Cardiac stress testing in patients with diabetes mellitus (DM) is a topic of much debate (1,2). The clinical heterogeneity and varied interpretation of atypical symptoms in this population may lead to significant variation in cardiac stress testing with downstream implications in health care expenditure. We evaluated facility-level variation in cardiac stress test use among patients with DM across the Veterans Affairs (VA) health care system.
We identified patients with DM aged ≥18 years with a primary care clinic visit during VA fiscal year 2014 at one of the 130 VA facilities and associated clinics. Patient demographics and medical history were identified using clinical data sources and ICD-9-CM codes. We calculated diagnostic cost group relative risk score (DCG-RRS), a validated surrogate for overall illness burden. Facility-level cardiac stress test use was defined as the number of stress tests performed per facility per 100 patients with DM in the preceding 365 days. Stress testing modalities evaluated included exercise treadmill test, stress echocardiography, and SPECT/PET MPI (myocardial perfusion imaging [single photon emission computed tomography or positron emission tomography]). Facilities with <10 studies/year were excluded. Median risk ratio (MRR), a well-established measure of facility-level variation (3), was derived by constructing multivariable hierarchical modified regression models adjusted for patient clustering and modeled patient characteristics as filter effects within each facility and individual facilities as a random effect (4). Unadjusted and adjusted MRRs (adjustment for patient, provider, and facility-level variables) were calculated for overall stress testing and individual stress modalities. MRR represents the likelihood of two random facilities differing in stress test use for two similar patients. For example, MRR of 1 corresponds to no facility-level variation, whereas MRR of 1.5 would suggest 50% variation in stress test use for two facilities with similar patients. We conducted sensitivity analyses to evaluate variation among patients with DM with and without ischemic heart disease (IHD). Lastly, geographic variation in stress test use was depicted (Fig. 1) using Federal Information Processing Standards (FIPS) codes. The Michael E. DeBakey VA Medical Center granted institutional review board approval.
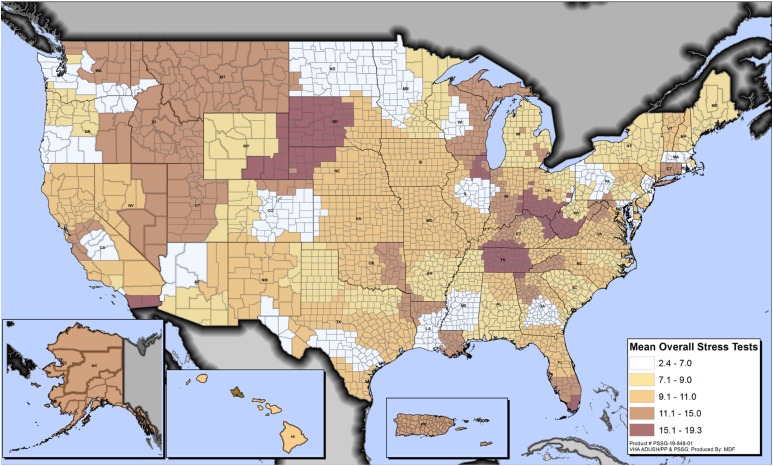
Figure 1.
Variation in cardiac stress test use rates among patients with diabetes by county across all VA health care facilities. Rates represent the number of stress tests performed per 100 patients with diabetes per county per year.
Our study included 1,448,906 patients with DM, who were predominantly white (65.1%) and male (96.3%) with high prevalence of hypertension (83.1%) and IHD (33.1%). The mean DCG-RRS was 1.69. Unadjusted facility-level rates of stress testing ranged from 2.40 to 19.32 studies per 100 patients with a mean ± SD of 9.77 ± 3.39 studies per 100 patients. Unadjusted MRR for overall stress test use was 1.52 (95% CI 1.44–1.60), which decreased to 1.38 (95% CI 1.32–1.43) after adjustment for patient-level variables (age, sex, race, hypertension, IHD, DCG-RRS, and use of insulin) and 1.37 (95% CI 1.32–1.43) after further adjustment for provider and facility-level variables (percutaneous coronary intervention accessibility and number of cardiology visits in preceding 12 months). This indicates a 37% variation in the probability of two similar patients with DM at two random facilities receiving a stress test. Similar magnitude of variation was observed across various stress modalities: exercise treadmill test (MRRadjusted 1.50, 95% CI 1.42–1.57), stress echocardiogram (MRRadjusted 2.67, 95% CI 2.24–3.10), and SPECT/PET MPI (MRRadjusted 1.44, 95% CI 1.38–1.51). Sensitivity analyses among patients with DM with or without IHD revealed an adjusted variation in overall stress test use of 39% in both subgroups. Fig. 1 shows interstate and intrastate facility-level variation in overall stress test use.
Our results suggest that despite adjustment for a multitude of covariates, significant residual variation in overall stress test use exists among veterans with DM with or without IHD. Several factors may explain these findings. Clinical heterogeneity among patients with DM and atypical or absent presenting symptoms creates a level of uncertainty that may lead to substantial variation in stress testing. Variation may also be attributed to misuse or overuse of stress testing due to insufficient knowledge about appropriate use criteria (5), simultaneous practice, or recent training in a non-VA health care system (where factors of financial incentives or “defensive medicine” may be at play). Underuse of stress testing by facilities with low use rates, secondary to constraints in facility-based resources or higher reliability on cardiac computed tomographic angiography, may also result in significant variation. A marked nationwide geographic variation may also be attributed to inefficient allocation of health care resources resulting in a supply-demand mismatch. Our results from a large health care system serve as benchmark data for variation in stress testing. Future studies are warranted to assess system-wide appropriateness of stress testing, assess patient-level symptom data, and conduct qualitative analyses to understand individual provider-level drivers behind such variation.
In conclusion, we demonstrated a 37% residual facility-level variation in stress test use. This level of unexplained variation across a nationwide health care system presents an opportunity for conglomeration of our methodology along with identification of provider-level variables, patient outcomes data, and appropriateness of stress testing in order to improve quality of stress testing.
Article Information
Funding. S.S.V. was supported by a Department of Veterans Affairs Health Services Research & Development Service Investigator-Initiated Grant (IIR 16-072), an American Heart Association Beginning Grant-in-Aid (14BGIA20460366), the American Diabetes Association Clinical Science and Epidemiology award (1-14-CE-44), and the Houston VA Health Services Research & Development Center for Innovations grant (CIN13-413). N.R.S. was supported by an Agency for Healthcare Research and Quality award (5K12HS022998). Support for VA/Centers for Medicare and Medicaid Services data was provided by the Department of Veterans Affairs, VA Health Services Research & Development Service, VA Information Resource Center (project numbers SDR 02-237 and 98-004).
The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs or the U.S. government.
Duality of Interest. K.N. serves on the advisory board of The Medicines Company and Regeneron. S.W.W. receives investigator-initiated research support to the Denver Research Institute from ABIOMED, Cardiovascular Systems Incorporated, and Merck Pharmaceuticals. C.M.B. receives grant/research support (all paid to institution, not individual) from Akcea, Amgen, Esperion, Novartis, Regeneron, and Sanofi-Synthelabo and serves as a consultant for Abbott Diagnostics, Akcea, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Intercept, Matinas BioPharma Inc., Merck, Novartis, Regeneron, and Sanofi-Synthelabo. S.S.V. receives an honorarium from the American College of Cardiology (Associate Editor for Innovations, acc.org) and serves on the steering committee of the Patient and Provider Assessment of Lipid Management Registry (PALM) at the Duke Clinical Research Institute (no financial remuneration). No other potential conflicts of interest relevant of this article were reported.
Author Contributions. D.M. researched data, wrote the manuscript, and revised the manuscript. S.T.A. wrote the manuscript. N.R.S. reviewed and edited the manuscript. D.J.R. compiled and analyzed the data. J.M.A. conceptualized the project and reviewed the manuscript. K.N. reviewed and edited the manuscript. I.R.H. reviewed and edited the manuscript. I.Y.E. reviewed and edited the manuscript. S.W.W. compiled data and reviewed and edited the manuscript. M.H.A.-M. reviewed and edited the manuscript. H.J. reviewed and edited the manuscript. C.M.B. reviewed and edited the manuscript. L.A.P. reviewed and edited the manuscript. S.S.V. conceptualized the project, researched data, reviewed and edited manuscript, and revised the manuscript. S.S.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Young LH, Wackers FJ, Chyun DA, et al.; DIAD Investigators . Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerc OF, Fuchs TA, Stehli J, et al. . Non-invasive screening for coronary artery disease in asymptomatic diabetic patients: a systematic review and meta-analysis of randomised controlled trials. Eur Heart J Cardiovasc Imaging 2018;19:838–846 [DOI] [PubMed] [Google Scholar]
- 3.Pokharel Y, Gosch K, Nambi V, et al. . Practice-level variation in statin use among patients with diabetes: insights from the PINNACLE registry. J Am Coll Cardiol 2016;68:1368–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein H. Multilevel Statistical Models. John Wiley & Sons, 2011
- 5.Wolk MJ, Bailey SR, Doherty JU, et al.; American College of Cardiology Foundation Appropriate Use Criteria Task Force . ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;63:380–406 [DOI] [PubMed] [Google Scholar]