Abstract
OBJECTIVE
To investigate factors related to glycemic management among members of a professional cycling team with type 1 diabetes over a 7-day Union Cycliste Internationale World Tour stage race.
RESEARCH DESIGN AND METHODS
An observational evaluation of possible factors related to glycemic management and performance in six male professional cyclists with type 1 diabetes (HbA1c 6.4 ± 0.6%) during the 2019 Tour of California.
RESULTS
In-ride time spent in euglycemia (3.9–10.0 mmol/L glucose) was 63 ± 11%, with a low percentage of time spent in level 1 (3.0–3.9 mmol/L; 0 ± 1% of time) and level 2 (<3.0 mmol/L; 0 ± 0% of time) hypoglycemia over the 7-day race. Riders spent 25 ± 9% of time in level 1 (10.1–13.9 mmol/L) and 11 ± 9% in level 2 (>13.9 mmol/L) hyperglycemia during races. Bolus insulin use was uncommon during races, despite high carbohydrate intake (76 ± 23 g ⋅ h−1). Overnight, the riders spent progressively more time in hypoglycemia from day 1 (6 ± 12% in level 1 and 0 ± 0% in level 2) to day 7 (12 ± 12% in level 1 and 2 ± 4% in level 2) (χ2[1] > 4.78, P < 0.05).
CONCLUSIONS
Professional cyclists with type 1 diabetes have excellent in-race glycemia, but significant hypoglycemia during recovery overnight, throughout a 7-day stage race.
Introduction
Athletes with type 1 diabetes have considerable challenges with glycemic control, particularly around training and competition (1). Despite these challenges, the Team Novo Nordisk (TNN) professional athletes compete in elite cycling stage races around the world. This study investigated the glycemic control and performance metrics of TNN athletes over a 7-day Union Cycliste Internationale World Tour stage race.
Research Design and Methods
Six riders from TNN (mean ± SD age 29 ± 3 years; duration of type 1 diabetes 13 ± 7 years; body mass 70.0 ± 5.3 kg; HbA1c 6.4 ± 0.6%; 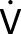 O2max 72.2 ± 5.0 mL ⋅ kg−1⋅ min−1 peak power 426 ± 36 W) cycled between 3 and 7 h and covered 128–219 km on each of the 7 days of the Tour of California (Table 1).
O2max 72.2 ± 5.0 mL ⋅ kg−1⋅ min−1 peak power 426 ± 36 W) cycled between 3 and 7 h and covered 128–219 km on each of the 7 days of the Tour of California (Table 1).
Table 1.
Rider and race characteristics over the 7-day Tour of California
| Stage of the tour | 7-Day mean | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Ride duration (min) | 194 ± 0 | 396 ± 15 | 360 ± 9 | 353 ± 0 | 298 ± 2 | 248 ± 9 | 174 ± 2 | 289 ± 86 |
| Distance (km) | 143 | 195 | 207 | 213 | 219 | 128 | 141 | 196 ± 44 |
| Total stage elevation (m) | 61 | 4,426 | 2,947 | 3,583 | 2,951 | 4,279 | 2,593 | 2,977 ± 1,461 |
| Ambient temperature (°C) | 25 | 20 | 21 | 21 | 18 | 12 | 11 | 18 ± 5 |
| Humidity (%) | 50 | 49 | 74 | 65 | 58 | 83 | 42 | 60 ± 15 |
| Energy expenditure (kcal) | 2,265 ± 499 | 4,955 ± 342* | 4,564 ± 284* | 4,334 ± 694* | 4,268 ± 283*£ | 3,691 ± 279*°§£ | 2,717 ± 300°§# | 3,828 ± 996 |
| In-ride energy intake (kcal) | 1,034 ± 324 | 2,138 ± 347* | 1,883 ± 406* | 2,075 ± 253* | 1,528 ± 390 | 1,704 ± 287 | 1,050 ± 545°§# | 1,630 ± 452 |
| In-ride energy change (kcal) | −1,230 ± 217 | −2,817 ± 499* | −2,681 ± 581* | −2,259 ± 684* | −2,740 ± 488* | −1,987 ± 541 | −1,666 ± 776°^ | −2,197 ± 602 |
| Power (W) | ||||||||
| Peak | 1,077 ± 183 | 939 ± 98 | 1,043 ± 100 | 981 ± 124 | 995 ± 106 | 897 ± 123*§ | 944 ± 106 | 982 ± 62 |
| Mean | 175 ± 41 | 219 ± 17* | 214 ± 13* | 205 ± 30* | 244 ± 17*°§# | 248 ± 14*°§# | 256 ± 17*°§# | 223 ± 2 |
| Normalized | 226 ± 28 | 246 ± 20* | 261 ± 12* | 247 ± 24* | 289 ± 18*°§# | 280 ± 16*°§# | 297 ± 17*°§# | 264 ± 19 |
| HR (bpm) | ||||||||
| Peak | 187 ± 3 | 189 ± 22 | 179 ± 3*° | 174 ± 6*° | 179 ± 15*° | 170 ± 2*°§^ | 175 ± 7*°$ | 179 ± 7 |
| Mean | 134 ± 8 | 144 ± 5* | 134 ± 5° | 127 ± 12° | 138 ± 3# | 138 ± 1# | 141 ± 5*§# | 137 ± 6 |
Data are presented as the mean ± SD or n.
Significant difference (P < 0.05) with respect to stage 1.
Significant difference (P < 0.05) with respect to stage 2.
Significant difference (P < 0.05) with respect to stage 3.
significant difference (P < 0.05) with respect to stage 4.
^ Significant difference (P < 0.05) with respect to stage 5.
Significant difference (P < 0.05) with respect to stage 6.
Significant difference (P < 0.05) with respect to stage 7.
Each rider was equipped with a mobile power meter (Pioneer, Aliso Viejo, CA), a G6 continuous glucose monitor (Dexcom, San Diego, CA), and a Wahoo cycle computer (Wahoo Fitness, Atlanta, GA) that monitored power output (Watts), cadence (revolutions per minute), temperature (degrees Celsius), speed (kilometers per hour), elevation (meters), grade (percentage), distance raced (kilometers), duration (hours, minutes, and seconds), and energy expenditure (kilocalories). Heart rate (HR) was measured by using a Wahoo chest strap (Wahoo Fitness). In-ride nutritional intake was logged by the research team and support staff, and the riders used NovoPen Echo Plus smart insulin pens (Novo Nordisk, Bagsværd, Denmark) to record insulin dosing.
The study was performed in accordance with the Declaration of Helsinki and was approved by a centralized institutional review board (Salus IRB, Austin, TX; ID no. DCR19-004). All participants provided both verbal and written informed consent.
Continuous glucose monitoring (CGM) data from each race, each night (2200–0600), and each 24-h period (0800–0800) were stratified by the percentage time spent within various glycemic ranges: 3.0–3.9 mmol/L (level 1 hypoglycemia), <3.0 mmol/L (level 2 hypoglycemia), 3.9–10.0 mmol/L (target range), 10.0–13.9 mmol/L (level 1 hyperglycemia), and >13.9 mmol/L (level 2 hyperglycemia), according to recent guidelines (2). The glucose targets for the TNN riders, as set by their clinical support team, are 6.7–12.2 mmol/L before a race in order to help minimize hypoglycemia, and between 3.9 and 10.0 mmol/L—ideally >6.7 mmol/L—during the ride for performance and to mitigate the risk of hypoglycemia. At all other times, the cyclists aim for a glucose concentration between 3.9 and 10.0 mmol/L.
Statistical Analysis
Average performance and diabetes-related metrics were compared between racing days by using one-way and two-way repeated-measures ANOVA, as appropriate. Pearson correlation coefficients were calculated in order to assess the association between in-ride glycemia and cycling metrics. Mean in-ride hourly carbohydrate consumption was compared against the international recommendations (60–90 g ⋅ h−1 for endurance athletes without diabetes [3,4]) by using a one-sample t test. Statistical analyses were performed by using RStudio version 1.1.447. Data are presented as the mean ± SD.
Results
Six of the seven TNN riders completed every stage of the Tour of California, with a total elevation of 20,840 m, covering 1,244 km over seven consecutive days. A seventh rider was excluded from the analysis because he withdrew on day 3 because of an accumulated delay in the race that did not seem to be related to diabetes. Overall, the team placed 14th among 19 teams, finishing ahead of three World Tour teams, with numerous individual successes, including TNN’s first rider in the top 10 for the final race stage.
There were significant differences between mean in-ride glucose (P < 0.01), power output (P < 0.001), and HR (P < 0.01) between race stages (Table 1). There were also differences between mean in-ride energy expenditure and energy intake (P < 0.001), as a function of race stage. Mean in-ride glucose was not correlated with energy expenditure (r = −0.17, P = 0.31), energy intake (r = −0.21, P = 0.21), or carbohydrate intake (r = −0.14, P = 0.41). Individual in-ride glucose data were not correlated with power (r = −0.01), HR (r = −0.02), speed (r = 0.08), or race distance (r = −0.09).
CGM data are presented in Supplementary Fig. 1 and Supplementary Table 1. During the races, riders spent 63 ± 11% of their time in the target range (3.9–10.0 mmol/L) and small percentages of time in level 1 (3.0–3.9 mmol/L; 0 ± 1% of time) and level 2 (<3.0 mmol/L; 0 ± 0% of time) hypoglycemia. Over the tour, there were two incidents of in-ride hypoglycemia (interstitial glucose ≤3.9 mmol/L for at least 15 min [2]). These occurred in the same rider and lasted 15 min each; in both cases, glucose did not fall below 3.8 mmol/L. Overall, during the rides the cyclists spent 25 ± 9% of time in level 1 hyperglycemia (10.1–13.9 mmol/L) and 11 ± 9% of time in level 2 hyperglycemia (>13.9 mmol/L). Overnight, between stages, the riders spent progressively more time in levels 1 and 2 hypoglycemia (Supplementary Fig. 2C), increasing from 6 ± 12% of time in level 1 and 0 ± 0% of time in level 2 on day 1 to 12 ± 12% in level 1 and 2 ± 4% in level 2 by day 7 (P < 0.05). The odds of being hypoglycemic overnight increased by 32% from day 1 to 7 (odds ratio = 1.32).
All riders were on a stable regimen of multiple daily doses of a range of rapid-acting/short-lasting and long-lasting insulins. Reliable insulin data were obtained from five of six riders. These five riders were on a split-dose basal regimen (two taking insulin glargine and three taking insulin detemir), and all riders used the same bolus insulin (Fiasp; Novo Nordisk). The total insulin dose administered over each 24-h period was 46 ± 37 IU on day 1; this reduced to 33 ± 30 IU on day 6 (Supplementary Fig. 1B).
In-ride nutrition consisted primarily of energy gels, high-carbohydrate energy bars, rice cakes, and bananas (Supplementary Table 2). Fluids consisted of water, a low-carbohydrate sports drink containing electrolytes, or cola. Mean in-ride carbohydrate intake was 76 ± 23 g ⋅ h−1, similar to that recommended by international guidelines (i.e., 60–90 g ⋅ h−1) (3,4).
Conclusions
This is the first report to our knowledge describing factors related to glycemic management over a World Tour stage race in members of a professional cycling team who have type 1 diabetes. Overall, the riders spent a large percentage of time in the target glycemic range (3.9–10 mmol/L) and spent little time with hypoglycemia. However, nocturnal hypoglycemia was noted, which seemed to worsen over the tour. These observations may be helpful for clinicians or exercise physiologists working with highly trained athletes with type 1 diabetes.
Overall, during races, riders spent a large percentage of time in the target glycemic range (63 ± 13%) and little time in level 1 (0 ± 1%) and level 2 (0 ± 0%) hypoglycemia (Supplementary Fig. 2A). There were only two episodes of mild hypoglycemia, suggesting that these riders are proficient at managing their nutrition and glucose levels using real-time CGM. However, the riders spent a large proportion of time in level 2 hyperglycemia (11 ± 9%), exceeding the recommended target of <5% over each 24-h period (5). It is unclear whether this level of hyperglycemia was detrimental to performance in this group of elite athletes. The decision for these athletes not to correct hyperglycemia via an insulin bolus may have been linked to their fear of developing hypoglycemia during the race.
During the nocturnal periods, the riders spent progressively more time in level 1 and level 2 hypoglycemia from day 1 (6 ± 12% of time at level 1 and 0 ± 0% at level 2) to day 7 (12 ± 12% of time at level 1 and 2 ± 4% at level 2). The progressively longer time in the hypoglycemic range overnight meant that by day 6, the riders had, on average, spent an amount of time in hypoglycemia that was well above the acceptable limit for time below target (i.e., <4% at level 1 and <1% at level 2 hypoglycemia) (5). These observations of elevated nocturnal hypoglycemia are concerning given the findings that nocturnal hypoglycemia can negatively impact cardiac autonomic regulation (6). Future work should examine the combined effect of nocturnal hypoglycemia and exhaustive exercise on overnight HR variability in athletes with type 1 diabetes and should further develop strategies to reduce the risk of nocturnal hypoglycemia by using nutritional interventions, automated insulin delivery systems, or both. Aside from the dangers of nocturnal hypoglycemia (7,8), hypoglycemia may impair recovery between race stages. Hypoglycemia has been shown to blunt neuroendocrine and metabolic responses during subsequent exercise (9), which may impact glycemia, fuel utilization, and thereby affect race performance. This race involved considerable distances both before the race (travel from different time zones) and between race stages, which may also impact glycemic control. Future research should investigate how the additional stresses of a cycling tour influence glycemic control.
Ingested carbohydrate is a primary fuel that affects race performance by cyclists without diabetes (10). Prior to this investigation, there was limited information on the nutritional behavior of elite athletes with type 1 diabetes. Mean in-ride carbohydrate intake in these cyclists with type 1 diabetes was 76 g ⋅ h−1 (range 30.5–124.8 g ⋅ h−1), which is in line with guidelines for endurance athletes without diabetes (i.e., 60–90 g ⋅ h−1) (3,4). These data demonstrate the importance of high carbohydrate intake to compete at a high level and that good glycemic control is possible.
To our knowledge, this is the first study to quantify habitual insulin doses and timing in a group of athletes with type 1 diabetes over a cycling stage race. Total insulin requirements reduced over the tour, whereas the basal-to-bolus ratio increased by ∼48%. The observation that riders did not typically inject bolus insulin during the races, even when in a state of level 2 hyperglycemia, suggests that they may fear developing hypoglycemia more than hyperglycemia.
Although other investigations of athleticism and type 1 diabetes exist (11–14), the strength of this study is the comprehensive range of in-ride glucose and performance measures collected from a team of elite athletes with type 1 diabetes over a multistage ultraendurance race (Supplementary Fig. 3). There are limitations, however, given that this was an observational study set in a race environment. The lack of nutrition data outside the race means that there is no information available on whether the riders reached a state of energy balance during recovery, the composition of meals, and what they consumed around bedtime and during the night. The sample size is in line with those described in previous reports of professional cyclists without type 1 diabetes (15,16), but the low participant number and the fact that data were collected during a single race means that caution must be taken when generalizing these results.
Supplementary Material
Article Information
Acknowledgments. The authors thank the riders of Team Novo Nordisk and the Team Novo Nordisk support staff for their willingness to let the authors use in this study the data collected during the 2019 Tour of California race.
Funding. Portions of this work were supported by Team Novo Nordisk, Atlanta, GA. Dexcom supplies were provided by Dexcom, San Diego, CA.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.N.S., M.P.C., F.Y.F., C.S., R.M.B., C.A.H., M.F., B.B., P.H.L., P.S., and M.C.R. contributed to the design of the study. S.N.S., M.P.C., F.Y.F., C.A.H., and P.H.L. contributed to data collection. S.N.S., M.P.C., F.Y.F., and M.C.R. contributed to data analysis. All authors contributed to the interpretation of study results. S.N.S., F.Y.F., and M.C.R. prepared the first draft of the manuscript, and all authors reviewed and approved the manuscript. S.N.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster at Schweizerischen Gesellschaft für Endokrinologie und Diabetologie (SGED), Bern, Switzerland, 14–15 November 2019, and at the 13th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD), Madrid, Spain, 19–22 February 2020.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-2302/-/DC1.
References
- 1.Keay N, Bracken RM. Managing type 1 diabetes in the active population. Br J Sports Med. 15 November 2019 [Epub ahead of print]. DOI: 10.1136/bjsports-2019-101368 [DOI] [PubMed] [Google Scholar]
- 2.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet 2016;116:501–528 [DOI] [PubMed] [Google Scholar]
- 4.Jeukendrup A. A step towards personalized sports nutrition: carbohydrate intake during exercise. Sports Med 2014;44(Suppl. 1):S25–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koivikko ML, Tulppo MP, Kiviniemi AM, et al. Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in patients with type 1 diabetes. Diabetes Care 2012;35:1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia 2009;52:42–45 [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly M, O’Sullivan EP, Davenport C, Smith D. “Dead in bed”: a tragic complication of type 1 diabetes mellitus. Ir J Med Sci 2010;179:585–587 [DOI] [PubMed] [Google Scholar]
- 9.Davis SN, Galassetti P, Wasserman DH, Tate D. Effects of antecedent hypoglycemia on subsequent counterregulatory responses to exercise. Diabetes 2000;49:73–81 [DOI] [PubMed] [Google Scholar]
- 10.Burke LM. Nutritional practices of male and female endurance cyclists. Sports Med 2001;31:521–532 [DOI] [PubMed] [Google Scholar]
- 11.Yardley JE, Zaharieva DP, Jarvis C, Riddell MC. The “ups” and “downs” of a bike race in people with type 1 diabetes: dramatic differences in strategies and blood glucose responses in the Paris-to-Ancaster Spring Classic. Can J Diabetes 2015;39:105–110 [DOI] [PubMed] [Google Scholar]
- 12.Adolfsson P, Mattsson S, Jendle J. Evaluation of glucose control when a new strategy of increased carbohydrate supply is implemented during prolonged physical exercise in type 1 diabetes. Eur J Appl Physiol 2015;115:2599–2607 [DOI] [PubMed] [Google Scholar]
- 13.Mattsson S, Jendle J, Adolfsson P. Carbohydrate loading followed by high carbohydrate intake during prolonged physical exercise and its impact on glucose control in individuals with diabetes type 1-an exploratory study. Front Endocrinol (Lausanne) 2019;10:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller-Korbsch M, Frühwald L, Heer M, Fangmeyer-Binder M, Reinhart-Mikocki D, Fasching P. Assessment of the “second day” exercise effect on glycemic control, insulin requirements, and CHO intake in type 1 diabetes adults. J Diabetes Sci Technol. 4 October 2019 [Epub ahead of print]. DOI: 10.1177/1932296819879419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saris WH, van Erp-Baart MA, Brouns F, Westerterp KR, ten Hoor F. Study on food intake and energy expenditure during extreme sustained exercise: the Tour de France. Int J Sports Med 1989;10(Suppl. 1):S26–S31 [DOI] [PubMed] [Google Scholar]
- 16.Ebert TR, Martin DT, Stephens B, McDonald W, Withers RT. Fluid and food intake during professional men’s and women’s road-cycling tours. Int J Sports Physiol Perform 2007;2:58–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


