Abstract
OBJECTIVE
The effect of early-life antibiotic treatment on the risk of type 1 diabetes is debated. This study assessed this question, applying a register-based design in children up to age 10 years including a large sibling-control analysis.
RESEARCH DESIGN AND METHODS
All singleton children (n = 797,318) born in Sweden between 1 July 2005 and 30 September 2013 were included and monitored to 31 December 2014. Cox proportional hazards models, adjusted for parental and perinatal characteristics, were applied, and stratified models were used to account for unmeasured confounders shared by siblings.
RESULTS
Type 1 diabetes developed in 1,297 children during the follow-up (median 4.0 years [range 0–8.3]). Prescribed antibiotics in the 1st year of life (23.8%) were associated with an increased risk of type 1 diabetes (adjusted hazard ratio [HR] 1.19 [95% CI 1.05–1.36]), with larger effect estimates among children delivered by cesarean section (P for interaction = 0.016). The association was driven by exposure to antibiotics primarily used for acute otitis media and respiratory tract infections. Further, we found an association of antibiotic prescriptions in pregnancy (22.5%) with type 1 diabetes (adjusted HR 1.15 [95% CI 1.00–1.32]). In general, sibling analysis supported these results, albeit often with statistically nonsignificant associations.
CONCLUSIONS
Dispensed prescription of antibiotics, mainly for acute otitis media and respiratory tract infections, in the 1st year of life is associated with an increased risk of type 1 diabetes before age 10 years, most prominently in children delivered by cesarean section.
Introduction
Type 1 diabetes is one of the most common chronic diseases in childhood. Several proposed early-life risk factors, including antibiotic treatment, are hypothesized to influence the risk of type 1 diabetes through alterations of the gut microbiome composition and subsequent effects on the immune system development (1). Children with type 1 diabetes are reported to have a lower microbial diversity of their gut flora than control children (2), and a marked decrease in diversity before type 1 diabetes onset is reported (3); however, whether the microbiota play an active role in the pathogenesis is unclear. Antibiotic exposure in early life delays the microbiota maturation (4). A recent report from The Environmental Determinants of Diabetes in the Young (TEDDY) study suggested decreased abundance of five Bifidobacterium species (including Bifidobacterium dentium) after antibiotic treatment and that children with signs of islet autoimmunity have a lower abundance of Bifidobacterium dentium than healthy control children (5). However, diabetes-prone rodent models have shown divergent results dependent on the type of antibiotics, with both accelerated (6,7) and reduced (8,9) type 1 diabetes development.
Although some epidemiological studies have failed to detect an association between prenatal (10,11) or early antibiotic exposure (10) and subsequent type 1 diabetes, others have found an association with multiple prescriptions and broad-spectrum antibiotics during early childhood (12,13). There is also some evidence that the association of antibiotics with type 1 diabetes could be modified by mode of delivery, with larger effects sizes in those with cesarean section births (14). Few studies have had sufficient statistical power to perform detailed analyses of the potential influence of antibiotics on type 1 diabetes. So far, only two overlapping Danish studies have reported >1,000 events, and the majority (71.8%) of the children in these studies were exposed to antibiotics during the first 2 years of life. Furthermore, many observational studies suffer from unmeasured confounding. This can partly be remedied through sibling analyses, which inherently account for unmeasured confounding by factors shared by full siblings such as paternal and maternal characteristics, genetic factors, and family environment (15). Sweden has among the highest type 1 diabetes incidence rates in the world and a relatively low antibiotic prescription rate, enabling a large register-based study design including a large proportion of nonexposed children and a sibling analysis as well as a description of the indications underlying the prescriptions.
The aim of this study was to assess the impact of prenatal or early childhood exposure to antibiotics on the risk of type 1 diabetes (up to age 10 years) in a nationwide setting using a conventional observational design complemented by a sibling analysis. We further investigated this association regarding type and number of prescriptions and interactions by mode of delivery, sex, birth year, and genetic predisposition to type 1 diabetes.
Research Design and Methods
Study Population
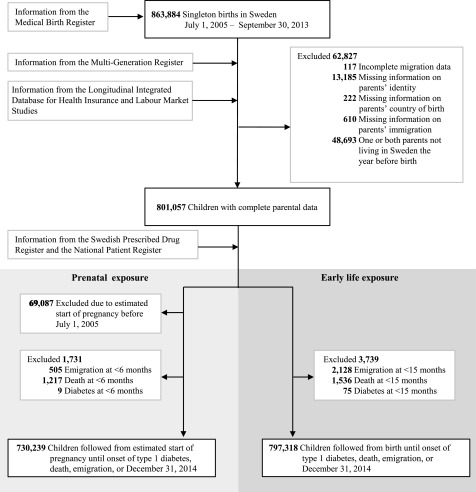
The study cohort consisted of all singleton children born in Sweden between 1 July 2005 and 30 September 2013 identified from the Medical Birth Register (MBR) (Fig. 1). The MBR includes 96–99% of all deliveries in Sweden (16). Linkage to other registers was enabled through the Swedish personal identity number. To avoid including events of neonatal diabetes, follow-up started at age 6 months in the analysis of prenatal exposure. As undiagnosed type 1 diabetes often leads to infections and sometimes increased use of antibiotics, which would lead to reverse causation, we started follow-up at 15 months when studying exposure in the 1st year of life. Thus, the analyses excluded children with at least one dispensed prescription of insulin (Anatomical Therapeutic Chemical [ATC] classification system: A10A) in the Swedish Prescribed Drug Register or who died or emigrated before age 6 months/15 months (Fig. 1). Additional information about the registers is available in Supplementary Table 1.
Figure 1.
Flow diagram of the study population.
Outcome
We identified type 1 diabetes events in data from the Swedish Prescribed Drug Register, validated for this purpose (17), and the National Patient Register (NPR). We obtained diagnoses, registered according to the ICD-10, from 1 July 2005 until 31 December 2013 from the NPR. The Swedish Prescribed Drug Register data were available from 1 July 2005 through 31 December 2014. Children were defined as having type 1 diabetes if they had at least one dispensed prescription of insulin (ATC: A10A). Date of onset was estimated as the date of type 1 diabetes diagnosis (ICD-10: E10) in the NPR or as 7 days before the first prescription of insulin in the Swedish Prescribed Drug Register if the NPR diagnosis was missing.
Exposure
We retrieved information on all dispensed systemic antibiotics (ATC: J01) from the Swedish Prescribed Drug Register from 1 July 2005 to 30 September 2013 in mothers during the pregnancy and to 30 September 2014 in children during their 1st year.
In Sweden there is no over-the-counter sale of antibiotics without a prescription from a physician. The antibiotics were classified into narrow-spectrum and broad-spectrum (18) and according to the fourth level of ATC as described in the Supplementary Data. We also created groups based on probable site of infection based on the type of antibiotics from ATC: 1) antibiotics with recommended use for otitis media and other respiratory tract infections (amoxicillin, penicillin, cephalosporin, and macrolides) and 2) antibiotics with recommended use for urinary tract or skin and soft tissue infection (pivmecillinam, trimethoprim, sulfonamide, ciprofloxacin, norfloxacin, nitrofurantoin, cloxacillin, flucloxacillin, and dicloxacillin) (19).
Free-text information about the reason for the prescription, from the prescribing physician, was available for approximately half of all prescriptions. For a descriptive validation of the classification of site of infection in children, we drew a random sample (n = 800) of the dispensed prescriptions and let two independent reviewers classify the site of infection based on the text. Infection site was classified as one of the following categories: ear, lower respiratory tract, upper respiratory tract, unspecified respiratory tract, urinary tract, skin, and gastrointestinal tract. All disagreements were resolved by consensus.
We set the start date of pregnancy as the date of birth of the child minus estimated gestational age at birth. Prenatal exposure was evaluated as any maternal dispensed prescription during the pregnancy as a whole and by trimester (1–91 days, 92–189 days, and ≥190 days, respectively) (20). This analysis was restricted to children from pregnancies with an estimated start date on or after 1 July 2005 (the Swedish Prescribed Drug Register start date).
Potential Confounders
Potential confounders were selected for adjustment based on directed acyclic graphs (DAGs) (21), taking into account prior knowledge regarding their effect on antibiotic exposure and type 1 diabetes (Supplementary Fig. 1). A DAG illustrates the assumptions about the relationship between the exposure and outcome and how this relationship is influenced by other variables. We used the DAGitty software that applies d-separation criteria to identify the confounders needed to be included in the model for estimating the total effect of antibiotics on type 1 diabetes (22). We collected information concerning the child’s birth and maternal BMI, smoking, and age from the MBR. Region of residence and parental income and education were collected from the Longitudinal Integration Database for Health Insurance and Labour Market Studies. Detailed information appears in the Supplementary Data.
Statistical Methods
We used Cox proportional hazards (PHs) models, with attained age as timescale, to assess the association between exposure to antibiotics in 1) prenatal life and 2) the 1st year of life with risk of type 1 diabetes. In the analysis of exposure in prenatal life, the follow-up started at age 6 months, and in the analysis of exposure in the 1st year of life, the follow-up started at age 15 months. Children were censored at emigration, death, or end of follow-up (31 December 2014), whichever came first. A robust sandwich estimator of variance was used to account for the dependence of children within the same family (23). Results are reported as hazards ratios (HRs) with 95% CIs. The PHs assumption was met.
Both models were adjusted for parity, smoking during early pregnancy, maternal type 1 diabetes, maternal age at delivery, parental country of birth, parental education, disposable income, birth year, birth season, region of residence, and population density. For prenatal exposure, we additionally adjusted for maternal BMI. For the exposure to antibiotics in early childhood, we additionally adjusted for mode of delivery, sex, gestational age, paternal type 1 diabetes, and small or large for gestational age. We used the Cox PHs model with standardization to estimate the number of children needed to be exposed to antibiotics to cause one additional case of type 1 diabetes diagnosed before age 9 years (24,25). Only individuals with complete data on covariates were included.
Sibling Analysis
A sibling analysis was performed with a stratified Cox PHs model, and the shared unmeasured confounders are thereby absorbed into an unspecified family-specific baseline hazard. Only families with at least one type 1 diabetes event and at least one individual free of type 1 diabetes at the age of event in the type 1 diabetes case contributed to the estimations in the sibling analyses; informative sample size is thus reported for sibling analyses.
Alternate Definitions of the Exposure
We compared narrow- and broad-spectrum antibiotics, site of infection based on type of antibiotic, and specific groups of antibiotics. To examine a possible dose-response relationship, the number of dispensed prescriptions was categorized as none, one, two to three, and four or more. Furthermore, we performed analyses to explore the association with antibiotics in fetal life split by trimester. All trimesters were included in the same model. We also fitted a model including exposure during the first 6 months of life.
Antibiotic use during hospitalization is not reported in the Swedish Prescribed Drug Register, and therefore, hospital-treated children without antibiotic prescriptions at discharge are likely to be misclassified as nonexposed. In a sensitivity analysis, we therefore investigated whether excluding preterm children (born <37 gestational weeks) and children hospitalized at any time in their 1st year of life without a dispensed prescription of antibiotics affected the results. Because repeated urinary tract infections and urinary tract anomalies may require prophylactic antibiotics, we also performed a sensitivity analysis excluding nitrofurantoin (J01XE01) and trimethoprim (J01EA01).
Validation of Classification of Site of Infection
Of 800 prescriptions dispensed in the 1st year of life, 372 (46%) were found to be informative and could be used for a descriptive validation. We report the distribution of site of infection based on the text on the prescription. A comparison of our ATC-based classification criteria with the text on the prescriptions was also performed, and we report the proportion of agreement.
Interactions
We performed subgroup analyses by adding one multiplicative interaction term at a time to the adjusted model. The subgroups were defined by mode of delivery, sex, genetic predisposition to type 1 diabetes (at least one parent with the diagnosis), and birth year (2005–2008 vs. 2009–2013).
Analyses were performed using SAS 9.4 software (SAS Institute) and R 3.3 software (The R Foundation). The regional ethical review board in Stockholm, Sweden, approved this study and allowed the researchers to waive the requirement for obtaining informed consent or parental permission (Dnr 2009/559-31/5).
Results
Cohort Description
The 797,318 children in the study cohort had a median follow-up of 4.0 years from age 15 months (range 1 day-8.3 years, total 3,212,023 person-years). There were 1,297 events of type 1 diabetes recorded at a mean age of 4.2 (SD 2.0) years. There were 730,221 children born from pregnancies estimated to be conceived on or after 1 July 2005 (Fig. 1). In total, 164,098 (22.5%) children were considered exposed to antibiotics in prenatal life and 189,682 (23.8%) children in the 1st year of life.
Of 800 prescriptions dispensed in the 1st year of life, 372 were found to be informative. A total of 69.9% (n = 259) were prescribed for ear infections, 11.6% (n = 43) for urinary tract infections, and 8.9% (n = 33) for infections in the skin and soft tissue. For β-lactamase–sensitive penicillin (J01CE) prescriptions (n = 207), 89.9% (n = 186) were prescribed for ear infections and 7.2% (n = 15) for upper respiratory tract. Applying the ATC-based classification criteria that we used in the analysis of the entire cohort, 293 of the 323 prescriptions (90.7%) classified as “otitis media and other respiratory tract infections” agreed with the text on the prescription and so did 48 of 50 prescriptions (96.0%) classified as urinary tract or skin and soft tissue infection antibiotics (Supplementary Table 2).
The most common dosage forms are presented in Supplementary Table 3. Child and family characteristics are described in Table 1 and Supplementary Table 4.
Table 1.
Child and family characteristics for children born in Sweden between 1 July 2005 and 30 September 2013 by antibiotic exposure
| Antibiotic exposure | ||||
|---|---|---|---|---|
| Prenatal life* | First year of life† | |||
| No | Yes | No | Yes | |
| Children, n | 566,141 | 164,098 | 607,636 | 189,682 |
| Parity, n (%) | ||||
| 1 | 253,677 (44.8) | 61,059 (37.2) | 284,118 (46.8) | 59,925 (31.6) |
| 2 | 209,774 (37.1) | 67,653 (41.2) | 217,512 (35.8) | 85,120 (44.9) |
| ≥3 | 102,690 (18.1) | 35,386 (21.6) | 106,006 (17.4) | 44,637 (23.5) |
| Mother’s BMI‡, median (IQR), kg/m2 | 23.6 (21.5, 26.6) | 23.9 (21.6, 27.3) | 23.5 (21.5, 26.6) | 23.9 (21.6, 27.1) |
| Missing | 23,211 (4.1) | 6,433 (3.9) | 27,098 (4.5) | 7,080 (3.7) |
| Cesarean delivery, n (%) | 91,491 (16.2) | 30,372 (18.5) | 99,694 (16.4) | 33,336 (17.6) |
| Elective before start of labor | 36,397 (6.4) | 13,410 (8.2) | 39,289 (6.5) | 14,966 (7.9) |
| Emergency | 53,887 (9.5) | 16,507 (10.1) | 58,948 (9.7) | 17,831 (9.4) |
| Missing | 1,207 (0.2) | 455 (0.3) | 1,457 (0.2) | 539 (0.3) |
| Type 1 diabetes, n (%) | ||||
| Mother | 3,292 (0.6) | 1,877 (1.1) | 4,133 (0.7) | 1,449 (0.8) |
| Father | 4,181 (0.7) | 1,319 (0.8) | 4,562 (0.8) | 1,417 (0.7) |
| Age at child’s birth, median (IQR), years | ||||
| Father | 33 (29, 37) | 33 (29, 37) | 33 (29, 37) | 33 (29, 37) |
| Mother | 30 (27, 34) | 31 (27, 34) | 30 (27, 34) | 31 (27, 34) |
| Mother born in Sweden, n (%) | 461,051 (81.4) | 132,739 (80.9) | 501,097 (82.5) | 149,730 (78.9) |
| Father born in Sweden, n (%) | 459,694 (81.2) | 130,998 (79.8) | 500,078 (82.3) | 147,370 (77.7) |
| Girls, n (%) | 274,825 (48.5) | 79,843 (48.7) | 303,342 (49.9) | 83,988 (44.3) |
| Gestational age (weeks), n (%) | ||||
| <38 | 51,469 (9.1) | 17,599 (10.7) | 56,181 (9.2) | 18,519 (9.8) |
| 38 | 77,073 (13.6) | 24,961 (15.2) | 83,098 (13.7) | 28,395 (15.0) |
| 39–40 | 296,155 (52.3) | 83,327 (50.8) | 315,667 (52.0) | 97,935 (51.6) |
| ≥41 | 141,444 (25.0) | 38,211 (23.3) | 152,512 (25.1) | 44,779 (23.6) |
| Missing | 0 (0.0) | 0 (0.0) | 178 (<0.1) | 54 (<0.1) |
| Birth weight, n (%) | ||||
| Small for gestational age | 11,693 (2.1) | 3,335 (2.0) | 12,671 (2.1) | 3,571 (1.9) |
| Normal | 534,206 (94.4) | 153,740 (93.7) | 573,158 (94.3) | 177,851 (93.8) |
| Large for gestational age | 19,533 (3.5) | 6,788 (4.1) | 20,890 (3.4) | 7,957 (4.2) |
| Missing | 709 (0.1) | 235 (0.1) | 917 (0.2) | 303 (0.2) |
IQR, interquartile range.
Children from pregnancies with an estimated start date on or after 1 July 2005. At least one antibiotic prescription during estimated duration of pregnancy.
At least one dispensed antibiotic prescription in the child’s 1st year of life.
At the first visit at the antenatal clinic.
Prenatal Exposure to Antibiotics
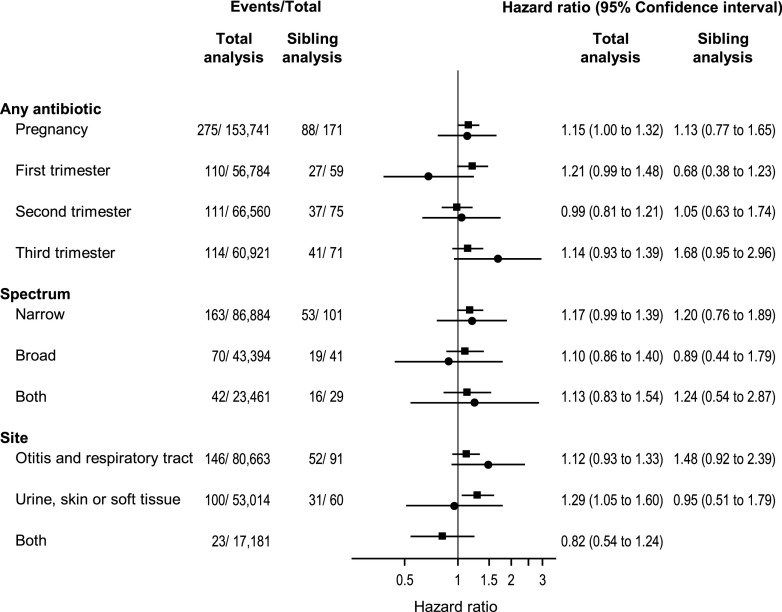
Type 1 diabetes incidence rate was 39.9/100,000 person-years among children exposed to antibiotics in prenatal life and 34.0/100,000 person-years among the nonexposed. Exposure to any antibiotics in prenatal life was associated with an increased risk of type 1 diabetes (adjusted [a]HR 1.15 [95% CI 1.00–1.32]. We also found an association with urinary tract or skin and soft tissue infection antibiotics (aHR 1.29 [95% CI 1.05–1.60]) (Fig. 2). However, both associations were nonsignificant in the sibling analysis (Fig. 2). Of all antibiotics, prescriptions with nitrofurantoin and trimethoprim constituted 20%, and exclusion of these prescriptions yielded an aHR of 1.08 (95% CI 0.94–1.25) for any antibiotics and 1.18 (95% CI 0.90–1.55) for antibiotics used for urinary tract or skin and soft tissue infections. Furthermore, there was evidence supporting an interaction with birth year (P = 0.031), with larger effect estimates during the second half of the study period (Supplementary Table 5).
Figure 2.
The association between exposure to antibiotics in prenatal life and childhood-onset type 1 diabetes in 685,002 children (with complete data). Only families with at least one type 1 diabetes event and at least one individual free of type 1 diabetes at the age of event in the individual with type 1 diabetes are included in the sibling analysis (n = 923). ▪, total analysis; ●, sibling analysis.
Exposure to Antibiotics During 1st Year of Life
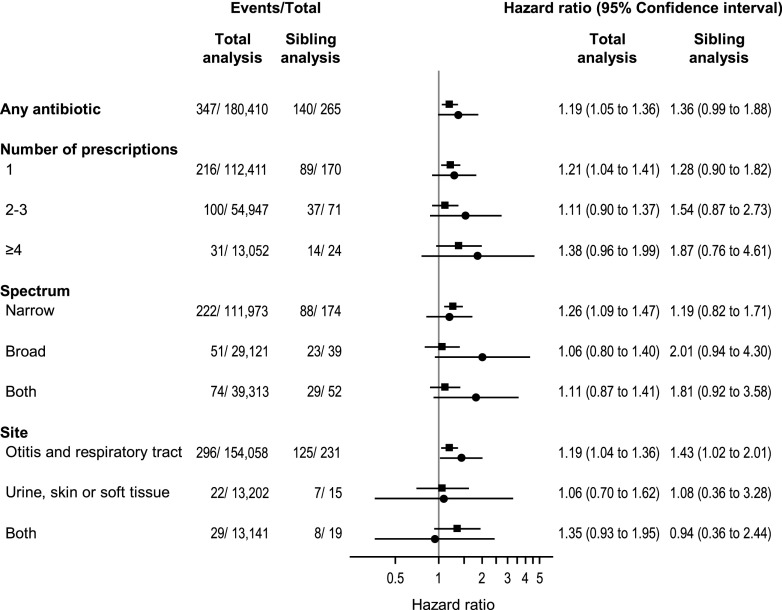
The type 1 diabetes incidence rate was 44.3/100,000 person-years among children exposed to antibiotics in the 1st year of life and 39.0/100,000 person-years among the nonexposed. Exposure to antibiotics in the 1st year of life was associated with an increased risk of type 1 diabetes (aHR 1.19 [95% CI 1.05–1.36]) (Fig. 3). If causal, the estimated number of children needed to be exposed to antibiotics to cause 1 additional case of type 1 diabetes before age 9 years was 1,475 (95% CI 356–2,594). The sibling analysis provided an aHR of 1.36 (95% CI 0.99–1.88) (Fig. 3). We found evidence supporting an interaction between antibiotic exposure and mode of delivery (P for interaction = 0.016), but not for sex (P = 0.54), genetic predisposition (P = 0.56), or birth year (P = 0.91) (Supplementary Table 5). The aHR was 1.10 (95% CI 0.96–1.28) in vaginally delivered children and 1.60 (95% CI 1.22–2.08) in children delivered by cesarean section. The most common complications of labor and delivery in the cesarean deliveries are presented in Supplementary Table 6.
Figure 3.
The association between exposure to antibiotics in the 1st year of life and childhood-onset type 1 diabetes in 760,907 children (with complete data). Only families with at least one type 1 diabetes event and at least one individual free of type 1 diabetes at the age of event in the individual with type 1 diabetes are included in the sibling analysis (n = 691). ▪, total analysis; ●, sibling analysis.
We found no dose-response relationship in the total cohort (Fig. 3). Although no statistically significant trend was observed, we noted that the risk estimates increased with the number of antibiotic prescriptions in the sibling analysis. Exposure to narrow-spectrum antibiotics was associated with an increased risk of type 1 diabetes in the analysis of the total cohort (Fig. 3). Exposure to antibiotics used to treat acute otitis media and other respiratory tract infections was associated with an increased risk in both the total (aHR 1.19 [95% CI 1.04–1.36]) and sibling analysis (aHR 1.43 [95% CI 1.02–2.01]). β-Lactamase–sensitive penicillin (J01CE) was dispensed to 72% (n = 136,426) of the exposed children and was associated with an increased risk of type 1 diabetes (aHR 1.23 [95% CI 1.06–1.42]) (Supplementary Table 7).
Restricting the exposure to the first 6 months yielded an aHR of 1.26 (95% CI 1.04–1.53) in the total cohort analysis and an aHR of 1.19 (95% CI 0.75–1.89) in the sibling analysis (Supplementary Fig. 2). Excluding preterm born children (n = 37,407) from the main analysis yielded an aHR of 1.20 (95% CI 1.06–1.36), and excluding children who were hospitalized sometime during their 1st year of life without an outpatient-dispensed prescription of antibiotics (n = 90,740) yielded an aHR of 1.20 (95% CI 1.06–1.37). Nitrofurantoin and trimethoprim comprised 3% of all antibiotic prescriptions in the 1st year of life, and exclusion of them from the main analysis yielded an aHR of 1.20 (95% CI 1.06–1.37).
Conclusions
In this large cohort study, dispensed antibiotic prescriptions in the 1st year of life were associated with an increased risk of type 1 diabetes before age 10 years. Estimates were similar, although not statistically significant, after additional adjustment for unmeasured confounding shared by siblings. The observed association seems to be driven by exposure to antibiotics used for otitis media and other respiratory tract infections. This association remained statistically significant in the sibling analysis. Furthermore, use of antibiotics was associated with an increased risk of diabetes among children delivered by cesarean section, but the association was less clear for children delivered vaginally. We further found some evidence that prenatal exposure to any antibiotics and to antibiotics used to treat urinary tract or skin and soft tissue infection was associated with an increased risk of type 1 diabetes in the analysis of the total cohort but not in the sibling analysis.
Other Studies
In contrast to our study, a Danish population-based study (1,578 events) (13) and a Norwegian population-based study (836 events) (10) found no overall association between early-life antibiotic exposure and type 1 diabetes. However, the Danish study reported an association with exposure to five or more courses of any (and broad-spectrum) antibiotics in the first 2 years of life (13). Similar results were observed in a Finnish case-control study (437 events) (12). Although suggested in our sibling analysis, no clear dose-response relationship was observed in our data. The Norwegian and Danish studies (10,13) were both nationwide population–based register studies in analogy with the current study. However, the Norwegian study (10) had a slightly different exposure window, and the Danish study (13) was a case-control study adjusting for few confounders. The differences in results could be due to study design or chance but also country-specific factors. Early exposure to antibiotics is more common in Denmark than in Sweden (26), with 49% of children treated during their 1st year of life compared with 21% in our study. Norway and Sweden have similar antibiotic prescription routines, but the incidence rate of type 1 diabetes in our study was almost twice as high as in the Norwegian study.
Another nationwide Danish cohort study (1,503 events, partially overlapping with the earlier study) reported an association with broad-spectrum antibiotics that was modified by mode of delivery (14). This is consistent with our findings, where antibiotics were associated with an increased risk of diabetes among children delivered by cesarean section but less so among children delivered vaginally. Children delivered by cesarean section have been found to have a different gut microbiota up to age 1 (4). We speculate that the gut microbiota of a child delivered by cesarean section might be less resilient to antibiotic treatment during early life.
A multinational cohort study of 8,495 children at high risk of type 1 diabetes found no association of early-life antibiotics and subsequent seroconversion with islet autoimmunity (27). By studying exposure before seroconversion, there was no risk for reverse causation. Similarly, we defined our exposure up to age 12 months and tracked type 1 diabetes from age 15 months. We therefore find it unlikely that the observed association in our study is caused by reverse causation. However, different length of follow-up, antibiotic-prescribing patterns, different genetic setup between the two countries, and the fact that not all children with islet autoimmunity will develop type 1 diabetes might explain the discrepancy between the study results.
Prenatal exposure to any, urinary tract, or skin and soft tissue infection antibiotics was associated with an increased risk of type 1 diabetes. Similarly, a recent cohort study showed that maternal urinary tract infection is associated with a higher risk of type 1 diabetes (28). That study included children born in Sweden from 1973 to 2013 and is thereby partly overlapping with our study. However, the main exposure—maternal urinary tract infections in their study—was based on data from the MBR and the NPR and not the Swedish Prescribed Drug Register. Together, the findings from the current study and the study by Waernbaum et al. (28) highlight a possible link between maternal urinary tract infection and type 1 diabetes, independent of the register-based criteria used for maternal urinary tract infection. However, because the material was overlapping, external replication is needed. As the association was not statistically significant in the sibling analysis or in the sensitivity analysis excluding antibiotics used for long-term prophylaxis of urinary tract infections, our study does not provide any firm evidence that maternal antibiotics use during pregnancy influences type 1 diabetes risk, consistent with other earlier studies (10–12).
Potential Mechanisms
A plausible explanation for the observed association between antibiotics and the risk of type 1 diabetes is through modification of the gut microbiota. Exposure to antibiotics in early life delays the microbiota maturation (4), and the composition of the microbiota is different in children who later develop type 1 diabetes compared with those who do not (3,5,29,30). As children delivered by cesarean section have a disturbed development of their microbiota profile compared with vaginally delivered children (4), effects of antibiotics could be more pronounced in children delivered by cesarean section.
It has been suggested that viral infections can trigger the immune process leading to type 1 diabetes (31–33). The association found in our study between antibiotic treatment in early life and later risk of type 1 diabetes could potentially be driven by viral infections causing symptoms mimicking a bacterial infection. Our data support that most children in the cohort were treated with antibiotics for ear, upper respiratory tract, and urinary tract infections. However, we cannot exclude that some of these children had indeed a viral infection alone or in combination with a bacterial infection. For instance, distinguishing between viral and bacterial otitis media infections is difficult, and here, most children <1 year will receive antibiotics (34), even if a proportion of children might actually have a viral infection. The use of antibiotics for viral infections is likely to decrease with more strict policies for antibiotic use. Antibiotic prescriptions are declining in Sweden (29% of the children born in the 1st year of our study period were exposed compared with 19% in the last year), and the seasonal variation of antibiotic prescriptions has also decreased, indicating less use for antibiotics for viruses such as cold and flu (35). This change in the antibiotic prescription pattern is likely a result of the national long-term program Strama (Swedish strategic program against antibiotic resistance) (36) aiming at restricting antibiotic prescription. We therefore assessed whether calendar period affected the association, and we did not find such effects, alleviating some concerns about viral infections driving the association.
We found an association with β-lactamase–sensitive penicillin (J01CE) but not with broad-spectrum antibiotics. This was unexpected, because broad-spectrum antibiotics likely have the highest impact on the microbiota. However, the use of broad-spectrum antibiotics was low in our study, which limited our power. Moreover, penicillin might have a larger impact on specific bacterial groups important for diabetes development. Given that other studies have not provided consistent associations of antibiotics with type 1 diabetes risk, we need to acknowledge the possibility that our results are due to chance alone. Furthermore, as the absolute risk is low and the number of children needed to be treated with antibiotics to cause 1 additional case of type 1 diabetes was estimated to be as many as 1,475 in our study, we conclude that benefits with antibiotic treatment when correctly used outweigh type 1 diabetes risk.
Strengths and Weaknesses of the Study
The primary strengths of our nationwide study are its population-based design and the use of data from high-quality registers with objectively and prospectively collected exposure data, potential confounders, a validated outcome, and linkage between siblings. The strength of a sibling analysis is its ability to adjust for shared unmeasured factors. However, it cannot rule out confounding factors that vary within families. A weakness with the sibling analysis is the lower power compared with the total population analysis, and although the effect estimates of exposure to any antibiotics were similar in both analysis, it was not statistically significant in the sibling analysis.
Antibiotic consumption during hospitalization is not reported in the Swedish Prescribed Drug Register. However, exclusions of hospitalized children did not change the result in our study. Another limitation is the lack of information on breastfeeding. However, in our sibling analysis we adjusted for confounding factors shared by families, which largely include breastfeeding patterns. Moreover, the Prescribed Drug Register does not contain any categorized information on indication, only free-text information, so we were unable to assess different types of infections. However, we manually reviewed a random sample of prescriptions and present a description of the indications underlying the prescriptions. Furthermore, there was a high agreement between our classification of the prescriptions using the ATC and free-text information. A limitation with the validation study is that only 46% of the prescriptions were found to be informative, which may introduce a selection bias. The TEDDY study includes information about occurrence of infections and antibiotics and may allow for further investigation of potential mechanisms by separating viral from bacterial infections with and without antibiotic treatment (37). Finally, our population-based design enables generalization to the Swedish population and likely to other similar populations with comparable antibiotic-prescribing cultures. However, the Swedish Prescribed Drug Register holds information on dispensed medication from mid-2005 and onwards. Hence, we could only study early-onset type 1 diabetes, and mean age at diagnosis in our study was about half that of the general child population with type 1 diabetes, which may limit generalizability to younger ages.
In conclusion, our study supports that dispensed prescription of antibiotics in the 1st year of life is associated with the risk of type 1 diabetes before age 10 years. The observed association was driven by treatment used for otitis media and other respiratory tract infections. The results further support an earlier observation suggestive of a larger effect size in the group of children delivered by cesarean section. The absolute risk is low, however, and antibiotics are likely to only make a small contribution to the overall risk of type 1 diabetes before age 10.
Supplementary Material
Article Information
Acknowledgments. The authors would like to thank Klara Andersson, Uppsala University, who reviewed free-text information on the reason for the prescription.
Funding. This work was supported by Vetenskapsrådet (Swedish Research Council) through the project grant 2015-03477, through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant no. 340-2013-5867, and by the Uppsala Antibiotic Center.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. The study was designed by M.-L.W., B.S., C.A., and T.F. M.-L.W. performed the statistical analysis, interpreted the data, and wrote the initial draft with supervision from T.F. K.F., B.S., J.F.L., A.S., and C.A. contributed with invaluable support for data analyses, interpretation of findings, and critical revision of the article. C.A. and T.F. obtained the financial support. All authors reviewed and approved the final version of the article submitted for publication. T.F. initiated the study. T.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
C.A. and T.F. report equal contribution.
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1162/-/DC1.
References
- 1.Gülden E. Lifestyle factors affecting the gut microbiota’s relationship with type 1 diabetes. Curr Diab Rep 2018;18:111. [DOI] [PubMed] [Google Scholar]
- 2.Leiva-Gea I, Sánchez-Alcoholado L, Martín-Tejedor B, et al. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care 2018;41:2385–2395 [DOI] [PubMed] [Google Scholar]
- 3.Kostic AD, Gevers D, Siljander H, et al.; DIABIMMUNE Study Group . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015;17:260–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016;8:343ra382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vatanen T, Franzosa EA, Schwager R, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018;562:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016;1:16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown K, Godovannyi A, Ma C, et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J 2016;10:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugman S, Klatter FA, Visser JT, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 2006;49:2105–2108 [DOI] [PubMed] [Google Scholar]
- 9.Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012;55:2285–2294 [DOI] [PubMed] [Google Scholar]
- 10.Tapia G, Størdal K, Mårild K, et al. Antibiotics, acetaminophen and infections during prenatal and early life in relation to type 1 diabetes. Int J Epidemiol 2018;47:1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haupt-Jørgensen M, Morgen CS, Jess T, et al. Maternal antibiotic use during pregnancy and type 1 diabetes in children—a national prospective cohort study. Diabetes Care 2018;41:e155–e157 [DOI] [PubMed] [Google Scholar]
- 12.Kilkkinen A, Virtanen SM, Klaukka T, et al. Use of antimicrobials and risk of type 1 diabetes in a population-based mother-child cohort. Diabetologia 2006;49:66–70 [DOI] [PubMed] [Google Scholar]
- 13.Mikkelsen KH, Knop FK, Vilsbøll T, Frost M, Hallas J, Pottegård A. Use of antibiotics in childhood and risk of Type 1 diabetes: a population-based case-control study. Diabet Med 2017;34:272–277 [DOI] [PubMed] [Google Scholar]
- 14.Clausen TD, Bergholt T, Bouaziz O, et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a nationwide Danish cohort study. PLoS One 2016;11:e0161654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health 2013;103(Suppl. 1):S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The National Board of Health and Welfare, Centre for Epidemiology The Swedish Medical Birth Register - a summary of content and quality [Internet], 2003. Available from https://www.socialstyrelsen.se/publikationer2003/2003-112-3. Accessed 21 March 2017.
- 17.Rawshani A, Landin-Olsson M, Svensson AM, et al. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia 2014;57:1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegård A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015;100:3633–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almqvist C, Wettermark B, Hedlin G, Ye W, Lundholm C. Antibiotics and asthma medication in a large register-based cohort study - confounding, cause and effect. Clin Exp Allergy 2012;42:104–111 [DOI] [PubMed] [Google Scholar]
- 20.Örtqvist AK, Lundholm C, Kieler H, et al. Antibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with sibling analysis. BMJ 2014;349:g6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48 [PubMed] [Google Scholar]
- 22.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 2016;45:1887–1894 [DOI] [PubMed] [Google Scholar]
- 23.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In Survival Analysis: State of the Art Nato Science (Series E: Applied Sciences). Klein JP, Goel PK, Eds. Springer, Dordrecht, the Netherlands, 1992, p. 237–247 [Google Scholar]
- 24.Laubender RP, Bender R. Estimating adjusted risk difference (RD) and number needed to treat (NNT) measures in the Cox regression model. Stat Med 2010;29:851–859 [DOI] [PubMed] [Google Scholar]
- 25.Sjölander A. Estimation of causal effect measures with the R-package stdReg. Eur J Epidemiol 2018;33:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pottegård A, Broe A, Aabenhus R, Bjerrum L, Hallas J, Damkier P. Use of antibiotics in children: a Danish nationwide drug utilization study. Pediatr Infect Dis J 2015;34:e16–e22 [DOI] [PubMed] [Google Scholar]
- 27.Kemppainen KM, Vehik K, Lynch KF, et al.; Environmental Determinants of Diabetes in the Young Study Group . Association between early-life antibiotic use and the risk of islet or celiac disease autoimmunity. JAMA Pediatr 2017;171:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waernbaum I, Dahlquist G, Lind T. Perinatal risk factors for type 1 diabetes revisited: a population-based register study. Diabetologia 2019;62:1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis-Richardson AG, Ardissone AN, Dias R, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cinek O, Kramna L, Lin J, et al. Imbalance of bacteriome profiles within the Finnish Diabetes Prediction and Prevention study: parallel use of 16S profiling and virome sequencing in stool samples from children with islet autoimmunity and matched controls. Pediatr Diabetes 2017;18:588–598 [DOI] [PubMed] [Google Scholar]
- 31.Beyerlein A, Donnachie E, Jergens S, Ziegler AG. Infections in early life and development of type 1 diabetes. JAMA 2016;315:1899–1901 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Calvo T. Enteroviral infections as a trigger for type 1 diabetes. Curr Diab Rep 2018;18:106. [DOI] [PubMed] [Google Scholar]
- 33.Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr 2013;167:800–807 [DOI] [PubMed] [Google Scholar]
- 34.Läkemedelsverket (Swedish Medical Products Agency) Otit (akut mediaotit - AOM) [Internet], 2018. Available from https://lakemedelsverket.se/otit. Accessed 14 October 2019.
- 35.Swedres-Svarm 2014 Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. Solna and Uppsala, Sweden, Public Health Agency of Sweden and National Veterinary Institute, 2015. (publ. no. ISSN 1650-6332) [Google Scholar]
- 36.Mölstad S, Löfmark S, Carlin K, et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull World Health Organ 2017;95:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.