Abstract
Insulin resistance is an underappreciated facet of type 1 diabetes that occurs with remarkable consistency and considerable magnitude. Although therapeutic innovations are continuing to normalize dysglycemia, a sizable body of data suggests a second metabolic abnormality—iatrogenic hyperinsulinemia—principally drives insulin resistance and its consequences in this population and has not been addressed. We review this evidence to show that injecting insulin into the peripheral circulation bypasses first-pass hepatic insulin clearance, which leads to the unintended metabolic consequence of whole-body insulin resistance. We propose restructuring insulin therapy to restore the physiological insulin balance between the hepatic portal and peripheral circulations and thereby avoid the complications of life-long insulin resistance. As technology rapidly advances and our ability to ensure euglycemia improves, iatrogenic insulin resistance will become the final barrier to overcome to restore normal physiology, health, and life in type 1 diabetes.
Introduction
Less than a decade after converting a janitor’s closet into a radioisotope laboratory in 1947, Yalow and Berson (1,2) revolutionized endocrinology research using radioiodine-labeled insulin to determine the hormone’s physiologic distribution. Noting that the liver was anatomically interposed between the pancreas and the remainder of the body, scientists in the late 1950s now possessed a valuable tool to quantify insulin clearance during transhepatic circulation. The work of Mortimore and Tietze (3) in rats as well as Madison et al. (4) and Samols and Ryder (5) in humans each showed that the liver extracted approximately half of plasma insulin on first pass. By the mid-1970s, additional investigations in canines (6) and humans (7,8) demonstrated that hepatic clearance maintained insulin at two- to threefold higher levels in the portal circulation than in the peripheral circulation.
As Yalow and Berson (9) themselves soon observed, however, injecting insulin into the peripheral circulation results in significantly higher insulin concentrations in the peripheral plasma than when the same amount of insulin is secreted by the pancreas. Indeed, because peripheral insulin delivery bypasses hepatic extraction, basal peripheral insulin levels are ∼2.5-fold higher in patients with type 1 diabetes than in individuals without diabetes with similar glycemia (10–12). So in treating hyperglycemia, peripheral insulin delivery creates a second, hidden deviation from the physiological norm: iatrogenic hyperinsulinemia.
In this Perspective, we review evidence to assert that an unintended but substantial consequence of iatrogenic hyperinsulinemia in type 1 diabetes is insulin resistance. The burden of insulin resistance is closely associated with cardiovascular risk, which is life-limiting in type 1 diabetes (13–15). For this reason, future therapies must overcome this obstacle to fully normalize the physiology of people with type 1 diabetes and promote optimal long-term outcomes.
Insulin Resistance Is Pronounced in Type 1 Diabetes
As early as the 1930s, the notion that diabetes could be differentiated into insulin sensitive (and insulin deficient) and insulin insensitive forms was first proposed by Sir Harold Himsworth (16) and was later supported by Yalow and Berson (2) with the advent of the insulin radioimmunoassay. The introduction by DeFronzo et al. (17) of the hyperinsulinemic, euglycemic clamp technique in 1979 provided what is now considered the gold-standard tool to quantify insulin’s ability to stimulate glucose disposal independent of endogenous insulin production. Up until that point, common clinical teaching maintained that insulin sensitivity was normal in type 1 diabetes. Three years later, however, DeFronzo et al. (18) used the clamp technique to show that insulin sensitivity was strikingly lower in these patients. Though the scientific community would draw far more attention to insulin insensitivity in type 2 diabetes over the next four decades, this malady was also observed in type 1 diabetes with remarkable consistency and considerable magnitude. In one meta-analysis, every one of 38 hyperinsulinemic, euglycemic clamp studies showed insulin sensitivity was significantly lower for patients with type 1 diabetes compared with matched, healthy control participants (19). In most hyperinsulinemic, euglycemic clamp studies, patients with type 1 diabetes have 30–50% lower insulin sensitivity than matched individuals without diabetes (20). Epidemiologic data from the Pittsburgh Epidemiology of Diabetes Complications study (21) and observational data from the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study (13,22) have linked insulin resistance independently with cardiovascular disease, which remains the major life-limiting complication of type 1 diabetes even when glucose is well controlled. Clearly, this underappreciated aspect of type 1 diabetes warrants greater consideration.
What makes insulin sensitivity so different between type 1 diabetes and matched control groups? The influential CACTI study that began in the early 2000s provided helpful information relating to this question. Although traditional risk factors for insulin insensitivity (e.g., BMI, waist circumference, triglyceridemia) inversely correlated with insulin sensitivity among type 1 diabetes participants as expected, this relationship was effectively “left-shifted” for the type 1 diabetes participants compared with the control participants (13). In other words, at a fixed value for a traditional insulin resistance risk factor, insulin sensitivity was even lower in a type 1 diabetes participant than a similar control participant. Thus, a more complete explanation for the difference was needed. Based on the earlier work of Yki-Järvinen et al. (23–26), the presence of hyperglycemia in type 1 diabetes seemed like a logical reason for the discrepancy. However, when the investigators analyzed the relationship between peripheral insulin sensitivity and either recent or chronic glycemia (measured by continuous glucose monitoring or glycosylated hemoglobin, respectively), no correlation was found. The CACTI investigators suggested that some aspect of nonphysiological insulin delivery might underlie the insulin resistance that seemed curiously consistent across differing levels of glycemic control (13). As the following studies in mice, canines, and humans show, a robust body of evidence indicates that, more than any other factor, nonphysiologic peripheral insulin delivery drives insulin resistance in type 1 diabetes.
Insulin Gene Expression Inversely Correlates With Insulin Sensitivity in Mice
Investigators have debated whether iatrogenic hyperinsulinemia is a primary cause of insulin resistance or instead reflects the impact of another process causing insulin resistance (27). The development and implementation of transgenic mice in the 1980s provided investigators with a tool to analyze the effect of hyperinsulinemia as a primary driver. Marban et al. established two lines of transgenic, nonobese mice overexpressing the human insulin gene, with one line carrying eight copies of the insulin gene and the other carrying thirty-two (28,29). Although fasting glucose levels were not different between control mice and the transgenic mice, basal insulin levels were two- and fourfold higher in the peripheral circulation of the two transgenic mice lines. Two-hour intraperitoneal glucose tolerance tests demonstrated that these mice were insulin resistant as evidenced by diminished glucose disposal; specifically, the areas of the curves above the baseline glucose for the two transgenic mice groups were approximately 1.3- and 2.0-fold higher than control mice, despite having 1.6- and 2.6-fold higher insulin levels. Likewise, when the mice were subjected to an insulin tolerance test, in which investigators injected 0.5 mU/g body weight of intraperitoneal insulin to provoke an immediate glucose drop, glucose levels fell by nearly 40% in the control animals versus only 15–20% in the hyperinsulinemic, transgenic mice, affirming their resistance to insulin action. To test alternative hypotheses to explain the insulin resistance in the nonobese, hyperinsulinemic mice, the investigators confirmed that neither anti-insulin antibodies nor biologically inactive insulin molecules were present. Using a different approach with NOD mice, Liu et al. (30) found that hyperglycemic mice treated with 2 weeks of saline and NOD mice without diabetes both had a 50% fall in glucose in response to an insulin tolerance test. By contrast, hyperglycemic NOD mice treated with 2 weeks of insulin detemir to normalize glycemia had a severely limited glycemic response to the same insulin tolerance test.
Whereas increasing insulin gene expression in transgenic mice brings about decreased insulin sensitivity, partially inactivating insulin gene expression in mice leads to increased insulin sensitivity (31). Templeman et al. conducted a series of experiments where the ancestral insulin gene Ins2 was either fully or partially expressed in female mice that were challenged with diets designed to stimulate relatively robust insulin production. Additionally, the investigators fully inactivated the rodent-specific Ins1 gene to prevent a compensatory increase in Ins1 expression. Compared with mice fully expressing Ins2, Ins2+/− mice had 25% and 34% lower basal insulin levels on average when fed moderate- and high-energy diets, respectively. Insulin sensitivity as estimated by HOMA of insulin resistance was approximately 40% lower in mice fully expressing Ins2 compared with those partially expressing Ins2 on the same diet. Further, when 0.75 mU/g body weight of intraperitoneal insulin was administered, the Ins2+/− animals had a significantly larger fall in glucose compared with the Ins+/+ mice on the same diet.
Collectively, these studies in euglycemic, transgenic mice show a reciprocal relationship exists: when insulin secretion is constitutively increased, insulin sensitivity decreases and when insulin secretion is constitutively decreased, insulin sensitivity increases.
Supraphysiological Intraportal Insulin Infusion Decreases Insulin Sensitivity in Dogs
Whereas some investigators exploited genetically modified mice to study the effect of hyperinsulinemia on insulin sensitivity, others in the 1980s used elegant surgical interventions in dogs to create hyperinsulinemia. Having noted previous in vitro studies of insulin action in human lymphocytes and cultured rat adipocytes involved very high insulin levels, McGuinness et al. (32) chronically catheterized canines to quantify how much mild hyperinsulinemia affected insulin sensitivity. For 4 weeks the investigators constantly infused saline or insulin into the hepatic portal vein. Whereas intraportal insulin infusion increased peripheral plasma insulin concentrations from 15 to 23 μU/mL, peripheral plasma concentrations decreased from 15 to 11 μU/mL with intraportal saline infusion. At the same time, basal glucose levels decreased from 113 mg/dL to 100 mg/dL with no significant increase in counterregulatory hormone levels measured every 2 hours over 24 h. Using the hyperinsulinemic, euglycemic clamp to quantify insulin sensitivity, the investigators found that insulin-mediated glucose disposal decreased by 39% after 4 weeks of mild intraportal hyperinsulinemia compared with a 2% decrease in the saline-infused dogs.
Peripheral Hyperinsulinemia to Levels Seen in Type 1 Diabetes Diminishes Insulin Sensitivity in Healthy Humans
Although the previous animal studies showed hyperinsulinemia begets insulin insensitivity, the hyperinsulinemia was induced by increased portal insulin delivery. In type 1 diabetes, bypassing first-pass hepatic extraction via peripheral insulin delivery is the cause of peripheral hyperinsulinemia. To evaluate the effect of hyperinsulinemia brought about by peripheral insulin delivery on insulin sensitivity, three separate hyperinsulinemic, euglycemic clamp studies were conducted in healthy humans in the 1980s and 1990s. A consistent theme emerged: sustained hyperinsulinemia of even shorter duration than in the canine studies also causes insulin insensitivity (Table 1). It is imperative to note that the sustained hyperinsulinemia in each of these three studies (20–30 μU/mL) is comparable to basal insulin levels seen in type 1 diabetes under euglycemic conditions (10,33,34).
Table 1.
Summary of three studies to quantify effect of modest, sustained hyperinsulinemia on insulin sensitivity in healthy humans
| Authors, year | Description of intervention | Duration of intervention | Key differences in glucose utilization (mg/kg/min) during hyperinsulinemic, euglycemic clamp studies* |
|---|---|---|---|
| Rizza et al., 1985 (35) | Induced hyperinsulinemia vs. saline infusion (plasma insulin 26 ± 2 vs. 9 ± 1 μU/mL, respectively). Paired studies. | 40 h | 17% lower insulin sensitivity after hyperinsulinemia compared with saline infusion |
| Marangou et al., 1986 (36) | Induced hyperinsulinemia vs. saline infusion (plasma insulin 30 ± 4 vs. 4 ± 1 μU/mL, respectively). Parallel studies. | 20 h | 19–28% decreases in glucose utilization from hyperinsulinemia vs. 5–8% decreases from saline infusion |
| Del Prato et al., 1994 (37) | Induced hyperinsulinemia raising plasma insulin from 8 ± 1 to 20 ± 2 μU/mL. Single intervention. | 72 h | 20% reduction in insulin sensitivity compared with baseline |
Comparisons made during a step of each clamp study where insulin was infused at 1.0 mU/kg/min.
Rizza et al. (35) conducted paired experiments under euglycemic conditions to assess insulin sensitivity after 40 h of saline versus insulin infusions. The latter intervention increased peripheral plasma insulin concentrations threefold above levels seen during the saline infusion. The investigators found glucose utilization was 17% lower following hyperinsulinemia than following saline infusion.
Marangou et al. (36) soon followed with parallel intervention studies to quantify insulin sensitivity before and after 20 h of insulin versus saline infusions. Here, the insulin infusion resulted in peripheral plasma insulin levels that were 7.5-fold higher than during the saline infusion. Inducing hyperinsulinemia led to 19–28% decreases in glucose utilization versus 5–8% decreases with the saline infusion. The investigators then conducted a second set of studies, again comparing the effect of 20 h of saline versus sustained peripheral hyperinsulinemia but using an intravenous glucose tolerance test minimal model to quantify insulin sensitivity. Here again, following 20 h of hyperinsulinemia, the rate of glucose disposal fell by 32% and the insulin sensitivity index fell by 53%, without any change in glucose-mediated glucose disposal or β-cell responsiveness. By comparison, saline control studies showed no significant change in any of these parameters.
Del Prato et al. (37) later conducted two studies to quantify and localize the defect in insulin action following chronic, sustained hyperinsulinemia and hyperglycemia in healthy volunteers. The first study quantified insulin sensitivity before and after 3 days of an insulin infusion that raised insulin 2.5-fold above basal levels. Plasma glucose levels were maintained at 87 mg/dL throughout the study. Hyperinsulinemic, euglycemic clamp studies showed the chronic insulin infusion was associated with a 20% reduction in glucose utilization. Further analysis revealed chronic insulin exposure caused distinct effects on oxidative versus nonoxidative glucose disposal. While chronic hyperinsulinemia diminished nonoxidative (i.e., glycogen synthetic) glucose disposal, oxidative glucose disposal was actually enhanced. This finding suggested the defect in insulin action occurred 1) downstream of glucose transport and phosphorylation and 2) not because of decreased insulin receptors or function or due to reduced glucose transport or phosphorylation. The second study quantified insulin sensitivity before and after 3 days of a glucose infusion that raised glucose concentrations from 85 mg/dL to 109 mg/dL. As a result of the glucose infusion, plasma insulin concentrations increased from 7 μU/mL to 20 μU/mL, matching the rise in insulin seen in the chronic insulin infusion study. During the hyperinsulinemic, euglycemic clamp studies, the chronic glucose infusion caused only a 4% decrease in the glucose utilization. Interestingly, the presence of hyperglycemia appeared to alter the intracellular partitioning of glucose between oxidative and nonoxidative pathways. The researchers found that while nonoxidative glucose disposal again declined, the addition of hyperglycemia was associated with a reciprocal increase in oxidative glucose disposal. This greater reciprocal increase in oxidative glucose disposal explained the difference between the 20% decrease in insulin sensitivity following chronic hyperinsulinemia and the 4% decrease following combined chronic hyperinsulinemia and hyperglycemia. Importantly, the study indicated that chronic hyperinsulinemia, whether brought about by chronic insulin infusion or by stimulation of endogenous insulin, leads to a pathway-specific defect in nonoxidative glucose disposal. A follow up study suggested decreased glycogen synthase activity was the key reason for this defect. The group concluded the decreased enzyme activity was likely attributable to a combination of allosteric inhibition from increased intramyocellular glycogen and posttranslational modification with phosphorylation by glycogen synthase kinase 3β (38). In addition to these changes in nonoxidative glucose disposal, different investigators using varying methodologies to assess intramuscular mitochondrial oxidative capacity suggested oxidative glucose disposal (but not necessarily glycolytic flux) may be decreased in type 1 diabetes in some (39–41) but not all (42,43) studies. Whether these steps in insulin signaling transduction represent potential therapeutic targets in type 1 diabetes remains to be seen.
Portal Insulin Delivery Corrects Insulin Sensitivity in Humans With Type 1 Diabetes
Whereas inducing sustained hyperinsulinemia via peripheral insulin delivery decreases insulin sensitivity in people without type 1 diabetes, reducing hyperinsulinemia via portal insulin delivery increases insulin sensitivity in people with type 1 diabetes.
In the early 1990s, Shishko et al. (44) divided 12 patients with poorly controlled type 1 diabetes to receive either continuous subcutaneous insulin infusion (CSII) or intraportal insulin infusion (IPII) via the umbilical vein for 4 months. While the 24-h serum insulin and plasma glucose profiles of patients receiving IPII approximated that of matched control subjects without diabetes, patients receiving CSII had two- to threefold higher peripheral insulin concentrations and 50% higher glucose levels. Additionally, the mean total daily insulin dose was 20% lower in the IPII group compared with the CSII group. Although IPII participants had lower doses and serum levels of insulin compared with CSII participants, glycosylated hemoglobin dropped much more in the IPII group than the CSII group (from 13.9 to 5.5% [128 to 37 mmol/mol] vs. 14.8% to 10.0% [138 to 86 mmol/mol], respectively). The fact that such a greater fall in glucose was seen with considerably less insulin implies whole-body insulin sensitivity was considerably greater with IPII than CSII.
Carpentier et al. (45) further investigated the effect of portal versus peripheral insulin delivery on insulin sensitivity in 16 patients with type 1 diabetes who were recipients of combined kidney-pancreas transplantation and had normal glucose levels. Nine patients had anastomosis of the pancreatic vein to the systemic circulation and seven had anastomosis to the portal circulation. Both groups had similar ages, BMIs, disease durations, renal function, antirejection therapy, and time between transplant and clamp studies. Despite having similar plasma glucose and C-peptide levels, insulin concentrations for participants with systemic anastomoses were twice as high as those with portal anastomoses and healthy control participants under both fasting and intravenous glucose–stimulated conditions. During hyperinsulinemic, euglycemic clamp studies, whole-body glucose utilization was 40% lower in the systemic anastomosis group than in the portal anastomosis group. Although basal nonesterified fatty acid (NEFA) levels were similar, the increase in insulin needed to lower NEFA levels by half was also twofold higher for participants with systemic anastomoses compared with participants with portal anastomoses and control participants. So although equipoise between groups existed for euglycemia, immunotherapeutic regimens, participant characteristics, and C-peptide responses to hyperglycemia, when first-pass hepatic extraction of insulin was bypassed, insulin sensitivity was a full 40% lower than when the physiologic portal-to-peripheral insulin gradient was restored.
In the 2000s, the development of protocols for intrahepatic islet transplantation provided another opportunity to assess how much targeting the liver as the primary site of insulin action affects sensitivity to the hormone. In 2006, Meier et al. (46) measured hepatic fractional extraction of insulin using hepatic vein angiocathers and found minimal difference between intrahepatic islet transplant recipients and healthy volunteers (84% vs. 78%, respectively). This finding indicated that the islet grafts released the majority of insulin directly into the liver sinusoids, as occurs with portal insulin delivery, rather than directly into the central hepatic vein and peripheral circulation. Seven years later, Rickels et al. (47) conducted hyperinsulinemic, euglycemic clamps before and 6 months after 12 patients with type 1 diabetes received intrahepatic islet transplants. After transplant, the participants received only glucocorticoid-free immunosuppression, required minimal exogenous insulin, and saw glycosylated hemoglobin decrease from 7.0% (53 mmol/mol) to 5.6% (38 mmol/mol). Immediately prior to the pre-transplant clamp study, the average basal peripheral insulin concentration was 27 μU/mL, owing to the peripheral intravenous insulin infusion used to normalize basal glycemia. By contrast, after the intrahepatic islet transplantation, the basal peripheral insulin concentration was 12 μU/mL, resembling levels in the control group (10 μU/mL). Between the pre-transplant and post-transplant studies, peripheral insulin sensitivity increased by 50%, nearly equaling that of control participants who were not receiving any immunosuppressive therapy. So a recurrent theme develops: peripheral insulin insensitivity in type 1 diabetes is mitigated when insulin is first delivered directly to the hepatic sinusoid.
Iatrogenic Hyperinsulinemia Has a Much Greater Association With Insulin Resistance Than Hyperglycemia in Type 1 Diabetes
When considering how best to ameliorate decreased insulin sensitivity in type 1 diabetes, we encountered a conundrum. The previously discussed research strongly suggested iatrogenic hyperinsulinemia was the key element behind insulin resistance in type 1 diabetes. Over the past four decades of research, however, other investigations had asserted hyperglycemia was the principal factor driving the insulin insensitivity. In these studies, an inverse correlation between glycemia and insulin sensitivity existed whether hyperglycemia was reduced with intensive insulin pump therapy (26,48,49), induced by intravenous glucose infusion (25,50), or partially resolved during the “honeymoon phase” (23). While many investigations analyzed the effect of hyperinsulinemia or hyperglycemia on insulin sensitivity in isolation, the two factors are not mutually exclusive. Indeed, in some of the studies that linked improved glycemia with enhanced insulin sensitivity, the improved glycemia was also accompanied by lower insulin doses (23,26) or levels (25,47). Furthermore, in one influential study by Yki-Järvinen and Koivisto (23), when participants with type 1 diabetes were grouped by disease duration (2–10 years, 11–20 years, and >20 years), average insulin sensitivity in all groups were nearly the same, with each group having ∼40% lower insulin sensitivity than the control group. Interestingly, each group had similar glycosylated hemoglobin levels and nearly the same daily insulin doses. Thus, we felt a study was needed to clarify which factor is more closely associated with insulin resistance in type 1 diabetes: hyperglycemia or iatrogenic hyperinsulinemia.
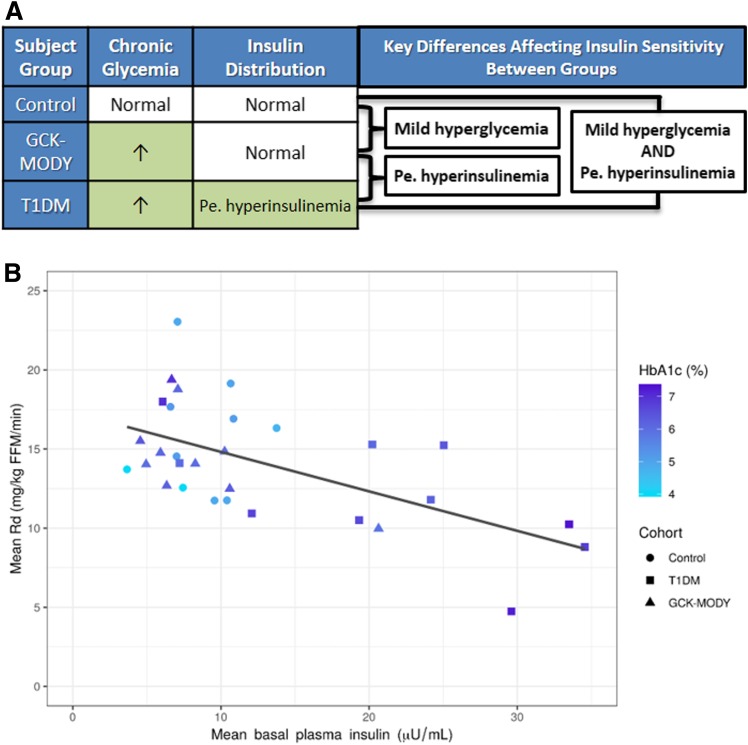
To help clarify whether iatrogenic hyperinsulinemia or hyperglycemia is the greater contributor to insulin insensitivity in type 1 diabetes, we studied three participant groups with differing conditions for hyperglycemia and iatrogenic hyperinsulinemia (10). These participant groups were 1) nondiabetic controls (with euinsulinemia and euglycemia), 2) glucokinase maturity-onset diabetes of the young (GCK-MODY) (with euinsulinemia and hyperglycemia owing to the mutation in GCK), and 3) type 1 diabetes (with hyperinsulinemia and selected to have hyperglycemia matching GCK-MODY) (Fig. 1A). To quantify tissue-specific insulin sensitivity, we conducted two-step hyperinsulinemic, euglycemic clamps. We found muscle tissue insulin sensitivity in subjects with type 1 diabetes was 22% lower than in GCK-MODY and 29% lower than in control subjects. Bivariate and multivariate linear regression analyses showed that iatrogenic hyperinsulinemia, not hyperglycemia, was the predominant factor associated with the variance in muscle insulin sensitivity (Fig. 1B). Further, we analyzed multiple potential confounders that could cause both hyperinsulinemia and insulin resistance by testing a series of adjusted and unadjusted multivariable linear regression models, but none appreciably altered the relationship between basal insulinemia and glucose uptake during the clamp. We additionally found that despite having hyperglycemia, the GCK-MODY group had the same degree of insulin-mediated NEFA and glycerol suppression as the control group. By contrast, more insulin was needed to suppress adipose tissue metabolism in the type 1 diabetes group. These data suggested hyperinsulinemia is the key player driving insulin resistance in both muscle and fat tissue.
Figure 1.
A: Key differences and similarities in chronic glycemia and insulin distribution affecting insulin sensitivity between participant groups. Pe. hyperinsulinemia, peripheral hyperinsulinemia. B: Scatterplot depicting correlation between mean basal insulin concentration (x-axis), glycosylated hemoglobin (HbA1c, darker shade of blue represents higher HbA1c), and mean rate of glucose disposal (Rd) during hyperinsulinemic, euglycemic clamp. Insulin was infused at 40 mU/m2/min when Rd was measured. The figure shows that participants with type 1 diabetes (T1DM) (squares) had higher basal insulinemia (further to the right on the x-axis), higher chronic glycemia (darker shade of blue), and lower Rd (further down on the y-axis). GCK-MODY participants (triangles) had glycemia similar to that of T1DM participants (similar shade of blue) but lower insulinemia (further to the left on the x-axis) and higher Rd (further up on the y-axis). Despite having differing glycemia (differing shades of blue), GCK-MODY and control (circles) participants generally had lower and similar insulinemia and higher Rd than T1DM participants. Coefficient of determination (R2) between basal insulin concentration and Rd was 0.36. R2 between HbA1c and Rd was 0.09. R2 for the multivariable linear regression model including both basal insulin concentration and HbA1c as dependent variables and Rd as an independent variable was 0.36. Thus, these linear regression analyses show that insulinemia alone explained 36% of the variance in Rd, a factor that was virtually unchanged with the addition of chronic glycemia in multivariable linear regression analysis. Collectively, these data suggest hyperinsulinemia stemming from peripheral insulin delivery plays a far greater role than hyperglycemia in causing peripheral insulin resistance in type 1 diabetes. FFM, fat-free mass.
We next considered hepatic insulin sensitivity. Based on our previous studies of equimolar portal versus peripheral insulin infusions in the canine (51,52), we estimated that at equivalent glycemia, hepatic sinusoidal insulin concentrations were similar between humans with endogenous insulin secretion and people with type 1 diabetes receiving peripherally delivered insulin. We reasoned that if local tissue insulin levels predominantly influenced insulin sensitivity, hepatic insulin sensitivity would be similar between groups with similar hepatic sinusoidal insulin levels. Although several previous studies reported the presence of hepatic insulin resistance in type 1 diabetes (11,18,26,53–55), we noted that glucagon concentrations were not reported in these studies. Thus, it remained unclear whether the liver was intrinsically less responsive to insulin or influenced by hyperglucagonemia. Indeed, in the only study we found that reported the presence of hepatic insulin resistance and also reported glucagon concentrations, the estimated hepatic sinusoidal glucagon-to-insulin ratio was twofold greater in type 1 diabetes participants than in control participants (56). For this reason, we elected to infuse somatostatin to disable the endocrine pancreas, glucagon to maintain basal concentrations, and insulin at a rate to induce a mild increase in hepatic sinusoidal insulin. In this way, hepatic sinusoidal insulin and glucagon were equivalent between all groups during the clamp, eliminating a potential confounder of insulin-mediated suppression of endogenous glucose production. When we analyzed insulin-mediated suppression of hepatic glucose production, we found almost no difference between the groups, despite differences in glycemia and peripheral insulinemia. This finding raised the possibility that when insulin and glucagon concentrations within the hepatic sinusoids are comparable between groups, hepatic insulin sensitivity is also similar between groups.
Collectively, our data suggested local tissue hyperinsulinemia, as occurs at muscle and fat tissue in type 1 diabetes but not in GCK-MODY or control or in any group at liver, is associated with tissue-specific insulin insensitivity. Furthermore, our study suggested iatrogenic hyperinsulinemia was much more associated with insulin insensitivity than hyperglycemia after adjustment for known confounders.
A Physiologic Restructuring of Insulin Therapy Is Needed to Rectify Cardiometabolic Risk in Type 1 Diabetes
These data indicate a therapeutic tightrope exists in type 1 diabetes. On one side, iatrogenic hyperinsulinemia primarily drives insulin resistance in type 1 diabetes. On the other side, reducing insulin doses would seemingly exacerbate hyperglycemia, which could also worsen cardiovascular outcomes and aggravate microvascular complications (57,58). To resolve this apparent dilemma, insulin therapy will need to more closely imitate physiological insulin delivery. More specifically, treatment should avoid overinsulinizing peripheral tissues by approximating the normal balance of insulin between hepatic and peripheral tissues. Restoring this equilibrium would benefit not only hyperinsulinemia-mediated insulin resistance but also hyperglycemia.
Compared with portal insulin delivery, peripheral insulin delivery in type 1 diabetes necessitates higher levels of insulin in the peripheral circulation to adequately restrain hepatic glucose production in the basal state and stimulate glucose uptake in the absorptive state (52). Consequently, skeletal muscle acts as an even larger “glucose sink,” and this alteration forces physicians to operate on a sharper portion of the dose-response curve that relates insulin dose to glucose uptake. By contrast, because of first-pass hepatic extraction, portal insulin delivery does not necessitate overinsulinization of peripheral tissues. This fact would allow physicians to operate on a flatter portion of the dose-response curve. Thus, physiological portal insulin delivery would reduce glycemic variability, thereby allowing for lowering of overall glycemia without worsening hypoglycemia. Generally speaking, two approaches to mimic portal insulin delivery, intraperitoneal insulin delivery (59) and hepatopreferential insulin analogs (60), reduce mean glycemia and glycemic variability compared with conventional insulin therapy. Table 2 outlines two categories of potential approaches to approximate aspects of portal insulin delivery: therapies attempting to replicate the physiologic insulin balance between the liver and peripheral tissues (e.g., hepatopreferential and oral insulin analogs, hepatic-directed vesicle insulin, intraperitoneal insulin delivery) and therapies to facilitate a reduction in peripheral hyperinsulinemia (e.g., hepatoselective glucokinase activators, glucagon receptor antagonists, sodium–glucose constransporter 2 inhibitors).
Table 2.
Key benefits and barriers associated with emerging therapies to recreate aspects of portal insulin delivery
| Therapies in development | Key benefits toward reducing cardiometabolic risk | Barriers and challenges to widespread use | |
|---|---|---|---|
| Therapies to replicate normal insulin balance between liver and peripheral tissues | Intraperitoneal/intraportal insulin delivery | Generally lowers hyperglycemia more than subcutaneous insulin (44,70–73) | Surgical complications (74) |
| Lessens severe hypoglycemia more than subcutaneous insulin to varying degrees (72–74) | Insulin aggregation and catheter occlusion in intraperitoneal pumps (77) | ||
| Tends to avoid weight gain (74) | |||
| Rapid absorption to approximate first-phase insulin release and reduce postprandial hyperglycemia (75,76) | |||
| Approximates insulin balance between portal and peripheral circulations (76) | |||
| Oral insulin delivery | Rapid absorption to approximate first-phase insulin release (78) | Limited bioavailability (78) | |
| Approximates insulin balance between portal and peripheral circulations (78) | Absorption affected by food in the gut (79) | ||
| Increases patient burden | |||
| Hepatopreferential insulin analogs and hepatic-directed vesicle insulin | Pharmacodynamic characteristics approximate balance in insulin action between portal and peripheral circulations (80–82) | Concern for potential hepatic toxicity (83) | |
| Tends to avoid weight gain (83) | |||
| Generally lowers hyperglycemia more than conventional insulin (83) | |||
| Encapsulated islet implantation in intraperitoneal space | Rapid absorption to approximate first-phase insulin release | Diminished engraftment | |
| Rapid pharmacokinetics could facilitate brisk decreases in plasma insulin during hypoglycemia | Early in development | ||
| Approximates insulin balance between portal and peripheral circulations | Requires surgery | ||
| Therapies to facilitate a reduction in peripheral hyperinsulinemia | Glucagon receptor antagonists | Decreases insulin requirements while improving mean glucose and time in range without increasing time in hypoglycemia (84) | Increases in weight, LDL cholesterol, and liver transaminases reported in type 2 diabetes (85) |
| May alter response to exogenous glucagon for severe hypoglycemia | |||
| Hepatoselective glucokinase activators | Tend to reduce hypoglycemia | Early in development | |
| Reduces chronic glycemia with lower doses of prandial insulin (86) | |||
| Sodium–glucose cotransporter 2 inhibitors | Reduces hyperinsulinemia by lowering total daily insulin doses by ∼15–20% (87–89) | Concern for diabetic ketoacidosis (90) | |
| Only modest reduction in hyperinsulinemia vs. therapies that normalize portal-peripheral insulin balance | |||
| Low-carbohydrate diet | Reduces hyperinsulinemia by lowering prandial insulin requirements | Only modest reduction in hyperinsulinemia vs. therapies that normalize portal-peripheral insulin balance |
Some have expressed concern that efforts to recreate portal insulin delivery could lead to increased hepatic steatosis. Indeed, previous investigations have linked hepatic steatosis with both intrahepatic islet transplantation (61) and the hepatopreferential basal insulin peglispro (62). If these approaches are attempting to mimic physiological insulin delivery, which is not associated with hepatic steatosis, why would hepatic steatosis occur? It seems most likely that the hepatic steatosis stems from the paracrine effect of the former intervention (61) and the profound hepatoselectivity of the latter intervention (63–65). For this reason, emerging therapies should aim to further replicate the physiologic state by cautiously protecting against overinsulinizing the liver.
Research by several leading investigators suggests hyperinsulinemia per se may exacerbate, sustain, and even initiate obesity and insulin insensitivity in the metabolic syndrome and type 2 diabetes (27,66,67). While the interplay between endogenous and iatrogenic hyperinsulinemia and insulin insensitivity in these patient populations is beyond the scope of this article, we believe this research is of considerable importance. Similarly, other investigators have observed parallel increases in the incidence of obesity and type 1 diabetes in industrialized countries. This observation has given rise to the accelerator hypothesis and the notion of “double diabetes.” These concepts posit that coexisting β-cell immune intolerance and insulin resistance related to increased weight work in concert to accelerate β-cell loss and thwart insulin therapy in type 1 diabetes (68,69). Although this research is also beyond the scope of the present discussion, we support efforts to better understand these observations as any means to salvage β-cell function may ultimately reduce the degree of requisite peripheral hyperinsulinemia for management.
In conclusion, we believe the available evidence indicates current insulin therapy will not fully mitigate cardiometabolic risk in type 1 diabetes as it induces an insulin resistance that is tied to increased cardiometabolic risk. This shortfall occurs because contemporary treatment strategies fail to emulate multiple characteristics of physiological insulin delivery. In this Perspective, we have specifically emphasized that well-intentioned efforts to correct hyperglycemia using peripheral insulin delivery departs from the physiological norm. This approach trades one cardiometabolic risk—hyperglycemia—for another—peripheral hyperinsulinemia. We believe that balancing the demands for euglycemia and insulin sensitivity by pursuing a physiological restructuring of hormone therapy will be required to achieve optimal long-term outcomes in type 1 diabetes. We look forward to new approaches to achieve this critical balance.
Article Information
Acknowledgments. The authors thank James C. Slaughter of the Vanderbilt University Department of Biostatistics for creating Fig. 1B in this article.
Funding. J.M.G. has received support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number K12HD087023 and from the Wallace Family Fund in Diabetes Research.
Duality of Interest. J.M.G. reports consulting fees from InClinica and advisory board fees from Eli Lilly and Medtronic. A.D.C. reports grants, personal fees, and other support from Metavention, Zafgen, and Abvance; grants and personal fees from Boston Scientific, Novo Nordisk, vTv Therapeutics, Merck, Eli Lilly, and Galvani Bioelectronics; personal fees from California Institute for Biomedical Research (Calibr) and MedImmune; and personal fees and other support from Fractyl, Thetis Pharmaceuticals, and Sensulin Laboratories. Additionally, A.D.C. has patented a method of treating overweight subjects that is issued to Zafgen, a method for treating diabetes issued to Abvance, and a method of controlling postprandial glucose levels in diabetes issued to Biocon. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest 1956;35:170–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest 1960;39:1157–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortimore GE, Tietze F. Studies on the mechanism of capture and degradation of insulin-1131 by the cyclically perfused rat liver. Ann N Y Acad Sci 1959;82:329–337 [DOI] [PubMed] [Google Scholar]
- 4.Madison LL, Combes B, Unger RH, Kaplan N. The relationship between the mechanism of action of the sulfonylureas and the secretion of insulin into the portal circulation. Ann N Y Acad Sci 1959;74:548–556 [DOI] [PubMed] [Google Scholar]
- 5.Samols E, Ryder JA. Studies on tissue uptake of insulin in man using a differential immunoassay for endogenous and exogenous insulin. J Clin Invest 1961;40:2092–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaden M, Harding P, Field JB. Effect of intraduodenal glucose administration on hepatic extraction of insulin in the anesthetized dog. J Clin Invest 1973;52:2016–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackard WG, Nelson NC. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes 1970;19:302–306 [DOI] [PubMed] [Google Scholar]
- 8.Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest 1975;55:1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yalow RS, Berson SA. Dynamics of insulin secretion in hypoglycemia. Diabetes 1965;14:341–349 [DOI] [PubMed] [Google Scholar]
- 10.Gregory JM, Smith TJ, Slaughter JC, et al. Iatrogenic hyperinsulinemia, not hyperglycemia, drives insulin resistance in type 1 diabetes as revealed by comparison with GCK-MODY (MODY2). Diabetes 2019;68:1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cree-Green M, Stuppy JJ, Thurston J, et al. Youth with type 1 diabetes have adipose, hepatic, and peripheral insulin resistance. J Clin Endocrinol Metab 2018;103:3647–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman BC, Howard D, Schauer IE, et al. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab 2012;97:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes 2011;60:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues TC, Biavatti K, Almeida FK, Gross JL. Coronary artery calcification is associated with insulin resistance index in patients with type 1 diabetes. Braz J Med Biol Res 2010;43:1084–1087 [DOI] [PubMed] [Google Scholar]
- 15.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himsworth HP. Diabetes mellitus: its differentiation into insulin-sensitive and insulin-insensitive types. Lancet 1936;227:127–130 [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 1982;31:795–801 [DOI] [PubMed] [Google Scholar]
- 19.Donga E, Dekkers OM, Corssmit EP, Romijn JA. Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: systematic review and meta-analysis. Eur J Endocrinol 2015;173:101–109 [DOI] [PubMed] [Google Scholar]
- 20.Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism 2015;64:1629–1639 [DOI] [PubMed] [Google Scholar]
- 21.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26:1374–1379 [DOI] [PubMed] [Google Scholar]
- 22.Dabelea D, Kinney G, Snell-Bergeon JK, et al.; Coronary Artery Calcification in Type 1 Diabetes Study . Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 2003;52:2833–2839 [DOI] [PubMed] [Google Scholar]
- 23.Yki-Järvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med 1986;315:224–230 [DOI] [PubMed] [Google Scholar]
- 24.Yki-Järvinen H, DeFronzo RA, Koivisto VA. Normalization of insulin sensitivity in type I diabetic subjects by physical training during insulin pump therapy. Diabetes Care 1984;7:520–527 [DOI] [PubMed] [Google Scholar]
- 25.Yki-Järvinen H, Helve E, Koivisto VA. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes 1987;36:892–896 [DOI] [PubMed] [Google Scholar]
- 26.Yki-Järvinen H, Koivisto VA. Continuous subcutaneous insulin infusion therapy decreases insulin resistance in type 1 diabetes. J Clin Endocrinol Metab 1984;58:659–666 [DOI] [PubMed] [Google Scholar]
- 27.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31(Suppl. 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- 28.Marban SL, DeLoia JA, Gearhart JD. Hyperinsulinemia in transgenic mice carrying multiple copies of the human insulin gene. Dev Genet 1989;10:356–364 [DOI] [PubMed] [Google Scholar]
- 29.Marbán SL, Roth J. Transgenic hyperinsulinemia: a mouse model of insulin resistance and glucose intolerance without obesity. In Lessons from Animal Diabetes VI. Shafrir E, Ed. Boston, Birkhäuser, 1996, p. 201–224 [Google Scholar]
- 30.Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem 2009;284:27090–27100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Templeman NM, Flibotte S, Chik JHL, et al. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Reports 2017;20:451–463 [DOI] [PubMed] [Google Scholar]
- 32.McGuinness OP, Friedman A, Cherrington AD. Intraportal hyperinsulinemia decreases insulin-stimulated glucose uptake in the dog. Metabolism 1990;39:127–132 [DOI] [PubMed] [Google Scholar]
- 33.Vlachokosta FV, Piper CM, Gleason R, Kinzel L, Kahn CR. Dietary carbohydrate, a Big Mac, and insulin requirements in type I diabetes. Diabetes Care 1988;11:330–336 [DOI] [PubMed] [Google Scholar]
- 34.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 35.Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia 1985;28:70–75 [DOI] [PubMed] [Google Scholar]
- 36.Marangou AG, Weber KM, Boston RC, et al. Metabolic consequences of prolonged hyperinsulinemia in humans. Evidence for induction of insulin insensitivity. Diabetes 1986;35:1383–1389 [DOI] [PubMed] [Google Scholar]
- 37.Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994;37:1025–1035 [DOI] [PubMed] [Google Scholar]
- 38.Shannon C, Merovci A, Xiong J, et al. Effect of chronic hyperglycemia on glucose metabolism in subjects with normal glucose tolerance. Diabetes 2018;67:2507–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cree-Green M, Newcomer BR, Brown MS, et al. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes 2015;64:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowther GJ, Milstein JM, Jubrias SA, Kushmerick MJ, Gronka RK, Conley KE. Altered energetic properties in skeletal muscle of men with well-controlled insulin-dependent (type 1) diabetes. Am J Physiol Endocrinol Metab 2003;284:E655–E662 [DOI] [PubMed] [Google Scholar]
- 41.Kacerovsky M, Brehm A, Chmelik M, et al. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J Intern Med 2011;269:189–199 [DOI] [PubMed] [Google Scholar]
- 42.Antonetti DA, Reynet C, Kahn CR. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. J Clin Invest 1995;95:1383–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Item F, Heinzer-Schweizer S, Wyss M, et al. Mitochondrial capacity is affected by glycemic status in young untrained women with type 1 diabetes but is not impaired relative to healthy untrained women. Am J Physiol Regul Integr Comp Physiol 2011;301:R60–R66 [DOI] [PubMed] [Google Scholar]
- 44.Shishko PI, Kovalev PA, Goncharov VG, Zajarny IU. Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes 1992;41:1042–1049 [DOI] [PubMed] [Google Scholar]
- 45.Carpentier A, Patterson BW, Uffelman KD, et al. The effect of systemic versus portal insulin delivery in pancreas transplantation on insulin action and VLDL metabolism. Diabetes 2001;50:1402–1413 [DOI] [PubMed] [Google Scholar]
- 46.Meier JJ, Hong-McAtee I, Galasso R, et al. Intrahepatic transplanted islets in humans secrete insulin in a coordinate pulsatile manner directly into the liver. Diabetes 2006;55:2324–2332 [DOI] [PubMed] [Google Scholar]
- 47.Rickels MR, Kong SM, Fuller C, et al. Improvement in insulin sensitivity after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2013;98:E1780–E1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonson DC, Tamborlane WV, Sherwin RS, Smith JD, DeFronzo RA. Improved insulin sensitivity in patients with type I diabetes mellitus after CSII. Diabetes 1985;34(Suppl. 3):80–86 [DOI] [PubMed] [Google Scholar]
- 49.Lager I, Lönnroth P, von Schenck H, Smith U. Reversal of insulin resistance in type I diabetes after treatment with continuous subcutaneous insulin infusion. Br Med J (Clin Res Ed) 1983;287:1661–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vuorinen-Markkola H, Koivisto VA, Yki-Jarvinen H. Mechanisms of hyperglycemia-induced insulin resistance in whole body and skeletal muscle of type I diabetic patients. Diabetes 1992;41:571–580 [DOI] [PubMed] [Google Scholar]
- 51.Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest 2006;116:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory JM, Kraft G, Scott MF, et al. Insulin delivery into the peripheral circulation: a key contributor to hypoglycemia in type 1 diabetes. Diabetes 2015;64:3439–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1982;23:313–319 [DOI] [PubMed] [Google Scholar]
- 54.Donga E, van Dijk M, Hoogma RP, Corssmit EP, Romijn JA. Insulin resistance in multiple tissues in patients with type 1 diabetes mellitus on long-term continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev 2013;29:33–38 [DOI] [PubMed] [Google Scholar]
- 55.Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 2003;285:E1174–E1181 [DOI] [PubMed] [Google Scholar]
- 56.Perseghin G, Lattuada G, De Cobelli F, et al. Reduced intrahepatic fat content is associated with increased whole-body lipid oxidation in patients with type 1 diabetes. Diabetologia 2005;48:2615–2621 [DOI] [PubMed] [Google Scholar]
- 57.The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 58.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 59.van Dijk PR, Logtenberg SJJ, Gans ROB, Bilo HJG, Kleefstra N. Intraperitoneal insulin infusion: treatment option for type 1 diabetes resulting in beneficial endocrine effects beyond glycaemia. Clin Endocrinol (Oxf) 2014;81:488–497 [DOI] [PubMed] [Google Scholar]
- 60.Jacober SJ, Prince MJ, Beals JM, et al. Basal insulin peglispro: overview of a novel long-acting insulin with reduced peripheral effect resulting in a hepato-preferential action. Diabetes Obes Metab 2016;18(Suppl. 2):3–16 [DOI] [PubMed] [Google Scholar]
- 61.Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes 2004;53:1311–1317 [DOI] [PubMed] [Google Scholar]
- 62.Buse JB, Rodbard HW, Trescoli Serrano C, et al.; IMAGINE 5 Investigators . Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with type 2 diabetes previously treated with basal insulin: IMAGINE 5. Diabetes Care 2016;39:92–100 [DOI] [PubMed] [Google Scholar]
- 63.Mudaliar S, Henry RR, Ciaraldi TP, et al. Reduced peripheral activity leading to hepato-preferential action of basal insulin peglispro compared with insulin glargine in patients with type 1 diabetes. Diabetes Obes Metab 2016;18(Suppl. 2):17–24 [DOI] [PubMed] [Google Scholar]
- 64.Henry RR, Mudaliar S, Ciaraldi TP, et al. Basal insulin peglispro demonstrates preferential hepatic versus peripheral action relative to insulin glargine in healthy subjects. Diabetes Care 2014;37:2609–2615 [DOI] [PubMed] [Google Scholar]
- 65.Moore MC, Smith MS, Sinha VP, et al. Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes 2014;63:494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 2012;61:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Templeman NM, Skovsø S, Page MM, Lim GE, Johnson JD. A causal role for hyperinsulinemia in obesity. J Endocrinol 2017;232:R173–R183 [DOI] [PubMed] [Google Scholar]
- 68.Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia 2013;56:1462–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 70.Logtenberg SJ, Kleefstra N, Houweling ST, et al. Improved glycemic control with intraperitoneal versus subcutaneous insulin in type 1 diabetes: a randomized controlled trial. Diabetes Care 2009;32:1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dassau E, Renard E, Place J, et al. Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab 2017;19:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Selam JL, Micossi P, Dunn FL, Nathan DM. Clinical trial of programmable implantable insulin pump for type I diabetes. Diabetes Care 1992;15:877–885 [DOI] [PubMed] [Google Scholar]
- 73.Haardt MJ, Selam JL, Slama G, et al. A cost-benefit comparison of intensive diabetes management with implantable pumps versus multiple subcutaneous injections in patients with type I diabetes. Diabetes Care 1994;17:847–851 [DOI] [PubMed] [Google Scholar]
- 74.Liebl A, Hoogma R, Renard E, et al.; European DiaPort Study Group . A reduction in severe hypoglycaemia in type 1 diabetes in a randomized crossover study of continuous intraperitoneal compared with subcutaneous insulin infusion. Diabetes Obes Metab 2009;11:1001–1008 [DOI] [PubMed] [Google Scholar]
- 75.Chakrabarty A, Gregory JM, Moore LM, et al. A new animal model of insulin-glucose dynamics in the intraperitoneal space enhances closed-loop control performance. J Process Contr 2019;76:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schade DS, Eaton RP, Davis T, et al. The kinetics of peritoneal insulin absorption. Metabolism 1981;30:149–155 [DOI] [PubMed] [Google Scholar]
- 77.van Dijk PR, Logtenberg SJ, Groenier KH, Haveman JW, Kleefstra N, Bilo HJ. Complications of continuous intraperitoneal insulin infusion with an implantable pump. World J Diabetes 2012;3:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gregory JM, Lautz M, Moore LM, Williams PE, Reddy P, Cherrington AD. Enterically delivered insulin tregopil exhibits rapid absorption characteristics and a pharmacodynamic effect similar to human insulin in conscious dogs. Diabetes Obes Metab 2019;21:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khedkar A, Lebovitz H, Fleming A, et al. Pharmacokinetics and pharmacodynamics of insulin tregopil in relation to premeal dosing time, between meal interval, and meal composition in patients with type 2 diabetes mellitus. Clin Pharmacol Drug Dev 2020;9:74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gregory JM, Kraft G, Scott MF, et al. Peripherally delivered hepatopreferential insulin analog insulin-406 mimics the hypoglycaemia-sparing effect of portal vein human insulin infusion in dogs. Diabetes Obes Metab 2019;21:2294–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edgerton DS, Scott M, Farmer B, et al. Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery. JCI Insight 2019;4:e126974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klonoff D, Bode B, Cohen N, Penn M, Geho WB, Muchmore DB. Divergent hypoglycemic effects of hepatic-directed prandial insulin: a six-month phase 2b study in type 1 diabetes. Diabetes Care 2019;42:2154–2157 [DOI] [PubMed] [Google Scholar]
- 83.Bergenstal RM, Lunt H, Franek E, et al.; IMAGINE 3 Trial Investigators . Randomized, double-blind clinical trial comparing basal insulin peglispro and insulin glargine, in combination with prandial insulin lispro, in patients with type 1 diabetes: IMAGINE 3. Diabetes Obes Metab 2016;18:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pettus J, Reeds D, Cavaiola TS, et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: A randomized controlled trial. Diabetes Obes Metab 2018;20:1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nunez DJ, D’Alessio D. Glucagon receptor as a drug target: a witches’ brew of eye of newt (peptides) and toe of frog (receptors). Diabetes Obes Metab 2018;20:233–237 [DOI] [PubMed] [Google Scholar]
- 86.Valcarce C, Freeman J, Dunn I, Dvergsten C, Soeder T, Buse J. Results from the sentinel and learning phase of the Simplici-T1 study, the first clinical trial to test activation of glucokinase as an adjunctive treatment for type 1 diabetes. Diabetologia 2019;62:1–60031384961 [Google Scholar]
- 87.Henry RR, Rosenstock J, Edelman S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 2015;38:412–419 [DOI] [PubMed] [Google Scholar]
- 88.Perkins BA, Cherney DZ, Soleymanlou N, et al. Diurnal glycemic patterns during an 8-week open-label proof-of-concept trial of empagliflozin in type 1 diabetes. PLoS One 2015;10:e0141085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pieber TR, Famulla S, Eilbracht J, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab 2015;17:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nathan DM. Adjunctive treatments for type 1 diabetes. N Engl J Med 2017;377:2390–2391 [DOI] [PubMed] [Google Scholar]