Abstract
Clinically, active surveillance involves continuous monitoring of patients who may be at risk for disease. Patients with low-grade and early-stage prostate cancer may benefit from active surveillance, rather than undergoing surgical and medical treatments that are associated with side effects. In these cases, the role of active surveillance is to ensure that there is no progression of the disease. However, active surveillance may be associated with a risk of under-diagnosis. Previously, the assignment of risk categories and patient monitoring were based on digital rectal examination, transrectal prostate biopsy, and monitoring of serum levels of prostate-specific antigen (PSA). Multiparametric magnetic resonance imaging (MRI) of the prostate gland has an estimated negative predictive value of 95% for the detection of prostate cancer, which makes this an effective imaging method for targeting biopsies and for monitoring patients over time. Also, multiparametric MRI-guided biopsy at the initial stage of the risk stratification for patients who are newly diagnosed with prostate cancer may reduce the number of underdiagnosed patients, improve long-term patient prognosis, and reduce the number of patients who are overtreated, which may reduce healthcare costs and reduce treatment morbidity. For these reasons, multiparametric MRI has become an accepted monitoring tool in patients who are enrolled in active surveillance programs. This review aims to present the current status of the use of multiparametric MRI in active surveillance of prostate cancer and to discuss future perspectives, supported by recent literature.
MeSH Keywords: Magnetic Resonance Imaging, Prostate, Prostatic Neoplasms
Background
Active surveillance is a strategy of management of a patient with low-risk prostate cancer based on monitoring of the disease and results in treatment only if disease progression is confirmed [1]. Alternative management following a diagnosis of prostate cancer includes radiotherapy (external or brachytherapy), and ablative therapies of the tumor foci, including cryotherapy, high-intensity focused ultrasound (HIFU), irreversible electroporation (IRE), and surgical removal of the whole prostate and seminal vesicles (radical prostatectomy). There are several protocols used to determine whether the patient is a good candidate for active surveillance (Tables 1, 2), none of which currently incorporate multiparametric magnetic resonance imaging (MRI) of the prostate gland.
Table 1.
Comparison of active surveillance and watchful waiting for prostate cancer.
| Active surveillance | Watchful waiting | |
|---|---|---|
| Treatment | Curative | Palliative |
| Markers | Digital rectal examination (DRE), prostate-specific antigen (PSA), prostate biopsy | Not defined |
| Follow-up | Schedule-based | Patient-dependant |
| Life expectancy | >10 years | <10 years |
| Tumor stage | Only low-risk patients | Patients at all stages |
| Aim | To reduce the side-effects of treatment witout compromising the survival rate | To reduce the side-effects of treatment |
Table 2.
Current active surveillance protocols for prostate cancer.
| Institution | Clinical stage | Gleason score (GS) | Number of positive biopsy cores | Single core involvement (%) | PSA (ng/ml) | PSA-density (PSAD) |
|---|---|---|---|---|---|---|
| JH | T1c | ≤6 | ≤2 | <50 | – | ≤0.15 |
| MSKCC | T1c–T2a | ≤6 | ≤3 | ≤50 | <10 | – |
| UCSF | T1c–T2 | ≤6 | ≤33% (at least 6) | ≤50 | <10 | – |
| PRIAS | T1c–T2 | ≤6 | ≤2 | – | <10 | ≤0.2 |
| UM | T1c–T2 | ≤6 | ≤2 | ≤20 | <15 | – |
JH – Johns Hopkins; MSKCC – Memorial Sloan Kettering Cancer Center; UCSF – University of California San Francisco; PRIAS – Prostate Cancer Research International Active Surveillance; UM – University of Michigan.
Active surveillance may help to resolve the problem of overtreatment of low-risk prostate cancer. Also, the implementation of multiparametric MRI can reduce the number of prostate biopsies and refine the early identification of occult higher-risk disease as well as reduce the number of diagnostic biopsies in low-risk patients with lesions in the anterior prostate [2]. The selection of candidates for active surveillance and disease re-classification during active surveillance using imaging of the prostate gland would be beneficial and increase diagnostic accuracy and improve patient management. Therefore, there is a need for additional clinical studies regarding the role of MRI in active surveillance programs. This review aims to present the current status of the use of multiparametric MRI in active surveillance of prostate cancer and to discuss future perspectives, supported by recent literature.
The Epidemiology of Prostate Cancer in the Context of Active Surveillance
The widespread use of screening for prostate cancer using the measurement of serum PSA levels has resulted in the increased treatment of cases of low-risk cancer of low grade, estimated as between 25–50% of newly diagnosed cases [3]. The high prevalence of incidental and asymptomatic prostate cancer found at autopsy has resulted in concerns regarding the need for treatment for all men diagnosed with prostate cancer [4,5].
However, there are no diagnostic or prognostic biomarkers that distinguish between indolent and aggressive tumors. Therefore, the prediction of tumor aggression and patient prognosis may require mathematical modeling.
The Concept of Active Surveillance for Prostate Cancer
Active surveillance in prostate cancer is a strategy of the management of patients with low-risk prostate cancer based on monitoring the disease. Active surveillance aims to undertake treatment if disease progression is confirmed. This approach is different from watchful waiting, which results in palliative treatment if malignancy progresses (Table 1). The intentional delay of the intervention in cases of clinically indolent tumors reduces the rate of overdiagnosis and overtreatment (Table 1).
The evaluation of several factors is required to determine whether active surveillance is suitable management for the patient. The clinical factors that require evaluation include the general clinical condition, life expectancy, possible treatment side effects, disease characteristics, and the wishes of the patient. However, current clinical assessments do not routinely involve imaging of the prostate gland imaging, for the localization of 12-core prostate biopsies, which has been reported to have an accuracy of less than 75% for identifying indolent cancer [6,7].
Patient Selection and Follow-Up Protocols in Active Surveillance for Prostate Cancer
Several protocols for active surveillance of prostate cancer have been proposed, and are summarized in Table 2 [8–10]. The National Comprehensive Cancer Network (NCCN) [11] have recommended the use of active surveillance in men with very low-risk prostate cancer. Very low-risk prostate cancer is defined as stage T1c, a Gleason score of ≤6, <3/12 positive biopsy cores, ≤50% cancer in each core biopsy, a PSA <10 ng/ml, and life expectancy >20 years [11]. The inclusion criteria of the European Association of Urology (EAU) for low-risk prostate cancer are similar and include stage T1–T2, a Gleason score of ≤6, ≤3/12 positive biopsy cores, <50% cancer in each core biopsy, and a PSA <10 ng/ml [12].
The NCCN follow-up protocol recommends a PSA measurement every six months (unless there is an earlier clinical indication), digital rectal examination every 12 months (unless there is an earlier clinical indication), re-biopsy every 12 months (unless there is an earlier clinical indication) [11]. The criteria that indicate cancer progression include increasing PSA (≥10 ng/ml) and an increase in Gleason score to ≥7 on repeat biopsy, which do not include imaging findings (Table 2) [11.12].
The benefits of active surveillance include avoidance of unnecessary treatment, including radical prostatectomy, which preserves the quality of life and reduces healthcare costs. The disadvantages of active surveillance include the risk of missing the optimal time to start definitive treatment, as well as the risk of tumor progression or metastasis before treatment. Also, if the tumor becomes more aggressive during surveillance, treatment will become more difficult.
Patients who have a diagnosis of prostate cancer may suffer from anxiety from the knowledge that they have cancer, which may impair their quality of life. A diagnosis of low-risk prostate cancer that is not likely to cause patient mortality may not be beneficial to the patient. According to the current protocols, there is also the need for repeat digital rectal examination and biopsies, which cause not also discomfort but also increase the risk of infections. Also, the natural history of untreated low-risk prostate cancer remains poorly understood.
The establishment of the role of imaging for early prostate cancer and the timing of repeat imaging studies remain to be accepted in clinical practice. The heterogeneity of inclusion criteria for active surveillance of patients, the definition of clinically significant disease, and agreement about what should be understood as radiologic progression are the main issues that affect the potential impact of magnetic resonance imaging (MRI) on active surveillance protocols [13].
The Role of Multiparametric MRI in Active Surveillance of Prostate Cancer
Multiparametric MRI enables prostate cancer detection, its localization, and further characterization in terms of tumor size and stage [13–19]. Multiparametric MRI may be useful in two stages of the active surveillance protocol, as the baseline examination at patient enrolment [14–19], and an alternative to follow-up prostate biopsy during the active surveillance program.
The 2019 update of the Prostate Imaging Reporting and Data System (PI-RADS) version 2.1 [20] diagnostic protocol incorporates T2-weighted MRI in the axial plane and at least one perpendicular plane, diffusion-weighted images (DWI] with ADC maps, and dynamic contrast-enhanced (DCE) images (Table 3). However, biparametric MRI that includes T2-weighted, DWI, and ADC maps, without DCE imaging, is an accepted method. However, there are no specific recommendations for the use of biparametric MRI in active surveillance for prostate cancer.
Table 3.
Characteristics of the magnetic resonance imaging (MRI) sequences.
| MRI sequences | Imaging characteristics |
|---|---|
| T2-weighted imaging | Allows differentiation of prostatic zones, localization of probable neoplastic lesion, identification of extra-prostatic disease |
| Diffusion-weighted imaging (DWI) | Reveals regions of restricted water diffusion as hyperintense (bright) on DWI, and hypointense (dark) on apparent diffusion coefficient (ADC) maps that point out probable neoplastic lesions |
| Dynamic contrast enhancement (DCE) | Focal contrast enhancement, faster than in surrounding tissues that may indicate the malignant nature of the lesion |
| T1-weighted imaging | Allows the assessment of post-biopsy hemorrhagic changes |
Multiparametric MRI is still preferred in men, where the balance between under-diagnosis and overdiagnosis favors the clinical priority of not missing significant cancer [21]. These patients include men with prior negative biopsies with unexplained raised PSA values, and those undergoing active surveillance who are being evaluated increased PSA levels or a change in clinicopathologic status. Recent studies have shown that confirmative multiparametric MRI following biparametric MRI could detect an increase in Gleason grade ≥2 prostate cancer lesions missed by biparametric MRI in 4% of patients, although this did not reach statistical significance [22,23]. Also, biparametric MRI was shown to have a similar diagnostic performance to multiparametric MRI, which supports its use as an alternative to the standard protocol for the detection of extraprostatic extension [22,23].
Diffusion-weighted MR imaging (DWI) has been the main sequence for tumor imaging in the peripheral zone of the prostate gland, and T2-weighted imaging is t main sequence for tumors in the transitional zone [24]. DWI detects the random movement of protons in the interstitial space. Water molecules, which are the main source of protons in the body, move freely in the normal tissue. Tumors, included prostate cancer, consist of densely packed cells and an abundance of cell membranes with restricted diffusion of water [20,21].
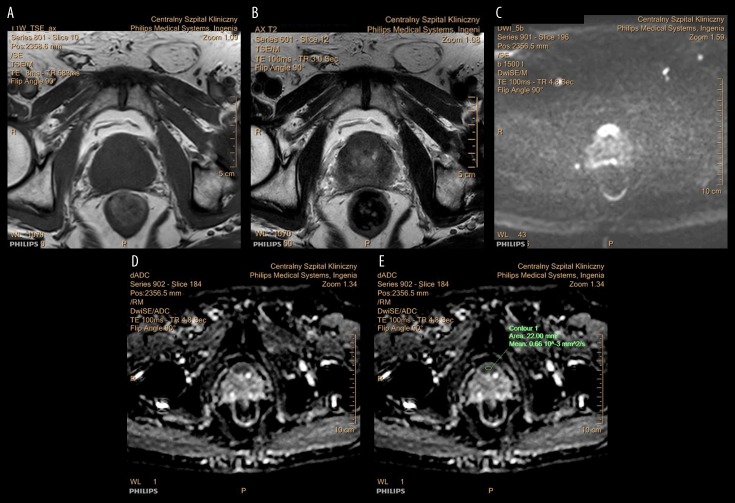
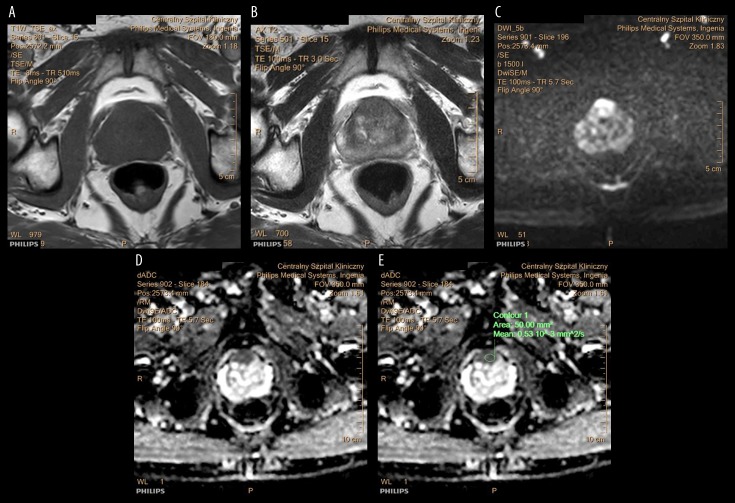
Figure 1A–1E show T1-weighted MRI images of the prostate gland that contains an anterior tumor, shown as an isointense area. In this example, the tumor involves the anterior fibromuscular stroma (AFMS). Figure 2A–2E show an anterior prostate carcinoma involving the AFMS, which is isointense on T1-weighted imaging, hypointense on T2-weighted imaging, hyperintense on DWI, and hypointense on the apparent diffusion coefficient (ADC) map. Previous studies have shown that a decreased ADC value when compared with previous MRI findings, indicates aggressive transformation (Table 3) [25–32].
Figure 1.
Magnetic resonance imaging (MRI) in a patient with prostate cancer. (A–E) Images of the prostate gland show a lesion located in the anterior aspect of the prostate gland that infiltrates the anterior fibromuscular stroma (AFMS). The tumor is isointense on T1-weighted (T1W) MRI with no signs of hemorrhage following trans-rectal biopsy. The tumor is hypointense on T2-weighted (T2W) MRI, hyperintense on diffusion-weighted imaging (DWI), and hypointense on the apparent diffusion coefficient (ADC) mapping. The ADC value is 0.66×10–3 mm2/s. The MRI features are in keeping prostate cancer.
Figure 2.
Follow-up magnetic resonance imaging (MRI) in a patient with prostate cancer. (A–E) Images of the prostate gland show a lesion located in the anterior aspect of the prostate gland that infiltrates the anterior fibromuscular stroma (AFMS). The tumor is isointense on T1-weighted (T1W) MRI, hypointense on T2-weighted (T2W) MRI, hyperintense on diffusion-weighted imaging (DWI), and hypointense on the apparent diffusion coefficient (ADC) mapping. The ADC value is 0.53×10–3 mm2/s. The MRI features are in keeping prostate cancer. The reduced ADC value, when compared with the previous MRI, indicates that the tumor has become more aggressive.
A non-significant or low-risk prostate carcinoma has a tumor volume <0.5 cm3, a Gleason score ≤3+3=6 without a Gleason pattern of 4 or 5 [11,12,25]. Currently, low-risk prostate carcinoma can only be confirmed based on the histological assessment of the prostatectomy specimen, often using large whole-mount tissue sections [25]. The standard preoperative assessment based on digital rectal examination, measurement of PSA) level, and repeat prostate biopsy have significant limitations, which cause reclassification in many cancers within two years in up to 20–30% of patients [25,26].
The combined use of MRI findings, clinical data, and biopsy results in active surveillance enrolment has been previously proposed, as early as 2010 [26]. According to previous studies, MRI-based models showed better results than clinical models (P<0,05) [26,27,33]. According to the current guidelines from the European Association of Urology (EAU) regarding men already on active surveillance and the treatment of patients with low-risk disease who qualified for active surveillance, there is a recommendation to perform multiparametric MRI before a confirmatory biopsy, if not done before the first biopsy [12].
Most of the occurrences of reclassification of prostate tumors are caused by under-sampling at first biopsy rather than the progression of an indolent tumor [28]. This finding indicates that the results of routine serial biopsies may be misleading. Therefore, there is a need for a method to reduce the risk of underestimation by targeting the biopsy needle to the most significant area of the prostate tumor. Targeting the biopsy needle on the index lesion corresponding with the most significant area in the prostate cancer visualized by the initial anatomical and functional imaging reduces the risk of underestimation of the stage of the prostate cancer.
One of the main disadvantages of serial biopsies is anterior prostate cancer underdiagnosis, which is a cause of reclassification of in 25% of patients undergoing active surveillance after two years [29,30]. The 95% negative predictive value of multiparametric prostate MRI highlights the opportunity to avoid repeat biopsies for active surveillance and monitoring.
The Identification of Significant Prostate Cancer
MRI shows a high degree of accuracy for the identification of significant prostate cancer, defined as a lesion >0.5 cm3, a combined Gleason score ≥7, and/or showing features of extraprostatic extension [11,12]. In 2009, Puech et al. showed that the histopathology of whole-mount prostatectomy specimens had a sensitivity and specificity of 86% and 94%, respectively [31]. In the same study, the mean volume of cancer tissue on MRI was 2.44 cm3 (range, 0.02–14.5 cm3), and the negative predictive value was 95% [31]. The findings from this study support the value of MRI for the detection of prostate cancer.
Studies have shown that MRI-guided prostate biopsy detects a further 52% of tumors in patients with prior negative serial biopsies [32,33]. MR-transrectal ultrasound (MR-TRUS) image fusion targeted biopsy can identify more cancers per core than serial transrectal ultrasound (TRUS)-guided biopsy, regardless of the location of the lesion (anterior or posterior).
The inadequate sampling of prostate tumors on serial biopsy is a reason for incorrect grading in between 23–25% of cases [33,34]. Multiparametric MRI provides data on the tumor volume and location and also on grade or behavior according to the Gleason score. Studies have identified a correlation between the apparent diffusion coefficient (ADC) values, and the histological Gleason grade [34,35]. Lower ADC values and a higher signal on DWI correspond with the more dense structure of less differentiated cancers [36–38]. This inverse relationship between the Gleason score and the ADC value was found for prostate cancers in the peripheral zone (PZ).
DWI imaging has resulted in the ability to distinguish between low-grade, intermediate-grade, and high-grade prostate cancer [39]. Also, the baseline ADC value is an independent predictor for unfavorable findings of control biopsy and time to radical prostatectomy. [37,40]
MRI is a useful tool for enrolling patients for active surveillance programs as well as a monitoring method for patients already under active surveillance. Patients under active surveillance who have a suspicion of malignancy on multiparametric MRI have an increased risk of the upgrading of the Gleason score when compared with patients who did not have imaging findings [41,42]. In 2012, Vargas et al. showed that the diagnosis of low-risk prostate cancer based on MRI had a negative predictive value of between 0.96 to 1.0 for upgrading, which means that these patients could have avoided repeat biopsy [42]. For lesions deemed high risk for significant prostate cancer, the positive predictive value was between 0.87 and 0.98, which means that, in these cases, repeat biopsy was strongly recommended [42].
The Limitations of Current Active Surveillance strategies for Prostate Cancer
Currently, patients who are candidates for active surveillance undergo an unguided transrectal prostate biopsy of between 10–12 transrectal needle biopsies. This approach results in an underestimation of cancer grade in 30% of cases, and an underestimation of the burden of cancer in 50% of patients diagnosed with low-risk malignancy [43]. Systematic errors in non-guided prostate biopsies are due to selective posterior zone sampling and inadequate transition and anterior zone biopsy [44]. Random errors occur because of the lack of awareness of the operator to the location of the tumor. The main strength of multiparametric MRI for active surveillance, regardless of the method of subsequent fusion of the images, is the use of targeting biopsies [45]. Therefore, a concept of MRI-targeted biopsy is a method of choice in active surveillance qualification [46]. MRI-targeted prostate biopsy in active surveillance is an alternative to repetitive biopsy sampling, and the use of three-dimensional (3D) transperineal mapping biopsy, which are invasive and expensive procedures, factors which limit their long-term use [47].
Anterior prostate cancer representing 20% of cases, are difficult to biopsy using the standard 12-core TRUS approach, and demand a different strategy. Because targeted prostate biopsy seems to be a method of choice, the choices are between cognitive fusion [32] or MRI/TRUS, using automated fusion as well as MRI-guided fusion [48]. Anterior prostate cancers are the most often missed or misclassified tumors. Anterior prostate cancer diagnosed with a 12-core TRUS biopsy require fewer cores than non-guided biopsies [49]. A comparison of the median biopsy core length between targeted systematic biopsy or non-targeted biopsy in anterior cancer cases was 8 mm compared with 1 mm (p<0.001) [45]. This consideration is important, as inaccuracies in tumor volume assessment and assessment of tumor grade have an impact on prognosis and choice of treatment [45].
Missed anterior cancers are the most common reclassified lesions as they have fewer cores that contain neoplastic tissue and a shorter core length than for posterior cancers [49]. The percentage of the patients enrolled in active surveillance protocols based on a negative prior 12-core TRUS biopsy who had cancer of the anterior portion of the gland indicated as a suspicious lesion on MRI performed before biopsy and diagnosed with a targeted biopsy was high, at up to 89% [30]. These findings support the value of baseline multiparametric MRI.
Perspectives and Future Studies
In men who are considered to be candidates for active surveillance for prostate cancer, the baseline multiparametric MRI may reduce the number of men under active surveillance and who have an overdiagnosis of insignificant cancers [50,51]. The accuracy of the detection of prostate cancer using a strategy of biopsy of significant lesions in men with increased PSA levels is greater than for serial biopsy (p<0.001) [50,51]. Targeted prostate biopsy identifies 16% more Gleason grade 4 and 5 prostate cancers. A high negative predictive value of multiparametric MRI may reduce the indications to rebiopsy. Therefore, there is a use for multiparametric MRI before inclusion in an active surveillance program followed by a targeted prostate biopsy to reduce the detection of insignificant prostate cancer and reduce the number of men undergoing active surveillance.
As previously discussed, there is a correlation between the apparent diffusion coefficient values and the Gleason score, and both may be considered as prognostic and predictive biomarkers for prostate cancer [50,51]. Also, PSA kinetics could be used to predict radiological progression, with a cut-off of PSA velocity of 0.75 ng/ml/year to define which patients would benefit from subsequent multiparametric MRI [52]. Therefore, multiparametric MRI can be of use for monitoring men under active surveillance, reducing the number of repeat prostate sampling, and change therapeutic management.
Recently, diverse applications of machine learning focusing on imaging of prostate cancer have been described [53]. The proposed solutions based on various algorithms may aid the main problems regarding segmentation of the prostate gland, and assessment of lesion aggressiveness to distinguish between indolent and clinically significant cancers, enrollment into active surveillance, detection and diagnosis, and identification of tumor invasion. Future developments of machine learning algorithms may also be used to identify transition zone and peripheral zone tumors, the use of PI-RADS, reproducibility of image interpretation, the differentiation of malignancy from benign conditions, such as benign prostatic hyperplasia and prostatitis, as well as local tumor staging.
Quantitative characterization of disease patterns defined as features extracted computationally from radiographic images supports the PI-RADS assessment of multiparametric MRI of the prostate gland and supports the future possibility of substitution of multiparametric MRI with biparametric MRI [54]. However, multiparametric MRI is not performed in every patient with a clinical suspicion for prostate cancer [55]. Biparametric MRI could increase the accessibility of prostate MRI with comparable accuracy of radiologic interpretation while reducing healthcare costs.
Conclusions
Multiparametric MRI of the prostate gland has been established as an imaging method of value in patients that require targeted prostate biopsy as it can identify higher grades and volume of prostate cancers than systematic 12-core prostate biopsy. Patients who are considered to be candidates for active surveillance for prostate cancer would benefit from multiparametric MRI as it enables better initial diagnosis and reduces the need for repeat biopsies. Multiparametric MRI is a valuable tool for monitoring patients who are already under active surveillance, resulting in a reduction in the need for repeat biopsies. Evidence-based clinical guidelines suggest that MRI will probably become the new triage test for men with suspicion of prostate cancer. Further large-scale prospective controlled studies are required to define the precise role of multiparametric MRI and biparametric MRI in active surveillance for prostate cancer.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Klotz L. Contemporary approach to active surveillance for favorable risk prostate cancer. Asian J Urol. 2019;6(2):146–52. doi: 10.1016/j.ajur.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg CJ, Habibian DJ, Katz AE, et al. Active holistic surveillance: The nutritional aspect of delayed intervention in prostate cancer. J Nutr Metab. 2016;2016 doi: 10.1155/2016/2917065. 2917065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–28. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Kattan MW. Nomograms for prostate cancer. BJU Int. 2006;98:39–46. doi: 10.1111/j.1464-410X.2006.06173.x. [DOI] [PubMed] [Google Scholar]
- 7.Serefoglu EC, Altinova S, Ugras NS, et al. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013;7:E293–98. doi: 10.5489/cuaj.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 9.Adamy A, Yee DS, Matsushita K, et al. Role of prostate-specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low-risk prostate cancer. J Urol. 2011;185:477–82. doi: 10.1016/j.juro.2010.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitson JM, Porten SP, Hilton JF, et al. The relationship between prostate-specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011;185:1656–60. doi: 10.1016/j.juro.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Carroll PR, Parsons JK, Andriole G, et al. NCCN Guidelines Insights: Prostate cancer early detection, Version 2. 2016. J Natl Compr Canc Netw. 2016;14:509–19. doi: 10.6004/jnccn.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottet N, Bellmunt J, Briers E, et al. Members of the EAU – ESTRO – ESUR –SIOG Prostate Cancer Guidelines Panel. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Edn. presented at the EAU Annual Congress; Copenhagen. Arnhem, The Netherlands: EAU Guidelines Office; 2018. 978-94-92671-02-8. https://uroweb.org/guideline/prostate-cancer/ [Google Scholar]
- 13.Barrett T, Haider M. The emerging role of MRI in prostate cancer active surveillance and ongoing challenges. Am J Roentgenol. 2017;208(1):131–39. doi: 10.2214/AJR.16.16355. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Jeong JY, Lee SW, et al. Diffusion-weighted magnetic resonance imaging for prediction of insignificant prostate cancer in potential candidates for active surveillance. Eur Radiol. 2015;25:1786–92. doi: 10.1007/s00330-014-3566-2. [DOI] [PubMed] [Google Scholar]
- 15.Jeong CW, Park YH, Hwang SI, et al. The role of 3-tesla diffusion-weighted magnetic resonance imaging in selecting prostate cancer patients for active surveillance. Prostate Int. 2014;2:169–75. doi: 10.12954/PI.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garisto JD, Klotz L. Active surveillance for prostate cancer: How to do it right. Oncology (Williston Park) 2017;31(5):333–40. 345. [PubMed] [Google Scholar]
- 17.Mullins JK, Bonekamp D, Landis P, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013;111:1037–45. doi: 10.1111/j.1464-410X.2012.11641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park BH, Jeon HG, Choo SH, et al. Role of multiparametric 3.0-Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int. 2014;113:864–70. doi: 10.1111/bju.12423. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Koo KC, Lee SH, et al. Tumor lesion diameter on diffusion-weighted magnetic resonance imaging could help predict insignificant prostate cancer in patients eligible for active surveillance: Preliminary analysis. J Urol. 2013;190:1213–17. doi: 10.1016/j.juro.2013.03.127. [DOI] [PubMed] [Google Scholar]
- 20.Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 Update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76(3):340–51. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Stanzione A, Ponsiglione A, Cuocolo R, et al. Abbreviated protocols versus multiparametric MRI for assessment of extraprostatic extension in prostatic carcinoma: A multireader study. Anticancer Res. 2019;39(8):4449–54. doi: 10.21873/anticanres.13617. [DOI] [PubMed] [Google Scholar]
- 22.Thestrup KD, Løgager V, Boesen L, Thomsen HS. Comparison of bi- and multiparametric magnetic resonance imaging to select men for active surveillance. Acta Radiol Open. 2019;8(8):2058460119866352. doi: 10.1177/2058460119866352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuocolo R, Stanzione A, Rusconi G, et al. PSA-density does not improve bi-parametric prostate MR detection of prostate cancer in a biopsy naïve patient population. Eur J Radiol. 2018;104:64–70. doi: 10.1016/j.ejrad.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Somford DM, Hambrock T, Hulsbergen-van de Kaa CA, et al. Initial experience with identifying high-grade prostate cancer using diffusion-weighted MR imaging (DWI) in patients with a Gleason score ≤3+3=6 upon schematic TRUS-guided biopsy: A radical prostatectomy correlated series. Invest Radiol. 2012;47(3):153–58. doi: 10.1097/RLI.0b013e31823ea1f0. [DOI] [PubMed] [Google Scholar]
- 25.Berglund RK, Masterson TA, Vora KC, et al. Pathological upgrading and upstaging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180:1964–67. doi: 10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 27.Shukla-Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012;109(9):1315–22. doi: 10.1111/j.1464-410X.2011.10612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29:2795–800. doi: 10.1200/JCO.2010.33.0134. [DOI] [PubMed] [Google Scholar]
- 29.Ayres BE, Montgomery BS, Barber NJ, et al. The role of transperineal template prostate biopsies in restaging men with prostate cancer managed by active surveillance. BJU Int. 2012;109(8):1170–76. doi: 10.1111/j.1464-410X.2011.10480.x. [DOI] [PubMed] [Google Scholar]
- 30.Lawrentschuk N, Haider MA, Daljeet N, et al. ‘Prostatic evasive anterior tumours’: The role of magnetic resonance imaging. BJU Int. 2010;105:1231–36. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 31.Puech P, Potiron E, Lemaitre L, et al. Dynamic contrast-enhanced MRI evaluation of intraprostatic prostate cancer. Correlation with radical prostatectomy specimens. Urology. 2009;74:1094–99. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- 32.Ploussard G, Beauval JB, Lesourd M, et al. Performance of systematic, MRI-targeted biopsies alone or in combination for the prediction of unfavourable disease in MRI-positive low-risk prostate cancer patients eligible for active surveillance. World J Urol. 2019 doi: 10.1007/s00345-019-02848-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Arabi A, Deebaja M, Yaguchi G, et al. Systematic biopsy does not contribute to disease upgrading in patients undergoing targeted biopsy for PI-RADS 5 lesions identified on magnetic resonance imaging in the course of active surveillance for prostate cancer. Urology. 2019;134:168–72. doi: 10.1016/j.urology.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Woodfield CA, Tung GA, Grand DJ, et al. Diffusion-weighted MRI of peripheral zone prostate cancer: Comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. Am J Roentgenol. 2010;194:W316–22. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 35.Itou Y, Nakanishi K, Narumi Y, et al. Clinical utility of apparent diffusion coefficient (ADC) values in patients with prostate cancer: Can ADC values contribute to assess the aggressiveness of prostate cancer? J Magn Reson Imaging. 2011;33:167–72. doi: 10.1002/jmri.22317. [DOI] [PubMed] [Google Scholar]
- 36.Langer DL, van der Kwast TH, Evans AJ, et al. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2-sparse versus dense cancers. Radiology. 2008;249:900–8. doi: 10.1148/radiol.2493080236. [DOI] [PubMed] [Google Scholar]
- 37.DeSouza NM, Riches SF, Vanas NJ, et al. Diffusion-weighted magnetic resonance imaging: A potential noninvasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol. 2008;63:774–82. doi: 10.1016/j.crad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimitsu K, Kiyoshima K, Irie H, et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: Correlation with stepwise histopathology. J Magn Reson Imaging. 2008;27:132–39. doi: 10.1002/jmri.21181. [DOI] [PubMed] [Google Scholar]
- 39.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent and diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–61. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 40.Van As NJ, de Souza NM, Riches SF, et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol. 2009;56:981–87. doi: 10.1016/j.eururo.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 41.Habibian DJ, Liu CC, Dao A, et al. Imaging characteristics of prostate cancer patients who discontinued active surveillance on 3-T multiparametric prostate MRI. Am J Roentgenol. 2017;208(3):564–69. doi: 10.2214/AJR.16.16822. [DOI] [PubMed] [Google Scholar]
- 42.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low-risk prostate cancer. J Urol. 2012;188(5):1732–38. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford ED, Wilson SS, Torkko KC, et al. Clinical staging of prostate cancer: A computer-simulated study of transperineal prostate biopsy. BJU Int. 2005;96:999–1004. doi: 10.1111/j.1464-410X.2005.05801.x. [DOI] [PubMed] [Google Scholar]
- 44.Presti JC. Prostate biopsy: Current status and limitations. Rev Urol. 2007;9:93–98. [PMC free article] [PubMed] [Google Scholar]
- 45.Del Monte M, Leonardo C, Salvo V, et al. MRI/US fusion-guided biopsy: Performing exclusively targeted biopsies for the early detection of prostate cancer. Radiol Med. 2018;123(3):227–34. doi: 10.1007/s11547-017-0825-8. [DOI] [PubMed] [Google Scholar]
- 46.Ouzzane A, Puech P, Lemaitre L, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011;78:1356–62. doi: 10.1016/j.urology.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed HU, Hu Y, Carter T, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol. 2011;186:458–64. doi: 10.1016/j.juro.2011.03.147. [DOI] [PubMed] [Google Scholar]
- 48.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189(1):86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bott SR, Young MP, Kellett MJ, Parkinson MC. Anterior prostate cancer: Is it more difficult to diagnose? BJU Int. 2002;89:886–89. doi: 10.1046/j.1464-410x.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 50.Giles SL, Morgan VA, Riches SF, et al. Apparent diffusion coefficient as a predictive biomarker of prostate cancer progression: Value of fast and slow diffusion components. Am J Roentgenol. 2011;196:586–91. doi: 10.2214/AJR.10.5016. [DOI] [PubMed] [Google Scholar]
- 51.Jayadevan R, Felker ER, Kwan L, et al. Magnetic resonance imaging-guided confirmatory biopsy for initiating active surveillance of prostate cancer. JAMA Netw Open. 2019;2(9):e1911019. doi: 10.1001/jamanetworkopen.2019.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens DJ, Moore C, Ahmed H, et al. 1096. The natural history of untreated prostate MRI lesions in an active surveillance prostate cancer population – 260 patient-years. European Urology Supplements. 2012;11(1):e1096. [Google Scholar]
- 53.Cuocolo R, Cipullo MB, Stanzione A, et al. Machine learning applications in prostate cancer magnetic resonance imaging. Eur Radiol Exp. 2019;3(1):35. doi: 10.1186/s41747-019-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Algohary A, Viswanath S, Shiradkar R, et al. Radiomic features on MRI enable risk categorization of prostate cancer patients on active surveillance: Preliminary findings. J Magn Reson Imaging. 2018 doi: 10.1002/jmri.25983. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walton EL, Deebajah M, Keeley J, et al. Barriers to obtaining prostate multi-parametric magnetic resonance imaging in African-American men on active surveillance for prostate cancer. Cancer Med. 2019;8(8):3659–65. doi: 10.1002/cam4.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]