Abstract
Diabetic keratopathy occurs in ∼70% of all people with diabetes. This study was designed to examine the effects of vitamin D receptor knockout (VDR−/−) and vitamin D deficiency (VDD) on corneal epithelial wound healing and nerve density in diabetic mice. Diabetes was induced using the low-dose streptozotocin method. Corneal epithelial wounds were created using an Algerbrush, and wound healing was monitored over time. Corneal nerve density was measured in unwounded mice. VDR−/− and VDD diabetic mice (diabetic for 8 and 20 weeks, respectively) had slower healing ratios than wild-type diabetic mice. VDR−/− and VDD diabetic mice also showed significantly decreased nerve density. Reduced wound healing ratios and nerve densities were not fully rescued by a supplemental diet rich in calcium, lactose, and phosphate. We conclude that VDR−/− and VDD significantly reduce both corneal epithelial wound healing and nerve density in diabetic mice. Because the supplemental diet did not rescue wound healing or nerve density, these effects are likely not specifically related to hypocalcemia. This work supports the hypothesis that low vitamin D levels can exacerbate preexisting ophthalmic conditions, such as diabetes.
Introduction
Diabetes can result in a number ophthalmic complications, including retinopathy, cataract, and uveitis (1,2). Diabetic keratopathy, with symptoms including reduced corneal sensitivity and impaired epithelial wound healing, occurs in ∼70% of all people with diabetes (3,4). Nerve changes in diabetic corneas have been well described and include reduced nerve density, thin nerve fibers, and impaired nerve migration (5–8). Neurotrophic keratopathy can lead to blindness (9–12). Clinical studies have demonstrated that corneal nerve regeneration can occur after interventions to improve diabetic control (13–15).
Vitamin D is a fat-soluble prohormone that is synthesized in the skin in response to sunlight (16) and is the precursor to the potent steroid hormone calcitriol [1,25(OH)2D3] (17). Vitamin D is primarily involved in mineral ion homeostasis (18) and has been demonstrated to regulate a wide range of physiological and pathological processes, including cell growth, migration, immune response modulation, and differentiation (19–22). 1,25(OH)2D3 is the naturally occurring ligand of the vitamin D receptor (VDR), a nuclear receptor and a clinically validated drug target. VDR has been found to be involved in controlling epidermal stem cells and progeny during cutaneous wound repair (20). Moreover, 1,25(OH)2D3 and synthetic analogs (e.g., calcipotriol) are approved treatments for numerous diseases, including psoriasis, a common autoimmune disorder (16).
Low serum vitamin D concentration levels have been detected in those with type 1 and type 2 diabetes (23–27). Reduced serum vitamin D binding protein levels are also associated with diabetes (28). An inverse association between 25-hydroxy vitamin D [25(OH)D] concentration and incident diabetes has been proven to be highly consistent (29). A number of trials have examined the possibility that vitamin D supplementation has the capacity to modify the development of diabetes. Early studies focused mainly on the effects of vitamin D deficiency (VDD) in animal models and humans on insulin secretion and glucose tolerance (30,31). Recently, vitamin D was found to improve glucose homeostasis and reduce weight (32). Vitamin D reduces diabetes incidence in nonobese diabetic mice (33) and may have a combined role with VDR in children at increased genetic risk for type 1 diabetes (25). Moreover, vitamin D–catabolizing CYP24A1 was found to be elevated in diabetic mouse kidneys, leading to lower vitamin D levels in these mice (34).
Extrarenal tissue–specific local production of vitamin D has been demonstrated in a number of tissues (35). Our laboratory has previously demonstrated that corneal epithelium, which is directly exposed to the sun in the same manner as the skin, has the enzymatic components and ability to generate and activate vitamin D. The VDR, CYP24A1, CYP27B1, and five vitamin D metabolites (36,37) have all been detected in the cornea and anterior segment. 1,25(OH)2D3 has been found to inhibit neovascularization and modulate angiogenesis, inflammatory responses (21), age-related macular degeneration (38), myopia (39), diabetic retinopathy (40,41), and antitumor activity in retinoblastoma (42). VDR knockout (VDR−/−) mice exhibit delayed skin wound healing (43–45). Our laboratory has demonstrated that VDR−/− mice have significantly slower corneal epithelial wound healing rates than wild-type (WT) mice (46). Interestingly, topically applied vitamin D has been found to decrease the corneal epithelial wound healing rate in a mouse model where acute inflammation is necessary for efficient wound closure (21).
Vitamin D is essential for calcium homeostasis. Serum calcium levels are reduced in VDR−/− mice (47). Calcium restriction has been found to enhance the deficit in skin wound healing caused by VDR deletion (43), and we found that a supplemental diet rich in calcium, lactose, and phosphate rescued the corneal wound healing deficit in VDR−/− mice (46). This same diet was found to rescue impaired mucin packaging in conjunctival goblet cells (48). The current study is the first in our knowledge to examine how VDD and VDR−/−, along with combined calcium supplementation, affect diabetic corneas.
Research Design and Methods
Animals
All animal studies were approved by the Augusta University institutional animal care and use committee, and animals were treated according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. VDR−/− mice were bred from breeding pairs ordered from The Jackson Laboratory (stock no. 006133; Ellsworth, ME). All animals were housed in standard conditions with a 12-h dark-light cycle. If not otherwise stated, at the end of the experiments, the mice were killed with CO2 inhalation and neck dislocation, and tissues were collected and frozen in liquid nitrogen.
Measurement of the Serum 25(OH)D
The serum level of 25(OH)D was measured using a 25(OH)D enzyme immunoassay kit (Immunodiagnostic Systems, Tyne and Wear, U.K.) according to the manufacturer’s protocol.
Measurement of the Serum Calcium Concentration
Serum calcium concentration was measured using a calcium colorimetric assay kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s protocol.
Animal Groups
The low-dose streptozotocin (STZ) injection method was used to induce diabetes in WT and VDR−/− mice. Males and females were used in our studies. Five sequential daily intraperitoneal injections of a freshly prepared solution of STZ in 0.1 mol/L citrate buffer (pH 4.5) at 60 mg/kg body weight were administered to 4-week-old mice. Blood glucose was measured 1 week after the final STZ injection. We limited the mice receiving corneal epithelial wounds to those with blood glucose levels >249 and <651 mg/dL (49).
The first group was composed of 8-week diabetic duration WT and VDR−/− mice. The second group was composed of 8-week diabetic VDR−/− mice fed a supplemental diet high in calcium, lactose, and phosphate (20% lactose, 2% calcium, 1.25% phosphate) (TD.96348 Diet; Envigo, Tampa, FL) previously shown to alleviate many of the VDR−/− phenotypical features (50,51). The supplemental diet was initiated at the end of the STZ protocol. The third group was composed of 10- and 20-week diabetic WT mice, which were fed a VDD diet (TD.89123 Diet; Envigo) initiated at the end of the STZ protocol. The control group for the VDD mice was fed the TD.89124 vitamin D control diet (Envigo).
Wound Healing
After anesthesia with isoflurane (Tec 4 vaporizer; Anesthesia Service and Equipment, Atlanta, GA), a 2-mm central epithelial wound was made with an Algerbrush. To monitor wound size, a drop of fluorescein sodium 0.25% with proparacaine hydrochloride 0.5% was applied to the wounded eye. An antibiotic ointment (bacitracin, neomycin, polymyxin) and 1 mg/kg buprenorphine (intramuscular) were used after every procedure. Wounds were photographed with a Topcon slit lamp (SL-D4; Topcon Medical Systems, Inc., Oakland, NJ) at 0, 18, 24, 40, 48, 72, and 96 h or until the wounds were healed. Wounds were manually traced using cellSens Dimension (Olympus, New Orleans, LA) software, and wound areas were measured at each time point. Wound areas were scaled to the initial wound area, and the wound healing ratio is defined as the percent change in wound area at a given time, where a ratio of 1 represents a fully healed wound.
Nerve Immunofluorescence Staining and Imaging
To stain corneas for sensory nerves (52), eyes were enucleated from nondiabetic 12-week-old WT and VDR−/− mice, 8-week diabetic WT and VDR−/− mice, 10-week diabetic VDD mice and their control counterparts WT mice. Eyes were fixed with Zamboni fixative (American MasterTech Scientific, Lodi, CA) for 75 min followed by washing with PBS (three times). Corneas were carefully excised along the sclera corneal rim and subjected to a rehydration series with increasing concentrations of Triton X-100 in PBS. To block nonspecific binding, corneas were incubated with 10% normal goat serum plus 0.1% Triton X-100 solution in PBS for 60 min at room temperature. Tissue was then incubated with primary rabbit polyclonal anti-β III tubulin (ab18207; Abcam, Cambridge, MA) (1:500) antibody in PBS containing 5% goat serum plus 0.1% Triton X-100 for 24 h at room temperature and constantly shaken. After washing with PBS (three times for 10 min each), the corneas were incubated with the secondary antibodies Alexa Fluor 488 goat anti-rabbit IgG (H+L) (Thermo Fisher Scientific, Norcross, GA) for 24 h at 4°C and washed thoroughly with PBS. To image the corneas, three radial cuts were made on each cornea, and the tissue was mounted flat on a slide with the epithelium side up. Images were taken with a Zeiss LSM 780 Confocal Microscope (Zeiss, Oberkochen, Germany). Stromal and basal epithelium z-stack images were collected, and nerves were analyzed from the zone just under the basal epithelium.
Nerve densities were measured using the method described by He et al. (7). Images were taken just below the central cornea epithelium using a confocal microscope (four pictures relative to the center: left, right, top, bottom). The most central half of each photo was traced in Adobe Photoshop. The average percentage of pixels traced, among the four central images, was defined as the nerve density value of the image.
Statistical Analysis
Wound healing was assessed using the parallelism test after curve fitting with the Weibull growth model (JMP Pro 14 software; SAS Institute, Cary, NC), and comparisons were made between genotypes and diet type using each data set. One-way ANOVA and independent t tests were used to analyze all other data (GraphPad Software, La Jolla, CA).
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Serum 25(OH)D Concentrations
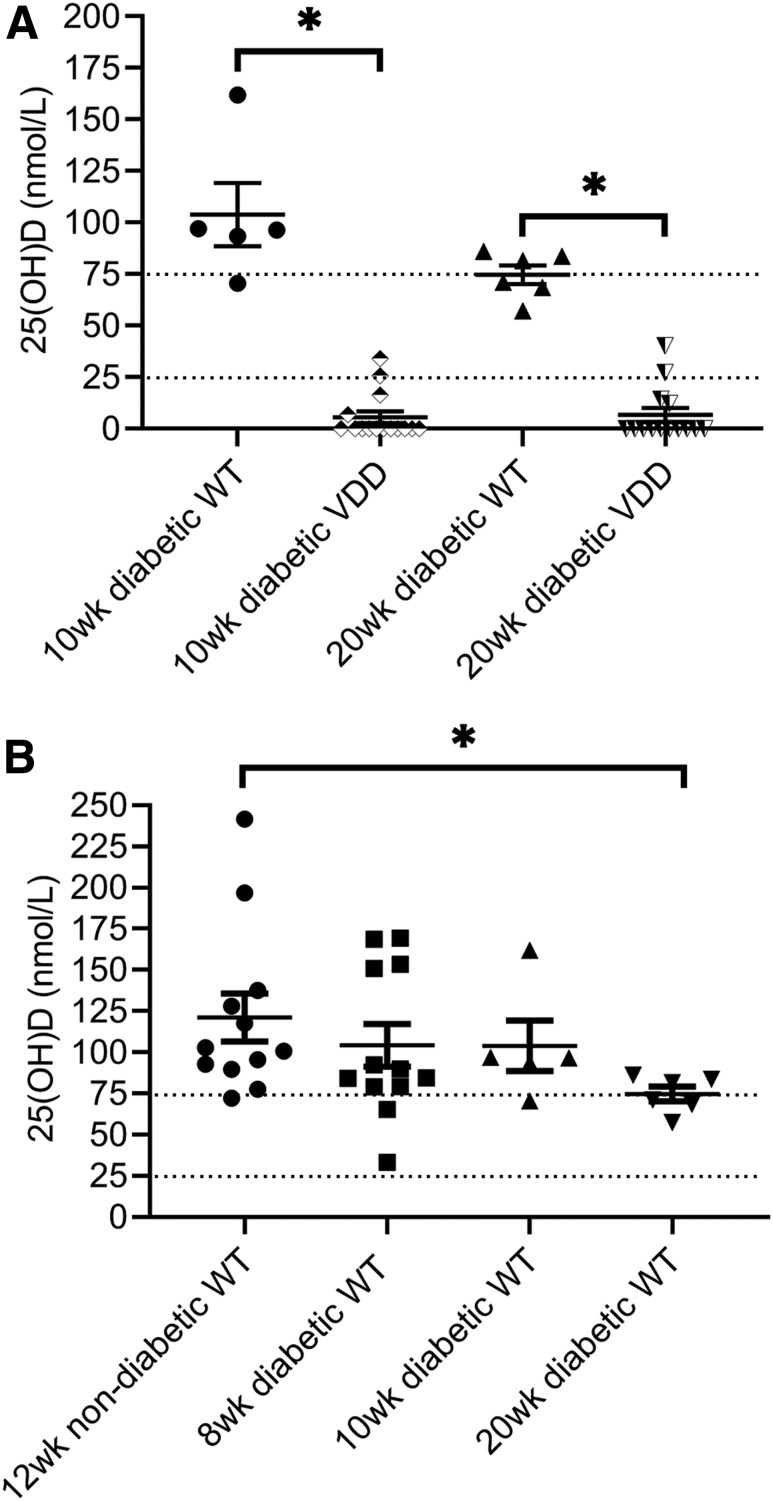
Figure 1A shows 25(OH)D concentrations in individual VDD mice and their WT controls. 25(OH)D concentrations were undetectable in 11 of 15 mice fed the vitamin D–free diet for 10 weeks and in 12 of 16 mice fed the same diet for 20 weeks (assay lower detection limit 16.3 nmol/L). Figure 1B shows the effect of diabetes on serum 25(OH)D concentrations. The mean ± SE serum 25(OH)D concentration in WT mice was 121.7 ± 14.57 nmol/L (n = 12). Mean 25(OH)D concentrations for 8-, 10-, and 20-week diabetic duration WT mice were 104.2 ± 12.88, 103.9 ± 15.31, and 74.73 ± 4.512 nmol/L (n = 12, 6, and 20), respectively. Only the mean 25(OH)D concentration of the 20-week diabetic WT group was significantly different from that of the normal WT group (t test, P < 0.05). The 20-week diabetic WT value of 74.73 nmol/L would be considered vitamin D insufficient for mice (53).
Figure 1.
Serum vitamin D concentrations in diabetic mice. A: The vitamin D concentration was significantly decreased in mice fed the VDD diet for 10 (n = 15) or 20 (n = 16) weeks (wk) and in the majority of mice was below the assay’s detection limit. B: Only the mean 25(OH)D concentration of the 20-week diabetic WT 25(OH)D concentration was significantly different from that of the normal WT value (n = 12, 6, and 20 for 8-, 10-, and 20-week diabetic duration WT mice, respectively). The dotted lines represent the vitamin D insufficiency range [serum 25(OH)D >25 nmol/L and <75 nmol/L]. *P < 0.05.
Serum Calcium Concentrations
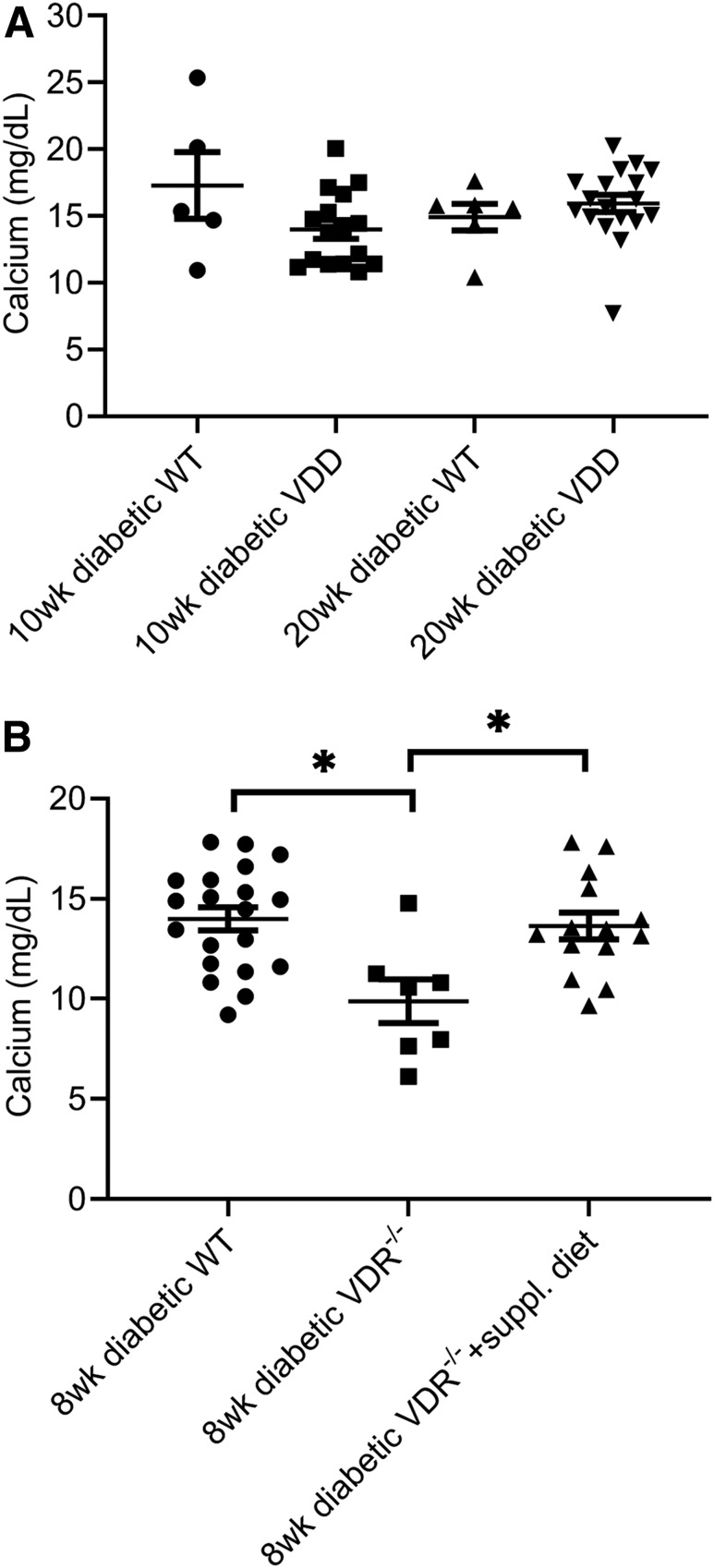
Figure 2A shows calcium concentrations in individual diabetic VDD mice, and Fig. 2B shows calcium concentrations in individual VDR−/− mice fed the supplemental mineral-rich diet. Mean ± SE values for VDD mice on the diet for 10 and 20 weeks were 13.99 ± 0.69 and 15.93 ± 0.65 mg/dL (n = 15 and 16), respectively (Fig. 2A). There were no significant differences in mean serum calcium concentration between 10- and 20-week diabetic VDD and WT mice or between 10- and 20-week diabetic WT mouse groups (n = 6 for both groups).
Figure 2.
Effect of VDD and VDR−/− on diabetic mouse serum calcium and vitamin D concentrations. (All mice in figure were diabetic.) A: Serum calcium concentrations were not significantly different between diabetic WT mice (n = 6) and diabetic VDD mice fed the VDD diet for 10 (n = 15) or 20 (n = 16) weeks (wk). B: The serum calcium concentration was significantly decreased in 8-week diabetic VDR−/− mice (n = 8) and was increased to the control diabetic level when VDR−/− mice were fed the supplemental diet (n = 15). *P < 0.05.
The mean ± SE 8-week-old VDR−/− mouse serum calcium concentration was 9.876 ± 1.09 mg/dL (n = 8), which was significantly lower than the age-matched diabetic WT group (14.00 ± 0.58 mg/dL, n = 20) (t test, P < 0.05). Diabetic VDR−/− mice on the supplemental diet had a mean serum calcium concentration of 13.64 ± 0.66 mg/dL (n = 15), which was not significantly different from that of the diabetic WT group (t test, P = 0.69) and was significantly higher than the diabetic VDR−/− group (t test, P < 0.05).
Effects of VDR−/− and Supplemental Diet on Diabetic Mouse Corneal Wound Healing
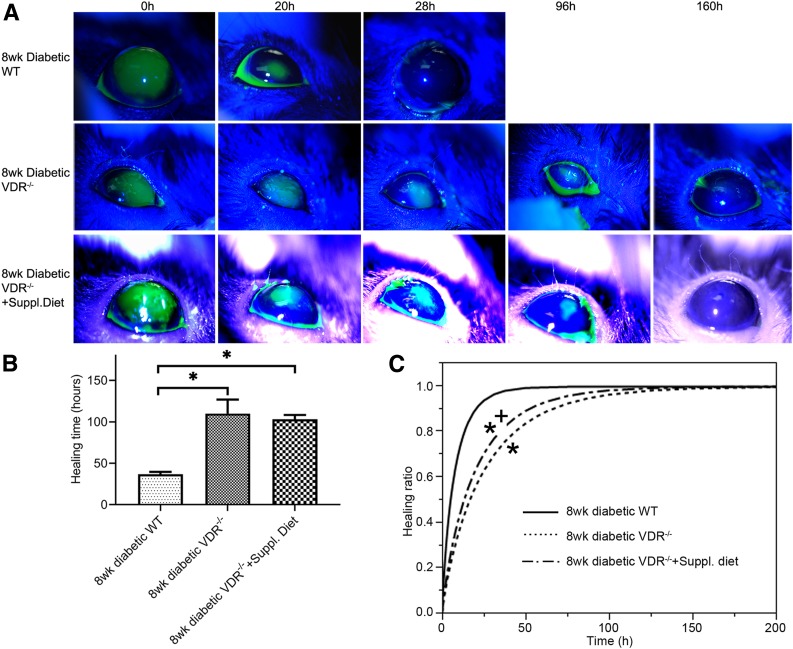
Figure 3A shows representative wound healing photos of the different diabetic WT and VDR–/– mouse groups. Diabetic WT mice typically healed on average in <48 h, while diabetic VDR−/− mice and the VDR−/− mice on the supplemental diet were often not completely healed at 96 h after surgery. Total healing times were significantly different between diabetic WT and diabetic VDR−/− mice and between diabetic WT mice and diabetic VDR−/− mice fed the supplemental diet (8-week diabetes duration) (Fig. 3B) (one-way ANOVA, P < 0.01, n = 10). There was no difference in total healing time between the diabetic VDR−/− mice and the VDR−/− mice fed the supplemental diet.
Figure 3.
Epithelial wound healing in diabetic VDR−/− and supplemental diet mice. A: Representative corneal wound healing slit lamp photographs of diabetic WT, VDR−/−, and VDR−/− mice on the supplemental diet (n = 10). B: Graph showing the mean time to complete wound healing in both diabetic VDR−/− and VDR−/− mice on the supplemental diet was slower than in diabetic WT controls. Data are mean ± SE. C: Healing ratio data were fitted with the Weibull growth model. Healing curves for both diabetic VDR−/− and VDR−/− mice on the supplemental diet were significantly different from the diabetic WT controls (*P < 0.05), while the VDR−/− and VDR−/− mice on the supplemental diet were significantly different from each other (+P < 0.05), indicating that the supplemental diet only partially reversed the effects of VDR−/−.
To analyze the wound healing process, the Weibull growth model was used to test parallelism of the different growth curves among different groups. The parallelism test did show significant differences between the healing curves of 8-week diabetic duration WT mice and the diabetic VDR−/− mice (P < 0.05, n = 10) (Fig. 3C). While the supplemental diet shifted the healing curve to the left, it was still significantly different from that of the diabetic WT mice. The diabetic VDR−/− and VDR−/− supplemental diet groups were significantly different from each other (P < 0.05, n = 10), indicating that normalizing calcium levels did result in some reversal of the wound healing deficit.
Effects of VDD on Diabetic Mouse Corneal Wound Healing
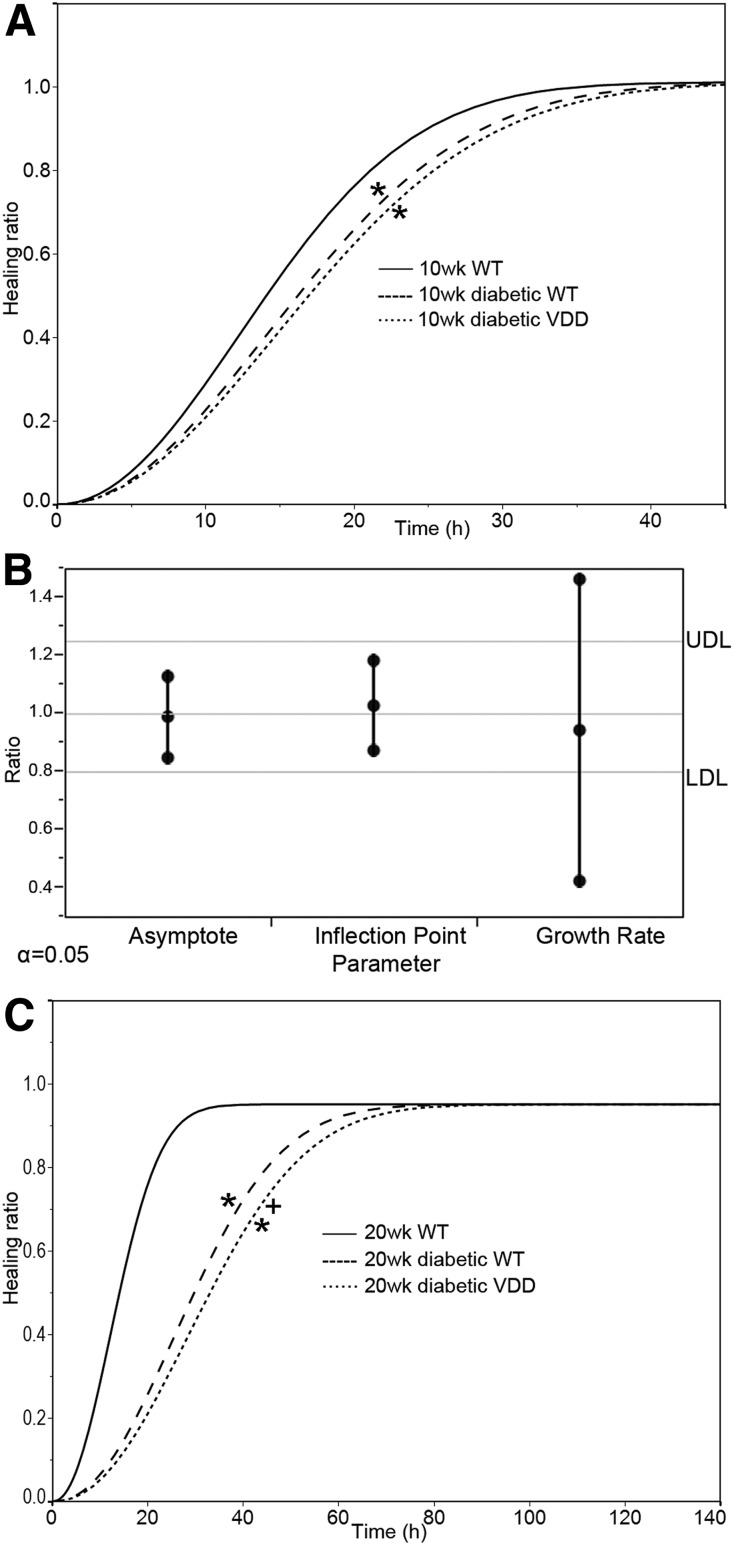
Figure 4 illustrates the effects of the duration of hyperglycemia and VDD on epithelial wound healing. There was a significant difference in the wound healing curves between the diabetic WT and WT groups, and between the VDD and the WT groups, with no significant difference between the diabetic WT and diabetic VDD groups (Fig. 4A). The 10-week diabetic group data were then analyzed with the equivalence test, which analyzes the asymptotes, inflection points, and growth rate parameters of the fitted curves when the parallelism test itself is not significantly different between groups. If a ratio value falls below the 0.8 (lower decision limit) or above the 1.2 (upper decision limit) ratio threshold, that specific parameter is considered significantly different between the curves. Figure 4B shows each parameter level of the fitted curves for the VDD diet versus the diabetic WT mice groups. The test results shown in the graph demonstrate that the growth rate of the diabetic 10-week VDD mice (n = 15) was significantly different from that of the diabetic WT mice (n = 10). Figure 4C demonstrates that parallelism testing of the wound healing curves of the 20-week diabetic VDD mice (n = 16) versus the diabetic WT mice (n = 10) showed significant differences between the curves (P < 0.05). Parallelism testing demonstrated significant differences between normoglycemic WT mice and all diabetic groups at both time points (Fig. 4A and C).
Figure 4.
The effects of hyperglycemia and VDD duration on epithelium wound healing. A: Wound healing ratio in WT, diabetic WT, and diabetic mice fed VDD diet for 10 weeks (wk) (n = 5, 10, and 15, respectively). Healing ratio data were fitted with the Weibull growth model. The parallelism test showed a significant difference in the overall wound healing curves (i.e., the curves were not considered parallel) for the diabetic WT or VDD group compared with the WT group, and no significant difference between the diabetic WT and diabetic VDD groups. B: Follow-up equivalence test on the parallelism data demonstrates a significant difference in the growth rate (equivalent to slope) between diabetic WT mice and 10-week diabetic VDD mice as indicated by both the lower decision limit (LDL) and the upper decision limit (UDL) being exceeded. C: Wound healing in diabetic WT mice fed the VDD diet for 20 weeks (n = 5, 10, and 16 for WT, diabetic WT, and VDD, respectively). Parallelism test results showed a significant difference in the wound healing curves of the diabetic WT and VDD groups compared with WT group (*P < 0.05), and a significant difference in the wound healing curves of diabetic mice and mice with the same duration of diabetes fed the VDD diet for 20 weeks (+P < 0.05).
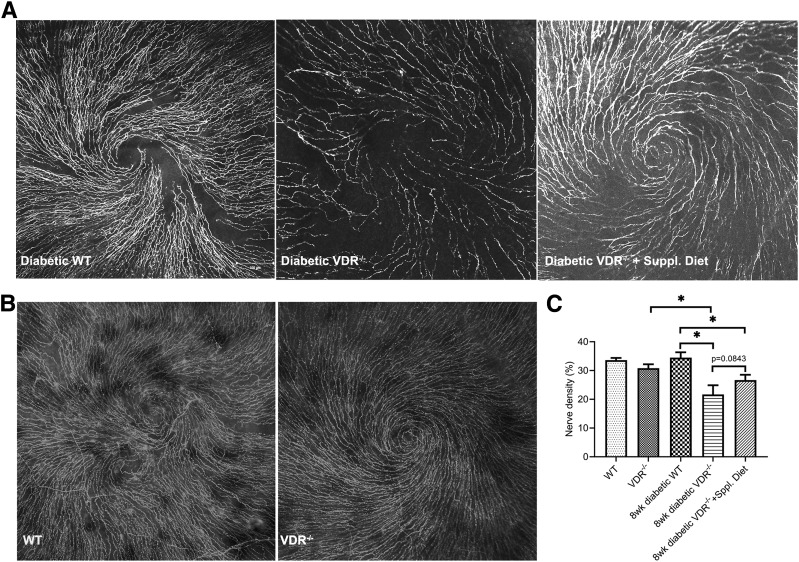
Effects of VDR−/− and Supplemental Diet on Diabetic Mouse Corneal Nerve Density
These was no significant difference in the nerve density of nondiabetic 12-week-old WT (n = 9) versus VDR−/− mice (n = 10) (Fig. 5B and C), indicating that VDR−/− alone may not affect corneal nerve density. However, for diabetic mice, the nerve density was significantly decreased in the VDR−/− (n = 6) versus WT (n = 7) groups. The nerve density was also significantly decreased in diabetic VDR−/− (n = 6) versus nondiabetic VDR−/− (n = 10) groups. Feeding the diabetic VDR−/− mice the supplemental diet (n = 10) did not rescue the reduced nerve density in these mice (Fig. 5A and C).
Figure 5.
Effect of diabetes, VDR−/−, and supplemental diet on mouse corneal nerve densities. A: Representative images showing corneal subbasal nerves of 8-week diabetic duration WT, diabetic VDR−/−, and diabetic VDR−/− mice on the supplemental diet. B: Representative images showing corneal subbasal nerves of 12-week-old nondiabetic WT and VDR−/− mice. C: There was no significant difference in nerve densities between WT (n = 9) and VDR−/− (n = 10) mice or WT and diabetic WT mice (n = 7). Diabetic VDR−/− mice (n = 6) had a significantly reduced nerve density compared with VDR−/− or diabetic WT mice, and the supplemental diet (n = 10) did not correct this reduced nerve density. Data are mean ± SE. *P < 0.05
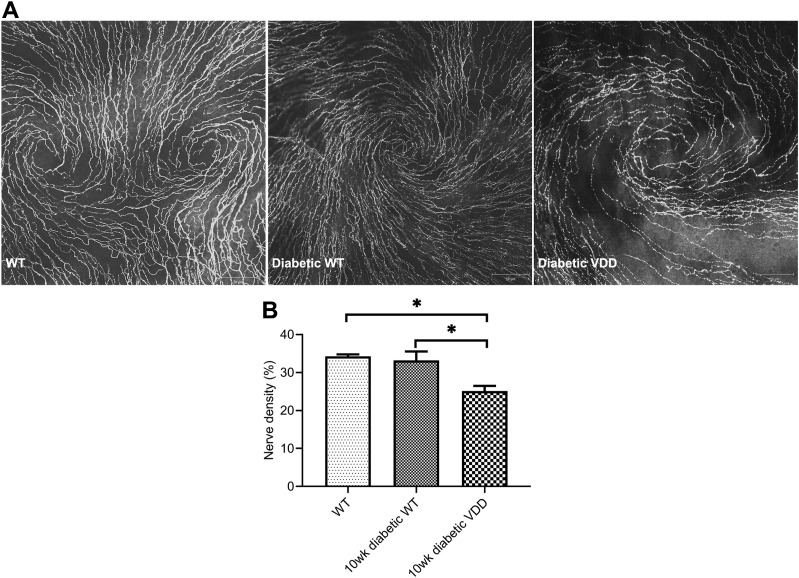
Effects of VDD Diet on Diabetic Mouse Corneal Nerve Density
Nerve density was significantly decreased in 10-week diabetic VDD mice (n = 8) compared with WT or diabetic WT controls (n = 6) (t test, P < 0.01) (Fig. 6A and B).
Figure 6.
Effects of VDD on mouse corneal nerve densities. A: Representative images showing corneal subbasal nerves of 10-week diabetic WT and diabetic VDD mice. B: Diabetic VDD mice (n = 8) had a significantly reduced average nerve density value compared with WT (n = 9) or diabetic WT (n = 6) mice. There was no difference observed between the WT and diabetic WT mice. Data are mean ± SE. *P < 0.05.
Discussion
There have been a number of clinical studies demonstrating a correlation between serum vitamin D levels and diabetes, and vitamin D replacement has been shown to have beneficial effects in people with type 2 diabetes (54). The Prospective Metabolism and Islet Cell Evaluation (PROMISE) study found that higher baseline 25(OH)D independently predicted better β-cell function and lower area under the curve (glucose) at follow-up, supporting a role for vitamin D in the type 2 etiology of diabetes (55). Cooper et al. (56) demonstrated that three key vitamin D metabolism genes show consistent evidence of association with type 1 diabetes risk, and Kodama et al. (57) provided evidence that vitamin D binding protein is an autoantigen in type 1 diabetes. The current study provides additional links between vitamin D and diabetes, demonstrating that diabetic VDR−/− and VDD mice have impaired corneal epithelial wound healing and reduced corneal nerve densities.
VDR−/− Impairs Diabetic Mouse Corneal Epithelial Wound Healing
A previous study from our laboratory found impaired corneal epithelial wound healing in VDR−/− mice that was rescued by the calcium-enriched supplemental diet examined in the current study (46). The same supplemental diet has also been shown to rescue mucin packaging problems found in VDR−/− mouse conjunctiva cells (48). VDR−/− has been found to delay cutaneous wound healing (20,58) and mucosal wound healing (59). In the current study, corneal epithelial wound healing in diabetic VDR−/− mice was significantly slower than in diabetic WT mice, and the supplemental diet only partially rescued the diabetic VDR−/− mouse healing ratio. This result in diabetic mice supports the hypothesis that loss of vitamin D will exacerbate primary corneal pathologies, in this case diabetic keratopathy. In addition, the partial rescue by the supplemental diet demonstrates that while some of the effects of VDR−/− result from the hypocalcemia associated with VDR−/− (47), there are also calcium-independent issues contributing to the wound healing deficit.
VDD Impairs Diabetic Mouse Corneal Wound Healing
VDD was examined in this study as a less dramatic and more realistic model of vitamin D interactions with diabetic keratopathy than the VDR−/− model. VDD is associated with clinical pathological conditions, including osteomalacia in adults and rickets in children, and much of the work exploring VDD has centered around bone repair and delayed bone mineralization and remodeling (60). The most recent estimates of the prevalence of the U.S. population at risk for VDD [serum 25(OH)D <30 nmol/L] is ∼5%, while the prevalence at risk for vitamin D inadequacy [serum 25(OH)D 30–49 nmol/L] is 17.7% (61). VDD was found to aggravate the decreased bone mineral density of diabetic mice (62). In our study, VDD significantly impaired the corneal epithelial healing ratio of 10-week diabetic mice, with a more significant impairment at 20 weeks diabetic duration. This demonstrates that the duration of VDD is an important contributor to the impact of VDD on corneal epithelial wound healing. Interestingly, for all these studies, the relatively short duration of diabetes in these mice was long enough to affect the vitamin D interaction with diabetes. Serum calcium was unchanged in these mice compared with their control counterparts, supporting the VDR−/− findings of a calcium-independent effect of low vitamin D and diabetes on corneal epithelial wound healing.
VDR−/− and VDD Reduces Nerve Density in Diabetic Mice
Corneal nerves have important trophic effects on the cornea and play a significant role in the maintenance of a healthy ocular surface through the stimulation of corneal wound healing after corneal injuries (4,63). Corneal nerves can stimulate epithelial cell growth, proliferation, differentiation, and type IV collagen formation through the release of neurotrophins and neuropeptides (64). Decreased corneal nerve function is closely related to poor epithelial healing, reduced lacrimal gland secretion, and epitheliopathy (65,66). Reduced nerve density in the diabetic cornea has been well documented and has been related to diabetic keratopathy (5–7). Human corneas from donors with type 1 diabetes show reduced nerve density that was significantly affected by the duration of diabetes (11). Ten-week diabetic mice have been shown to have a significant decrease in epithelial nerve density with stromal nerve neuropathies (7). Interestingly, the current study found no difference in the nerve density between WT and 8-week diabetic mice (Fig. 5) or in 10-week diabetic mice (Fig. 6). One difference in the previous study was the age of STZ treatment (8 weeks) versus the current study (4 weeks). It is possible that the earlier age of diabetes initiation could have allowed for more plasticity and capacity to produce nerves. It is also possible that this difference is the result of the congenic strain of these mice because all were bred from VDR heterozygotes. The current study did reveal that nerve density was significantly reduced in diabetic VDR−/− and VDD mice compared with their diabetic control counterparts. It is likely that this reduced nerve density contributed to the delayed wound healing in these mice. Additional studies will be required to provide data to further support this possibility.
VDR is present in neurons and glial cells (47,67), and 1,25(OH)2D3 has been shown to be a potent inducer of nerve growth factor, glial cell line–derived neurotropic factor, and neurotropin-3 (68,69). In addition, the expression of nerve growth factor and glial cell line–derived neurotrophic factor were both found to be reduced in VDD newborn rats (70), and gene expression of nerve growth factor, neurofilament, and γ-aminobutyric acid-A was reduced in the brains of rats that were transiently VDD (47,71). In the current study, there was no significant change in the nerve density of nondiabetic VDR−/− mice compared with their WT controls.
Vitamin D and the VDR play a significant role in calcium homeostasis, and a phenotypical characteristic of VDR−/− mice is hypocalcemia (47). A number of studies have found that placing VDR−/− mice on the supplemental diet high in calcium, lactose, and phosphate used in the current study can reverse this hypocalcemia along with a number of other phenotypical characteristics of these mice (72,73).
In the current study, WT diabetic mice were found to have elevated serum calcium levels compared with their nondiabetic counterparts. Interestingly, the 10-week diabetic mice fed the VDD diet had lower calcium levels than the controls, while the 20-week diabetic mice had normal calcium levels. Diabetic VDR−/− mice on the supplemental diet had significantly higher serum calcium levels than did the diabetic VDR−/− mice on the normal diet. Both the diabetic VDR−/− and VDD diet mice had lower nerve densities than their respective control groups, and the supplemental diet did not reverse the low nerve density in the diabetic VDR−/− mice. We conclude that as in the wound healing response, serum calcium is not a major contributor to the reduced nerve density found in diabetic VDR−/− or VDD mice.
In summary, corneal epithelial wound healing is negatively affected by VDD and VDR−/− in diabetic mice. These results may be related to the reduced nerve density in the diabetic versus nondiabetic mice, which is a widely used clinical marker for early detection of diabetic neuropathy. The impaired wound healing and reduced nerve densities appear to be independent of serum calcium levels. These results support the hypothesis that VDD can exacerbate pathologies associated with primary ophthalmic pathologies, such as diabetic keratopathy, suggesting that vitamin D levels should be assayed and normalized in patients with corneal pathologies.
Article Information
Funding. This work was supported by National Eye Institute grant EY-021747-06.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.L. carried out experiments and drafted the manuscript. S.V. carried out the nerve analysis. Z.C. carried out detection of calcium and vitamin D. J.C. performed the biostatistics analyses and edited the manuscript. M.A.W. designed the study and edited and revised the manuscript. All authors approved the final manuscript. M.A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Vancouver, British Columbia, Canada, 28 April–2 May 2019.
References
- 1.Sun H, Lee P, Yan C, et al. Inhibition of soluble epoxide hydrolase 2 ameliorates diabetic keratopathy and impaired wound healing in mouse corneas. Diabetes 2018;67:1162–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Threatt J, Williamson JF, Huynh K, Davis RM, Hermayer K. Ocular disease, knowledge and technology applications in patients with diabetes. Am J Med Sci 2013;345:266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikbova G, Oshitari T, Baba T, Bikbov M, Yamamoto S. Diabetic corneal neuropathy: clinical perspectives. Clin Ophthalmol 2018;12:981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res 2019;73:100762. [DOI] [PubMed] [Google Scholar]
- 5.Yu FS, Yin J, Lee P, Hwang FS, McDermott M. Sensory nerve regeneration after epithelium wounding in normal and diabetic cornea. Expert Rev Ophthalmol 2015;10:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards K, Pritchard N, Poole C, et al. Development of a novel technique to measure corneal nerve migration rate. Cornea 2016;35:700–705 [DOI] [PubMed] [Google Scholar]
- 7.He J, Pham TL, Kakazu A, Bazan HEP. Recovery of corneal sensitivity and increase in nerve density and wound healing in diabetic mice after PEDF plus DHA treatment. Diabetes 2017;66:2511–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao N, Yan C, Lee P, Sun H, Yu FS. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J Clin Invest 2016;126:1998–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midena E, Brugin E, Ghirlando A, Sommavilla M, Avogaro A. Corneal diabetic neuropathy: a confocal microscopy study. J Refract Surg 2006;22(Suppl.):S1047–S1052 [DOI] [PubMed] [Google Scholar]
- 10.Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci 2013;54:ORSF81–ORSF87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Bazan HE. Mapping the nerve architecture of diabetic human corneas. Ophthalmology 2012;119:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res 2018;66:107–131 [DOI] [PubMed] [Google Scholar]
- 13.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 2011;28:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efron N. The Glenn A. Fry award lecture 2010: ophthalmic markers of diabetic neuropathy. Optom Vis Sci 2011;88:661–683 [DOI] [PubMed] [Google Scholar]
- 15.Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf 2018;16:45–57 [DOI] [PubMed] [Google Scholar]
- 16.Schmitz AA, Hackethal S, Schulz A, et al. Sharing pharma compounds with academia: experiences with providing vitamin D receptor ligands. Nat Rev Drug Discov 2015;14:294. [DOI] [PubMed] [Google Scholar]
- 17.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–357 [DOI] [PubMed] [Google Scholar]
- 18.Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol 2017;13:466–479 [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Chen Z, Mylarapu N, Watsky MA. Effects of 1,25 and 24,25 vitamin D on corneal epithelial proliferation, migration and vitamin D metabolizing and catabolizing enzymes. Sci Rep 2017;7:16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oda Y, Hu L, Nguyen T, et al. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. J Invest Dermatol 2018;138:2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reins RY, Hanlon SD, Magadi S, McDermott AM. Effects of topically applied vitamin D during corneal wound healing. PLoS One 2016;11:e0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wimalawansa SJ, Razzaque MS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol 2018;180:4–14 [DOI] [PubMed] [Google Scholar]
- 23.Grammatiki M, Rapti E, Karras S, Ajjan RA, Kotsa K. Vitamin D and diabetes mellitus: causal or casual association? Rev Endocr Metab Disord 2017;18:227–241 [DOI] [PubMed] [Google Scholar]
- 24.Kayaniyil S, Harris SB, Retnakaran R, et al. Prospective association of 25(OH)D with metabolic syndrome. Clin Endocrinol (Oxf) 2014;80:502–507 [DOI] [PubMed] [Google Scholar]
- 25.Norris JM, Lee HS, Frederiksen B, et al.; TEDDY Study Group . Plasma 25-hydroxyvitamin D concentration and risk of islet autoimmunity. Diabetes 2018;67:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Belle TL, Gysemans C, Mathieu C. Vitamin D and diabetes: the odd couple. Trends Endocrinol Metab 2013;24:561–568 [DOI] [PubMed] [Google Scholar]
- 27.Pozzilli P, Manfrini S, Crinò A, et al.; IMDIAB Group . Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res 2005;37:680–683 [DOI] [PubMed] [Google Scholar]
- 28.Blanton D, Han Z, Bierschenk L, et al. Reduced serum vitamin D-binding protein levels are associated with type 1 diabetes. Diabetes 2011;60:2566–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angellotti E, Pittas AG. The role of vitamin D in the prevention of type 2 diabetes: to D or not to D? Endocrinology 2017;158:2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980;209:823–825 [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 1984;73:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisley SR, Arble DM, Chambers AP, et al. Hypothalamic vitamin D improves glucose homeostasis and reduces weight. Diabetes 2016;65:2732–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takiishi T, Ding L, Baeke F, et al. Dietary supplementation with high doses of regular vitamin D3 safely reduces diabetes incidence in NOD mice when given early and long term. Diabetes 2014;63:2026–2036 [DOI] [PubMed] [Google Scholar]
- 34.Aatsinki SM, Elkhwanky MS, Kummu O, et al. Fasting-induced transcription factors repress vitamin D bioactivation, a mechanism for vitamin D deficiency in diabetes. Diabetes 2019;68:918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 2007;103:316–321 [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Ubels JL, Schotanus MP, et al. Enhancement of vitamin D metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr Eye Res 2012;37:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, Elizondo RA, Nielsen R, et al. Vitamin D in tear fluid. Invest Ophthalmol Vis Sci 2015;56:5880–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parekh N, Chappell RJ, Millen AE, Albert DM, Mares JA. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch Ophthalmol 2007;125:661–669 [DOI] [PubMed] [Google Scholar]
- 39.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res 2013;114:58–68 [DOI] [PubMed] [Google Scholar]
- 40.Aksoy H, Akçay F, Kurtul N, Baykal O, Avci B. Serum 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy vitamin D (25(OH)D) and parathormone levels in diabetic retinopathy. Clin Biochem 2000;33:47–51 [DOI] [PubMed] [Google Scholar]
- 41.Reins RY, McDermott AM. Vitamin D: implications for ocular disease and therapeutic potential. Exp Eye Res 2015;134:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhoeff FH. Retinoblastoma undergoing spontaneous regression. Calcifying agent suggested in treatment of retinoblastoma. Am J Ophthalmol 1966;62:573–574 [DOI] [PubMed] [Google Scholar]
- 43.Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol 2016;164:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao H, Rieger S, Abe K, Hewison M, Lisse TS. DNA damage-inducible transcript 4 is an innate surveillant of hair follicular stress in vitamin D receptor knockout mice and a regulator of wound re-epithelialization. Int J Mol Sci 2016;17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury. Endocrinology 2013;154:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elizondo RA, Yin Z, Lu X, Watsky MA. Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Invest Ophthalmol Vis Sci 2014;55:5245–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paz HB, Tisdale AS, Danjo Y, Spurr-Michaud SJ, Argüeso P, Gipson IK. The role of calcium in mucin packaging within goblet cells. Exp Eye Res 2003;77:69–75 [DOI] [PubMed] [Google Scholar]
- 49.Yamakawa I, Kojima H, Terashima T, et al. Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab 2011;301:E844–E852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kollenkirchen U, Fox J, Walters MR. Normocalcemia without hyperparathyroidism in vitamin D-deficient rats. J Bone Miner Res 1991;6:273–278 [DOI] [PubMed] [Google Scholar]
- 51.Li YC, Amling M, Pirro AE, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 1998;139:4391–4396 [DOI] [PubMed] [Google Scholar]
- 52.Kneer K, Green MB, Meyer J, Rich CB, Minns MS, Trinkaus-Randall V. High fat diet induces pre-type 2 diabetes with regional changes in corneal sensory nerves and altered P2X7 expression and localization. Exp Eye Res 2018;175:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seldeen KL, Pang M, Rodríguez-Gonzalez M, et al. A mouse model of vitamin D insufficiency: is there a relationship between 25(OH) vitamin D levels and obesity? Nutr Metab (Lond) 2017;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Issa CM. Vitamin D and type 2 diabetes mellitus. Adv Exp Med Biol 2017;996:193–205 [DOI] [PubMed] [Google Scholar]
- 55.Kayaniyil S, Retnakaran R, Harris SB, et al. Prospective associations of vitamin D with β-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes 2011;60:2947–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 2011;60:1624–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kodama K, Zhao Z, Toda K, et al. Expression-based genome-wide association study links vitamin D-binding protein with autoantigenicity in type 1 diabetes. Diabetes 2016;65:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oda Y, Hu L, Nguyen T, Fong C, Tu CL, Bikle DD. Combined deletion of the vitamin D receptor and calcium-sensing receptor delays wound re-epithelialization. Endocrinology 2017;158:1929–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008;294:G208–G216 [DOI] [PubMed] [Google Scholar]
- 60.Gorter EA, Hamdy NA, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone 2014;64:288–297 [DOI] [PubMed] [Google Scholar]
- 61.Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr 2019;110:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao L, Tamura Y, Kawao N, et al. Influence of diabetic state and vitamin D deficiency on bone repair in female mice. Bone 2014;61:102–108 [DOI] [PubMed] [Google Scholar]
- 63.Suuronen EJ, Nakamura M, Watsky MA, et al. Innervated human corneal equivalents as in vitro models for nerve-target cell interactions. FASEB J 2004;18:170–172 [DOI] [PubMed] [Google Scholar]
- 64.Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci 1993;34:137–144 [PubMed] [Google Scholar]
- 65.Byun YS, Kang B, Yoo YS, Joo CK. Poly(ADP-ribose) polymerase inhibition improves corneal epithelial innervation and wound healing in diabetic rats. Invest Ophthalmol Vis Sci 2015;56:1948–1955 [DOI] [PubMed] [Google Scholar]
- 66.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res 2003;76:521–542 [DOI] [PubMed] [Google Scholar]
- 67.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13:100–105 [DOI] [PubMed] [Google Scholar]
- 68.Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 1996;7:2171–2175 [DOI] [PubMed] [Google Scholar]
- 69.Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res 1991;28:110–114 [DOI] [PubMed] [Google Scholar]
- 70.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641–653 [DOI] [PubMed] [Google Scholar]
- 71.Féron F, Burne TH, Brown J, et al. Developmental vitamin D3 deficiency alters the adult rat brain. Brain Res Bull 2005;65:141–148 [DOI] [PubMed] [Google Scholar]
- 72.Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr 2001;131:1787–1791 [DOI] [PubMed] [Google Scholar]
- 73.Rummens K, van Cromphaut SJ, Carmeliet G, et al. Pregnancy in mice lacking the vitamin D receptor: normal maternal skeletal response, but fetal hypomineralization rescued by maternal calcium supplementation. Pediatr Res 2003;54:466–473 [DOI] [PubMed] [Google Scholar]