Abstract
The coordinated electrical activity of β-cells within the pancreatic islet drives oscillatory insulin secretion. A recent hypothesis postulates that specially equipped “hub” or “leader” cells within the β-cell network drive islet oscillations and that electrically silencing or optically ablating these cells suppresses coordinated electrical activity (and thus insulin secretion) in the rest of the islet. In this Perspective, we discuss this hypothesis in relation to established principles of electrophysiological theory. We conclude that whereas electrical coupling between β-cells is sufficient for the propagation of excitation across the islet, there is no obvious electrophysiological mechanism that explains how hyperpolarizing a hub cell results in widespread inhibition of islet electrical activity and disruption of their coordination. Thus, intraislet diffusible factors should perhaps be considered as an alternate mechanism.
Introduction
Plasma insulin oscillates with a period of 5–10 min (1). A similar pulsatility is observed in isolated islets obtained from healthy human donors (2). There is evidence that the loss of the insulin pulses, via permanent elevation of the insulin receptors, can contribute to insulin receptor desensitization and insulin resistance (1).
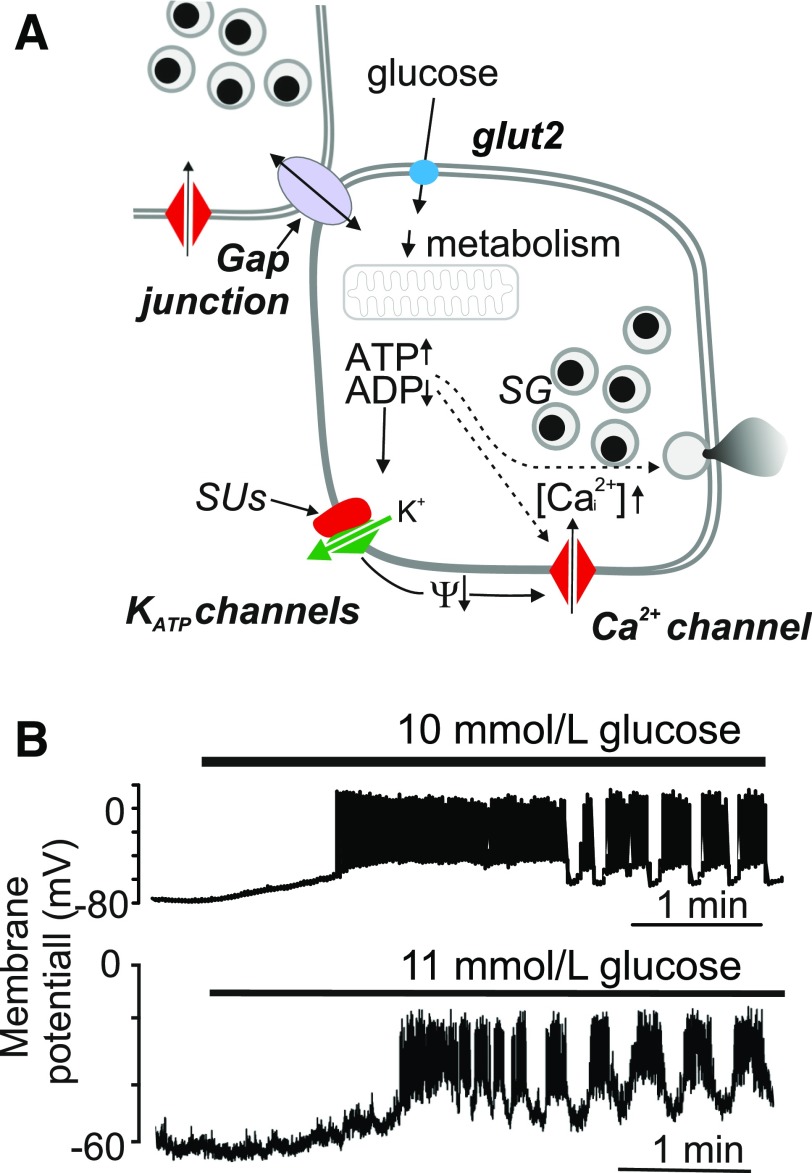
At the level of the β-cell, pulsatile insulin secretion is driven by a stereotypical pattern of electrical activity that consists of groups of action potentials superimposed on slower depolarized plateaus that are separated by hyperpolarized/electrically silent intervals (3). According to the “consensus model” of glucose-induced insulin secretion, glucose uptake and metabolism culminate in an increase in the cytoplasmic ATP/ADP ratio that in turn closes ATP-sensitive potassium (KATP) channels. The activity of the KATP channels maintains a negative membrane potential (approximately −80 to −70 mV) in the β-cell under low-glucose conditions, so closure of KATP channels by elevated glucose metabolism is required for membrane depolarization (i.e., a less negative membrane potential). When the membrane potential is more positive than −50 mV, voltage-sensitive Ca2+ channels open, and the associated increase in cytoplasmic Ca2+ concentration ([Ca2+]i) triggers the exocytosis of insulin-containing secretory granules (4) (Fig. 1A). After an initial phase of spiking activity, the islet settles into a rhythmical bursting pattern (Fig. 1B) that drives pulses of insulin exocytosis. Most of the characterization of β-cell electrical activity was carried out on mouse cells, but it appears that similar processes operate in human β-cells and increasing glucose elicits bursting electrical activity also in β-cells in intact human pancreatic islets (5) (Fig. 1B). Although the exact correlation between the bursts of electrical activity and insulin secretion seen in individual islets and the appearance of plasma insulin oscillations in vivo remains enigmatic, studies of islet rhythmicity can provide insights that can help to explain why plasma insulin oscillations are either smaller in amplitude or not observed at all in patients with type 2 diabetes (6).
Figure 1.
A: Stimulus-secretion coupling of the pancreatic β-cell (“consensus model”). The arrows (↑,↓) indicate an increase or decrease of the indicated parameter. Glucose initiates insulin secretion following its metabolism with a resultant increase in cytoplasmic ATP-to-ADP ratio, closure of KATP channels, membrane depolarization, opening of voltage-gated Ca2+ channels, and initiation of Ca2+-dependent exocytosis of the insulin-containing secretory granules (SG). In addition, the β-cells are electrically coupled to neighboring β-cells via gap junctions consisting of connexin-36. Ψ, membrane potential. Sulfonylureas (SUs) stimulate insulin secretion by closing the KATP channels. B: Glucose-induced electrical activity recorded from a β-cell in intact mouse (top) or human (bottom) islets when the glucose concentration was increased from 1 to 10 or 11 mmol/L (as indicated by horizontal bars).
In 2016, Johnston et al. (7) proposed that glucose-dependent oscillations in β-cell cytosolic free calcium ([Ca2+]i), which are driven by glucose-dependent electrical bursting activity and linked to pulsatile insulin secretion, are initiated by the activation of specially equipped “hub cells,” pacemaker-like cells that initiate and coordinate the oscillatory network of the islet β-cell syncytium. In a subsequent study, these cells were renamed “leader cells” (8). Activation of these hub/leader cells (for example, using the light-activated channel channelrhodopsin) may trigger [Ca2+]i waves that extend to the other cells within the islets (“follower cells”) (9). The propagation of the [Ca2+]i waves depends on the metabolic state of the β-cell, KATP channel activity, and cell coupling (10). These coordinated increases and decreases in [Ca2+]i drive pulses of insulin secretion from individual islets (11).
In this article, we will review the existence and role of hub/leader cells in islets from an electrophysiological viewpoint and highlight some of the earlier studies (some of which were conducted ∼40 years ago), which are difficult to reconcile with the leader/follower cell concept and that we feel have not received the recent attention they deserve. As we shall point out, some aspects of the hub/leader cell concept are incompatible with electrophysiological theory and it may therefore be necessary to consider other possibilities (including intraislet diffusible factors).
Evidence for the Existence of Hub Cells in Pancreatic Islets
Johnston et al. (7) reported that a subset (10%) of “superconnected” β-cells controls electrical activity (and thus [Ca2+]i and secretion) in the β-cells of the remainder of the islet. This “connectedness” was inferred from pairwise correlation analysis of glucose-induced [Ca2+]i oscillations. Depolarization of thus identified hub cells (using the light-activated ion channel channelrhodopsin) evoked electrical activity across the entire network of connected β-cells. A key observation of this article was the demonstration that hyperpolarizing these “hub cells” (i.e., making the β-cell membrane potential more negative by photoactivating the light-sensitive Cl− pump halorhodopsin) disrupted the entire network of oscillating follower β-cells. Notably, photoinhibition of the leader cell did not abolish the [Ca2+]i oscillations in the followers but they became uncoordinated. In a follow-up study, photoablation of the leader cells (but not follower cells) using laser light reduced [Ca2+]i in the small islets of zebrafish larvae (8). The hub cells so identified were also reported to exhibit a characteristic metabolic signature suggesting that they are highly active metabolically with increased ATP production and increased glucokinase expression. In addition, they had reduced insulin content and reduced expression of β-cell–specific “markers” (like Pdx1 and Nkx6.1), suggesting they may be less mature than the other β-cells of the islet. The possible significance of these hub or leader cells for diabetes is that if their activity was important for glucose-induced insulin secretion, their selective dysfunction could in turn contribute to the loss of glucose homeostasis that is a hallmark of type 2 diabetes (12,13). However, it is not immediately evident how hub/leader cells would participate in the synchronization of the pulsatile secretion of 1,000 (mouse) (14) or 1,000,000 (human) (15) islets within the whole pancreas (16), although how well synchronized the electrical bursts of different islets are in vivo is not clear (17).
Cell Coupling
In elevated glucose, widespread oscillations in [Ca2+]i that correlate with electrical activity and insulin secretion are observed (18–20). The [Ca2+]i oscillations seen in islets are synchronized by electrical coupling between adjacent β-cells (21). Thus, electrical activity that occurs in one β-cell results in the injection of current into its neighboring β-cells, and a membrane depolarization that may be sufficient to trigger their electrical activity results from this cell-cell communication. Electrical coupling is mediated by proteins known as gap junctions (Fig. 1A). Connexin-36 is the only connexin expressed at significant levels in either mouse or human islets (21). The central role of the electrical coupling and the connexins in the coordination of the [Ca2+]i waves is illustrated by the complete loss of their synchronization and loss of pulsatile insulin secretion after genetic ablation of connexin-36 (21). Importantly, individual cells continue to oscillate.
Electrophysiology allows quantitative measurements of the electrical coupling between islet β-cells. As discussed above, glucose stimulation leads to the initiation of bursting electrical activity in the β-cells. This electrical activity measured with patch-clamp or sharp intracellular electrodes reports the behavior of the single β-cell within the islet that impaled with or connected to the recording electrode and can be suppressed by injecting negative current to clamp the membrane potential at −70 mV regardless of any currents flowing through the membrane (a technique called “voltage-clamp”). This membrane potential is too negative to activate any of the voltage-gated ion channels of the β-cell membrane. Nevertheless, oscillations in the holding current that look like inverted bursts of electrical activity remain observable when the prevailing glucose concentration is >7 mmol/L or so. The interpretation of this phenomenon is that the current oscillations occur because voltage-clamping can only suppress electrical activity in the cell connected to the recording electrode but is insufficient to control the membrane potential of the neighboring β-cells. Thus, the other cells “escape” voltage control and are free to fire action potentials that can spread into the voltage-clamped β-cell via the gap junctions (22,23) (Fig. 2A and B).
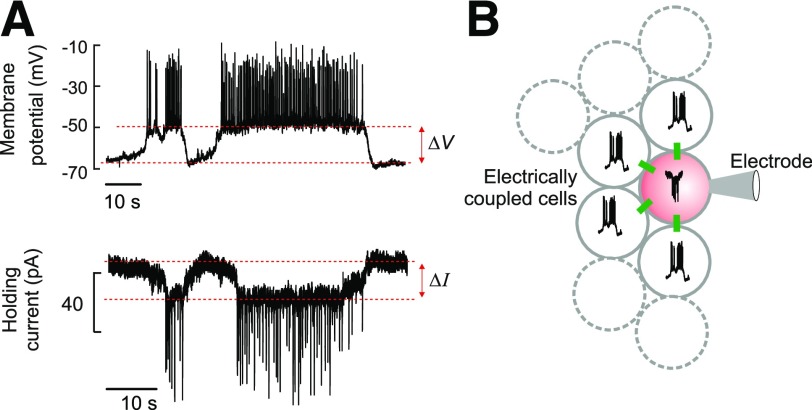
Figure 2.
A: Membrane potential (top) and holding current (bottom) recorded from the same β-cell under current- and voltage-clamp conditions, respectively. The voltage-clamp recording was obtained at −70 mV. B: Schematic of electrical coupling between β-cells in an intact islet. The green rectangles indicate gap junctions. One β-cell within the islet is voltage-clamped at −70 mV through the recording electrode (pink), and regenerative electrical activity in this cell is therefore prevented. Spontaneous electrical activity in neighboring electrically coupled cells results in an inward current that resembles an inverted burst of action potentials in the voltage-clamped cell. Assuming that electrical activity recorded in the β-cell connected to the patch electrode prior to voltage-clamping approximates that of its neighbors, the total gap-junctional conductance (Gj) can be estimated from the equation Gj = ΔI/ΔV, where ΔV and ΔI represent the current and voltage differences between the plateau current/potential and the most repolarized voltage/least negative current, respectively (as indicated in A).
Comparing membrane potential and membrane current recordings from the same β-cell allows us to quantitate the extent of the cell-to-cell coupling. The plateaus that are observed are ∼20 mV depolarized relative to the most repolarized membrane potentials observed and inject ∼20 pA of current into these neighboring cells. From these values, we can estimate that the total gap-junctional conductance (Gj) experienced by a β-cell in an intact islet is ∼1 nS (22) (Fig. 2B). Relating this value to the coupling conductance measured between an isolated pair of β-cells (∼200 pS [24,25]) suggests that a β-cell within an intact mouse islet is coupled to an average of approximately five other β-cells.
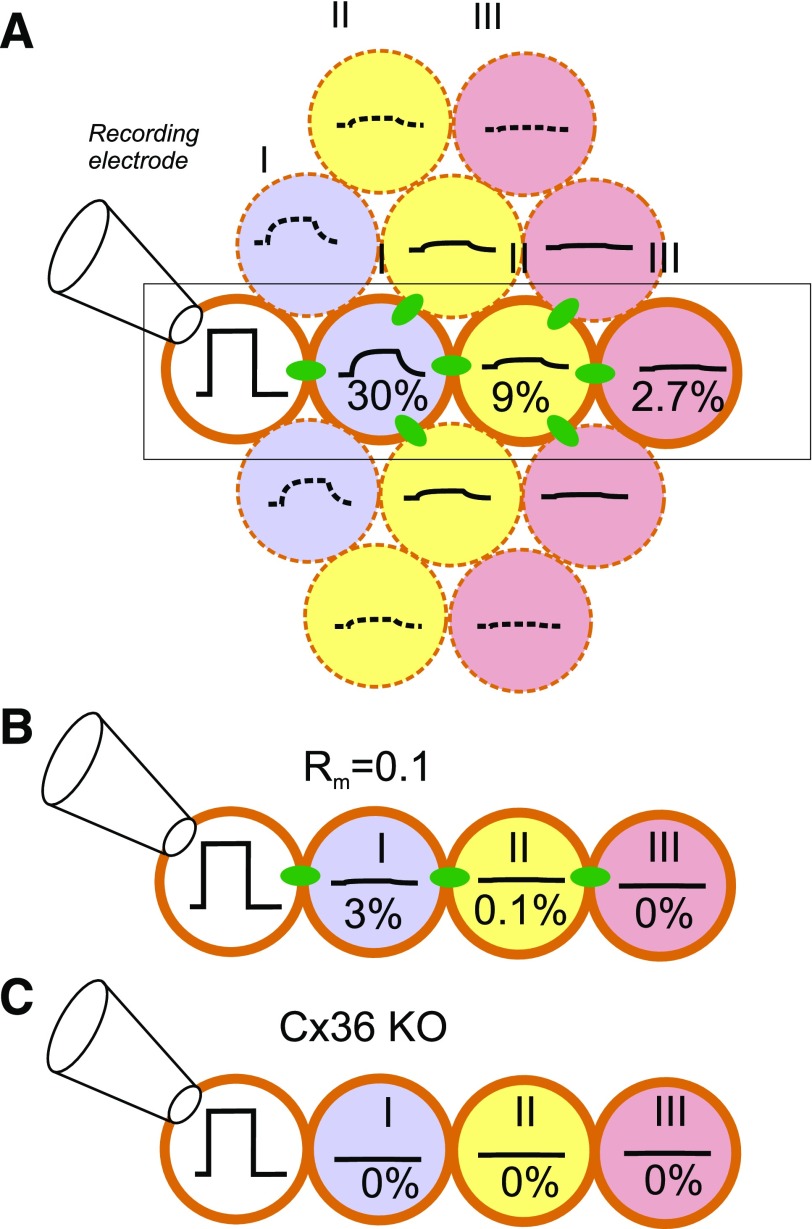
What do these biophysical data mean, and what are their implications for the hub cell concept? They suggest that although β-cells are unarguably electrically coupled, this coupling is not very strong. Thus, only 30% of the voltage change imposed on the voltage-clamped cell will extend to its neighbors, and there will be a similar degree of attenuation for every layer of β-cells that is situated away from the recording electrode; 9% and 2.7% of the command will propagate to the 2nd and 3rd tier of β-cells, respectively (Fig. 3A). This argument is consistent with direct measurements made by Eddlestone et al. in the early 1980s (26). In those experiments, the same mouse islet was impaled with two fine-tipped glass microelectrodes placed 10–100 μm apart. It was observed that current injected into one cell only elicited a change in membrane potential in the second cell if the distance between them was less than ∼35 μm (i.e., equivalent to ∼2–3 β-cell diameters).
Figure 3.
Attenuation of electrical signaling via gap junctions. A: A voltage step is applied to a voltage-clamped β-cell in an islet (white) (connected to recording electrode). The change in membrane potential enters neighboring cells (blue) (layer II) via the gap junctions but is attenuated by 70%, and its time course is “filtered” by the resistive-capacitive properties of the β-cell. The voltage change in layer I extends into layer II (yellow) but undergoes additional attenuation, and only 9% remains. In layer III (pink), the signal has declined to <3% of the original value. B: Impact of increased KATP channel activity in a cell within layer I (due to, for example, expression of a gain-of-function mutant KATP channel or impaired ATP production). If the input resistance in layer I is reduced by 90%, then the voltage change in this and cells in layers II and III will be correspondingly smaller. C: Genetic ablation of connexin-36 (Cx36 KO) (the β-cell gap junctions) will abolish electrical coupling. B and C highlight the part of A indicated by rectangle.
Importantly, the efficiency of electrical coupling (measured as Gj [see above]) also depends on the resting conductance (Gr) of the β-cell membrane (that at low glucose concentrations principally reflects KATP channel activity): the greater the Gr, the weaker the impact of Gj will be. For example, in a cell with a Gr of 1 nS (corresponding to a resistance [R] of 1GΩ), a 20-pA current (I) injected into a β-cell from its electrically active neighbors via the gap junctions will produce a 20-mV change in membrane potential (V) by Ohm’s law (V = I × R), which would be just sufficient to trigger electrical activity in the neighbor. However, if Gr increases to 3.5 nS (equal to 0.3GΩ: equivalent to what would be seen in the absence of glucose, when KATP channel activity is high), the current will only produce a 6-mV depolarization in its neighbor, which would be insufficient to induce regenerative electrical activity in an electrically inactive (i.e., hyperpolarized) β-cell. These considerations explain an observation made in mice expressing a gain-of-function mutation in the KATP channel that was expressed in 60% of the β-cells (27). Although the fraction of β-cells (∼40%) that expressed normal KATP channels displayed glucose-induced [Ca2+]i responses, these were not synchronized across the islet. This is so because the cells with high KATP channel activity (i.e., those cells expressing the mutant gain-of-function channels) function as electrical shunts that prevent propagation of the depolarizing wave (Fig. 3B). Similarly, loss of gap-junctional coupling in connexin-36 knockout mice blocks the spread of active currents (28) (Fig. 3C).
Two important conclusions can be drawn from these observations. First, the electrical activity of electrically “insulated” β-cells occurs independently and does not require hub cells. This is in agreement with studies of dispersed islet cells, which show that 80% of the cells (similar to the percentage of islet cells that are identified as β-cells) respond to glucose with electrical activity and/or an increase in [Ca2+]i (29). Given that nearly every β-cell in the islet has the capacity of producing spontaneous glucose-induced electrical activity, it would be peculiar if silencing a single β-cell would suffice to silence the entire islet. Indeed, as remarked above, optical silencing of leader/hub cells did not abolish glucose-induced [Ca2+]i oscillations in the individual cells—it merely led to the disruption of synchronicity. Second, the coordination of electrical activity across the islet syncytium requires electrical connections between β-cells.
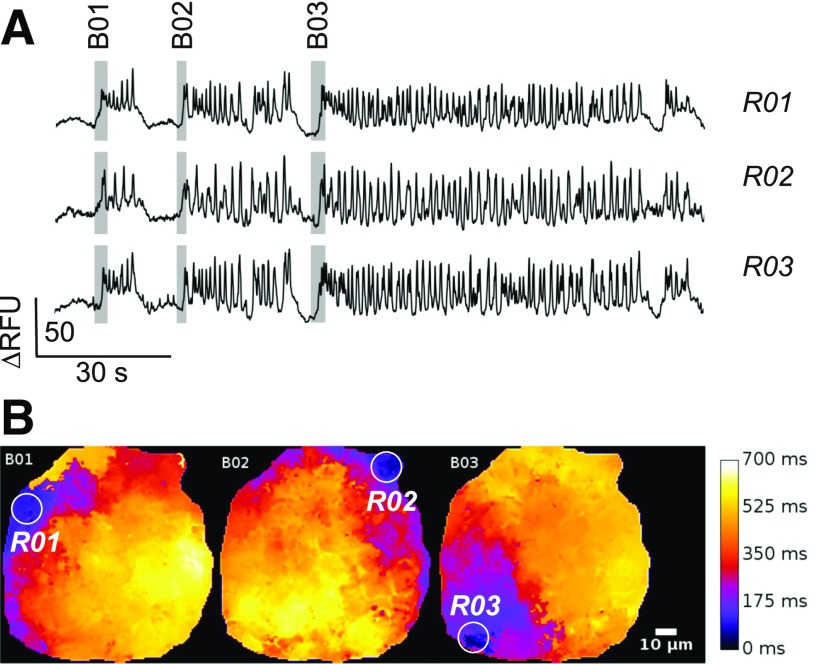
To establish whether hub cells exist will require the simultaneous measurement of electrical activity in all of many β-cells in an islet. This feat may eventually be achieved using multiarray electrode measurements of islet β-cell electrical activity (30,31) or optical measurements of membrane potential. Figure 4A shows an example where we have monitored spontaneous electrical oscillations in an islet expressing the membrane potential sensor Optopatch2 (32) in its β-cells. The example shown is representative of several measurements made from multiple islets obtained from several mice (J. Tolö, H. Dou, and P.R., unpublished observations). The islet in this example was exposed to 11 mmol/L glucose, and the three measurements shown are taken from three different regions of the islet (R01–R03). It is clear that the β-cells in this case generate bursts of electrical activity that closely resemble those recorded using patch pipettes (compared with Fig. 1B). We analyzed the spread of electrical activity during three bursts (shown as B01–B03). The pseudocolor images shown in Fig. 4B depict the delay that occurs between burst initiation and electrical activity in different parts of the islet. During burst B01, for example, electrical activity was first detected in RO1 and then spread across the islet within 700 ms. However, the next burst (B02) was initiated in R02 (>60 μm away from R01) and the third burst (B03) was first detected in R03 (>100 μm away from R02). Clearly, in this case at least, there is no hub cell having a fixed location, demonstrating that even if there are tendencies for certain regions or certain individual β-cells to fire first in response to glucose stimulation, such roles are not absolute. Ultimately, the resolution to the question of whether hub cells exist will require rapid three-dimensional imaging of electrical activity in every β-cell within the entire islet.
Figure 4.
No evidence of hub cells from optical imaging of β-cell electrical activity. A: Electrical activity in three regions (R01–R03) within an islet expressing the membrane potential sensor Optopatch2. Responses are shown as the changes in relative fluorescence units (RFU). The gray rectangles superimposed on the traces highlight three successive bursts of electrical activity (B01–B03). B: Spread of electrical activity across the islet. The delay with respect to the first point at which electrical activity was detected is displayed in pseudocolors with black corresponding to no delay and white to delay of 700 ms. Note that bursts B01, B02, and B03 are initiated in three different spatially separated areas of the islet (R01–R03: white circles). The measurements in A were taken from these regions.
Cell Coupling and the Propagation of Electrical Activity/[Ca2+]i Waves in Islets
As discussed above, electrical activity in a “leader” cell will inject a depolarizing current into neighboring cells via the gap junctions. In those neighbors with sufficiently low Gr, this may produce sufficient depolarization to exceed the voltage threshold for action potential initiation, which is approximately −50 mV (22). Around this voltage, there is an ∼0.2 s delay, which largely reflects the time between depolarization and the initiation of an action potential (only 5% is due to the time needed for the gap junctions to charge the neighboring cell). Given that β-cells have a diameter of ∼15 μm, we can estimate an upper limit of the spread of electrical activity/[Ca2+]i as 75 μm/s for mouse islets, in good agreement with what we observed experimentally (22) and similar to the 50–75 μm/s reported by others (7,9). Electrical activity in the “new” cell amplifies the “signal” to its original full amplitude, as action potentials are self-regenerative so that they can be propagated to the next coupled cell, etc. (22), in a way similar to the use of relays in early telegraphs or to the nodes of Ranvier that display saltatory action potential propagation. Thus, unless the signal is continuously regenerated (i.e., by the initiation of new electrical activity) it will decay to very low values within 1–2 cell diameters and thus die out. It is implicit from this argument that the follower cells have to be close to threshold and likely would have fired soon anyway. This is consistent with the weak spread of [Ca2+]i waves at substimulatory glucose concentrations (9).
Oscillatory Electrical Activity Can Be Reset by Current Injection
The concept that electrical activity in leader cells propagates across the islet via gap junctions and successive electrical activation of follower cells explains why it is possible to reset the rhythmicity of glucose-induced electrical activity by experimentally injecting large currents into islets (33). It was shown by Cook et al. (33) in the early 1980s that the injection of exogenous currents into the islet while simultaneously recording electrical activity from a β-cell within it could either prematurely trigger an oscillation during the silent phase between bursts or prematurely hasten its deactivation and repolarization by applying depolarizing or hyperpolarizing currents, respectively. Notably, the currents required to influence β-cell electrical activity in these experiments were very large in amplitude (∼10 μA, i.e., >100,000-fold larger than those used to identify hub cells) and had to be applied over a large area of the islet to be effective. This means that many islet cells had to be electrically stimulated or inhibited to significantly alter the observed bursting pattern.
The finding that membrane depolarization can induce widespread Ca2+ waves (now confirmed in islets by optogenetic means [7]) is uncontroversial, and these waves are initiated and propagated as we discussed above. However, the finding that a 50-mV hyperpolarization applied to a single hub cell can abolish the activity of the rest of the islet is difficult to reconcile with what is known about β-cell coupling and electrical activity, as this hyperpolarization would be largely restricted to the cell to which it is applied. This is because there is no obvious mechanism that can regenerate a hyperpolarization as it spreads across the islet, and the effect on membrane potential would quickly decline to very low values: the 50-mV hyperpolarization in the leader cell produced by activation of halorhodopsin would decrease to 15 mV in the first layer neighbors, <5 mV in the second layer, and ∼1 mV in the third layer, etc.
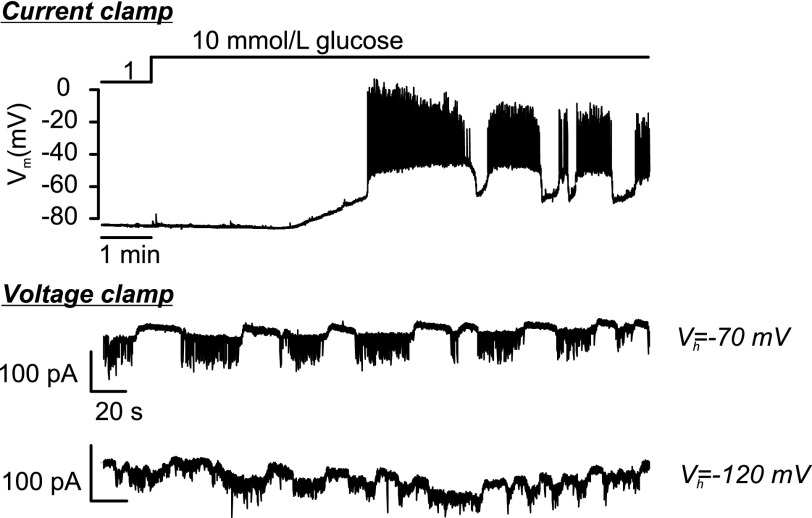
We used the oscillations in holding current seen in voltage-clamped cells described above to indirectly monitor electrical activity in the neighbors when the β-cell connected to the recording electrode is experimentally hyperpolarized (Fig. 5). We found that in islets exposed to 10–20 mmol/L glucose, when the β-cells are firing action potentials from a plateau potential of approximately −40 mV, hyperpolarizing the β-cells’ membrane potential to −70 (n = 33 [20 mmol/L glucose]) or less than or equal to −110 mV (n = 10 [10 mmol/L glucose]) had no effect on islet electrical activity. From these observations, we can conclude with 99% certainty that hub cells do not operate via electrical coupling; there is a 1% chance (100 × [1 − 0.1]43%) that we would have missed the “hub” cells in 43 trials if they represented 10% of the islet cells.
Figure 5.
Hyperpolarization of β-cells does not affect electrical activity in neighbors. Changes in β-cell membrane potential when increasing glucose from 1 to 10 mmol/L recorded under current-clamp conditions (top) and under voltage-clamp conditions when holding the membrane potential at −70 mV (middle) or −120 mV (bottom). The current oscillations reflect glucose-induced electrical activity in neighboring (unclamped) β-cells. The bottom trace appears noisier at −120 mV because of membrane breakdown (partial seal rupture) around the recording electrode at this unphysiologically negative membrane potential.
Possible Role of Diffusible Factors
Collectively, the findings reviewed above argue that the disruption of the coordination observed upon optical (halorhodopsin) hyperpolarization is unlikely to rely solely on an electrical signal and may perhaps (at least in part) reflect diffusible paracrine signals released within the islet. Photoactivation of β-cells in islets exposed to 5 mmol/L glucose has previously been shown to evoke action potential firing in δ-cells and stimulate somatostatin secretion (34). Possibly, hyperpolarization of β-cells may hyperpolarize δ-cells electrically connected to the hub cell, which may result in a paradoxical stimulation of δ-cell electrical activity and somatostatin secretion by reactivation of channels that have undergone voltage-dependent inactivation. Thus, studies using somatostatin receptor antagonists may be rewarding.
In more recent work, it was reported that photoablation of leader cells abolishes glucose-induced β-cell [Ca2+]i oscillations in zebrafish islets (8). However, in other studies the β-cell glucose-induced [Ca2+]i oscillations in zebrafish islets were poorly correlated, and thus islets in this species might lack strong gap-junctional coupling (35). It is therefore of interest that connexin-35b, the zebrafish ortholog of connexin-36, is not detected in zebrafish islets (35). It would be interesting to know whether the suppression of the [Ca2+]i oscillations was permanent of if they resumed if the islets were allowed sufficient time to recover. It is also noteworthy that in mouse islets, ablation of the “1st responder” (the leader cell) did not affect the capacity of the islet to respond to glucose with an increase in [Ca2+]i and a new 1st responder emerged (36). If these preliminary data can be confirmed, it would reinforce our reservations on electrophysiological grounds about the hub/leader cell concept.
Coda
We have reviewed the role of leader/hub cells from an electrophysiological perspective. Whereas depolarization of these cells may suffice to trigger electrical activity and [Ca2+]i waves in the rest of the islet, it is difficult for one cell to supply enough current to repolarize the entire islet.
While there is no direct evidence yet, an alternative hypothesis might be that diffusible factors and not electrical coupling may be preferentially released by specialized cells or groups of cells within islets. Future research should concentrate on the molecular identification of such putative diffusible factor(s). Evidence has appeared for possible roles of released nitric oxide (37), carbon monoxide (38), and GABA (39,40), but these will need to be evaluated much more thoroughly within the context of hub cells and β-cell–β-cell synchronicity.
Article Information
Acknowledgments. The authors thank Dr. David Hodson (Birmingham, U.K.) for helpful discussions. The authors are indebted to Dr. Haiqiang Dou (Gothenburg, Sweden) for the membrane potential recording shown in human islets and Dr. Johan Tolö (Gothenburg, Sweden) for the optical imaging of islet electrical activity. The authors are grateful for Drs. Arthur Sherman (National Institutes of Health) and Richard Bertram (Florida State University) for comments and suggestions regarding the manuscript.
Funding. The research laboratory of L.S.S. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (RO1DK46409), JDRF, and funds from the FastForward Research Program of the University of Michigan. Studies in the laboratories of P.R. are supported by Wellcome, the Swedish Research Council, and The Leona M. and Harry B. Helmsley Charitable Trust. Q.Z. holds a Diabetes UK RD Lawrence Fellowship.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med 2015;42:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JM, Fabregat ME, Gomis R, Bergsten P. Pulsatile insulin release from islets isolated from three subjects with type 2 diabetes. Diabetes 2002;51:988–993 [DOI] [PubMed] [Google Scholar]
- 3.Henquin JC, Meissner HP. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia 1984;40:1043–1052 [DOI] [PubMed] [Google Scholar]
- 4.Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 2018;98:117–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013;75:155–179 [DOI] [PubMed] [Google Scholar]
- 6.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes 1981;30:435–439 [DOI] [PubMed] [Google Scholar]
- 7.Johnston NR, Mitchell RK, Haythorne E, et al. . Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 2016;24:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem V, Silva LD, Suba K, et al. Leader β-cells coordinate Ca2+ dynamics across pancreatic islets in vivo. Nature Metabolism 2019;1:615–629 [DOI] [PubMed] [Google Scholar]
- 9.Westacott MJ, Ludin NWF, Benninger RKP. Spatially organized β-cell subpopulations control electrical dynamics across islets of Langerhans. Biophys J 2017;113:1093–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilon P, Henquin JC. Distinct effects of glucose on the synchronous oscillations of insulin release and cytoplasmic Ca2+ concentration measured simultaneously in single mouse islets. Endocrinology 1995;136:5725–5730 [DOI] [PubMed] [Google Scholar]
- 11.Bergsten P, Hellman B. Glucose-induced amplitude regulation of pulsatile insulin secretion from individual pancreatic islets. Diabetes 1993;42:670–674 [DOI] [PubMed] [Google Scholar]
- 12.Rosengren AH, Braun M, Mahdi T, et al. . Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes 2012;61:1726–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Guerra S, Lupi R, Marselli L, et al. . Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 2005;54:727–735 [DOI] [PubMed] [Google Scholar]
- 14.Alanentalo T, Asayesh A, Morrison H, et al. . Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat Methods 2007;4:31–33 [DOI] [PubMed] [Google Scholar]
- 15.Hellman B. Actual distribution of the number and volume of the islets of Langerhans in different size classes in non-diabetic humans of varying ages. Nature 1959;184(Suppl. 19):1498–1499 [DOI] [PubMed] [Google Scholar]
- 16.Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RKP. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 2012;61:1700–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdeolmillos M, Gomis A, Sánchez-Andrés JV. In vivo synchronous membrane potential oscillations in mouse pancreatic beta-cells: lack of co-ordination between islets. J Physiol 1996;493:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdeolmillos M, Santos RM, Contreras D, Soria B, Rosario LM. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett 1989;259:19–23 [DOI] [PubMed] [Google Scholar]
- 19.Gilon P, Henquin JC. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem 1992;267:20713–20720 [PubMed] [Google Scholar]
- 20.Gosak M, Stožer A, Markovič R, et al. . Critical and supercritical spatiotemporal calcium dynamics in beta cells. Front Physiol 2017;8:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravier MA, Güldenagel M, Charollais A, et al. . Loss of connexin36 channels alters β-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005;54:1798–1807 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Galvanovskis J, Abdulkader F, et al. . Cell coupling in mouse pancreatic beta-cells measured in intact islets of Langerhans. Philos Trans A Math Phys Eng Sci 2008;366:3503–3523 [DOI] [PubMed] [Google Scholar]
- 23.Mears D, Sheppard NF Jr, Atwater I, Rojas E. Magnitude and modulation of pancreatic beta-cell gap junction electrical conductance in situ. J Membr Biol 1995;146:163–176 [DOI] [PubMed] [Google Scholar]
- 24.Andreu E, Soria B, Sanchez-Andres JV. Oscillation of gap junction electrical coupling in the mouse pancreatic islets of Langerhans. J Physiol 1997;498:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Armendariz M, Roy C, Spray DC, Bennett MV. Biophysical properties of gap junctions between freshly dispersed pairs of mouse pancreatic beta cells. Biophys J 1991;59:76–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddlestone GT, Gonçalves A, Bangham JA, Rojas E. Electrical coupling between cells in islets of Langerhans from mouse. J Membr Biol 1984;77:1–14 [DOI] [PubMed] [Google Scholar]
- 27.Girard CA, Wunderlich FT, Shimomura K, et al. . Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest 2009;119:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 2007;56:1078–1086 [DOI] [PubMed] [Google Scholar]
- 29.Rocheleau JV, Remedi MS, Granada B, et al. . Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol 2006;4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer T, Kraushaar U, Düfer M, et al. . Rapid functional evaluation of beta-cells by extracellular recording of membrane potential oscillations with microelectrode arrays. Pflugers Arch 2011;462:835–840 [DOI] [PubMed] [Google Scholar]
- 31.Raoux M, Bornat Y, Quotb A, Catargi B, Renaud S, Lang J. Non-invasive long-term and real-time analysis of endocrine cells on micro-electrode arrays. J Physiol 2012;590:1085–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochbaum DR, Zhao Y, Farhi SL, et al. . All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods 2014;11:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook DL, Crill WE, Porte D Jr. Plateau potentials in pancreatic islet cells are voltage-dependent action potentials. Nature 1980;286:404–406 [DOI] [PubMed] [Google Scholar]
- 34.Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. δ-Cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J Physiol 2018;596:197–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emfinger CH, Lőrincz R, Wang Y, et al. . Beta-cell excitability and excitability-driven diabetes in adult Zebrafish islets. Physiol Rep 2019;7:e14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kravets V, Dwulet JM, Schleicher WE, Piscopio RA, Benninger RKP. Beta cells subpopulations: do they control islet function? (Abstract). Abstract 264, presented at the 55th Annual Meeting of the European Association for the Study of Diabetes, 16–20 September 2019, Barcelona, Spain.
- 37.Nunemaker CS, Buerk DG, Zhang M, Satin LS. Glucose-induced release of nitric oxide from mouse pancreatic islets as detected with nitric oxide-selective glass microelectrodes. Am J Physiol Endocrinol Metab 2007;292:E907–E912 [DOI] [PubMed] [Google Scholar]
- 38.Lundquist I, Alm P, Salehi A, Henningsson R, Grapengiesser E, Hellman B. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am J Physiol Endocrinol Metab 2003;285:E1055–E1063 [DOI] [PubMed] [Google Scholar]
- 39.Braun M, Ramracheya R, Bengtsson M, et al. . γ-Aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic β-cells. Diabetes 2010;59:1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menegaz D, Hagan DW, Almaça J, et al. . Mechanism and effects of pulsatile GABA secretion from cytosolic pools in the human beta cell. Nat Metab 2019;1:1110–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]