Abstract
Vitamin D deficiency has been associated with increased incidence of diabetes, both in humans and in animal models. In addition, an association between vitamin D receptor (VDR) gene polymorphisms and diabetes has also been described. However, the involvement of VDR in the development of diabetes, specifically in pancreatic β-cells, has not been elucidated yet. Here, we aimed to study the role of VDR in β-cells in the pathophysiology of diabetes. Our results indicate that Vdr expression was modulated by glucose in healthy islets and decreased in islets from both type 1 diabetes and type 2 diabetes mouse models. In addition, transgenic mice overexpressing VDR in β-cells were protected against streptozotocin-induced diabetes and presented a preserved β-cell mass and a reduction in islet inflammation. Altogether, these results suggest that sustained VDR levels in β-cells may preserve β-cell mass and β-cell function and protect against diabetes.
Introduction
Vitamin D deficiency has been associated with diabetes, both in individuals with type 1 diabetes (T1D) and insulin resistance/type 2 diabetes (T2D) (1–3). In addition, a parallel increase in the prevalence of diabetes and in vitamin D deficiency incidence worldwide has been observed, which may result from both deficient sun exposure, involved in vitamin D synthesis by the skin, and inadequate dietary supply (4). Vitamin D exerts its actions mainly through its binding to vitamin D receptor (VDR), and VDR gene polymorphisms have also been associated with the risk of T2D in different ethnic populations (5). Likewise, two single nucleotide polymorphisms in the VDR gene have been associated with T1D (6). This strongly suggests an essential role of the vitamin D/VDR axis in diabetes, although the mechanisms have not yet been elucidated.
VDR belongs to the steroid hormone receptor superfamily, and it is widely expressed in several cell types where it is known to regulate key cellular processes such as proliferation, differentiation, apoptosis, and immunomodulation (7). In addition, genome-wide VDR-binding chromatin immunoprecipitation sequencing (ChIP-seq) data revealed that an important part of target genes is involved in metabolism (8–10). Moreover, VDR is expressed in a wide variety of immune cells, and vitamin D is a known immunomodulator in both the innate and adaptive arms of the immune system (11). In particular, vitamin D can promote immune tolerance and has immunosuppressive properties (11,12). Vitamin D exposure also causes T cells to change their cytokine production from a proinflammatory to an anti-inflammatory profile (13). It has been hypothesized that vitamin D may have a protective role in diabetes since the immune system is involved in the development of both T1D and T2D (14,15).
VDR is also expressed in several insulin-responsive metabolic tissues, such as the liver, skeletal muscle, or adipose tissue, and it has been reported that vitamin D may improve insulin sensitivity of these tissues (16). Vitamin D may directly increase insulin receptor expression, and thereby enhance insulin stimulation of glucose transport, or indirectly decrease insulin resistance by decreasing inflammatory responses, one of the causes of insulin resistance (16).
Pancreatic islets also express VDR and can metabolize inactive 25-hydroxyvitamin D3 to active 1,25 (OH)2D3 (17). Vitamin D has been reported to exert beneficial effects on glucose tolerance enhancing β-cell function (18,19). Studies in cultured rat islets demonstrated that synthesis and release of insulin may be enhanced by treatment with high doses of vitamin D (20). Moreover, mice with VDR deficiency presented impaired glucose tolerance, defective insulin secretion, and a reduction in insulin mRNA content, suggesting that VDR is a key factor in β-cell function (21). However, the role of VDR, specifically in β-cells, during the development of diabetes remains unknown.
Thus, here we aimed to study the role of VDR in the β-cell in the pathophysiology of diabetes. We found that Vdr expression is decreased in islets from both T1D and T2D mouse models. We also demonstrated that overexpression of VDR in β-cells of transgenic (Tg) mice counteracted experimental diabetes, providing evidence that sustained VDR levels in β-cells may preserve β-cell mass and function and protect against diabetes.
Research Design and Methods
Animals
Female NOD/LtJ and male BKS.Cg-+Leprdb/+Leprdb OlaHsd (db/db), BKS.Cg-m+/+Leprdb/OlaHsd (db/+), and C57Bl6/SJL mice were used. Heterozygous male Tg mice expressing mouse Igf2 under the control of the rat insulin promoter-I (RIP-I) were used (22). Diabetes was induced by streptozotocin (STZ) as previously described (23). All mice were fed ad libitum with a standard chow diet (2018S Teklad Global; Harlan) and maintained under conditions of controlled temperature and light (12-h light/dark cycles). Where stated, mice were fasted for 16 h. Animal care and experimental procedures were approved by the Ethics Committee in Animal and Human Experimentation of the Universitat Autònoma de Barcelona, Bellaterra, Spain.
Generation of Tg Mice
The RIP-I/Vdr chimeric gene was obtained by introduction of a 3.3-kb EcoRV-EclXI fragment containing the entire mouse Vdr cDNA (Open Biosystems INC, Huntsville, AL) (ref. 3710866, GeneBankBC006716) at the EcoRI site in RIP-I/β-globin expression vector (22). This chimeric gene was microinjected into fertilized mouse eggs from a C57BL6/SJL background. The general procedures used for microinjection and detection were as described (24).
Immunohistochemistry and Histopathology
For immunohistochemical detection of VDR, insulin, glucagon, somatostatin, pancreatic polypeptide, TUNEL, and Ki67, pancreas were fixed for 12–24 h in formalin, embedded in paraffin, and sectioned. Sections were then incubated overnight at 4°C with the following antibodies: rat anti-mouse VDR (clone 9A7; Merck KGaA, Darmstadt, Germany), guinea pig anti-porcine insulin (Sigma Chemical, St Louis, MO), rabbit anti-human glucagon (Signet Laboratories, Dedham, MA), rabbit anti-somatostatin (Serotec, Oxford, U.K.), rabbit anti-human pancreatic polypeptide (ICN Biomedicals), and anti-Ki67 (BD Pharmingen). As secondary antibodies, peroxidase-conjugated rabbit anti-guinea pig IgG (Dako, Glostrup, Denmark), biotinylated goat anti-rabbit (Pierce, Rockford, IL), tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-guinea pig (Molecular probes, Leiden, the Netherlands), biotinyilated goat anti-rabbit (Molecular Probes), biotinylated rabbit anti-rat (Dako), and biotinylated horse anti-mouse (Vector Laboratories, Burlingame, CA) antibodies were used. Streptavidin-conjugated Alexa 488 (Molecular Probes) or streptavidin-conjugated Alexa 568 (Molecular Probes) were used as fluorochromes. Images were obtained with a Nikon Eclipse 90i microscope (Nikon, Tokyo, Japan).
Morphometric Analysis
β-Cell and α-cell mass, β-cell replication, and apoptosis determination were performed as previously described (23,25).
Islet Isolation and Culture
Pancreatic islets were isolated as previously described (23) and cultured overnight to recuperate from isolation stress in RPMI-1640 (2.5 mmol/L glucose), supplemented with 1% BSA, 2 mmol/L glutamine, and penicilin/streptomycin at 37°C in an atmosphere of 95% humidified air/5% CO2. To study the effects of glucose on Vdr expression on β-cells, after overnight culture, pools of islets were treated with 2.5 or 9 mmol/L of glucose and 9 mmol/L 2-deoxy-d-glucose. Some of the pools were also cultured with recombinant INS (2 ng/mL) (Sigma). After 8 h of treatment, islets were hand-picked and processed to obtain RNA.
Gene Expression Analysis
For quantitative PCR analysis, total RNA was extracted from isolated islets using Tripure Isolation Reagent (Roche Molecular Biochemicals) and Rneasy Micro Kit (Qiagen, Hilden, Germany). Total RNA (1 µg) was reverse-transcribed for 1 h at 37°C with Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative PCR was performed in a Light Cycler 480 II (Roche) using Light Cycler 480 SYBR Green I Master mix (Roche). Values were normalized to Rplp0 as housekeeping. The primers listed in Table 1 (Supplementary Data) were used for murine islets.
Table 1.
Primer list
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Mm.RplpO | TCC-CAC-CTT-GTC-TCC-AGT-CT | ACT-GGT-CTA-GGA-CCC-GAG-AAG |
| Mm.Ins | GCG-ATT-GTG-GAT-CAG-TGC-T | AGG-TGG-GCC-TTA-GTT-GCA-C |
| Mm.Gck | ATG-ACA-GAG-CCA-GGA-TGG-AG | CGG-CTC-ATC-ACC-TTC-TTC-AG |
| Mm.Slc2a2 | CTG-GAG-CCC-TCT-TGA-CGG-GA | CCA-GTC-CTG-AAA-TTA-GCC-CAC-A |
| Mm.H2-Aa | CTC-TGA-TTC-TGG-GGG-TCC-T | ACC-ATA-GGT-GCC-TAC-GTG-GT |
| Mm.Ucp2 | ACT-GTC-AGT-TCC-GCC-CTC-G | ATG-GCT-GGG-AGA-CGA-AAC-AC |
| Mm.Tnfa | GAT-CGG-TCC-CCA-AAG-GGA-TG | TTT-GCT-ACG-ACG-TGG-GCT-AC |
| Mm.Tgfb | CTG-CTG-ACC-CCC-ACT-GAT-AC | GTG-AGC-GCT-GAA-TCG-AAA-GC |
| Mm.Pcna | GAG-CTT-GGC-AAT-GGG-AAC-AT | GGA-GAC-AGT-GGA-GTG-GCT-TTT |
| Mm.Vdr | GCA-TCC-AAA-AGG-TCA-TCG-GC | AGC-GCA-ACA-TGA-TCA-CCT-CA |
| Mm.Tnfaip3/A20 | CCT-GCC-CAG-GAG-TGT-TAC-AG | TCA-AAC-CTA-CCC-CGG-TCT-CT |
| Mm.Cdk2 | TGG-AGT-CCC-TGT-CCG-AAC-TT | CGG-GTC-ACC-ATT-TCA-GCA-AA |
| Mm.Gcg | ATC-TTG-CCA-CCA-GGG-ACT-TC | AAG-TGA-CTG-GCA-CGA-GAT-GT |
Hormone and Metabolite Assays
Blood glucose levels and serum insulin concentrations were measured as previously described (23). A glucose tolerance test was performed as previously described (23). For insulin release determination, glucose (3 g/kg body weight) was injected intraperitoneally, and venous blood from the tail vein was collected at 0, 2, 5, 15, and 30 min in prechilled tubes (Microvette CB 300; SARSTEDT), which was immediately centrifuged to separate plasma and was stored at −20°C. Insulin levels from an insulin release test were measured by ELISA (Crystal Chemical, Chicago, IL).
Statistical Analysis
All values are expressed as the mean ± SEM. Differences between two means were compared by Student t test, and differences between three or more means were analyzed by one-way ANOVA or two-way ANOVA tests using GraphPad Prism software (version 7.00; GraphPad Software). A P value <0.05 was considered statistically significant. Correlations were determined by nonparametric Spearman correlation test using the computer program GraphPad Prism (version 7.00; GraphPad Software).
Data and Resource Availability
To characterize the expression in human β-cells of different genes, such as VDR, INS, or SLC2A2, we used publicly available data sets from Gene Expression Omnibus (GEO) (26) and ArrayExpress databases (27): GSE20966 (28) and E-CBIL-20 (29). For each data set, the publicly available normalized expression levels for each gene were used. When different probes were detecting the expression of the same gene, their genomic locations were determined to assess its relevance. Data were then plotted as relative signal expression.
Results
VDR Expression Is Reduced in Islets From Diabetic Mice
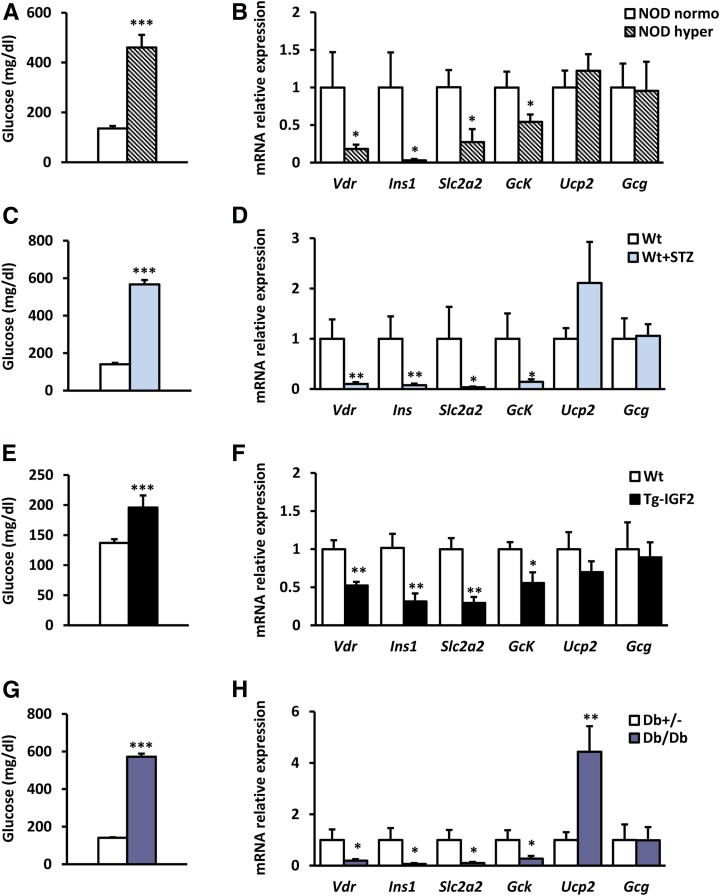
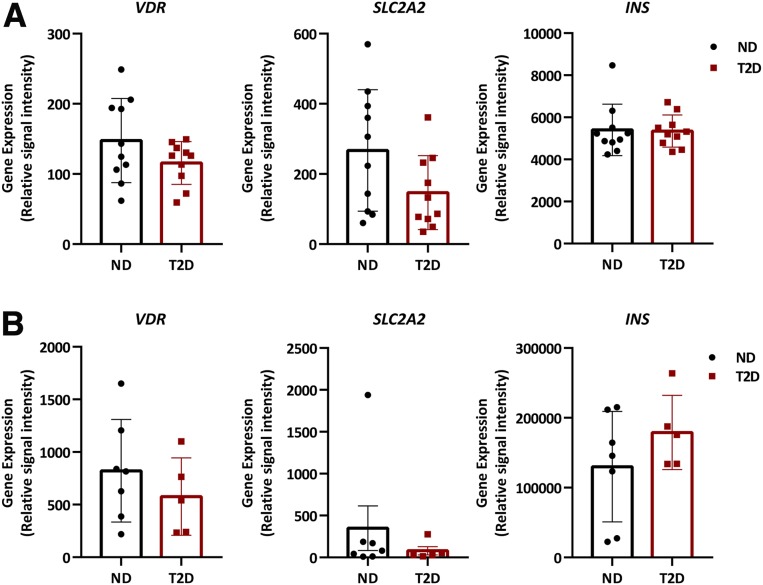
To study the regulation of Vdr gene expression in islets during the diabetic process, Vdr mRNA levels were measured in islets of several diabetic animal models. In islets from nonobese diabetic (NOD) mice (Fig. 1A), the most common type 1 diabetic mouse model, which shows infiltrated islets and β-cell reduction related to glycemia (Supplementary Fig. 1A), Vdr mRNA levels were decreased in hyperglycemic mice compared with normoglycemic littermates (Fig. 1B). This reduction was observed in parallel with a decline in the expression levels of β-cell marker genes, such as insulin (Ins), solute carrier family member 2 (Slc2a2 or Glut2), and glucokinase (Gck) (Fig. 1B). In addition, islet glucagon (Gcg) and uncoupling protein 2 (Ucp2) expression remained unchanged (Fig. 1B). Similarly, in islets from STZ-induced diabetic mice (Fig. 1C), expression of Vdr and β-cell gene markers was also reduced (Fig. 1D). In contrast, Gcg expression was unaltered, and Ucp2 expression presented a clear trend to increase in STZ islets (Fig. 1D). Moreover, Vdr expression was measured in islets from a Tg T2D mouse model in which islets were hyperplasic because of IGF2 overexpression specifically in β-cells (22,25). These Tg mice (Tg-IGF2) develop a prediabetic state with disrupted islet structure, β-cell dysfunction, altered glucose homeostasis, and islet hyperplasia (25) (Supplementary Fig. 1B). Similar to those in the T1D models, islets from prediabetic Tg-IGF2 mice also showed a clear decrease in Vdr expression together with a reduction in β-cell gene markers (Fig. 1E and F). Likewise, a reduction in Vdr expression levels and a decrease in β-cell gene markers were also observed in the well-established T2D model db/db mice, which also displayed unchanged islet Gcg expression and a significant increase of Ucp2 mRNA levels (Fig. 1G and H). Moreover, the correlation between Vdr expression levels and glycemia was examined in T1D and T2D models. Clearly, Vdr expression levels in islets correlated with glucose levels in all analyzed T1D and T2D mice models (Supplementary Fig. 1C–F). It is noteworthy that analysis of human islets from healthy and T2D patients from two public available gene expression data set GSE20966 (28) (Fig. 2A) and E-CBIL-20 (29) (Fig. 2B) revealed a nonsignificant trend toward a reduction in the expression levels of VDR and SLC2A2 in islets from T2D patients. These results from different diabetic mouse models and human samples provided evidence that Vdr expression in islets was reduced in conditions of hyperglycemia.
Figure 1.
Islet gene expression from diabetic mouse models. A and B: Glycemia, Vdr gene expression analysis, and β-cell gene profile in islets from NOD mice. Blood glucose levels (A) and gene expression in islets (B) from NOD hyperglycemic and NOD normoglycemic female mice (26 weeks old). NOD normoglycemic mice (white bars, n = 7) and NOD hyperglycemic mice (striped bars, n = 9). Results are mean ± SEM. *P < 0.05 and ***P < 0.001 vs. NOD normoglycemic. C and D: Glycemia, Vdr expression analysis, and β-cell gene profile in islets after experimental diabetes induction. Blood glucose levels (C) and gene expression in islets (D) from 3-month-old mice treated with multiple doses of STZ (50 mg/kg body weight) 40 days after treatment. Wt untreated mice (white bars) and Wt STZ-treated mice (blue bars); n = 7 per group. Results are mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. untreated mice. E and F: Glycemia, Vdr expression analysis and β-cell gene profile in islets from Tg-IGF2 mice. Blood glucose levels (E) and gene expression in islets (F) from hyperglycemic and insulin-resistant Tg mice that overexpress IGF2 specifically in β-cells. Wt mice (white bars) and Tg-IGF2 mice (black bars); n = 6 per group. Results are mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. Wt. G and H: Glycemia, Vdr gene expression analysis, and β-cell gene profile in islets from db/db mice. Blood glucose levels (G) and gene expression in islets (H) from diabetic db/db and normoglycemic db+/− male mice (19 weeks old). db+/− mice (white bars) and db/db mice (purple bars); n = 8 per group. Results are mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. db+/−.
Figure 2.
Transcriptomic analysis of human β-cells. A: Expression levels of VDR, SLC2A2, and INS in islets from nondiabetic (ND) and T2D patients obtained from the raw data deposited in a Minimum Information About a Microarray Experiment (MIAME) compliant database (GEO accession number: GSE20966); n = 10 per group. B: Expression levels of VDR, SLC2A2, and INS in islets from ND (n = 7) and T2D (n = 5) patients obtained from complete microarray data sets available from ArrayExpress E-CBIL-20.
VDR Expression Is Controlled by Glucose
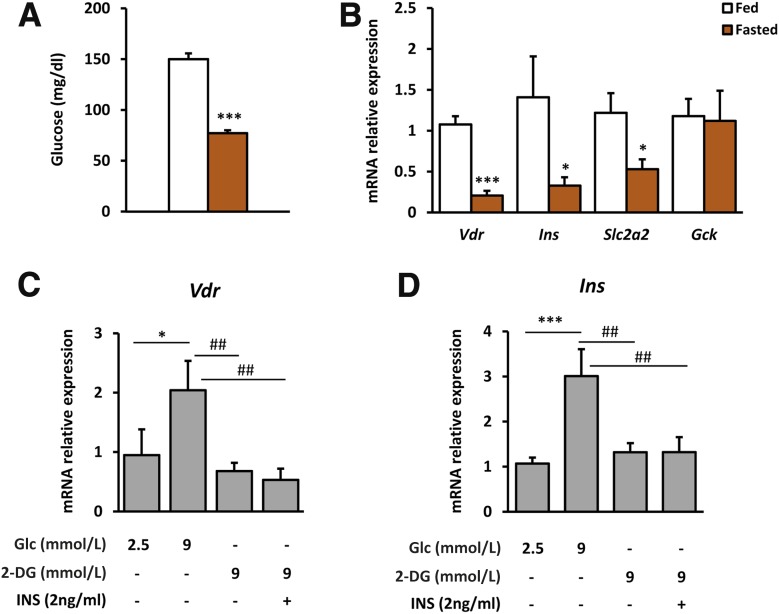
To further explore the regulation of Vdr expression by changes in glucose levels, Vdr expression was measured in islets from fed and fasted wild-type (Wt) mice (Fig. 3A). Unexpectedly, our results showed a marked reduction in Vdr mRNA levels in islets from fasted mice compared with islets from fed mice (Fig. 3B). The decrease in Vdr expression in fasted conditions was also parallel to a significant reduction in Ins and Slc2a2 gene expression (Fig. 3B). We next evaluated the effects of changes in glucose concentration on Vdr gene expression in cultured islets. In islets incubated with high glucose, Vdr mRNA levels were increased, whereas this increase was blunted in islets treated with nonmetabolizable 2-deoxy-d-glucose (Fig. 3C), suggesting that glucose metabolization is required to induce Vdr expression. In addition, to examine the effects of insulin on Vdr expression, islets were treated with both insulin and 2-deoxy-d-glucose. Insulin in the presence of nonmetabolizable glucose did not modify either Vdr or Ins mRNA levels (Fig. 3C and D).
Figure 3.
Islet gene expression from fed and fasted Wt mice. A and B: Glycemia, Vdr expression analysis, and β-cell gene expression profile in islets from fed and fasted mice. Blood glucose levels (A) and gene expression in islets (B) from mice in fed and overnight-fasted conditions. Fed mice (white bars), fasted mice (orange bars). n = 6 per group. Results are mean ± SEM. *P < 0.05, ***P < 0.001 vs. fed group. C and D: Glucose and insulin effect on Vdr expression in culture islets. C: Vdr expression in islets incubated for 8 h with 2.5 or 9 mmol/L of glucose (Glc), 9 mmol/L of 2-deoxy-d-glucose (2-DG), and both 9 mmol/L 2-DG and 2 ng/mL of insulin (INS). D: Insulin expression in islets incubated for 8 h with 2.5 or 9 mmol/L of Glc, 9 mmol/L of 2-DG, and both 9 mmol/L 2-DG and 2 ng/mL of INS. Results shown represent the data obtained for at least six wells (100 islets per well)/condition and from three independent experiments. Data are expressed as mean ± SEM. A one-way ANOVA with Tukey post hoc analysis was used to determine statistical significance *P < 0.05, ***P < 0.001 vs. 2.5 mmol/L Glc and ##P < 0.01 vs. 9 mmol/L Glc.
Altogether, these results indicate that Vdr gene expression in islets is controlled by glucose, which supports the idea that VDR may play a role in β-cell function.
β-Cell VDR Overexpression Ameliorates Diabetes in Mice
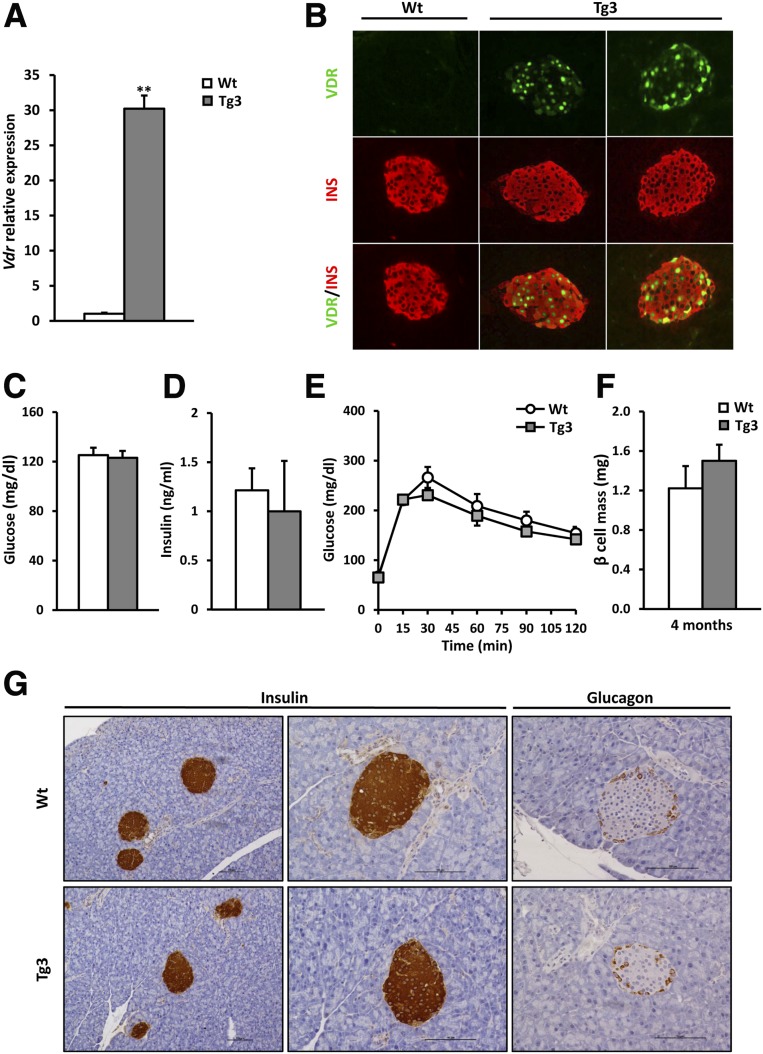
To unravel the role of VDR in β-cells, Tg mice overexpressing VDR, specifically in β-cells, were generated. Three lines of Tg mice (Tg1, Tg3, and Tg4) were obtained that overexpressed murine Vdr under the control of RIP-I. Islets from Tg mice presented higher levels of Vdr mRNAs than Wt littermates (Fig. 4A and Supplementary Fig. 2A). Immunohistochemical analysis revealed that VDR overexpression was detected, specifically in insulin-positive cells (Fig. 4B and Supplementary Fig. 2B). In addition, glycemia and insulinemia remained unchanged in Tg mice when compared with Wt mice (Fig. 4C and D and Supplementary Fig. 2C and D). Since similar results were obtained in the three lines for a number of analyses and to avoid an unnecessary increase in the number of mice studied, Tg3 was selected for a further phenotyping. In accordance with normal glucose and insulin levels, both 4- and 8-month-old Tg mice exhibited normal glucose tolerance (Fig. 4E and Supplementary Fig. 3A). In addition, glucose-stimulated insulin release in 4-month-old mice was not significantly altered (Supplementary Fig. 3B), indicating that VDR overexpression did not modify β-cell function. To evaluate if VDR overexpression affected islet cell mass and distribution, β- and α-cell morphometric analyses were performed. Both 4- and 10-month-old Tg3 mice presented nonsignificant changes in β-cell mass compared with Wt littermates (Fig. 4F and Supplementary Fig. 3C). Immunohistochemical analysis against insulin revealed no differences in islet number (0.12 ± 0.01 and 0.08 ± 0.02 islet number/mg pancreas in Wt and Tg3, respectively) and β-cell distribution in the pancreas between Wt and Tg3 mice (Fig. 4G). Similarly, no alterations in α-cell mass and distribution were observed in 4- and 10-month-old mice (Fig. 4G and Supplementary Fig. 3D and E).
Figure 4.
Generation of VDR Tg mice. Tg mice overexpressing murine Vdr cDNA under the control of the RIP-I were obtained. A: Islets Vdr gene expression. Vdr mRNA levels in islets from 2-month-old Wt (white bars) and Tg3 (gray bars) mice; n = 6 per group. Results are mean ± SEM. **P < 0.01 vs. Wt. B: Detection of VDR specifically in β-cell. Immunohistochemical analysis of insulin (red) and VDR (green) in pancreas of 2-month-old mice revealed β-cell–specific VDR expression. Original magnification ×20. C: Fed glycemia in Tg mice. Wt (white bars, n = 15) and Tg3 (gray bars, n = 10) mice. Results are mean ± SEM. D: Serum insulin levels. Insulin concentration was determined in fed conditions by radioimmunoassay. Wt (white bars, n = 15) and Tg3 (gray bars, n = 10) mice. Results are mean ± SEM. E: Glucose tolerance test (1 g/kg glucose), which was performed in 4-month-old Wt mice (white circles) and Tg3 mice (gray squares). F: β-cell mass in VDR Tg mice. β-cell mass was determined in Wt and Tg3 mice pancreas at the age of 4 months. Results are mean ± SEM. n = 4 per group. G: Pancreas immunohistochemical analysis. Immunohistochemical analysis of insulin and Gcg expression in Wt and Tg3 islets from 4-month-old mice. Scale bars, 100 μm.
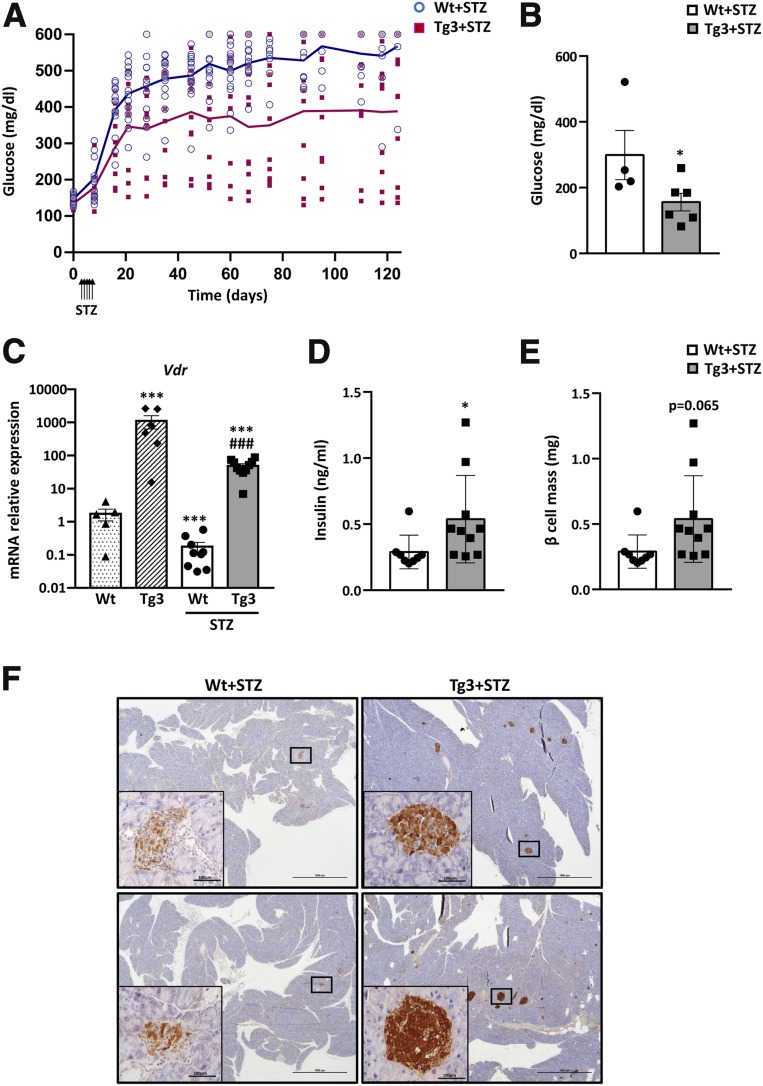
Next, to examine the effects of VDR overexpression during the diabetic process, Wt and Tg3 mice received five daily consecutive injections of a standard dose of STZ (50 mg/kg) to induce diabetes. After STZ treatment, Wt mice displayed severe hyperglycemia, and 100% of mice developed overt diabetes. However, only 60% of treated Tg mice were diabetic and presented a less severe hyperglycemia at the end of the study (Fig. 5A and Supplementary Fig. 4A and B). In Tg3 mice, glycemia improvement was even more evident in fasting conditions (Fig. 5B). Similar results were obtained when STZ treatment was administered to Tg1 mice (Supplementary Fig. 4C and D). As previously shown, Vdr expression declined in islets from Wt mice after STZ treatment (Fig. 5C). In contrast, although Tg3 mice presented a similar Vdr reduction after STZ treatment, they sustained high Vdr expression levels (Fig. 5C).
Figure 5.
Glycemia before and after STZ treatment (5 × 50 mg/kg). A: Evolution of fed glycemia. Glycemia before and after STZ treatment was measured. Wt (blue circles, n = 14) and Tg3 (pink squares, n = 13) mice. A two-way ANOVA with Tukey post hoc analysis was used to determine statistical significance. P < 0.01 was found in time, genotype, and time × genotype. B: Fasted glycemia 60 days after STZ treatment in Wt (white bars, n = 14) and Tg3 (gray bars, n = 13) mice. Results are mean ± SEM. *P < 0.05 vs. Wt. C: Islet Vdr gene expression. Vdr mRNA levels in islets from non-STZ-treated Wt (dotted bars) and Tg3 (striped bars) mice and from STZ-treated Wt (white bars) and Tg3 (dark gray bars) mice. Results are mean ± SEM. ***P < 0.001 vs. Wt. ###P < 0.001 vs. STZ-treated Wt . D: Serum insulin levels. Insulin was determined in fed conditions by radioimmunoassay. Wt (white bars) and Tg3 (gray bars) mice. Results are mean ± SEM. *P < 0.05 vs. Wt. E: β-cell mass in VDR Tg mice after STZ treatment. β-cell mass was determined 4 months after STZ treatment in Wt and Tg3 mice pancreas. Results are mean ± SEM. F: Pancreas immunohistochemical analysis. Immunohistochemical analysis of insulin expression in Wt and Tg3 islets 3 months after STZ treatment. Scale bars, 100 μm.
Immunohistochemical analysis against insulin also clearly revealed that after STZ treatment, Tg mice presented a higher number and area of insulin-positive islets in the pancreas compared with Wt mice, which showed few insulin-positive β-cells (Fig. 5E and F). These results correlated with higher insulin levels in Tg3 compared with Wt mice (Fig. 5D). The observed β-cell mass maintenance was parallel to a lower decrease in mRNA levels of β-cell gene markers such as Ins1, Slc2a2, and GcK after STZ treatment in Tg3 islets (Supplementary Fig. 4E).
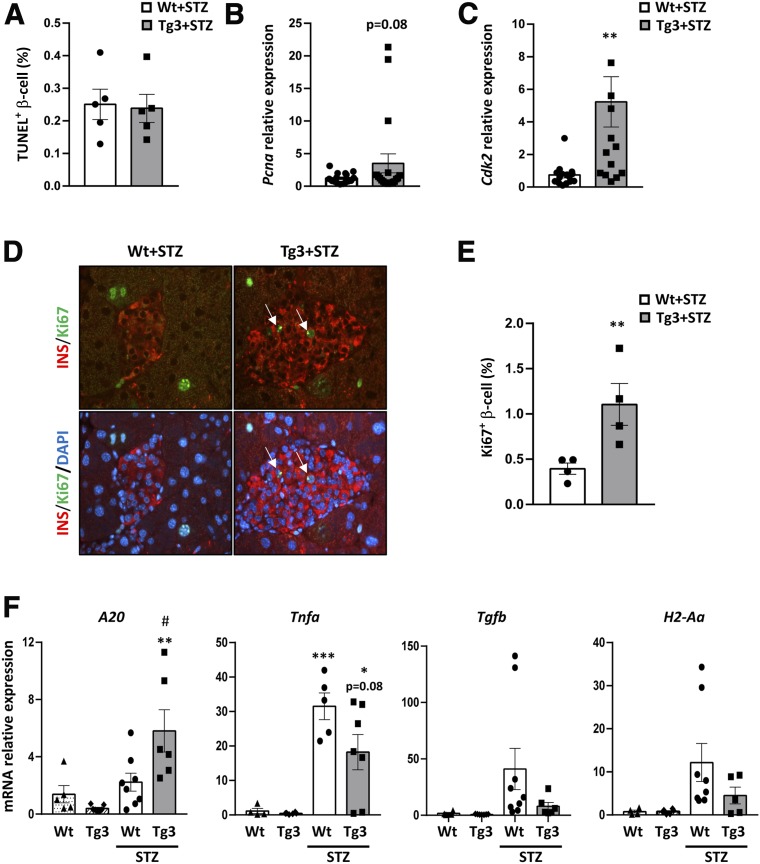
The maintenance of pancreatic β-cell mass basically depends on two mechanisms: apoptosis and replication (30). Both mechanisms were explored in mice treated with STZ by morphometric analysis. TUNEL-positive β-cell percentage, an index of apoptosis, was similar in Wt and Tg3 mice, indicating that VDR overexpression did not protect β-cell from apoptosis (Fig. 6A). In contrast, Tg3 islets showed a clear increase in proliferation cell markers Pcna and CdK2 (Fig. 6B and C). In addition, a higher Ki67-positive β-cell percentage, which is a classical β-cell replication index, was detected in Tg3 islets compared with Wt (Fig. 6D and E). Thus, these results indicated that maintenance of the proliferation capacity may be involved in β-cell preservation in Tg3 mice after STZ damage.
Figure 6.
β-cell mass in VDR Tg mice. A: β-cell apoptosis analysis. Quantification of apoptotic β-cells. The percentage of apoptotic β-cells was determined in Wt and Tg3 mice 40 days after STZ treatment. B and C: Islet cell cycle gene expression analysis. Pcna (B) and Cdk2 (C) gene expression was analyzed in islets from Wt and Tg3 mice 40 days after STZ treatment. Wt STZ-treated mice (white bars) and Tg3 STZ-treated mice (gray bars). Results are mean ± SEM. **P < 0.01 vs. Wt+STZ. D: Immunohistochemical detection of β-cell replication. Analysis of β-cell replication by double immunostaining with Ki67 replication marker (green) and insulin (red) in Wt and Tg3 islets 40 days after STZ treatment. Original magnification ×20. E: β-cell replication analysis. Quantification of β-cell replication. The percentage of replicative β-cell was determined in Wt and Tg3 mice 40 days after STZ treatment. Results are mean ± SEM. **P < 0.01 vs. Wt+STZ. F: β-cell gene profile analysis in islets after experimental diabetes induction. Gene expression in islets from 3-month-old mice 40 days after treatment with multiple doses of STZ (50 mg/kg body weight). Wt (white-dotted bars) and Tg3 (white-striped bars) non-STZ-treated mice; Wt STZ-treated (white bars) and Tg3 STZ-treated (dark gray bars) mice. Results are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Wt, and #P < 0.05 vs. Wt+STZ.
The presence of inflammation was examined in islets by measuring islet proinflammatory and anti-inflammatory markers. Tg3 mice showed higher levels of anti-inflammatory A20 mRNA and a reduction of proinflammatory Tnfa, Tgfb, and H2-Aa mRNA levels related to Wt mice (Fig. 6F). In addition, Ucp2, which has been reported to be an inhibitor of A20 gene expression, was downregulated in Tg3 compared with Wt islets before and after STZ treatment (Supplementary Fig. 4F). Altogether, these data suggested that sustained VDR expression levels protected Tg mice to develop severe hyperglycemia, partially preserving β-cell mass and reducing local inflammation and diabetic consequences.
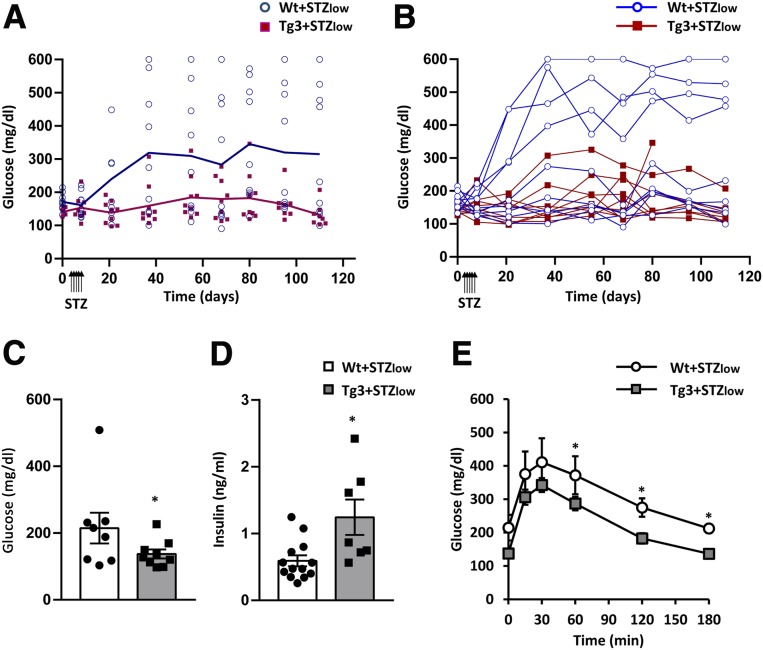
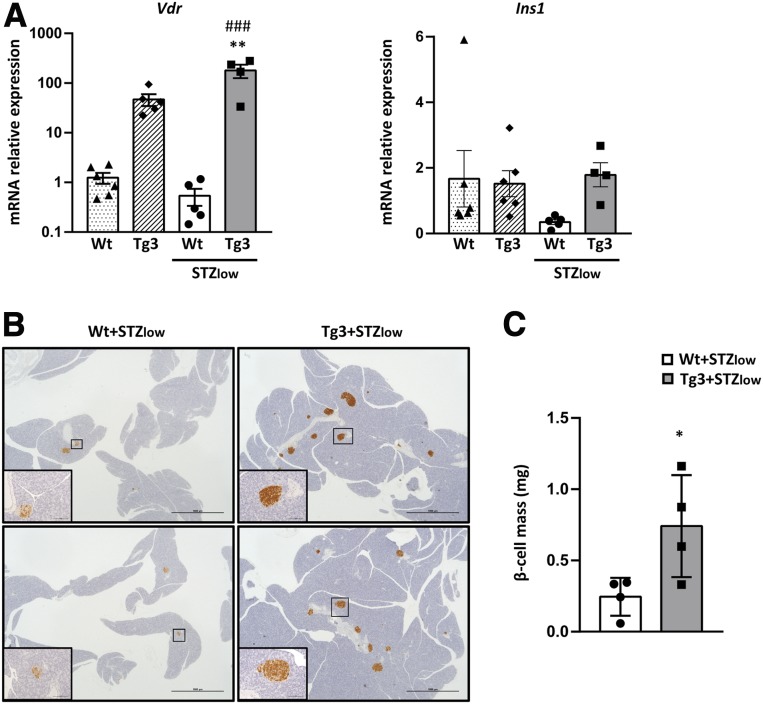
Since standard doses of STZ may have very toxic consequences (31), the effects of VDR overexpression were also explored in diabetic mice induced by very low doses of STZ (30 mg/kg) (five doses consecutive days) (+STZlow). After STZlow treatment, Wt mice developed hyperglycemia, and about 50% of them became overtly diabetic (Fig. 7A and B). In contrast, Tg3+STZlow mice maintained normoglycemia during all the study with no signs of diabetes (Fig. 7A and B). This improvement was also observed in fasted glycemia, which was clearly lower in Tg3+STZlow mice (Fig. 7C), and in insulin levels that were higher in Tg3+STZlow mice compared with Wt+STZlow mice (Fig. 7D) and were similar to Wt healthy mice (Fig. 4D). In addition, 2 months after STZlow treatment, Tg3+STZlow mice showed an improvement in glucose tolerance compared with Wt+STZlow mice (Fig. 7E). Gene expression analysis of islets at the end of the study revealed a decrease in Vdr expression in Wt+STZlow as observed previously (Figs. 5C and 8A), whereas Tg3+STZlow mice maintained high levels of Vdr, correlating with sustained levels of Ins expression (Fig. 8A). β-cell mass was also higher in Tg3+STZlow mice, accordingly to glucose and insulin values. Likewise, immunohistochemical analysis of the pancreas revealed few insulin-positive islets in Wt+STZlow mice, while Tg3+STZlow mice presented a normal number of insulin-positive islets according to β-cell mass data (Fig. 8B and C).
Figure 7.
Glycemia before and after STZlow treatment (5 × 30 mg/kg). A: Evolution of fed glycemia (mean). B: Evolution of individual fed glycemia. Glycemia before and after STZlow treatment was measured. Wt (blue circles n = 9) and Tg3 (pink squares, n = 9) mice. In both A and B, two-way ANOVA with Tukey post hoc analysis was used to determine statistical significance. P < 0.05 (days 8, 21, and 37), P < 0.01 (days 55, 68, 80, 95, and 110) Wt responders vs. Tg3. C: Fasted glycemia. Fasted glycemia 60 days after STZlow treatment in Wt (white bars) and Tg3 (gray bars) mice. *P < 0.05 vs. Wt+STZlow. D: Serum insulin levels. Insulin was determined in fed conditions by radioimmunoassay. Wt (white bars) and Tg3 (gray bars) mice. Results are mean ± SEM. *P < 0.05 vs. Wt+STZlow. E: Glucose tolerance test (1 g/kg glucose), which was performed in Wt mice (white circles, n = 9) and Tg3 mice (gray squares, n = 9) 60 days after STZlow treatment. Results are mean ± SEM. *P < 0.05 vs. Wt+STZlow.
Figure 8.
Gene expression analysis. Vdr and Ins gene expression in islets after experimental diabetes induction. A: Gene expression in islets from 3-month-old mice 40 days after treatment with multiple doses of STZ (STZlow) (30 mg/kg body weight). Wt (dotted bars) and Tg3 (striped bars) non-STZlow-treated mice, Wt (white bars) and Tg3 (dark gray bars) STZlow-treated mice. Results are mean ± SEM. A one-way ANOVA with Tukey post hoc analysis was used to determine statistical significance. **P < 0.01 vs. Wt untreated mice, ###P < 0.001 vs. Wt+STZlow-treated mice. B: Pancreas immunohistochemical analysis of β-cell mass in VDR STZ-treated mice. Immunohistochemical analysis of insulin expression in Wt and Tg3 islets 4 months after STZ treatment. Scale bars, 100 μm. C: β-cell mass, which was determined 40 days after STZlow treatment (30 mg/kg body weight) in Wt and Tg3 mice pancreas. Results are mean ± SEM. *P < 0.05 vs. Wt + STZlow.
Overall, these results provide evidence that sustained levels of VDR during the diabetic process revert hyperglycemia onset, improve glucose tolerance, and maintain β-cell mass.
Discussion
The role of vitamin D in the protection against diabetes is now widely accepted, although the underlying mechanisms are mainly unknown. In agreement with a protective role of vitamin D, vitamin D deficiency has been associated with diabetes (1). Similarly, polymorphisms in the Vdr gene, which lead to a significant decrease of mRNA and VDR protein levels, are a risk factor for diabetes development (32). Here, we clearly demonstrate that Vdr expression in mice is downregulated in islets during the development of both T1D and T2D. It is noteworthy that our results obtained from publicly available data sets also showed a trend toward a reduction in VDR expression in human islets from T2D patients. These results were consistent with previous reports showing a reduction in Vdr expression in pancreas from STZ-treated mice and rats (17). In this work, we provided evidence that Vdr reduction also occurs in islets from NOD mice that spontaneously develop diabetes and share many features with human T1D (33). In addition, we observed decreased Vdr levels in two different mouse models of T2D with β-cell hyperplasia (25,34).
Altogether, our results suggested that Vdr downregulation in β-cells may be a common feature of diabetes and that this reduction, along with a reduction in the expression of other β-cell markers, may be the result of β-cell loss/dedifferentation in diabetic islets, one of the common features of both T1D and T2D. VDR decrease was also associated with hyperglycemia, the major hallmark of diabetes. Indeed, Vdr expression was negatively correlated to circulating glucose levels in all the diabetic models studied here. Unexpectedly, we also showed that Vdr expression was decreased when circulating glucose levels were physiologically low, i.e., during fasting. Our results obtained from an in vitro study in cultured islets further indicated that Vdr expression was stimulated by glucose. Thus, although we cannot discard that decreased Vdr expression was due to β-cell loss during diabetes, our results may also be explained by the fact that diabetes is associated with low intracellular glucose levels resulting from decreased glucose uptake and a decrease in glucose sensing players, such as GLUT2 in β-cells (35).
Likewise, in fasted conditions, β-cells present similar low intracellular glucose. Thus, we can hypothesize that despite hyperglycemia, the low intracellular glucose levels may be responsible for Vdr downregulation. Accordingly, in islets from all of the diabetic mouse models analyzed, a reduction in expression of genes involved in glucose uptake in β-cells, the glucose transporter Slc2a2 and the glucose-phosphorylating enzyme Gck, was parallel to Vdr downregulation. In human islets from T2D patients, a parallel nonsignificant trend to decrease of SLC2A2 and VDR expression has also been observed. Thus, in humans, decreased glucose uptake in β-cells may also lead to a reduction in VDR expression. However, the mechanisms of regulation of β-cell VDR expression in humans remain to be elucidated. In addition, although our results suggest for the first time, that glucose metabolism may modulate positively Vdr expression, further studies are necessary to carefully explore this issue. It is also noteworthy that Vdr expression in islets declined in fasted compared with fed state, corroborating the importance of glucose metabolism for physiological regulation of Vdr expression. Accordingly, it has been reported recently that fasting-induced transcription factors repress vitamin D bioactivation (36). Thus, our findings reveal that both physiological acute changes in glucose concentration and pathophysiological disruption of glucose uptake alter Vdr gene expression in islets and may be responsible for Vdr decreased expression during diabetes.
Our results also support that VDR deficiency may have an impact on β-cell function alterations during diabetes. Results obtained from mice lacking functional Vdr indicate that VDR loss leads to a reduction in insulin mRNA levels and a deficit in insulin secretion (21). In accordance with this, we observed a decrease in insulin gene expression levels parallel to Vdr reduction in islets from diabetic mice. Moreover, treatment with vitamin D increased Ins1 mRNA expression in control animals, but this effect was lost in STZ-treated mice, possibly because of a decrease in Vdr expression in islets (17). These results suggest that VDR may be involved in Ins1 transcription in mouse pancreas (17). However, so far, no vitamin D response elements have been identified in the human or mouse insulin gene promoters. VDR-deficient mice are glucose intolerant, probably due in part to defects of the β-cell function, but the absence of VDR in other tissues may also contribute to their phenotype (21).
In this study, we found that Tg mice overexpressing VDR specifically in β-cells were resistant to the development of STZ-induced diabetes. Our results show a clear protective effect of sustained VDR levels in β-cell in front of STZ damage. It has been described that multiple doses of STZ induce diabetes through an increase in inflammatory cytokines, β-cell functional defects, and finally β-cell loss (37). Tg mice overexpressing VDR presented a preservation of β-cell mass, at least in part by maintaining β-cell replication capacity in front of STZ damage. In agreement with these results, it has recently been reported that VDR activation leads to an induction of β-cell replication (38). In addition, VDR overexpression partially protected Tg mice from islet inflammation, reducing the expression of inflammatory markers in islets. This reduction in inflammation and the subsequent protection against diabetes may have been mediated by VDR, as suggested by the fact that vitamin D, or its nonhypercalcemic analog, significantly inhibits insulitis and prevents or delays the onset of diabetes in NOD mice (13,39,40). In addition, the activation of VDR in human β-like cells is able to counteract the inflammatory response induced by cytokines and maintain β-cell functionality (38). In contrast, knockdown of VDR led to an increased cytokine-induced cell death in human β-like cells and to a reduction in the expression of key β-cell genes in cytokine-treated rat β-cells INS1 (38). All of these data suggest that VDR may reduce the inflammatory milieu in islets and therefore maintain β-cell mass and function resulting in a protection against diabetes development. Therefore, we demonstrated that sustained VDR levels in β-cells may protect against diabetes-induced damages by increasing β-cell functional target genes, decreasing inflammation and maintaining β-cell proliferation capacity.
Data from animal studies show that although vitamin D administration may delay the onset of diabetes in NOD mice, no or limited benefit has been observed by the administration of a vitamin D analog on β-cell damage after STZ treatment in mice (38). Interestingly, pharmacologically induced VDR signaling by a synthetic ligand in combination with a VDR-downstream modulator is able to partially restore β-cell function and glucose homeostasis in various T2D and T1D mouse models. Nevertheless, these effects were lost when the VDR synthetic ligand was used alone (38). In light of our results, this lack of effectiveness of vitamin D analogs or VDR ligands may be due to reduced Vdr expression levels in diabetic mice.
In humans, clinical data of effectiveness of vitamin D supplements are controversial. Although some data reveal beneficious effects of vitamin D supplementation on glucose metabolism (41,42), others report no effect of vitamin D supplements (43,44). The results of supplementation may be influenced by factors such as baseline vitamin D status, variability in dosages and forms of vitamin D used, duration of the intervention, or heterogeneity of the patients (45). It has also been suggested that genetic variation in VDR may influence metabolic effects of vitamin D supplementation in T2D (46). Our data clearly point out that variations in VDR expression in diabetic patients may also influence the outcomes of vitamin D supplementation. Therefore, a better knowledge of the VDR regulation in diabetes is necessary to define appropriate strategies of supplementation to improve glycemic control and metabolic alterations.
Although various studies described genetic variations in the VDR gene as a risk factor for T1D and T2D development, contradictory results about the association between VDR single nucleotide polymorphisms and T2D have been reported (47–49). While genetic variations may not be responsible for diabetes development, a pathophysiological decrease in VDR expression may lead to a loss of protection against β-cell damage. As evidenced here, VDR expression maintenance might be essential to counteract β-cell damage in the diabetic process and to protect against diabetes development. Thus, future strategies for treatment of diabetes should be based on a better knowledge of mechanisms underlying VDR downregulation during diabetes and address restoration of VDR levels.
Supplementary Material
Article Information
Acknowledgments. The authors thank Malcolm Watford (Rutgers University, New Brunswick, New Jersey) and Ivet Elias (Universitat Autònoma de Barcelona, Bellaterra, Spain), for helpful discussion and Aina Bonet, Marc Leal, Albert Ribera, Marta Moya, Jennifer Barrero, and Lidia Hernández (Universitat Autònoma de Barcelona, Bellaterra, Spain) for technical support.
Funding. This work was supported by grants from FEDER/Ministerio de Ciencia, Innovación y Universidades – Agencia Estatal de Investigación (SAF2017-86166-R), Generalitat de Catalunya (ICREA Academia Award to F.B.), Spain. G.E. received a predoctoral fellowship from Generalitat de Catalunya, Spain.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.Mor. and L.V. designed experiments, generated reagents, performed experiments, and wrote and edited the manuscript. S.F. designed experiments and wrote and edited the manuscript. C.M. generated reagents, performed experiments, and contributed to discussions. G.E., T.F., M.Mol., and E.C. generated reagents and performed experiments. J.R. analyzed human data and contributed to discussion. A.P. generated transgenic mice and contributed to discussion. N.T. designed experiments and contributed to discussions. F.B. and A.C. designed experiments and wrote and edited the manuscript. A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at https://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0757/-/DC1.
M.Mor. and L.V. contributed equally to this work.
References
- 1.Grammatiki M, Rapti E, Karras S, Ajjan RA, Kotsa K. Vitamin D and diabetes mellitus: causal or casual association? Rev Endocr Metab Disord 2017;18:227–241 [DOI] [PubMed] [Google Scholar]
- 2.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes 2008;57:298–305 [DOI] [PubMed] [Google Scholar]
- 3.Dong J-Y, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients 2013;5:3551–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahota O. Understanding vitamin D deficiency. Age Ageing 2014;43:589–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bid HK, Konwar R, Aggarwal CG, et al. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J Med Sci 2009;63:187–194 [PubMed] [Google Scholar]
- 6.Mukhtar M, Batool A, Wajid A, Qayyum I. Vitamin D receptor gene polymorphisms influence T1D susceptibility among Pakistanis. Int J Genomics 2017;2017:4171254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005;26:662–687 [DOI] [PubMed] [Google Scholar]
- 8.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol 2014;5:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008;10:185–197 [DOI] [PubMed] [Google Scholar]
- 10.Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013;153:601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010;10:482–496 [DOI] [PubMed] [Google Scholar]
- 12.Adorini L, Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. In Dendritic Cells. Handbook of Experimental Pharmacology. Lombardi G, Riffo-Vasquez Y, Eds. Berlin, Springer, 2009, p. 251–273 [DOI] [PubMed] [Google Scholar]
- 13.Takiishi T, Van Belle T, Gysemans C, Mathieu C. Effects of vitamin D on antigen-specific and non-antigen-specific immune modulation: relevance for type 1 diabetes. Pediatr Diabetes 2013;14:81–89 [DOI] [PubMed] [Google Scholar]
- 14.Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol 2011;347:106–120 [DOI] [PubMed] [Google Scholar]
- 15.Lee M-S. Role of innate immunity in the pathogenesis of type 1 and type 2 diabetes. J Korean Med Sci 2014;29:1038–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung C-C, Liao M-T, Lu K-C, Wu C-C. Role of vitamin D in insulin resistance. BioMed Res Int 2012;2012:634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozeki J, Choi M, Endo-Umeda K, Sakurai K, Amano S, Makishima M. Enhanced transcription of pancreatic peptide YY by 1α-hydroxyvitamin D3 administration in streptozotocin-induced diabetic mice. Neuropeptides 2013;47:329–332 [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 1984;73:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 1986;29:142–145 [DOI] [PubMed] [Google Scholar]
- 20.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am 2010;39:419–446 [DOI] [PubMed] [Google Scholar]
- 21.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 2003;17:509–511 [DOI] [PubMed] [Google Scholar]
- 22.Devedjian J-C, George M, Casellas A, et al. Transgenic mice overexpressing insulin-like growth factor-II in β cells develop type 2 diabetes. J Clin Invest 2000;105:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casellas A, Salavert A, Agudo J, et al. Expression of IGF-I in pancreatic islets prevents lymphocytic infiltration and protects mice from type 1 diabetes. Diabetes 2006;55:3246–3255 [DOI] [PubMed] [Google Scholar]
- 24.Costantini F, Lacy E. Gene transfer into the mouse germ-line. J Cell Physiol Suppl 1982;1:219–226 [DOI] [PubMed] [Google Scholar]
- 25.Casellas A, Mallol C, Salavert A, et al. Insulin-like growth factor 2 overexpression induces β-cell dysfunction and increases beta-cell susceptibility to damage. J Biol Chem 2015;290:16772–16785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. doi: 10.1093/nar/gks1193. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res 2012;41:D991–D995. [DOI] [PMC free article] [PubMed]
- 27.Athar A, Füllgrabe A, George N, et al. ArrayExpress update - from bulk to single-cell expression data. Nucleic Acids Res 2019;47:D711–D715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marselli L, Thorne J, Dahiya S, et al. Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One 2010;5:e11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunton JE, Kulkarni RN, Yim S, et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 2005;122:337–349 [DOI] [PubMed] [Google Scholar]
- 30.Hanley SC, Austin E, Assouline-Thomas B, et al. β-cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology 2010;151:1462–1472 [DOI] [PubMed] [Google Scholar]
- 31.Bolzán AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res 2002;512:121–134 [DOI] [PubMed] [Google Scholar]
- 32.Ogunkolade B-W, Boucher BJ, Prahl JM, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002;51:2294–2300 [DOI] [PubMed] [Google Scholar]
- 33.Jayasimhan A, Mansour KP, Slattery RM. Advances in our understanding of the pathophysiology of type 1 diabetes: lessons from the NOD mouse. Clin Sci (Lond) 2014;126:1–18 [DOI] [PubMed] [Google Scholar]
- 34.Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav 2010;101:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger RH. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science 1991;251:1200–1205 [DOI] [PubMed] [Google Scholar]
- 36.Aatsinki SM, Elkhwanky MS, Kummu O, et al. Fasting-induced transcription factors repress vitamin D bioactivation, a mechanism for vitamin D deficiency in diabetes. Diabetes 2019;68:918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang-Fischer Y, Garyantes T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J Diabetes Res 2018;2018:8054073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Z, Yoshihara E, He N, et al. Vitamin D switches BAF complexes to protect β cells. Cell 2018;173:1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002;51:1367–1374 [DOI] [PubMed] [Google Scholar]
- 40.Driver JP, Foreman O, Mathieu C, van Etten E, Serreze DV. Comparative therapeutic effects of orally administered 1,25-dihydroxyvitamin D(3) and 1alpha-hydroxyvitamin D(3) on type-1 diabetes in non-obese diabetic mice fed a normal-calcaemic diet. Clin Exp Immunol 2008;151:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathieu C. Vitamin D and diabetes: where do we stand? Diabetes Res Clin Pract 2015;108:201–209 [DOI] [PubMed] [Google Scholar]
- 42.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Boer IH, Tinker LF, Connelly S, et al.; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008;31:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avenell A, Cook JA, MacLennan GS, McPherson GC; RECORD Trial Group . Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2009;38:606–609 [DOI] [PubMed] [Google Scholar]
- 45.Santos RKF, Brandão-Lima PN, Tete RMDD, Freire ARS, Pires LV. Vitamin D ratio and glycaemic control in individuals with type 2 diabetes mellitus: a systematic review. Diabetes Metab Res Rev 2018;34:e2969. [DOI] [PubMed] [Google Scholar]
- 46.Al-Daghri NM, Mohammed AK, Al-Attas OS, et al. Vitamin D receptor gene polymorphisms modify cardiometabolic response to vitamin D supplementation in T2DM patients. Sci Rep 2017;7:8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Wu B, Liu JY, Yang LB. Vitamin D receptor gene polymorphisms and type 2 diabetes: a meta-analysis. Arch Med Res 2013;44:235–241 [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Xi B, Reilly KH, Liu M, Fu M. Quantitative assessment of the associations between four polymorphisms (FokI, ApaI, BsmI, TaqI) of vitamin D receptor gene and risk of diabetes mellitus. Mol Biol Rep 2012;39:9405–9414 [DOI] [PubMed] [Google Scholar]
- 49.Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 2011;377:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.