an increase in schizotypal traits. Psychiatry Res 249:30–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.