Abstract
OBJECTIVE
Most individuals with type 2 diabetes also have obesity, and treatment with some diabetes medications, including insulin, can cause further weight gain. No approved chronic weight management medications have been prospectively investigated in individuals with overweight or obesity and insulin-treated type 2 diabetes. The primary objective of this study was to assess the effect of liraglutide 3.0 mg versus placebo on weight loss in this population.
RESEARCH DESIGN AND METHODS
Satiety and Clinical Adiposity—Liraglutide Evidence (SCALE) Insulin was a 56-week, randomized, double-blind, placebo-controlled, multinational, multicenter trial in individuals with overweight or obesity and type 2 diabetes treated with basal insulin and ≤2 oral antidiabetic drugs.
RESULTS
Individuals were randomized to liraglutide 3.0 mg (n = 198) or placebo (n = 198), combined with intensive behavioral therapy (IBT). At 56 weeks, mean weight change was −5.8% for liraglutide 3.0 mg versus −1.5% with placebo (estimated treatment difference −4.3% [95% CI −5.5; −3.2]; P < 0.0001). With liraglutide 3.0 mg, 51.8% of individuals achieved ≥5% weight loss versus 24.0% with placebo (odds ratio 3.41 [95% CI 2.19; 5.31]; P < 0.0001). Liraglutide 3.0 mg was associated with significantly greater reductions in mean HbA1c and mean daytime glucose values and less need for insulin versus placebo, despite a treat-to-glycemic-target protocol. More hypoglycemic events were observed with placebo than liraglutide 3.0 mg. No new safety or tolerability issues were observed.
CONCLUSIONS
In individuals with overweight or obesity and insulin-treated type 2 diabetes, liraglutide 3.0 mg as an adjunct to IBT was superior to placebo regarding weight loss and improved glycemic control despite lower doses of basal insulin and without increases in hypoglycemic events.
Introduction
Obesity is a chronic, progressive disease (1) associated with multiple complications that, individually and in combination, confer morbidity and mortality risk (2). The risk of developing type 2 diabetes increases with adiposity and increasing BMI (3,4), and the global rise in the prevalence of this disease closely follows that of obesity (5,6). In turn, obesity in individuals with type 2 diabetes can exacerbate deterioration of glycemic control (7).
There is substantial evidence that weight loss interventions can lower blood glucose (BG) levels, and, although weight loss remains a key recommendation in diabetes guidelines (8–10), it is frequently poorly implemented (11). Type 2 diabetes is a progressive disease, and despite improved oral and injectable glucose-lowering agents available today, many individuals with long-standing type 2 diabetes eventually require insulin (12).
Weight gain following initiation of insulin or sulfonylureas (SUs) is common, with increases of ∼4 kg often observed with insulin and ∼2 kg with SUs (13). Given that insulin use is associated with weight gain (14), weight management in individuals with coexistent obesity and type 2 diabetes requiring insulin is particularly challenging. This population would benefit from greater availability of pharmacotherapeutic agents that address obesity. Accordingly, the American Association of Clinical Endocrinologists diabetes guidelines, Endocrine Society obesity guidelines, and the latest European Association for the Study of Diabetes/American Diabetes Association (ADA) consensus report advise that the effect on weight should be considered when choosing diabetes treatment (8–10,15), and given their glucose- and weight-lowering effects, glucagon-like peptide 1 (GLP-1) receptor agonists have an advantage over many glucose-lowering agents in this regard.
Liraglutide is an analog of GLP-1 and in doses up to 1.8 mg is approved for use in combination with insulin (16). It is also approved as a fixed-ratio combination with insulin degludec (17), as an adjunct to diet and exercise for type 2 diabetes treatment. Liraglutide 3.0 mg (18) is approved for chronic weight management in individuals with overweight or obesity and has been investigated in individuals with type 2 diabetes as part of the Satiety and Clinical Adiposity—Liraglutide Evidence (SCALE) phase 3a program. SCALE Diabetes was a 56-week trial of liraglutide 1.8 mg and 3.0 mg in individuals with overweight or obesity and diabetes treated with ≤2 oral antidiabetic drugs (OADs) but excluded insulin-treated individuals. In this previous study, weight loss of 4.7% and 6.0% was observed with liraglutide 1.8 mg and 3.0 mg, respectively, versus 2.0% with placebo (19). While liraglutide 1.8 mg is indicated in combination with insulin for diabetes treatment, liraglutide 3.0 mg combined with insulin for weight management has not previously been studied. Furthermore, to our knowledge, no medications approved for chronic weight management have been prospectively investigated in individuals with overweight or obesity and insulin-treated type 2 diabetes.
The current study aimed to evaluate the efficacy and safety of liraglutide 3.0 mg for weight management in individuals with overweight (BMI ≥27 kg/m2) or obesity (BMI ≥30 kg/m2) and type 2 diabetes treated with basal insulin and up to two OADs.
Research Design and Methods
Study Overview
SCALE Insulin (reg. no. NCT02963922, ClinicalTrials.gov) was conducted from February 2017 to September 2018 at 53 sites globally. The trial protocol was approved by local ethics committees or institutional review boards, and the trial was conducted in accordance with the principles of the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice guidelines (20). The sponsor, Novo Nordisk A/S, developed the study protocol, planned and performed the statistical analyses, provided editorial and writing assistance, and provided the trial medications.
Study Objective
The primary objective was to confirm superiority of liraglutide 3.0 mg versus placebo, as an adjunct to intensive behavioral therapy (IBT), on weight loss efficacy in individuals with overweight or obesity and type 2 diabetes treated with basal insulin and up to two OADs. Secondary objectives aimed to investigate the liraglutide 3.0 mg effects on other relevant efficacy end points and to establish the safety and tolerability of liraglutide 3.0 mg versus placebo as an adjunct to IBT.
Participants
Eligible individuals were aged ≥18 years with a BMI of ≥27 kg/m2, stable body weight (maximum 5 kg self-reported weight change within 90 days before screening), diagnosed with type 2 diabetes with an HbA1c ≥6.0 to ≤10% (42–86 mmol/mol) at screening, and receiving stable treatment with any basal insulin (≥90 days; no requirement for minimum or maximum dose) and ≤2 OADs. Individuals were excluded if they had type 1 diabetes, recurrent severe hypoglycemic episodes within the last year, or use of dipeptidyl peptidase 4 inhibitors, GLP-1 receptor agonists, bolus insulin, or medications known to induce significant weight change in the previous 90 days. Other exclusion criteria included a recent history of cardiovascular event; history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2; pregnancy, breast-feeding, or intention to become pregnant; or a history of pancreatitis.
Study Design
SCALE Insulin was a 56-week, randomized, double-blind, placebo-controlled, multinational, multicenter trial (Supplementary Fig. 1). A total of 396 individuals were randomized centrally using an interactive voice/web response system to receive either liraglutide 3.0 mg or placebo (1:1) as adjunct to IBT. Individuals treated with SUs were stratified between the two arms. Liraglutide 3.0 mg or placebo was administered once daily by subcutaneous injection. During the first 4 weeks postrandomization, the dose was escalated by 0.6 mg weekly to reach the maintenance dose of 3.0 mg. A 4-week follow-up period was included after the 56-week treatment period. To promote individual retention and improve data quality, individuals were permitted to stop and restart the study drug without re-escalating the dose, or with re-escalation if three consecutive doses had been missed.
IBT
IBT consisted of a hypocaloric diet, increased physical activity, and behavioral therapy delivered in frequent counseling sessions and is described in detail elsewhere (21) and in the Supplementary Data. Individuals attended a total of 23 individual or group counseling sessions during the 56-week period, delivered by a registered dietitian or similarly qualified health care professional.
Concomitant Diabetes Medication
It was recommended that, after randomization and at the investigator’s discretion, individuals should reduce their dose of SUs by 50% to lower the likelihood of SU-induced hypoglycemia. In individuals with HbA1c ≤8% (64 mmol/mol) at randomization, it was recommended to reduce the dose of basal insulin by 15–20% owing to anticipated glycemic improvements. Insulin doses were adjusted based on self-measured BG (SMBG) values to ensure that similar levels of fasting glucose were maintained between the two arms, regardless of background medication (Supplementary Table 1). In individuals using once-daily basal insulins, weekly adjustments were based on the mean of three prebreakfast SMBG values with a target range of 4 to 5 mmol/L (71–90 mg/dL). In individuals using twice-daily basal insulins, adjustment was based on the mean of three prebreakfast and predinner SMBG measurements. Basal insulin dose was not to exceed the entry dose within the first 5 weeks. Furthermore, the initiation of bolus insulin was permitted after the 5-week period and only after optimization of basal insulin dose. The type and dose of other OADs were kept constant throughout the trial, unless unacceptable hypoglycemia occurred that could not be managed by a reduction of basal insulin.
Hypoglycemia Classification
Hypoglycemia was defined using the ADA classification (22). Severe hypoglycemia was defined as an episode requiring assistance of another person to actively administer carbohydrate or glucagon or take other corrective actions. Documented symptomatic and asymptomatic hypoglycemia were defined as a plasma glucose concentration ≤70 mg/dL (3.9 mmol/L) with and without typical symptoms of hypoglycemia, respectively. Hypoglycemia was also assessed using Novo Nordisk’s classification, which, together with ADA criteria, is included in the Supplementary Data.
Study End Points
Coprimary end points were change in body weight (percentage) from baseline to week 56 and proportion of individuals losing ≥5% of baseline body weight at week 56. Confirmatory secondary end points included the proportion of individuals losing >10% of baseline body weight at week 56 and the change from baseline to week 56 in waist circumference, HbA1c, fasting plasma glucose (FPG), SF-36 version 2.0 acute physical functioning score, and Impact of Weight on Quality of Life-Lite for Clinical Trial Version (IWQOL-Lite for CT) physical function domain (five-items) score.
Key supportive end points were change from baseline to week 56 in total daily insulin dose (units), mean daytime glucose value (based on seven-point SMBG values), systolic and diastolic blood pressure, lipids, SF-36 physical component summary, SF-36 mental component summary, SF-36 subdomains, IWQOL-Lite for CT subdomains, and the weight-related sign and symptom measure total score. Additionally, two composite end points of HbA1c <7% (53 mmol/mol) plus weight loss ≥5% and HbA1c <7% (53 mmol/mol) plus weight loss ≥5% plus no documented symptomatic hypoglycemia were prespecified. A post hoc analysis of the change in the seven-point SMBG profile was also carried out.
Safety was assessed by adverse events (AEs) and hypoglycemic episodes, physical examination, resting pulse, electrocardiogram, and laboratory measurements. Two different observation periods were used: the on-drug period, used for all safety end points, with the exception of neoplasms, for which the in-trial period was used. The in-trial period included time from randomization to the final follow-up visit (or date of last contact) regardless of trial product discontinuation. On-drug safety was assessed from the first treatment day to 14 days after the last treatment day, excluding potential off-drug time intervals triggered by at least 2 weeks of consecutive missed doses.
Statistical Considerations
The planned sample size for this trial of 400 participants, along with a 1:1 randomization and assuming a 30% discontinuation rate, resulted in a combined power of 95.2%, which was estimated to be adequate to evaluate the two coprimary end points. Power for the continuous end point, percentage weight change, was calculated with a two-group Satterthwaite test; the power for the categorical end point, ≥5% responders, was calculated using a Pearson χ2 test, both at a 5% significance level. The two coprimary end points were tested in hierarchal order with change in body weight at week 56 as a percentage of baseline body weight first, followed by proportion of individuals losing ≥5% of baseline body weight at week 56.
To estimate the intervention effect, the treatment policy estimand (primary estimand) was defined for each efficacy end point. The treatment policy estimand evaluated the effect of liraglutide 3.0 mg versus placebo at week 56 for all randomized individuals, regardless of premature discontinuation of trial product. This estimand reflects the intention-to-treat principle as defined in the ICH E9. Missing values at week 56 were imputed from the placebo arm using a jump-to-reference multiple imputation approach based on 100 iterations of the data set (23).
For the coprimary end points and confirmatory secondary end points, the trial product estimand (secondary estimand), based on a mixed model for repeated measurements, evaluated the treatment effect of liraglutide 3.0 mg versus placebo at week 56 for all randomized individuals with the assumption that all individuals had remained on trial product for the entire planned trial duration, using assessments only from individuals who were taking the randomized treatment until end of trial or at first discontinuation (see Supplementary Data for detailed description). The treatment policy and trial product estimands correspond to the updated ICH Good Clinical Practice regulatory guidelines on quantifying treatment effects of medications (24).
Continuous primary and secondary end points were analyzed using ANCOVA with randomized treatment, BMI and sex as factors, and baseline end point as a covariate. The estimates and SDs were pooled using Rubin’s formula. All categorical end points were assessed at week 56 and analyzed using logistic regression with the same factors and covariate as the continuous end point analysis.
For analyses of end points, the estimated treatment difference (ETD) for continuous and the estimated odds ratio (OR) for the categorical end points are reported with the associated two-sided 95% CI and corresponding P value. All analyses were undertaken using UNIX SAS (version 9.4) on the Statistical Computing Environment.
Results
Trial Population
A total of 551 individuals were screened, and 396 were randomized: 198 to liraglutide 3.0 mg combined with IBT and 198 to placebo combined with IBT. Baseline demographics were similar between treatment arms (Table 1).
Table 1.
Baseline demographics and medications
| Liraglutide 3.0 mg (n = 198) | Placebo (n = 198) | |
|---|---|---|
| Male sex, n (%) | 90 (45.5) | 99 (50.0) |
| Mean age, years (SD) | 55.9 (11.3) | 57.6 (10.4) |
| Race, n (%) | ||
| White | 174 (87.9) | 180 (90.9) |
| Black | 17 (8.6) | 11 (5.6) |
| Asian | 3 (1.5) | 5 (2.5) |
| Ethnicity not Hispanic or Latino, n (%) | 155 (78.3) | 169 (85.4) |
| Mean body weight, kg (SD)* | 100.6 (20.8) | 98.9 (19.9) |
| Mean BMI, kg/m2 (SD) | 35.9 (6.5) | 35.3 (5.8) |
| Mean waist circumference, cm (SD) | 114.8 (13.7) | 114.2 (13.2) |
| Mean HbA1c, % (SD) | 7.9 (1.1) | 8 (1.0) |
| Mean HbA1c, mmol/mol (SD) | 63.0 (11.5) | 63.6 (11.3) |
| Mean FPG, mmol/L (SD) | 7.8 (2.2) | 8.1 (2.5) |
| Mean FPG, mg/dL (SD) | 141 (40) | 146 (46) |
| Mean diabetes duration, years (SD) | 11.4 (6.8) | 12.8 (6.9) |
| Mean heart rate, bpm (SD)† | 74.0 (10.0) | 75.0 (11.0) |
| Mean SBP, mmHg (SD) | 129.0 (14.0) | 132.0 (16.0) |
| Mean DBP, mmHg (SD) | 78.0 (9.0) | 78.0 (9.0) |
| Mean total cholesterol, mmol/L (SD) | 4.5 (1.0) | 4.4 (0.9) |
| Mean total cholesterol, mg/dL (SD) | 172 (39) | 171 (36) |
| Mean LDL cholesterol, mmol/L (SD) | 2.4 (0.9) | 2.4 (0.8) |
| Mean LDL cholesterol, mg/dL (SD) | 94 (33) | 94 (29) |
| Mean HDL cholesterol, mmol/L (SD) | 1.2 (0.3) | 1.2 (0.3) |
| Mean HDL cholesterol, mg/dL (SD) | 45 (12) | 45 (11) |
| Mean VLDL cholesterol, mmol/L (SD) | 0.9 (0.4) | 0.8 (0.4) |
| Mean VLDL cholesterol, mg/dL (SD) | 33 (16) | 32 (15) |
| Mean triglycerides, mmol/L (SD) | 2.0 (1.2) | 1.9 (1.0) |
| Mean triglycerides, mg/dL (SD) | 174 (105) | 168 (89) |
| Mean free fatty acids, mmol/L (SD) | 0.6 (0.2) | 0.6 (0.3) |
| Mean free fatty acids, mg/dL (SD) | 15.9 (6.9) | 15.5 (7.3) |
| Antidiabetic medications at screening, n (%) | ||
| Biguanides | 175 (88.4) | 176 (88.9) |
| SUs | 68 (34.3) | 71 (35.9) |
| SGLT2i | 44 (22.2) | 44 (22.2) |
| Thiazolidinediones | 4 (2.0) | 6 (3.0) |
| Combination BG-lowering drugs (oral) | 4 (2.0) | 3 (1.5) |
| α-Glucosidase inhibitors | 2 (1.0) | 0 (0.0) |
| Other BG-lowering drugs, excluding insulins | 1 (0.5) | 5 (2.5) |
| Insulins/analogs (injection), n (%) | ||
| Long-acting | 180 (90.9) | 184 (92.9) |
| Intermediate-acting | 18 (9.1) | 14 (7.1) |
DBP, diastolic blood pressure; SBP, systolic blood pressure; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Body weight measurements include both fasting and nonfasting measures.
Safety analysis set; liraglutide, n = 195; placebo, n = 197.
A high proportion of individuals returned for the final evaluation at week 56 (96.5% in the liraglutide 3.0 mg arm and 97.5% in the placebo arm) and remained on study drug up to week 56 (83.8% and 84.8%, respectively). Two individuals on liraglutide 3.0 mg and four on placebo were lost to follow-up (Supplementary Fig. 2 and Supplementary Table 2).
Primary, confirmatory, and supportive secondary end points relating to the treatment policy estimand are presented in Table 2. Corresponding trial product estimand end points are presented in Supplementary Table 3 where applicable.
Table 2.
Change in primary and secondary end points from baseline to week 56: treatment policy estimand
| Liraglutide 3.0 mg (n = 198) | Placebo (n = 198) | ETD/OR* (95% CI) | P value | |
|---|---|---|---|---|
| Primary end points | ||||
| Change in body weight from baseline, % | −5.8 | −1.5 | −4.3 (−5.5; −3.2) | <0.0001 |
| Proportion of individuals achieving ≥5% weight loss,* % | 51.8 | 24.0 | 3.4 (2.2; 5.3) | <0.0001 |
| Secondary confirmatory end points | ||||
| Proportion of individuals achieving >10% weight loss,* % | 22.8 | 6.6 | 4.2 (2.2; 8.2) | <0.0001 |
| Change in waist circumference from baseline, cm | −5.3 | −2.6 | −2.7 (−3.9; −1.5) | <0.0001 |
| Change in HbA1c from baseline, % | −1.1 | −0.6 | −0.5 (−0.8; −0.3) | <0.0001 |
| Change in HbA1c from baseline, mmol/mol | −11.9 | −6.0 | −5.8 (−8.3; −3.4) | <0.0001 |
| Change in FPG from baseline, mmol/L | −1.0 | −0.6 | −0.4 (−0.9; 0.1) | 0.1502 |
| Change in FPG from baseline, mg/dL | −18.4 | −11.5 | −6.9 ( −16.4; 2.5) | 0.1502 |
| Change in SF-36 physical functioning score from baseline | 2.7 | 2.3 | 0.4 (−1.0; 1.8) | 0.5716 |
| Change in IWQOL-Lite for CT physical function domain score from baseline | 8.2 | 5.7 | 2.5 (−1.5; 6.4) | 0.2218 |
| Secondary supportive end points | ||||
| Change in total daily insulin dose from baseline, units | 2.8 | 17.8 | −15.0 (−22.0; −8.0) | <0.0001 |
| Change in mean daytime glucose value from baseline, mmol/L | −2.2 | −1.5 | −0.7 (−1.1; −0.2) | 0.0032 |
| Change in mean daytime glucose value from baseline, mg/dL | −39.6 | −27.3 | −12.4 (−20.6; −4.1) | 0.0032 |
| Individuals achieving ≥5% weight loss and HbA1c <7% at week 56* | 39.0 | 13.9 | 3.9 (2.4; 6.5) | <0.0001 |
| Individuals achieving ≥5% weight loss, HbA1c <7%, and no documented symptomatic hypoglycemia at week 56* | 17.8 | 6.2 | 3.3 (1.66; 6.48) | 0.0006 |
| Change in systolic blood pressure from baseline, mmHg | −5.6 | −1.6 | −4.0 (−6.4; −1.5) | 0.0014 |
| Change in diastolic blood pressure from baseline, mmHg | −2.3 | −0.9 | −1.4 (−3.0; 0.2) | 0.0905 |
| Total cholesterol† | 0.97 | 1.01 | 0.97 (0.94; 1.00) | 0.0463 |
| LDL cholesterol† | 0.97 | 1.01 | 0.96 (0.91; 1.01) | 0.1027 |
| HDL cholesterol† | 1.04 | 1.02 | 1.02 (0.99; 1.04) | 0.2778 |
| VLDL cholesterol† | 0.89 | 0.94 | 0.94 (0.88; 1.01) | 0.0830 |
| Triglycerides† | 0.88 | 0.94 | 0.94 (0.87; 1.01) | 0.0715 |
| Free fatty acids† | 0.79 | 0.84 | 0.95 (0.85; 1.07) | 0.3936 |
Baseline to week 56 vs. placebo. Full analysis set. Statistical analysis is ANCOVA with jump-to-reference multiple imputation.
The end point is analyzed in a logistic regression model.
Data are treatment ratios (liraglutide 3.0 mg/placebo).
Body Weight and Waist Circumference
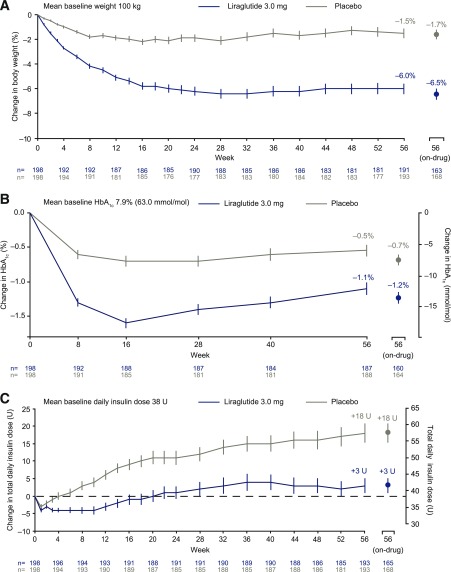
Figure 1A shows observed mean weight loss over time in the two groups. For the treatment policy estimand (intention-to-treat principle), mean weight loss at 56 weeks was −5.8% with liraglutide 3.0 mg and −1.5% with placebo (ETD −4.3% [95% CI −5.5; −3.2]; P < 0.0001) (Table 2 and Supplementary Fig. 3A). For the trial product estimand (if-all-adhered principle), estimated mean weight change at 56 weeks was −6.4% for liraglutide 3.0 mg and −1.3% for placebo (ETD −5.1% [95% CI −6.3; −3.9]; P < 0.0001) (Supplementary Table 3 and Supplementary Fig. 3B). For individuals on the trial product at 56 weeks, mean observed change in weight was −6.5% (n = 163) with liraglutide 3.0 mg and −1.7% (n = 168) with placebo (Fig. 1A).
Figure 1.
Change in body weight and glycemic control over time. A: Change in body weight (percentage); observed mean data ± SEM. n values refer to all individuals who attended visit regardless of treatment status; on-drug n values refer to individuals who were still on active treatment at time of visit. B: Change in HbA1c; observed mean data ± SEM. n values refer to all individuals who attended visit regardless of treatment status; on-drug n values refer to individuals who were still on active treatment at time of visit. C: Change in total daily insulin dose; graph shows observed mean data ± SEM. n values refer to all individuals who attended visit regardless of treatment status; on-drug n values refer to individuals who were still on active treatment at time of visit. U, units.
The proportion of individuals who achieved ≥5% weight loss was 51.8% with liraglutide 3.0 mg and 24.0% with placebo (OR 3.41 [95% CI 2.19; 5.31]; P < 0.0001). The proportion who lost >10% was 22.8% and 6.6%, respectively (OR 4.21 [95% CI 2.2; 8.2]; P < 0.0001) (Supplementary Fig. 4). A significant decrease was observed in waist circumference at 56 weeks in the liraglutide 3.0-mg group versus placebo (−5.28 cm vs. −2.56 cm [ETD −2.71 (95% CI −3.90; −1.53); P < 0.0001]) (Table 2).
Glycemic Parameters
Figure 1B shows observed changes in HbA1c over time in the two groups. For the treatment policy estimand at 56 weeks, a significantly greater reduction in mean HbA1c was observed with liraglutide 3.0 mg (−1.1% [−11.9 mmol/mol]) versus placebo (−0.6% [−6.0 mmol/mol]; ETD −0.5% [95% CI −0.8; −0.3]; P < 0.0001). There was no significant difference in the reduction of mean FPG (mmol/L) between the liraglutide 3.0 mg (−1.0 mmol/L) and the placebo group (−0.6 mmol/L; ETD −0.4 [95% CI −0.9; 0.1]; P = 0.1502) (Table 2), in keeping with the trial design to target the same FPG in both groups. Change in FPG over time is shown in Supplementary Fig. 5. Liraglutide 3.0 mg was associated with lower pre- and postprandial glucose values over the course of the day, as evident in the mean daytime glucose value (Supplementary Fig. 6A) and the seven-point SMBG profile at week 16 and week 56 (Supplementary Fig. 6B–D). Relative to placebo, there was a significantly smaller increase in insulin dose required to achieve target fasting BG (+3 units with liraglutide 3.0 mg vs. +18 units with placebo; ETD −15.0 [95% CI −22.0; −8.0]; P < 0.0001) (Fig. 1C). A total of 24 individuals who had completed the trial (21 with liraglutide and 3 with placebo) were no longer using insulin at the study end.
Cardiometabolic Parameters and Quality of Life
Mean systolic blood pressure decreased significantly with liraglutide 3.0 mg (−5.6 mmHg) versus placebo (−1.6 mmHg; ETD −4.0 [95% CI −6.4; −1.5]; P = 0.0014), and changes in diastolic blood pressure were not significant (Table 2). There was a trend for improved lipids with liraglutide 3.0 mg versus placebo, although with the exception of total cholesterol, no significant differences between treatment arms were observed at 56 weeks. Individuals on liraglutide 3.0 mg and placebo both reported increased physical functioning at week 56 as determined by the SF-36 physical functioning domain score (Supplementary Fig. 7A) and the IWQOL-Lite for CT physical function domain score (Supplementary Fig. 7B), but there were no significant differences between treatment groups.
Composite End Points
The proportion of individuals achieving ≥5% weight loss plus ADA HbA1c target of <7% (53 mmol/mol) was 39.0% with liraglutide 3.0 mg and 13.9% with placebo (OR 3.94 [95% CI 2.38; 6.53]; P < 0.0001). The proportion of individuals achieving ≥5% weight loss plus ADA HbA1c target of <7% (53 mmol/mol) plus who did not report any documented symptomatic hypoglycemia was 17.8% with liraglutide 3.0 mg and 6.2% with placebo (OR 3.28 [95% CI 1.66; 6.48]; P = 0.0006).
Safety
Liraglutide 3.0 mg in combination with IBT was generally well tolerated, with no new safety signals identified. Safety data are summarized in Table 3. AE incidence was similar for liraglutide 3.0 mg and placebo, except for gastrointestinal AEs, which had a greater incidence with liraglutide 3.0 mg (62.1% vs. 46.7%). AEs reported by ≥5% of participants and more frequently by participants in the liraglutide arm than the placebo arm included nausea, nasopharyngitis, diarrhea, headache, and upper respiratory tract infection (Supplementary Table 4). The incidence of nausea was greater with liraglutide 3.0 mg (29.7%) than with placebo (11.7%), and most events were mild or moderate in severity. There was no significant difference in change in heart rate for liraglutide versus placebo. The proportion of individuals reporting a serious AE was 8.2% (23 events in 16 individuals) with liraglutide 3.0 mg and 9.6% (25 events in 19 individuals) with placebo. There were no AEs with fatal outcomes in the trial.
Table 3.
Safety data (on-drug)
| Liraglutide 3.0 mg | Placebo | |||||
|---|---|---|---|---|---|---|
| n (%) | E | R | n (%) | E | R | |
| Number of individuals | 195 | — | — | 197 | — | — |
| Total AEs | 180 (92.3) | 1,139 | 578.3 | 175 (88.8) | 1,053 | 531.2 |
| Serious AEs | 16 (8.2) | 23 | 11.7 | 19 (9.6) | 25 | 12.6 |
| Fatal AEs | 0 (0.0) | 0 | 0.0 | 0 (0.0) | 0 | 0.0 |
| Events leading to treatment discontinuation | 15 (7.7) | 17 | 8.6 | 6 (3.0) | 6 | 3.0 |
| GI AEs | 121 (62.1) | 408 | 207.1 | 92 (46.7) | 202 | 101.9 |
| Nausea | 58 (29.7) | 105 | 53.3 | 23 (11.7) | 27 | 13.6 |
| Constipation | 28 (14.4) | 36 | 18.3 | 17 (8.6) | 21 | 10.6 |
| Diarrhea | 45 (23.1) | 77 | 39.1 | 30 (15.2) | 54 | 27.2 |
| Vomiting | 32 (16.4) | 53 | 26.9 | 12 (6.1) | 13 | 6.6 |
| Abdominal discomfort | 11 (5.6) | 17 | 8.6 | 8 (4.1) | 11 | 5.5 |
| Hypoglycemic episodes | 140 (71.8) | 1,462 | 742.3 | 140 (71.1) | 1,859 | 937.9 |
| ADA classified | ||||||
| Severe | 3 (1.5) | 3 | 1.5 | 2 (1.0) | 2 | 1.0 |
| Asymptomatic | 116 (59.5) | 742 | 376.7 | 116 (58.9) | 988 | 498.4 |
| Documented symptomatic | 92 (47.2) | 662 | 336.1 | 102 (51.8) | 816 | 411.7 |
| Pseudohypoglycemia | 17 (8.7) | 42 | 21.3 | 14 (7.1) | 31 | 15.6 |
| Probable symptomatic | 8 (4.1) | 10 | 5.1 | 18 (9.1) | 22 | 11.1 |
| Unclassifiable | 2 (1.0) | 3 | 1.5 | 0 (0.0) | 0 | 0 |
Safety analysis set. Hypoglycemic episodes were classified using ADA criteria and recorded in individual diaries. Data are from individuals on-drug.
E, number of events; GI, gastrointestinal; n, number of individuals experiencing at least one event; %, percentage of individuals experiencing at least one event; R, event rate per 100 patient-years of exposure.
Using ADA criteria, ≥1 episode of hypoglycemia occurred in 71.8% (742.3 events per 100 patient-years) of the liraglutide 3.0-mg group, compared with 71.1% (937.9 events per 100 patient-years) of the placebo group (Table 3). Documented symptomatic hypoglycemia occurred at rates of 336.1 and 441.7 events per 100 patient-years of exposure with liraglutide 3.0 mg and placebo, respectively. There were three severe hypoglycemic episodes (requiring assistance of another person) with liraglutide 3.0 mg versus two episodes with placebo. Rates of hypoglycemia determined by NovoNordisk criteria are shown in Supplementary Table 5. A greater number of hypoglycemic episodes occurred in individuals treated with SUs in both arms (Supplementary Table 6).
Three acute gallstone disease events occurred in the trial, two with liraglutide 3.0 mg (both cholelithiasis) and one with placebo (gallbladder disorder); none of these were serious AEs. Two cases of pancreatitis in one individual (acute pancreatitis and pancreatitis, both serious) were reported with placebo. A similar number of neoplasm events were reported with liraglutide 3.0 mg (23 events in 19 individuals) and placebo (21 events in 17 individuals) during the in-trial period. Six neoplasm events with liraglutide 3.0 mg and two with placebo were serious (Supplementary Table 7). With the exception of one case of thyroid adenoma, all serious neoplasm events were assessed as malignant; all remaining neoplasm events were benign. There were no reports of breast cancer or medullary thyroid carcinoma with liraglutide 3.0 mg. There were a similar number of cases of depression and suicidal ideation/behavior AEs with liraglutide 3.0 mg and placebo (eight events in seven individuals vs. eight events in eight individuals with placebo).
Conclusions
Weight loss therapy as a primary treatment approach in individuals with overweight or obesity and type 2 diabetes goes beyond glucose control in favor of a more holistic approach addressing the full range of complications and underlying pathophysiological mechanisms driving weight gain. Recently published European Association for the Study of Diabetes/ADA guidelines recommend considering the effect on weight when choosing diabetes treatment (8,9), and the American Association of Clinical Endocrinologists obesity guidelines regard the objective of weight loss therapy to be prevention and treatment of weight-related complications, including type 2 diabetes (10). Weight loss medications have been shown to have a highly favorable therapeutic profile in individuals with obesity/overweight and type 2 diabetes; however, efficacy has not been examined in all subgroups of individuals with diabetes as a function of specific concomitant diabetes medication.
To our knowledge, SCALE Insulin is the first randomized clinical trial to specifically investigate the efficacy and safety of an approved antiobesity medication in individuals with overweight or obesity and type 2 diabetes treated with basal insulin. Our findings demonstrate superiority of liraglutide 3.0 mg versus placebo regarding both percentage of weight loss (ETD −4.3% [95% CI −5.5; −3.2]; P < 0.0001) and proportion of individuals reaching a clinically relevant ≥5% weight loss at week 56 (liraglutide 3.0 mg, 51.8%; placebo, 24.0%; P < 0.0001). Thus, the two primary objectives of the trial were met.
The weight loss findings in the SCALE Insulin trial are in line with those observed in the previously described SCALE Diabetes trial, in which insulin-treated individuals were excluded (19). In SCALE Diabetes, placebo-adjusted weight loss in individuals with overweight or obesity and type 2 diabetes was 2.7% and 4.0% with liraglutide 1.8 mg and 3.0 mg, respectively. Similarly, the proportion of individuals reaching ≥5% weight loss with liraglutide 3.0 mg and placebo in SCALE Insulin was also comparable to SCALE Diabetes (liraglutide 3.0 mg, 54.3%; placebo, 21.4%) (19). Notably, the placebo arm in SCALE Insulin demonstrated greater weight loss than in the SCALE Diabetes trial, despite the fact that the trial population was older, had a greater number of complications, and was on weight-promoting insulin. This is most likely attributable to a more intensive lifestyle intervention. Improvements in several cardiovascular risk factors such as waist circumference, systolic blood pressure, and total cholesterol were also in line with findings of the SCALE Diabetes trial. Direct comparisons between these two trials should take into account differences in trial designs and statistical analyses, including the more intensive lifestyle intervention in the current trial (IBT).
In the Dual Action of Liraglutide and Insulin Degludec (DUAL) II trial, the contribution of liraglutide in a fixed-ratio combination with insulin degludec was investigated regarding efficacy and safety (25). The trial demonstrated that insulin degludec alone had a minimal effect on weight; however, when combined with liraglutide, it resulted in an ETD of −2.5 kg (95% CI −3.2; −1.8; P < 0.0001). While comparisons of these findings to SCALE Insulin should be cautious given the differences in liraglutide doses and study designs, the studies demonstrate that basal insulin combined with liraglutide can result in clinically significant weight loss relative to treatment with insulin alone.
Regarding the parameters of glycemic control in SCALE Insulin, individuals in the liraglutide 3.0-mg group achieved statistically significant and clinically meaningful improvements from baseline to week 56 in HbA1c and mean daytime glucose values. At 56 weeks, there was no significant difference between treatment groups concerning improvements in FPG. Given that all individuals were actively treated with basal insulin to achieve the same glycemic targets in both treatment arms (Supplementary Table 1), this was expected. Importantly, however, similar fasting glucose levels were achieved with an average of 15 units/day lower insulin requirement in the liraglutide group compared with placebo. Superiority with liraglutide 3.0 mg versus placebo was confirmed for reductions in mean HbA1c and mean daytime glucose values despite lower basal insulin requirements. Given the broad range of baseline HbA1c (6–10% [42–86 mmol/mol]), these glycemic improvements are likely the result of the preferential effects of liraglutide on postprandial, rather than preprandial, glucose (as indicated by daily seven-point SMBG profiles) (Supplementary Fig. 6) combined with the significantly greater weight loss versus placebo.
Although treatment with insulin often results in weight gain, the extent to which the weight loss observed with liraglutide 3.0 mg in the present trial was the result of the direct action of liraglutide on feelings of hunger and satiety and possible delay in gastric emptying (26,27), or the result of indirectly reducing insulin requirements and use of SUs, requires further investigation. Furthermore, while individuals on SUs were stratified between the two treatment arms and insulin doses were titrated to achieve similar levels of glycemic control, we are unable to quantify the effect of other OADs on weight. Despite the marked reduction in insulin dose needed to meet glycemic targets with liraglutide 3.0 mg versus placebo, both groups required an increase in total daily insulin dose, as evident at week 56. This was expected in these actively treated individuals with long-standing type 2 diabetes, given the trial duration and the purposeful titration of basal insulin to achieve the same glycemic targets in all individuals. Given the superior weight loss and beneficial glycemic effects of liraglutide 3.0 mg, this treatment group experienced a substantially reduced need for exogenous insulin compared with placebo-treated individuals. It is of interest to consider that being accustomed to treatment with injectable insulins may have had a positive influence on treatment adherence in this trial (84.3% of individuals were on drug at week 56).
In SCALE Insulin, no new safety signals were observed, and the safety profile observed with liraglutide 3.0 mg was in line with that reported in previous trials, with the most common AEs being gastrointestinal in nature. As trial participants were informed about possible gastrointestinal side effects related to treatment prior to the start of the trial, we cannot exclude the possibility of a “precebo effect” having been observed. Gastrointestinal AE findings are also subject to limitations of self-reporting used in current and other SCALE trials. Fewer serious AEs occurred in the liraglutide 3.0-mg group compared with placebo. Improvements in glycemic outcomes in the present trial were achieved with fewer hypoglycemic events per 100 patient-years of exposure compared with the placebo group. This may be related to the higher insulin dose required to achieve glycemic targets in the placebo group when compared with those randomized to liraglutide 3.0 mg and/or the ability of liraglutide to reduce glycemic variability (28). Taken together, liraglutide 3.0 mg had a favorable therapeutic profile; namely, greater weight loss with better glycemic control, with less need for basal insulin and without any increase in hypoglycemia.
Conclusion
In individuals with overweight or obesity and basal insulin-treated type 2 diabetes, liraglutide 3.0 mg was superior to placebo with respect to mean and categorical weight loss at 56 weeks, as well as improvements in glycemic control despite a lower need for basal insulin. No new safety or tolerability issues were observed during the trial, and fewer hypoglycemic events were observed with liraglutide 3.0 mg versus placebo.
Supplementary Material
Article Information
Acknowledgments. The authors thank the people who participated in this study and Jamie Cozens, Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc (supported by Novo Nordisk), for writing assistance. A complete list of the SCALE Insulin trial’s investigators can be found in the Supplementary Data online.
Duality of Interest. The study was sponsored by Novo Nordisk A/S. W.T.G. reports institutionally sponsored research grants from Merck/Pfizer, AstraZeneca, Sanofi, Novo Nordisk, and Lexicon Pharmaceuticals, Inc. and consulting fees and/or honoraria for advisory board participation from Novo Nordisk, American Medical Group Association, BOYDSense, Gilead Sciences, Inc., Amgen, and Sanofi. A.L.B. reports funds to conduct the trial, global panel honoraria, and travel expenses for meetings from Novo Nordisk during the conduct of the study, and personal fees for educational talks from Sanofi, AstraZeneca, Eli Lilly and Company, and Novo Nordisk. D.D. reports consulting fees and/or honoraria for advisory board participation and speaking from Merck Sharp & Dohme, Boehringer Ingelheim, Novo Nordisk, Sanofi, AstraZeneca, and Teva Pharmaceutical Industries Ltd. and institutionally sponsored research grants from Boehringer Ingelheim. G.M. has received grants from Novo Nordisk and consulting fees and/or honoraria for advisory board participation and speaking for Fractyl Laboratories Inc. and Johnson & Johnson. S.D.P. reports grants, personal fees, and nonfinancial support from Novo Nordisk during the conduct of this study; personal fees and nonfinancial support including advisory boards participation, speaker fees, and travel fees from Novo Nordisk and Bausch Health/Valeant Pharmaceuticals International, Inc.; personal fees and nonfinancial support from Janssen Pharmaceuticals; grants, personal fees, and nonfinancial support from Eli Lilly and Company, AstraZeneca, Boehringer Ingelheim, and Sanofi; personal fees from Merck, Prometic BioTherapeutics Inc., and Pfizer; and grants and personal fees from Abbott Laboratories. A.S., D.Sk., and C.J. are employees of Novo Nordisk. D.Su. reports research grants from Novo Nordisk. O.M. reports fees related to advisory boards from Novo Nordisk, Eli Lilly and Company, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, and AstraZeneca; grants paid to institution as study physician from AstraZeneca and Bristol-Myers Squibb; research grant support through Hadassah Hebrew University Hospital from Novo Nordisk; and speaker bureau participation for AstraZeneca, Bristol-Myers Squibb, Novo Nordisk, Eli Lilly and Company, Sanofi, Novartis, Merck Sharp & Dohme, Boehringer Ingelheim, and Teva Pharmaceutical Industries Ltd. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors were involved in the analysis of the data, the preparation of the manuscript, and the decision to submit it for publication and verify the accuracy and completeness of the data and analyses. W.T.G. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 26th European Congress on Obesity 2019, Glasgow, U.K., 28 April–1 May 2019.
Footnotes
Clinical trial reg. no. NCT02963922, clinicaltrials.gov
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1745/-/DC1.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Bray GA, Kim KK, Wilding JPH; World Obesity Federation . Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017;18:715–723 [DOI] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–486 [DOI] [PubMed] [Google Scholar]
- 4.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529 [DOI] [PubMed] [Google Scholar]
- 5.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: analysis of physician electronic health records in the US from 2009-2011. J Diabetes Complications 2016;30:212–220 [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–2498 [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvey WT, Mechanick JI, Brett EM, et al.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22(Suppl. 3):1–203 [DOI] [PubMed] [Google Scholar]
- 11.Pi-Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care 2005;28:1526–1527 [DOI] [PubMed] [Google Scholar]
- 12.Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges? Diabetes Care 2014;37:1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 14.Kenkre J, Tan T, Bloom S. Treating the obese diabetic. Expert Rev Clin Pharmacol 2013;6:171–183 [DOI] [PubMed] [Google Scholar]
- 15.Apovian CM, Aronne LJ, Bessesen DH, et al.; Endocrine Society . Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–362 [DOI] [PubMed] [Google Scholar]
- 16. Victoza (liraglutide [rDNA origin] injection), solution for subcutaneous use [package insert]. Plainsboro, NJ, Novo Nordisk, 2010.
- 17. Xultophy 100/3.6 (insulin degludec and liraglutide injection), for subcutaneous use [package insert]. Plainsboro, NJ, Novo Nordisk, 2016.
- 18. Saxenda (liraglutide) injection, for subcutaneous use [package insert]. Plainsboro, NJ, Novo Nordisk, 2018.
- 19.Davies MJ, Bergenstal R, Bode B, et al.; NN8022-1922 Study Group . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687–699 [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki, 2004. Available from https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/doh-oct2004/. Accessed 27 February 2020
- 21.Wadden TA, Tronieri JS, Sugimoto D, et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring) 2020;28:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter JR, Roger JH, Kenward MG. Analysis of longitudinal trials with protocol deviation: a framework for relevant, accessible assumptions, and inference via multiple imputation. J Biopharm Stat 2013;23:1352–1371 [DOI] [PubMed] [Google Scholar]
- 24.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Guideline: Estimands and Sensitivity Analysis in Clinical Trials E9(R1) [Internet], 2017. Available from https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical_en.pdf. Accessed 27 February 2020
- 25.Buse JB, Vilsbøll T, Thurman J, et al.; NN9068-3912 (DUAL-II) Trial Investigators . Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014;37:2926–2933 [DOI] [PubMed] [Google Scholar]
- 26.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017;2:890–899 [DOI] [PubMed] [Google Scholar]
- 27.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes 2014;38:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj HS, Venn K, Ye C, et al. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION study). Diabetes Care 2017;40:194–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.