Abstract
Background:
In multiple clinical studies, teduglutide reduced parenteral support (PS) with a consistent safety profile in adults with short bowel syndrome–associated intestinal failure (SBS–IF). The objective of this study was to assess adverse events (AEs) from a pooled data set.
Methods:
Safety data from four prospective clinical trials of teduglutide in patients with SBS–IF were assimilated. AEs were evaluated in patient groups based on treatment received in each study and in populations stratified to create distinct subgroups based on aetiology, bowel anatomy and baseline PS volume requirements.
Results:
Safety data are reported for up to 2.5 years, totalling 222 person-years exposure to teduglutide. In most patients, AEs were reported as mild or moderate in severity in all patient groups and occurred at comparable rates between patients who received teduglutide or placebo. Several common gastrointestinal AEs, including abdominal pain, nausea and abdominal distension, were reported more frequently earlier in the course of treatment, with their frequency declining over time. Fewer gastrointestinal AEs were reported in patients with vascular causes of SBS–IF and patients with most of their colon-in-continuity than in other patient subgroups. Across the patient stratification subgroups, the predominant treatment-emergent AEs for which patients receiving teduglutide had a significantly increased relative risk were abdominal distension and gastrointestinal stoma complication compared with patients receiving placebo.
Conclusions:
Teduglutide had a safety profile consistent with prior adult data and no new safety concerns were identified. The most frequently reported AEs were gastrointestinal in origin, consistent with the underlying disease condition and intestinotrophic actions of teduglutide.
Clinical Trial Registry information:
NCT00081458/EudraCT, 2004-000438-35; NCT00798967/EudraCT, 2008-006193-15; NCT00172185/EudraCT, 2004-000439-27; NCT00930644/EudraCT, 2009-011679-65
Keywords: diarrhoea, gastrointestinal cancer, gastrointestinal polyps, inflammatory bowel disease, large intestine, malabsorption, nutrition, small intestine, teduglutide
Introduction
Short bowel syndrome (SBS) is a rare disorder caused by extensive surgical resection of the small intestine or congenital diseases of the small bowel, resulting in decreased intestinal length and compromised function, leading to markedly decreased absorption of fluids and nutrients.1,2 Patients with SBS comprise a heterogeneous population with varying causes of SBS and differences in residual bowel anatomy and function.1,2 Patients with chronic intestinal failure associated with SBS (SBS–IF) remain dependent on parenteral support (PS; parenteral nutrition and/or intravenous fluids) to maintain adequate calorie intake and fluid, electrolyte and micronutrient balance, despite the adaptive response of the intestine that may manifest after resection with expansion of the mucosal surface area to increase absorptive capacity.3,4 PS requirements can be used to classify chronic intestinal failure into functional categories based on volume or energy content of parenteral nutrition.5,6 Although PS provides vital support for patients with SBS–IF, its use may be associated with potentially life-threatening metabolic and central venous catheter complications and reduced quality of life.7–11
Adaptation of the intestine after resection involves expansion of the mucosal surface area and modulation of intestinal blood flow, secretions, permeability and motility that together increase absorptive capacity.12,13 Glucagon-like peptide-2 (GLP-2) is a hormone secreted postprandially by L cells located in the distal ileum and proximal colon, sections of the bowel that are frequently removed in patients with SBS.14 GLP-2 is involved in maintaining normal nutrient and energy absorption in the intestine and is associated with intestinal adaptation and epithelial proliferation after resection.13,15 Teduglutide is an analogue of GLP-2 and is approved in the United States and Europe for the treatment of patients at least 1 year of age with SBS who are dependent on PS.16,17 In two randomized, placebo-controlled phase III studies (CL0600-004 and STEPS [Study of Teduglutide Effectiveness in Parenteral Nutrition-Dependent Short Bowel Syndrome Subjects])18,19 and their respective open-label extension studies (CL0600-005 and STEPS-2),20,21 teduglutide substantially reduced PS requirements in adults with SBS–IF. Many of the observed treatment-emergent adverse events (TEAEs) were consistent with the known mechanism of action of teduglutide, factors underlying SBS or complications associated with PS. In patients receiving teduglutide and patients receiving placebo, the most frequently reported categories of TEAEs were of gastrointestinal origin.18–21
Teduglutide has also demonstrated clinical effectiveness in real-world studies. In a single-centre, retrospective study of anatomically heterogeneous patients with SBS–IF, 15 of 19 (79%) achieved a decrease of at least 20% in PS volume with teduglutide after treatment durations varying between 1 and 45 weeks; 2 of these patients (11%) obtained complete independence from PS.22 Reductions in PS requirements were accompanied by significant increases in mean villus height and crypt depth in this cohort as assessed by direct measurement of biopsies obtained during endoscopy. In a second single-centre retrospective study of 18 patients with SBS–IF receiving teduglutide, 14 (78%) had reduced PS requirements by at least 1 day per week, and 11 (61%) achieved independence from PS after 3–36 months of treatment.23
To better understand patient characteristics possibly associated with response to teduglutide, Jeppesen and colleagues recently conducted a post hoc analysis of defined patient subgroups in the STEPS study.24 The greatest effects of teduglutide in terms of reducing absolute PS volume requirements were observed in patients who had inflammatory bowel disease (IBD) as the underlying cause of SBS–IF, in patients with stoma who lacked colon-in-continuity and in patients with the highest baseline PS volumes.
The objective of this comprehensive pooled analysis of clinical trial data was to assess the safety of teduglutide in terms of adverse event (AE) frequency, severity, duration, reversibility and potential effect on drug discontinuation, as well as within patient subgroups defined by aetiology, bowel anatomy and baseline PS volume requirements, in the largest data set available to date of patients with SBS–IF from combined, prospective clinical trials.
Methods
Patients and included studies
Pooled safety data were analysed from four clinical studies of teduglutide in patients with SBS–IF conducted from May 2004 through January 2013 and described previously.18–21 CL0600-004 and STEPS were 24-week, double-blind, randomized, placebo-controlled, multicentre and multinational phase III studies in which patients received teduglutide 0.05 mg/kg/day (approved dose) or 0.10 mg/kg/day (CL0600-004 only) or placebo. The phase III randomized controlled trials (RCTs) for teduglutide enrolled adult patients with SBS who required PS at least three times weekly for at least 12 continuous months. Patients with recent or active cancer were excluded from participation. Upon completion of the primary study, patients could elect to enrol in the related extension study. CL0600-005 was a 28-week, double-blind extension of CL0600-004. Patients who received teduglutide in the primary study continued at their original dose (0.05 or 0.10 mg/kg/day); patients who received placebo in the primary study were randomized to receive teduglutide 0.05 or 0.10 mg/kg/day in the extension. STEPS-2 was a 24-month, open-label extension for patients who completed STEPS. In addition, 12 patients who were screened and optimized for STEPS but not randomized because of full study enrolment entered STEPS-2 directly. All patients enrolled in the STEPS-2 extension received teduglutide 0.05 mg/kg/day. The maximum treatment duration was 1 year for patients who received teduglutide in studies CL0600-004 and CL0600-005 and 30 months for patients who received teduglutide in STEPS and completed STEPS-2.
All studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guidelines, and Good Clinical Practice. All patients provided written informed consent, and study protocols were approved by local institutional review boards or medical ethics committees, as described previously.18,19
Analysis populations
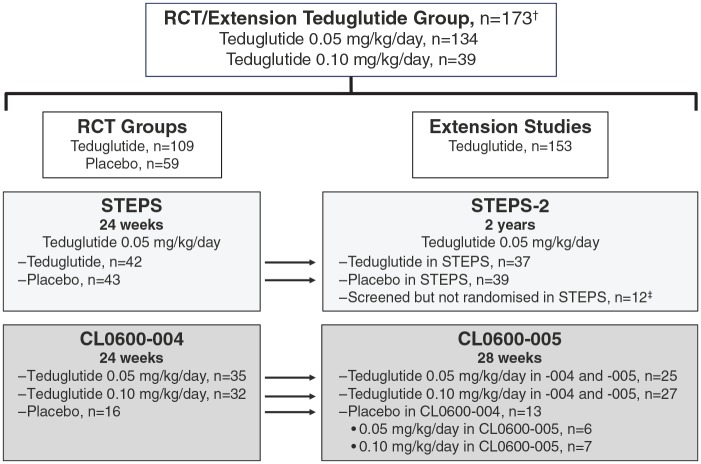
The analysis populations (Figure 1) included the RCT teduglutide group, consisting of patients who received teduglutide in the two RCTs (STEPS and CL0600-004); the RCT placebo group, consisting of patients who received placebo in the two RCTs; the RCT/extension teduglutide group, consisting of patients who received teduglutide in the two RCTs and/or the subsequent extension studies.
Figure 1.
Patient disposition in the RCTs and extension studies.
†Includes 64 patients who either received placebo in an RCT or were not treated in STEPS.
‡Entered STEPS-2 directly because of full enrolment in STEPS.
RCT, randomized controlled trial.
The analysis population was stratified three times to create three distinct populations based on aetiology, bowel anatomy, and baseline PS requirements, by analogy to a previous post hoc efficacy analysis.24 The aetiology subgroups consisted of patients with IBD as the underlying cause of SBS–IF, patients with vascular disease (Vasc) as the underlying cause of SBS–IF, and patients with other underlying causes of SBS–IF. The bowel anatomy subgroups were subgroup 1: patients with no colon remaining, stoma present and no colon-in-continuity; subgroup 2: patients with at least 50% colon remaining, no stoma present and colon-in-continuity; and subgroup 3: patients with less than 50% colon remaining or with colostomy. The baseline PS volume subgroups were patients who received ⩽9 l per week PS, >9 to 18 l per week PS and >18 l per week PS at baseline, respectively.
Safety outcomes
Pooled safety data are reported for up to 2.5 years of exposure to teduglutide. AEs were coded using system, organ, class terms, and preferred terms from the Medical Dictionary for Regulatory Activities version 12.0. In some cases, preferred terms representing medically similar terms were combined into groupings of AEs, as indicated in the text and tables and figures. Preferred terms that comprise each AE grouping are shown in Supplementary Table 1. Safety outcomes were summarized and included change in weight and body mass index (BMI), TEAEs, TEAEs leading to discontinuation, severity of TEAEs, TEAEs by duration of treatment with teduglutide, duration of TEAEs considered to be related to treatment by the investigator, treatment-emergent serious AEs (TESAEs), TESAEs by duration of exposure to teduglutide and TEAEs in patient subgroups defined by aetiology, bowel anatomy and baseline PS volume. AEs were reported as mild, moderate or severe. Mild AEs were usually transient, requiring no special treatment and generally did not interfere with daily activities. Moderate AEs impaired usual activities and required simple therapeutic action. Severe AEs resulted in an interruption of usual activities and required vigorous therapeutic intervention. Rates of central line-associated blood stream infections (CLABSI) were estimated by calculating the number of catheter-related sepsis and catheter-related bacteraemia events (preferred terms) per 1000 catheter-days during the study period. For determination of CLABSI rates, it was assumed that patients had central lines throughout the study period and that other catheter-related TEAE categories were not blood stream infections.
Statistical analysis
Data were summarized using descriptive statistics and are reported as mean (SD) unless indicated otherwise. TEAEs were reported by frequency without correction for duration of exposure to study drug. Patients in the RCT teduglutide and RCT placebo groups were treated with teduglutide or placebo for equivalent periods of time, facilitating direct comparisons of safety outcomes; the RCT/extension teduglutide group was included in the analysis to permit assessment of the cumulative spectrum and frequency of TEAEs over a longer exposure period. In patient groups stratified by anatomic presentation of the SBS, aetiology and baseline PS volume requirements, the common relative risk between patients receiving teduglutide and patients receiving placebo after adjustment for each stratification factor was derived using the Cochran–Mantel–Haenszel test.
Results
Patients and study drug exposure
Patient participation in the included studies is shown in Figure 1. The RCT teduglutide group included 109 patients who received teduglutide in the two initial RCTs (for up to 24 weeks); 77 patients received teduglutide 0.05 mg/kg/day and 32 received teduglutide 0.10 mg/kg/day. Mean duration of exposure to teduglutide was 22.2 (6.62) weeks. The RCT placebo group consisted of 59 patients who received placebo in the two initial RCTs; mean duration of exposure to placebo was 23.1 (4.46) weeks. The RCT/extension teduglutide group included 173 patients who received teduglutide in the two RCTs and/or their respective extension studies (for up to 30 months). In the RCT/extension group, 134 patients were treated with 0.05 mg/kg/day and 39 patients were treated with 0.10 mg/kg/day. Mean duration of exposure to teduglutide was 66.9 (42.11) weeks (range, 0.6–143.3 weeks). Of the 173 patients in the RCT/extension teduglutide group, 107 (61.8%) were treated for at least 48 weeks. Patient demographics and baseline clinical characteristics were generally balanced among the three pooled analysis populations (Table 1).
Table 1.
Patient demographics and baseline SBS characteristics.
| Parameter | RCT teduglutide group, n = 109 | RCT/extension teduglutide group, n = 173 | RCT placebo group, n = 59 |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 59 (54.1) | 95 (54.9) | 33 (55.9) |
| Age, mean (SD), years | 49.7 (13.5) | 49.8 (14.0) | 49.6 (15.4) |
| Reason for major intestinal resection, n (%) | |||
| Crohn’s disease | 33 (30.3) | 47 (27.2) | 15 (25.4) |
| Vascular disease | 35 (32.1) | 55 (31.8) | 19 (32.2) |
| Injury | 9 (8.3) | 13 (7.5) | 5 (8.5) |
| Volvulus | 9 (8.3) | 21 (12.1) | 8 (13.6) |
| Cancer | 1 (0.9) | 3 (1.7) | 2 (3.4) |
| Other | 19 (17.4) | 34 (19.7) | 10 (16.9) |
| Stoma present, n (%) | 45 (41.3) | 68 (39.3) | 22 (37.3) |
| Stoma type, n (%)† | |||
| Jejunostomy | 21 (46.7) | 31 (45.6) | 9 (40.9) |
| Ileostomy | 15 (33.3) | 23 (33.8) | 10 (45.5) |
| Colostomy | 9 (20.0) | 12 (17.6) | 1 (4.5) |
| Other | 0 | 2 (2.9) | 2 (9.1) |
| Colon-in-continuity, n (%) | 70 (64.2) | 109 (63.0) | 34 (57.6) |
| % Colon remaining, mean (SD) | 64.9 (23.1)‡ | 65.9 (24.9)§ | 69.3 (25.3)¶ |
| Patients with >50% colon-in-continuity, n (%) | 56 (51.4) | 89 (81.7) | 30 (50.8) |
| Estimated length of small intestine remaining, mean (SD), cm | 72.2 (53.8)|| | 68.0 (50.7)# | 71.0 (60.8)∆ |
| <60 cm, n (%) | 44 (45.4) | 80 (51.6) | 31 (56.4) |
| ⩾60 cm, n (%) | 53 (54.6) | 75 (48.4) | 24 (43.6) |
| Distal/terminal ileum present, n (%) | 24 (22.4) | 43 (25.1) | 17 (28.8) |
| Ileocecal valve present, n (%) | 11 (45.8) | 22 (51.2) | 11 (64.7) |
RCT, randomized controlled trial; SBS, short bowel syndrome.
Percentages calculated based on number of patients with a stoma, ‡n = 69, §n = 113, ¶n = 36, ||n = 97, #n = 155, ∆n = 55.
Mean BMI in the three study groups remained stable through the end of treatment. At baseline, mean BMI was 21.9 (2.96) kg/m2 in the RCT teduglutide group, 22.0 (3.10) kg/m2 in the RCT/extension teduglutide group and 22.2 (3.12) kg/m2 in the RCT placebo group. At the end of treatment, mean change from baseline in BMI was +0.4 (1.03) kg/m2 in the RCT teduglutide group (n = 106), +0.02 (1.52) kg/m2 in the RCT/extension teduglutide group (n = 168) and −0.1 (1.01) kg/m2 in the RCT placebo group.
Safety
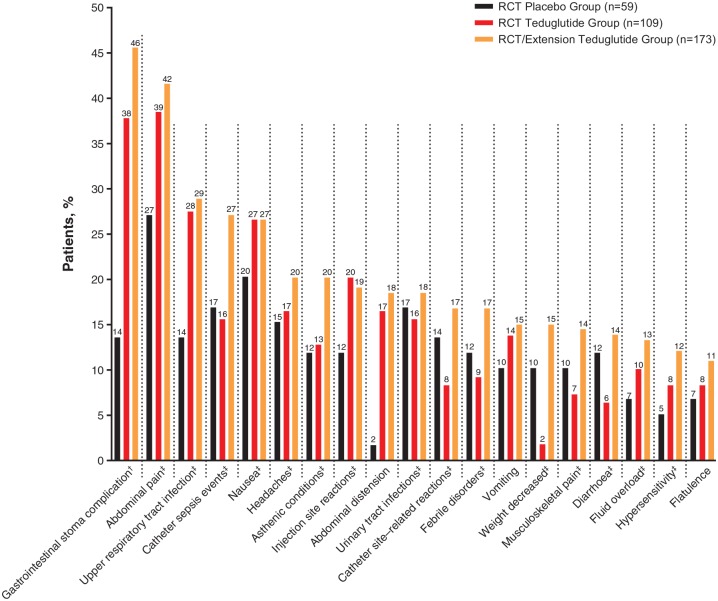
TEAEs were reported in most patients in all three study groups; most TEAEs were mild or moderate in severity (Table 2 and Supplementary Table 2). TEAEs led to study discontinuation for 10 of 109 patients (9.2%) in the RCT teduglutide group and 34 of 173 patients (19.7%) in the RCT/extension teduglutide group (Table 2). The only TEAEs that led to discontinuation in more than 2.0% of patients were abdominal pain (n = 8; 4.6%) and gastrointestinal stoma complication (n = 3 of 68 patients with stoma; 4.4%; Table 3). The most commonly reported TEAEs (by AE grouping or preferred term) in the 173 patients in the RCT/extension teduglutide group were gastrointestinal stoma complication (n = 31 of 68 patients with stoma; 45.6%), abdominal pain (n = 72; 41.6%), upper respiratory tract infection (n = 50; 28.9%) and nausea (n = 46; 26.6%; Table 4 and Figure 2). TEAEs that tended to be reported more frequently in the RCT teduglutide group versus the RCT placebo group were abdominal pain (38.5% versus 27.1%), gastrointestinal stoma complications (37.8% versus 13.6% in patients with stoma [n = 45 and n = 22, respectively]), upper respiratory tract infection (27.5% versus 13.6%) and abdominal distension (16.5% versus 1.7%). Rates of CLABSI (preferred terms of catheter-related sepsis and catheter-related bacteraemia) per 1000 catheter-days were 1.18 for the RCT teduglutide group, 0.32 for the RCT placebo group and 0.68 for the RCT/extension teduglutide group. In post hoc exploratory analyses, there was no correlation between the presence of stoma and occurrence of catheter sepsis events (AE grouping), or between TEAEs of stoma complication (preferred term) and catheter sepsis events in any study group, with or without adjustment for aetiology, bowel anatomic presentation or baseline PS volume stratification factors (data not shown).
Table 2.
Overall summary of TEAEs and TESAEs according to severity and discontinuation of treatment.
| Parameter | RCT teduglutide group, n = 109 |
RCT/extension teduglutide group, n = 173 |
RCT placebo group, n = 59 |
|||
|---|---|---|---|---|---|---|
| n (%) | Number of events | n (%) | Number of events | n (%) | Number of events | |
| Any TEAE | 99 (90.8) | 778 | 167 (96.5) | 2235 | 49 (83.1) | 372 |
| TEAE severity | ||||||
| Mild | 84 (77.1) | 441 | 151 (87.3) | 1179 | 45 (76.3) | 184 |
| Moderate | 74 (67.9) | 268 | 140 (80.0) | 849 | 34 (57.6) | 145 |
| Severe | 31 (28.4) | 69 | 83 (48.0) | 207 | 16 (27.1) | 43 |
| Any TESAE | 39 (35.8) | 80 | 101 (58.4) | 259 | 17 (28.8) | 34 |
| TESAE severity | ||||||
| Mild | 13 (11.9) | 17 | 29 (16.8) | 47 | 5 (8.5) | 6 |
| Moderate | 18 (16.5) | 28 | 59 (34.1) | 114 | 7 (11.9) | 9 |
| Severe | 16 (14.7) | 35 | 56 (32.4) | 98 | 8 (13.6) | 19 |
| TEAE leading to discontinuation | 10 (9.2) | 17 | 34 (19.7) | 52 | 4 (6.8) | 5 |
| AEs leading to death | 0 | 0 | 3 (1.7) | 3 | 0 | 0 |
AE, adverse event; RCT, randomized controlled trial; TEAE, treatment-emergent AE; TESAE, treatment-emergent serious AE.
Table 3.
TEAEs leading to discontinuation in more than one patient.
| AE preferred term, n (%) | RCT teduglutide group, n = 109 | RCT/extension teduglutide group, n = 173 | RCT placebo group, n = 59 |
|---|---|---|---|
| Any TEAE leading to discontinuation | 10 (9.2) | 34 (19.7) | 4 (6.8) |
| Abdominal pain | 1 (0.9) | 8 (4.6) | 0 |
| Gastrointestinal stoma complication† | 0 | 3 (4.4) | 0 |
| Nausea | 1 (0.9) | 3 (1.7) | 0 |
| Vomiting | 1 (0.9) | 3 (1.7) | 0 |
| Abdominal distension | 2 (1.8) | 2 (1.2) | 0 |
| Asthenia | 1 (0.9) | 2 (1.2) | 0 |
| Constipation | 2 (1.8) | 2 (1.2) | 0 |
RCT, randomized controlled trial; TEAE, treatment-emergent adverse event.
Percentages calculated based on number of patients with a stoma (n = 68 for the RCT/extension teduglutide group).
Table 4.
Frequency of TEAEs reported in at least 5.0% of patients in the RCT/extension teduglutide group.
| AE grouping† or AE preferred term, n (%) | RCT teduglutide group, n = 109 | RCT/extension teduglutide group, n = 173 | RCT placebo group, n = 59 |
|---|---|---|---|
| Gastrointestinal stoma complications‡ | 17 (37.8) | 31 (45.6) | 3 (13.6) |
| Abdominal pain† | 42 (38.5) | 72 (41.6) | 16 (27.1) |
| Upper respiratory tract infection† | 30 (27.5) | 50 (28.9) | 8 (13.6) |
| Catheter sepsis events† | 17 (15.6) | 47 (27.2) | 10 (16.9) |
| Nausea† | 29 (26.6) | 46 (26.6) | 12 (20.3) |
| Headaches† | 18 (16.5) | 35 (20.2) | 9 (15.3) |
| Asthenic conditions† | 14 (12.8) | 35 (20.2) | 7 (11.9) |
| Injection site reactions† | 22 (20.2) | 33 (19.1) | 7 (11.9) |
| Abdominal distension | 18 (16.5) | 32 (18.5) | 1 (1.7) |
| Urinary tract infections† | 17 (15.6) | 32 (18.5) | 10 (16.9) |
| Catheter site–related reactions† | 9 (8.3) | 29 (16.8) | 8 (13.6) |
| Febrile disorders† | 10 (9.2) | 29 (16.8) | 7 (11.9) |
| Vomiting | 15 (13.8) | 26 (15.0) | 6 (10.2) |
| Weight decreased† | 2 (1.8) | 26 (15.0) | 6 (10.2) |
| Musculoskeletal pain† | 8 (7.3) | 25 (14.5) | 6 (10.2) |
| Diarrhoea† | 7 (6.4) | 24 (13.9) | 7 (11.9) |
| Fluid overload† | 11 (10.1) | 23 (13.3) | 4 (6.8) |
| Hypersensitivity† | 9 (8.3) | 21 (12.1) | 3 (5.1) |
| Flatulence | 9 (8.3) | 19 (11.0) | 4 (6.8) |
| Cognition and attention disorders and disturbances† | 5 (4.6) | 17 (9.8) | 4 (6.8) |
| Dehydration | 4 (3.7) | 17 (9.8) | 5 (8.5) |
| Arthralgia | 7 (6.4) | 15 (8.7) | 3 (5.1) |
| Muscle spasms | 4 (3.7) | 15 (8.7) | 4 (6.8) |
| Appetite disorders† | 8 (7.3) | 14 (8.1) | 2 (3.4) |
| Biliary tract disorders† | 4 (3.7) | 14 (8.1) | 1 (1.7) |
| Lower respiratory tract infection† | 6 (5.5) | 13 (7.5) | 3 (5.1) |
| Skin haemorrhage† | 5 (4.6) | 13 (7.5) | 1 (1.7) |
| Gastrointestinal stenosis and obstruction† | 6 (5.5) | 12 (6.9) | 0 |
| Sleep disturbances† | 6 (5.5) | 10 (5.8) | 0 |
| Depressive disorders† | 2 (1.8) | 10 (5.8) | 1 (1.7) |
| Coughing and associated symptoms† | 5 (4.6) | 9 (5.2) | 0 |
| Hepatic enzyme increased† | 4 (3.7) | 9 (5.2) | 2 (3.4) |
| Pancreatic disorders NEC† | 3 (2.8) | 9 (5.2) | 1 (1.7) |
| Contusion | 2 (1.8) | 9 (5.2) | 0 |
| Peripheral embolism and thrombosis† | 1 (0.9) | 9 (5.2) | 2 (3.4) |
| Hot flush | 1 (0.9) | 9 (5.2) | 0 |
| Blood bicarbonate decreased | 0 | 9 (5.2) | 0 |
AE, adverse event; NEC, not elsewhere classified; RCT, randomized controlled trial; TEAE, treatment-emergent AE.
The preferred terms in the AE groupings represent medically similar terms.
Percentages calculated based on number of patients with a stoma (n = 45 for the RCT teduglutide group; n = 68 for the RCT/extension teduglutide group; n = 22 for the RCT placebo group).
Figure 2.
Frequency of TEAEs reported in at least 10.0% of patients in the RCT/extension teduglutide group.
†Percentages calculated based on number of patients with a stoma (n = 45 for the RCT teduglutide group; n = 68 for the RCT/extension teduglutide group; n = 22 for the RCT placebo group).
‡AE grouping; the preferred terms in the AE groupings represent medically similar terms.
AE, adverse event; RCT, randomized controlled trial; TEAE, treatment-emergent AE.
In the RCT/extension teduglutide group, colonic polyps were reported for three patients (1.7%), rectal polyps were reported for two patients (1.2%), and intestinal polyp and duodenal polyp were reported for one patient (0.6%) each (Supplementary Table 2). All AEs of polyps were mild in severity. The duodenal polyp was detected in a 64-year-old man during an upper endoscopy performed as part of a workup for non-small cell lung cancer. In addition, a patient in the RCT placebo group reported a severe TEAE of intestinal polyp that resulted in study discontinuation.
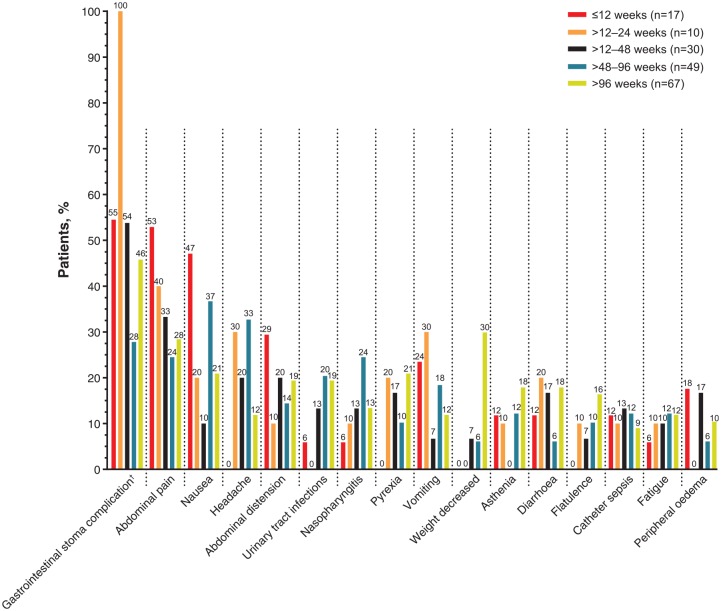
TEAEs by treatment duration in the RCT/extension teduglutide group are shown in Table 5 and Figure 3. Most gastrointestinal TEAEs tended to be reported with greater frequency in patients in the first 12 or 24 weeks after treatment initiation. In contrast, TEAEs of urinary tract infection, decreased weight, muscle spasms, catheter-related infection, central line infection and catheter site infection tended to be reported more frequently in patients who had been treated for more than 48 weeks. The duration of TEAEs (by AE grouping or preferred term) that were considered by the investigator to be related to teduglutide and were reported at least three times in the RCT/extension teduglutide group is shown in Supplementary Table 3. Among these, the related AEs with the longest median durations were flatulence (63 days; n = 7 events), appetite disorders (23 days; n = 5 events), and asthenic conditions (22 days, n = 5 events). Among the related TEAEs that were reported at least 10 times in patients in the RCT/extension teduglutide group, only fluid overload and nausea had a median duration of at least 10 days [20 days (n = 12 events) and 15 days (n = 24 events), respectively].
Table 5.
Frequency of TEAEs by teduglutide treatment duration.
| Preferred term, n (%) | Duration of teduglutide treatment in the RCT/extension teduglutide group, n = 173 |
||||
|---|---|---|---|---|---|
| ⩽12 weeks (n = 17) | >12–24 weeks (n = 10) | >24–48 weeks (n = 30) | >48–96 weeks (n = 49) | >96 weeks (n = 67) | |
| Gastrointestinal disorders | |||||
| Abdominal pain | 9 (52.9) | 4 (40.0) | 10 (33.3) | 12 (24.5) | 19 (28.4) |
| Nausea | 8 (47.1) | 2 (20.0) | 3 (10.0) | 18 (36.7) | 14 (20.9) |
| Abdominal distension | 5 (29.4) | 1 (10.0) | 6 (20.0) | 7 (14.3) | 13 (19.4) |
| Vomiting | 4 (23.5) | 3 (30.0) | 2 (6.7) | 9 (18.4) | 8 (11.9) |
| Diarrhoea | 2 (11.8) | 2 (20.0) | 5 (16.7) | 3 (6.1) | 12 (17.9) |
| Flatulence | 0 | 1 (10.0) | 2 (6.7) | 5 (10.2) | 11 (16.4) |
| Abdominal pain upper | 1 (5.9) | 1 (10.0) | 2 (6.7) | 6 (12.2) | 3 (4.5) |
| Abdominal discomfort | 0 | 3 (30.0) | 3 (10.0) | 2 (4.1) | 3 (4.5) |
| Dyspepsia | 1 (5.9) | 0 | 1 (3.3) | 5 (10.2) | 2 (3.0) |
| Other most common TEAEs† | |||||
| Gastrointestinal stoma complication‡ | 6 (54.5) | 2 (100.0) | 7 (53.8) | 5 (27.8) | 11 (45.8) |
| Headaches | 0 | 3 (30.0) | 6 (20.0) | 16 (32.7) | 8 (11.9) |
| Urinary tract infections | 1 (5.9) | 0 | 4 (13.3) | 10 (20.4) | 13 (19.4) |
| Nasopharyngitis | 1 (5.9) | 1 (10.0) | 4 (13.3) | 12 (24.5) | 9 (13.4) |
| Pyrexia | 0 | 2 (20.0) | 5 (16.7) | 5 (10.2) | 14 (20.9) |
| Weight decreased | 0 | 0 | 2 (6.7) | 3 (6.1) | 20 (29.9) |
| Asthenia | 2 (11.8) | 1 (10.0) | 0 | 6 (12.2) | 12 (17.9) |
| Catheter sepsis | 2 (11.8) | 1 (10.0) | 4 (13.3) | 6 (12.2) | 6 (9.0) |
| Fatigue | 1 (5.9) | 1 (10.0) | 3 (10.0) | 6 (12.2) | 8 (11.9) |
| Peripheral oedema | 3 (17.6) | 0 | 5 (16.7) | 3 (6.1) | 7 (10.4) |
| Dehydration | 1 (5.9) | 1 (10.0) | 1 (3.3) | 3 (6.1) | 11 (16.4) |
| Injection site haematoma | 0 | 2 (20.0) | 0 | 11 (22.4) | 2 (3.0) |
| Muscle spasms | 0 | 0 | 1 (3.3) | 6 (12.2) | 8 (11.9) |
| Arthralgia | 0 | 1 (10.0) | 3 (10.0) | 5 (10.2) | 6 (9.0) |
| Injection site erythema | 0 | 2 (20.0) | 2 (6.7) | 6 (12.2) | 3 (4.5) |
| Back pain | 0 | 0 | 3 (10.0) | 5 (10.2) | 5 (7.5) |
| Decreased appetite | 2 (11.8) | 3 (30.0) | 0 | 5 (10.2) | 2 (3.0) |
| Catheter-related infection | 0 | 0 | 2 (6.7) | 1 (2.0) | 9 (13.4) |
| Pain in extremity | 0 | 0 | 3 (10.0) | 1 (2.0) | 8 (11.9) |
| Influenza | 1 (5.9) | 1 (10.0) | 0 | 6 (12.2) | 3 (4.5) |
| Central line infection | 1 (5.9) | 0 | 0 | 0 | 9 (13.4) |
| Catheter bacteraemia | 0 | 0 | 3 (10.0) | 3 (6.1) | 4 (6.0) |
| Catheter site infection | 0 | 0 | 1 (3.3) | 2 (4.1) | 7 (10.4) |
| Hot flush | 0 | 1 (10.0) | 0 | 2 (4.1) | 6 (9.0) |
| Depression | 0 | 0 | 3 (10.0) | 1 (2.0) | 5 (7.5) |
| Contusion | 0 | 0 | 2 (6.7) | 5 (10.2) | 2 (3.0) |
| Cough | 0 | 0 | 2 (6.7) | 5 (10.2) | 2 (3.0) |
| Dizziness | 0 | 0 | 0 | 5 (10.2) | 4 ( 6.0) |
| Blood bicarbonate decreased | 1 (5.9) | 0 | 1 (3.3) | 1 (2.0) | 6 (9.0) |
AE, adverse event; RCT, randomized controlled trial; TEAE, treatment-emergent AE.
Most common TEAEs (by AE preferred term) occurring in at least 5.0% of patients overall in the RCT/extension teduglutide group.
Percentages calculated based on number of patients with a stoma (n = 11 for ⩽12 weeks; n = 2 for >12–24 weeks; n = 13 for >24–48 weeks; n = 18 for >48–96 weeks; n = 24 for >96 weeks).
Figure 3.
Frequency of TEAEs reported in at least 10.0% of patients in the RCT/extension teduglutide group by treatment duration.
†Percentages calculated based on number of patients with a stoma (n = 11 for ⩽12 weeks; n = 2 for >12–24 weeks; n = 13 for >24–48 weeks; n = 18 for >48–96 weeks; n = 24 for >96 weeks).
RCT, randomized controlled trial; TEAE, treatment-emergent adverse event.
The percentage of patients reporting TESAEs was comparable between the RCT teduglutide group and the RCT placebo group (35.8% versus 28.8%, respectively). The percentage of patients reporting TESAEs in the RCT/extension teduglutide group was higher (58.4%; Table 2); however, the person-years of exposure in this group was approximately 5 times greater owing to the longer treatment time in the extension studies (221.86 person-years versus 46.42 person-years in the RCT teduglutide group and 26.10 person-years in the RCT placebo group). The most commonly reported TESAEs (by AE grouping or preferred term) in the RCT/extension teduglutide group were catheter sepsis (n = 43; 24.9%), gastrointestinal stenosis and obstruction (n = 8; 4.6%) and biliary tract disorder (n = 8; 4.6%; Table 6). TESAEs by treatment duration are shown in Supplementary Table 4.
Table 6.
Frequency of TESAEs occurring in ⩾1.5% of patients in the RCT/extension teduglutide group.
| AE grouping† or AE preferred term, n (%) | RCT teduglutide group, n = 109 | RCT/extension teduglutide group, n = 173 | RCT placebo group, n = 59 |
|---|---|---|---|
| Catheter sepsis events† | 15 (13.8) | 43 (24.9) | 9 (15.3) |
| Gastrointestinal stenosis and obstruction† | 5 (4.6) | 8 (4.6) | 0 |
| Biliary tract disorder† | 3 (2.8) | 8 (4.6) | 0 |
| Gastrointestinal stoma complication‡ | 1 (2.2) | 3 (4.4) | 0 |
| Catheter site–related reaction† | 2 (1.8) | 7 (4.0) | 1 (1.7) |
| Febrile disorders† | 2 (1.8) | 7 (4.0) | 0 |
| Lower respiratory tract infection† | 3 (2.8) | 7 (4.0) | 1 (1.7) |
| Peripheral embolism and thrombosis† | 1 (0.9) | 6 (3.5) | 0 |
| Urinary tract infections† | 3 (2.8) | 6 (3.5) | 1 (1.7) |
| Abdominal pain† | 1 (0.9) | 3 (1.7) | 0 |
| Cognition and attention disorders and disturbances† | 2 (1.8) | 3 (1.7) | 0 |
| Cholestasis and jaundice† | 0 | 3 (1.7) | 1 (1.7) |
| Device dislocation | 2 (1.8) | 3 (1.7) | 2 (3.4) |
| Intestinal haemorrhages† | 1 (0.9) | 3 (1.7) | 0 |
| Pancreatic disorders NEC† | 1 (0.9) | 3 (1.7) | 0 |
AE, adverse event; NEC, not elsewhere classified; RCT, randomized controlled trial; TESAE, treatment-emergent serious AE.
The AE preferred terms in the AE groupings represent medically similar terms.
Percentages calculated based on number of patients with a stoma (n = 45 for the RCT teduglutide group; n = 68 for the RCT/extension teduglutide group; n = 22 for the RCT placebo group).
No cases of malignancy or death were reported in either of the placebo-controlled trials; however, as reported previously by Schwartz and colleagues21 three cases of malignancy and three deaths occurred during STEPS-2. A 48-year-old man with a history of Hodgkin disease (diagnosed 22 years earlier), cecal necrosis caused by radiation and primary liver disease was diagnosed with a metastatic adenocarcinoma 11 months after the start of teduglutide. A computed tomography (CT) examination of the patient’s abdomen was performed 6 months before starting teduglutide and had shown liver enlargement without focal lesions. The patient initially entered the STEPS study and, as per protocol, had undergone a colonoscopy of the remaining colon before randomization that showed no changes. Because STEPS was fully enrolled by the time the patient was eligible for randomization, he enrolled directly into STEPS-2. Subsequent magnetic resonance imaging and CT scans done after the onset of back pain reported 313 days after starting teduglutide revealed extensive heterogeneous solid tumour masses in the liver (not reported in the initial CT), a metastatic lesion on the third lumbar vertebra, and numerous enlarged lymph nodes in the thoracic cavity. Teduglutide was discontinued. Liver biopsy taken 8 days before death was consistent with metastatic adenocarcinoma; the primary tumour was considered likely to be in the gastrointestinal tract, without a precise origin determined. The autopsy report concluded the patient died a ‘natural death caused by the disease’ and described the cause of death as generalized malignancy of intestinal cancer. The event was considered by the investigator to be related to teduglutide.
Two additional events of cancer that were not considered by the investigator to be related to teduglutide were reported during STEPS-2. A 64-year-old man with a history of smoking (approximately 30 cigarettes per day for 30 years) and asbestos exposure was diagnosed with non-small cell lung cancer 85 days after initiating treatment with teduglutide. Teduglutide was discontinued at diagnosis, and the patient died 5 months later. A 74-year-old man with a history of smoking 10 cigarettes per day for 5 years (smoking ceased 25 years earlier) was diagnosed with lung squamous cell carcinoma approximately 17 months after starting teduglutide. The patient withdrew participation from the study, and the event was ongoing as of the last study visit.
In addition to the two events with fatal outcome of adenocarcinoma and non-small cell lung cancer described previously, a third death occurred during STEPS-2. A 70-year-old man died during a hospitalisation for catheter-related sepsis and urinary tract infection after 28 months of treatment with teduglutide. The event was not considered related to teduglutide.
Safety in patient subgroups
The distribution of TEAEs was analysed in patient subgroups defined by aetiology, bowel anatomy and baseline PS volume (Supplementary Tables 5–7). When patients in the RCT/extension teduglutide group were stratified by aetiology, teduglutide-associated gastrointestinal disorders tended to be reported more frequently in patients with IBD as the cause of SBS (n = 16/20; 80.0%) and other causes of SBS (n = 71/95; 74.7%) than in patients with vascular causes of SBS (n = 32/58; 55.2%; Supplementary Table 5). Among the most common gastrointestinal-associated TEAEs, each was reported more frequently in patients with IBD as the cause of SBS (n = 20; abdominal pain, 50.0%; nausea, 50.0%; abdominal distension, 35.0%; vomiting, 20.0%) than in patients with vascular causes (n = 58; abdominal pain, 19.0%; nausea, 13.8%; abdominal distension, 13.8%; vomiting, 6.9%) or other causes of SBS (n = 95; abdominal pain, 34.7%; nausea, 28.4%; abdominal distension, 17.9%; vomiting, 18.9%), respectively. Gastrointestinal-associated TEAEs in patients with IBD as the cause of SBS tended to occur more frequently in earlier periods after treatment initiation and declined over time. In the RCT/extension teduglutide group, gastrointestinal TEAEs reported in SBS–IBD patients treated for at most 12 weeks were abdominal pain (all patients), nausea (all patients), abdominal distension (33.3%), and vomiting (33.3%). In SBS–IBD patients treated for >96 weeks, the reported gastrointestinal TEAEs were abdominal pain (37.5%), nausea (37.5%), abdominal distension (37.5%) and vomiting (12.5%).
When patients in the RCT/extension teduglutide group were stratified by remnant bowel anatomy, patients in subgroup 1 (no remaining colon with stoma and no colon-in-continuity) tended to report more gastrointestinal TEAEs [n = 48/57 patients (84.2%)] compared with patients in subgroup 2 [⩾50% colon remaining without stoma and with colon-in-continuity; n = 52/89 (58.4%)] or subgroup 3 [<50% colon remaining or with colostomy; n = 19/27 (70.4%); Supplementary Table 6]. When patients in the RCT/extension teduglutide group were stratified by baseline PS volume requirements, dehydration, fluid retention and peripheral oedema tended to be reported more frequently in the subgroup of patients with the highest baseline PS volume needs [>18 l/week; n = 6/25 (24.0%), n = 4/25 (16.0%) and n = 5/25 (20.0%), respectively] compared with the subgroup of patients with the lowest PS volume needs [⩽9 l/week; n = 7/66 (10.6%), n = 1/66 (1.5%) and n = 9/66 (13.6%), respectively; Supplementary Table 7]. In the RCT teduglutide group, rates of TEAEs of infections and infestations indicated a possible trend for occurring more commonly in patients with baseline PS volume >18 l/week [n = 10/14 (71.4%)] compared with patients with baseline PS volume ⩽9 l/week [n = 25/42 (59.5%)]. However, this trend was not as apparent in the overall RCT/extension teduglutide group [n = 18/25 (72.0%) compared with n = 45/66 (68.2%) for patients with baseline PS volume >18 l/week and ⩽9 l/week, respectively].
After adjustment for aetiology stratification, bowel anatomy stratification and baseline PS volume stratification, the predominant TEAEs for which patients receiving teduglutide had a significantly increased relative risk (RR) compared with patients receiving placebo were abdominal distension (RR, 9.641–9.760; p = 0.003–0.004) and gastrointestinal stoma complication (RR, 3.132–3.394; p = 0.019–0.033) (Supplementary Tables 5–7). In addition, the RR for nasopharyngitis was greater for patients receiving teduglutide after adjustment for bowel anatomy, baseline PS volume, and for aetiology stratification [RR, 3.765 (p = 0.048), 3.725 (p = 0.047) and 3.632; (p = 0.05), respectively]. In contrast, patients receiving teduglutide had a significantly reduced risk of decreased weight after adjustment for aetiology and baseline PS volume stratification [RR, 0.090 (p = 0.005) and 0.088 (p = 0.006), respectively] compared with patients receiving placebo. The RR for CLABSI for patients receiving teduglutide compared with patients receiving placebo after adjustment stratification for bowel anatomy, aetiology and baseline PS volume was 2.718 (p = 0.16), 2.636 (p = 0.17) and 2.878 (p = 0.15), respectively.
Discussion
Integrated safety data for 222 person-years of exposure to teduglutide in four clinical trials demonstrated that teduglutide has a safety profile in adult patients with SBS–IF consistent with prior studies. The spectrum of commonly reported TEAEs in this analysis was similar to that in the teduglutide paediatric studies, with the exception of TEAEs common in children (e.g. cough and pyrexia), which occurred more frequently in the paediatric trials.25,26 The overall occurrence of TEAEs was comparable between the RCT teduglutide group and the RCT placebo group (90.8% and 83.1% of patients reporting TEAEs, respectively). Several of the most common TEAEs with teduglutide were gastrointestinal events that were consistent with the underlying disease state and its management. For example, use of opioids, often prescribed to patients with SBS to decrease intestinal motility, is associated with abdominal complaints independent of teduglutide treatment.27 Specific TEAEs may be associated with the mode of action of teduglutide. Gastrointestinal stoma complication, in the relevant subgroup of patients with a stoma, was the most commonly reported TEAE in the RCT/extension teduglutide group and tended to be reported more frequently in the RCT teduglutide group compared with the RCT placebo group (37.8% versus 13.6%). After adjustment for stratification factors, teduglutide was associated with a significantly increased risk of gastrointestinal stoma complication compared with placebo. Stoma enlargement was observed in clinical trials and in real-world clinical practice20,23,28 and may be driven by teduglutide-induced hypertrophy or increases in intestinal blood flow.29,30
The enhancement of intestinal absorptive capacity by teduglutide has the potential to lead to fluid overload. In this integrated safety analysis, the AE grouping of fluid overload was reported by 13.3% of patients in the RCT/extension group. Fluid overload can be a sign of insufficient weaning off PS and can be managed through adherence to a PS weaning algorithm. In STEPS and STEPS-2, reductions in PS volumes by 10% to 30% of baseline levels were permitted if the 48-hour urinary volumes exceeded baseline values by more than 10%.19,21 Conversely, improper PS management can result in signs of dehydration, including headache and decreased weight.19 European Society for Clinical Nutrition and Metabolism guidelines recommend careful monitoring of patients for symptoms of dehydration and fluid balance to prevent chronic renal failure.2
Gastrointestinal disorders, including abdominal pain, abdominal distension, vomiting and abdominal discomfort, tended to be reported more frequently in patients earlier in the course of treatment, suggesting that many of the more common gastrointestinal-related issues may diminish or resolve with prolonged treatment with teduglutide. In contrast, some TEAEs may occur more frequently in patients with longer duration of treatment. Decreased weight, pyrexia, dehydration and headache were reported in no patients with at most 12 weeks of treatment with teduglutide and 29.9%, 20.9%, 16.4% and 11.9% of patients with more than 96 weeks of treatment with teduglutide, respectively. Although individual patients may have been vulnerable to weight loss or TEAEs related to fluid imbalance during long-term treatment, mean BMI remained stable in the overall study population. A similar result was reported in a single-centre, real-world clinical study in which BMI was maintained in patients treated with teduglutide, despite PS volume reductions of at least 20% in 79% of patients. In addition, bioelectrical impedance analysis of body composition showed stability of nutritional status in the study cohort.22 Instances of fluid/electrolyte imbalance or malnutrition require careful monitoring and may necessitate a switch to personalized parenteral nutrition formulas in patients receiving commercial parenteral nutrition admixtures for which the decreases/increases of water, proteins and energy are proportional.18,19
The AE grouping of catheter sepsis events was the most commonly reported TESAE in the study. The frequency of serious catheter sepsis events was similar in the RCT teduglutide and RCT placebo groups (13.8% and 15.3%, respectively). The incidence of serious catheter sepsis events increased with long-term treatment (24.9% in patients in the RCT/extension teduglutide group treated for up to 2.5 years), highlighting the importance of best practices for catheter maintenance and patient monitoring. Rates of CLABSI (preferred terms of catheter-related sepsis and catheter-related bacteraemia) TEAEs with teduglutide were 0.68 to 1.18 events per 1000 catheter-days, which is within the ranges reported in the literature for standard of care.31 However, CLABSI rates reported here may be underestimated because the methodology assumed that no interruptions in catheter placement occurred during the study period. In a systematic review of catheter-related infections in patients receiving home parenteral nutrition, duration of treatment and daily PS infusion were identified as risk factors associated with increased catheter sepsis.31 Treatment with teduglutide is associated with decreased PS volume requirements and frequency of administration, which may help mitigate some of the risk associated with catheter sepsis.20,21
Three cases of cancer occurred during the phase III studies, as reported previously.21 Two were cases of lung cancer in patients with a medical history of smoking and were considered unrelated to teduglutide. The third was a metastatic adenocarcinoma in a patient with many comorbidities, including a history of Hodgkin disease treated with radiation. Hodgkin disease treated with chemotherapy or radiation is associated with an increased risk of difficult-to-treat secondary malignancy, even many years after initial treatment.32,33 No malignancies were reported in a 1-year follow-up study to STEPS-2 conducted in 14 patients who were treated with teduglutide for up to 42 months, representing the longest reported patient exposure to teduglutide.34 An ongoing, long-term, multinational observational registry is expected to provide more information on potential risk of malignancy with teduglutide in patients with SBS in a real-world setting (ClinicalTrials.gov identifier: NCT01990040).35
When TEAEs were analysed in patient subgroups, patients in the SBS–IBD subgroup and patients with an end jejunostomy (subgroup 1) tended to report more gastrointestinal AEs than patients in the SBS–Vasc group and patients with most of their colon-in-continuity (subgroup 2). In a recent analysis of baseline characteristics and clinical response to teduglutide in these same patient subgroups, patients in the SBS–IBD subgroup and patients with an end jejunostomy (subgroup 1) had higher baseline PS volumes and greater decreases in PS requirements with teduglutide than patients in the SBS–Vasc subgroup and patients with most of their colon-in-continuity (subgroup 2).24 In the SBS–IBD subgroup, diseased parts of the remaining intestine, concomitant medications, altered patient pain perception and higher baseline PS volumes may contribute to the higher rates of gastrointestinal events reported in this population. The increased frequency of gastrointestinal AEs in bowel anatomy subgroup 1 is likely driven by a more challenging bowel anatomy (no colon remaining and stoma) and higher baseline PS volumes. Nonetheless, because many gastrointestinal TEAEs tend to occur early in treatment and decline over time, comprehensive patient education in recognising and managing gastrointestinal TEAEs before the initiation of treatment with teduglutide and careful monitoring throughout treatment are essential for optimal outcomes.
Inflammatory bowel disease is a disorder involving aberrant immune responses,36 and patients with IBD as the cause of SBS who have residual activity or postinflammatory IBD may be predisposed to infectious complications that may be unrelated to exposure to teduglutide. Indeed, in the RCT placebo group, the SBS–IBD subgroup had the highest incidence of infections and infestations (75.0%, compared with 45.0% in the SBS–Vasc subgroup and 51.6% in the other subgroup). Educating patients on the importance of reporting infections is warranted, and investigations are required to better clarify the causes of infections in all patients treated with teduglutide.
The subgroup of patients with the greatest baseline PS volume requirements (>18 l/week) reported more TEAEs of dehydration, fluid retention and peripheral oedema than the subgroups with lower baseline PS volumes. Higher PS volume requirements necessitate longer infusion times or frequency and may predispose patients to fluid imbalances. Furthermore, patients with higher baseline PS volumes reported greater reductions in PS requirements with teduglutide;24 insufficient PS reductions after treatment with teduglutide may result in fluid retention or oedema.
The inherent challenges presented by the heterogeneity of patients with SBS–IF, including varying aetiology, anatomy and PS requirements require subgroup-specific approaches toward anticipating, monitoring and managing AEs, as necessary. Patients with SBS–IBD and patients with high PS volume requirements may be at higher risk for infections irrespective of treatment. These patients may have hydration status ‘imbalances’ that may be related to the management of PS while on teduglutide and require close monitoring. Patients with SBS–IBD should be educated regarding gastrointestinal-associated AEs that occur most often in the early period after initiation of treatment. Gastrointestinal complaints should be proactively monitored and managed, including modification of concomitant medications, as necessary. Specialist institutions that coordinate multidisciplinary teams are recommended in order to obtain optimal outcomes in patients with SBS.5,37,38 An expert facility/multidisciplinary team approach is recommended when initiating treatment with teduglutide in order to optimally manage the patient, particularly in the first 6 months for adverse reactions that are specific to the mechanism of action and response to treatment, and beyond that for any TEAEs.
Strengths of this integrated analysis of phase III trials include the large, pooled sample of patients treated with teduglutide for up to 2.5 years in a clinical trial programme with quality data for a rare disease state. A major study limitation was the relatively high patient discontinuation rate due to TEAEs. Patient withdrawal subsequent to TEAEs may have confounded outcomes, particularly with respect to the analysis of TEAEs by treatment duration. Conversely, however, this study was also limited by a potential for reporting bias, particularly for mild events, because patients receiving teduglutide may be more closely monitored during the open-label extension trials. In addition, detailed histology that would enable grading of polyps and provide further information regarding malignancy is not available.
Conclusion
No new safety concerns were identified in the pooled analysis of the four clinical trials. The most frequently reported TEAEs were gastrointestinal in origin, consistent with the underlying disease condition and intestinotrophic action of teduglutide.
Supplemental Material
Supplemental material, TAG-19-09-246_Pape_et_al_revised_supplementary_file_final for Teduglutide for the treatment of adults with intestinal failure associated with short bowel syndrome: pooled safety data from four clinical trials by Ulrich-Frank Pape, Kishore R. Iyer, Palle B. Jeppesen, Marek Kunecki, Loris Pironi, Stéphane M. Schneider, Douglas L. Seidner, Hak-Myung Lee and John Caminis in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors are grateful to all participating patients and their families and the clinical investigators and staff at all participating centres for their contributions to the entire adult teduglutide clinical trial programme. We thank Ihor Turkevych and Tom Organ (Shire, a member of the Takeda group of companies) for providing safety information related to this publication. Editorial support, funded by Shire International GmbH, Zug, Switzerland, a member of the Takeda group of companies, was provided by Heather Heerssen of Complete Healthcare Communications, LLC, a CHC Group company (North Wales, PA, USA).
Footnotes
Authors’ note: The present address of John Caminis is Sanofi Genzyme, Bridgewater, NJ, USA.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Shire International GmbH, Zug, Switzerland, a member of the Takeda group of companies.
Conflict of interest statement: The authors declare the following conflicts of interest: Shire is a member of the Takeda group of companies. U-FP and SMS have served as advisory board members for Shire and have received honoraria and research funding from Shire. KRI has served as a consultant for Shire and as a scientific/medical advisory board member for Zealand Pharma. PBJ has served as a speaker’s bureau member and consultant for Shire and Zealand Pharma. DLS has served as a consultant for Shire and Zealand Pharma. MK received honoraria as a speaker from Shire. LP has served as a consultant for Baxter, B. Braun, Fresenius-Kabi and Shire. H-ML is an employee of Shire, and JC is a former employee of Shire, a member of the Takeda group of companies.
ORCID iD: Ulrich-Frank Pape  https://orcid.org/0000-0002-5238-8348
https://orcid.org/0000-0002-5238-8348
Data Sharing Statement: The datasets, including redacted study protocol, redacted statistical analysis plan, and individual participant data behind the results reported in this article, will be available 3 months after the submission of a request to researchers who provide a methodologically sound proposal after de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Data requests should follow the process outlined in the Data Sharing section on Shire’s website (www.shiretrials.com) and should be directed to clinicaltrialdata@shire.com. For approved requests, the researchers will be provided access to de-identified/anonymized data on a password-protected website upon Shire’s receipt of a signed Data Sharing Agreement.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ulrich-Frank Pape, Department of Internal Medicine and Gastroenterology, Asklepios Klinik St. Georg, Lohmühlenstr. 5, Hamburg, 20099 Germany; Department of Hepatology & Gastroenterology, Charité University Medicine, Berlin, Germany.
Kishore R. Iyer, Department of Surgery, Mount Sinai Medical Centre, New York, NY, USA
Palle B. Jeppesen, Department of Gastroenterology and Hepatology, Rigshospitalet, Copenhagen, Denmark
Marek Kunecki, Department of Nutrition and Department of General and Vascular Surgery, M. Pirogow Hospital, Lódz, Poland.
Loris Pironi, Department of Digestive System, St. Orsola Hospital, University of Bologna, Bologna, Italy.
Stéphane M. Schneider, Gastroentérologie et Nutrition Clinique, Université Côte D’Azur, Nice, France
Douglas L. Seidner, Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition, Vanderbilt University Medical Centre Nashville, TN, USA
Hak-Myung Lee, Biostatistics & Statistical Programming, Shire Human Genetic Therapies, Inc., Lexington, MA, USA, a member of the Takeda group of companies.
John Caminis, Global Drug Safety, Shire, Cambridge, MA, USA, a member of the Takeda group of companies.
References
- 1. Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr 2014; 38(Suppl. 1): 8S–13S. [DOI] [PubMed] [Google Scholar]
- 2. Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr 2016; 35: 247–307. [DOI] [PubMed] [Google Scholar]
- 3. Buchman AL. The medical and surgical management of short bowel syndrome. MedGenMed 2004; 6: 12. [PMC free article] [PubMed] [Google Scholar]
- 4. Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003; 124: 1111–1134. [DOI] [PubMed] [Google Scholar]
- 5. Pironi L, Corcos O, Forbes A, et al. Intestinal failure in adults: recommendations from the ESPEN expert groups. Clin Nutr 2018; 37(6 Pt A): 1798–1809. [DOI] [PubMed] [Google Scholar]
- 6. Pironi L, Konrad D, Brandt C, et al. Clinical classification of adult patients with chronic intestinal failure due to benign disease: an international multicenter cross-sectional survey. Clin Nutr 2018; 37: 728–738. [DOI] [PubMed] [Google Scholar]
- 7. Carlsson E, Bosaeus I, Nordgren S. Quality of life and concerns in patients with short bowel syndrome. Clin Nutr 2003; 22: 445–452. [DOI] [PubMed] [Google Scholar]
- 8. DeLegge M, Alsolaiman MM, Barbour E, et al. Short bowel syndrome: parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci 2007; 52: 876–892. [DOI] [PubMed] [Google Scholar]
- 9. Jeppesen PB, Langholz E, Mortensen PB. Quality of life in patients receiving home parenteral nutrition. Gut 1999; 44: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J Parenter Enteral Nutr 2014; 38(Suppl. 1): 32S–37S. [DOI] [PubMed] [Google Scholar]
- 11. Bluthner E, Bednarsch J, Stockmann M, et al. Determinants of quality of life in patients with intestinal failure receiving long-term parenteral nutrition using the SF-36 questionnaire: a German single-center prospective observational study. JPEN J Parenter Enteral Nutr. Epub ahead of print 13 March 2019. DOI: 10.1002/jpen.1531. [DOI] [PubMed] [Google Scholar]
- 12. Jeppesen PB. The long road to the development of effective therapies for the short gut syndrome: a personal perspective. Dig Dis Sci 2019; 64: 2717–2735. [DOI] [PubMed] [Google Scholar]
- 13. Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr 2014; 38(Suppl. 1): 23S–31S. [DOI] [PubMed] [Google Scholar]
- 14. Drucker DJ. Gut adaptation and the glucagon-like peptides. Gut 2002; 50: 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janssen P, Rotondo A, Mule F, et al. Review article: a comparison of glucagon-like peptides 1 and 2. Aliment Pharmacol Ther 2013; 37: 18–36. [DOI] [PubMed] [Google Scholar]
- 16. Shire Pharmaceuticals Ireland Limited. Revestive® (package insert). Dublin, Ireland: Shire Pharmaceuticals Ireland Limited, 2019. [Google Scholar]
- 17. Shire-NPS Pharmaceuticals, Inc. GATTEX® (package insert). Lexington, MA: Shire-NPS Pharmaceuticals, Inc, 2019. [Google Scholar]
- 18. Jeppesen PB, Gilroy R, Pertkiewicz M, et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011; 60: 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012; 143: 1473–1481.e1473. [DOI] [PubMed] [Google Scholar]
- 20. O’Keefe SJ, Jeppesen PB, Gilroy R, et al. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol 2013; 11: 815–823. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz LK, O’Keefe SJ, Fujioka K, et al. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol 2016; 7: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pevny S, Maasberg S, Rieger A, et al. Experience with teduglutide treatment for short bowel syndrome in clinical practice. Clin Nutr 2019; 38: 1745–1755. [DOI] [PubMed] [Google Scholar]
- 23. Lam K, Schwartz L, Batisti J, et al. Single-center experience with the use of teduglutide in adult patients with short bowel syndrome. JPEN J Parenter Enteral Nutr 2018; 42: 225–230. [DOI] [PubMed] [Google Scholar]
- 24. Jeppesen PB, Gabe SM, Seidner DL, et al. Factors associated with response to teduglutide in patients with short-bowel syndrome and intestinal failure. Gastroenterology 2018; 154: 874–885. [DOI] [PubMed] [Google Scholar]
- 25. Carter BA, Cohran VC, Cole CR, et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr 2017; 181: 102–111.e105. [DOI] [PubMed] [Google Scholar]
- 26. Kocoshis SA, Merritt RJ, Hill S, et al. Safety and efficacy of teduglutide in pediatric patients with intestinal failure due to short bowel syndrome: a 24-week, phase III study. JPEN J Parenter Enteral Nutr. Epub ahead of print 8 September 2019. DOI: 10.1002/jpen.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujioka K, Jeejeebhoy K, Pape UF, et al. Patients with short bowel on narcotics during 2 randomized trials have abdominal complaints independent of teduglutide. JPEN J Parenter Enteral Nutr 2017; 41: 1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005; 54: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bremholm L, Hornum M, Andersen UB, et al. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept 2011; 168(1–3): 32–38. [DOI] [PubMed] [Google Scholar]
- 30. Bremholm L, Hornum M, Henriksen BM, et al. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol 2009; 44: 314–319. [DOI] [PubMed] [Google Scholar]
- 31. Dreesen M, Foulon V, Spriet I, et al. Epidemiology of catheter-related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr 2013; 32: 16–26. [DOI] [PubMed] [Google Scholar]
- 32. Bhuller KS, Zhang Y, Li D, et al. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol 2016; 172: 757–768. [DOI] [PubMed] [Google Scholar]
- 33. Eichenauer DA, Becker I, Monsef I, et al. Secondary malignant neoplasms, progression-free survival and overall survival in patients treated for Hodgkin lymphoma: a systematic review and meta-analysis of randomized clinical trials. Haematologica 2017; 102: 1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seidner DL, Fujioka K, Boullata JI, et al. Reduction of parenteral nutrition and hydration support and safety with long-term teduglutide treatment in patients with short bowel syndrome-associated intestinal failure: STEPS-3 study. Nutr Clin Pract 2018; 33: 520–527. [DOI] [PubMed] [Google Scholar]
- 35. ClinicalTrials.gov. A prospective, multi-center registry for patients with short bowel syndrome (NCT01990040), https://clinicaltrials.gov/ct2/show/NCT01990040. (2016, accessed 11 February, 2020).
- 36. Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol 2006; 12: 4807–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown CR, DiBaise JK. Intestinal rehabilitation: a management program for short-bowel syndrome. Prog Transplant 2004; 14: 290–296; quiz 297–298. [DOI] [PubMed] [Google Scholar]
- 38. Matarese LE, Jeppesen PB, O’Keefe SJ. Short bowel syndrome in adults: the need for an interdisciplinary approach and coordinated care. JPEN J Parenter Enteral Nutr 2014; 38(Suppl. 1): 60S–64S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAG-19-09-246_Pape_et_al_revised_supplementary_file_final for Teduglutide for the treatment of adults with intestinal failure associated with short bowel syndrome: pooled safety data from four clinical trials by Ulrich-Frank Pape, Kishore R. Iyer, Palle B. Jeppesen, Marek Kunecki, Loris Pironi, Stéphane M. Schneider, Douglas L. Seidner, Hak-Myung Lee and John Caminis in Therapeutic Advances in Gastroenterology