Abstract
Ferritins are evolutionarily conserved proteins that regulate cellular iron metabolism. It is the only intracellular protein that is capable of storing large quantities of iron. While the ratio of different subunits determines the iron content of each ferritin molecule, the exact mechanism that dictates organization of these subunits is still unclear. In this review, we addressed renal ferritin expression and its implication in kidney disease. Specifically, we addressed the role of ferritin subunits in preventing kidney injury and also promoting tolerance against infection-associated kidney injury. We describe newly identified functions for ferritin that are independent of its ability to ferroxidize and store iron. We further discuss the implications of ferritin in body fluids, including blood and urine during inflammation and kidney disease. While there are several in-depth review articles on ferritin in the context of iron metabolism, we chose to focus on the role of ferritin particularly in kidney health and disease and highlight unanswered questions in the field.
Keywords: ferritin, iron, ferritinophagy, kidney, injury, ferroptosis
Discovery and function
Ferritin was discovered by Victor Laufberger in 1937. Lauferberger isolated a protein from a horse spleen and found that it contained over 20% iron by dry weight. He named the protein Ferritin, which is derived from the Latin word “ferratus,” meaning “bound by iron.” It has since been defined as a major iron storage protein, and a key player in iron metabolism.1–3 Ferritin is an evolutionarily conserved globular protein, composed of 24 polypeptide chains. It forms a spherical shape that is approximately 8 nm in diameter, allowing it to store approximately 4,500 Fe atoms.3–5 It has been shown that this protein originated during early phylogenesis and is present in most organisms, ranging from archeobacteria to mammals. Ferritin has been isolated from humans, horses and mice as well as chitons, insects, parasites and plants.6–9 Due to its high conservation through evolution, all ferritins have the ability to interact readily with ferrous iron (Fe2+), inducing iron oxidation and aggregation inside the spherical cavity. However, some differences can be found among ferritin between different species. Ferritin from bacteria and plants are composed of 24 subunits of the same type, whereas ferritin from vertebrates is composed of two distinct ferritin subunits, the ferritin heavy chain (FtH) and the ferritin light chain (FtL).1, 2, 10, 11 Recently, a third subunit that is expressed specifically in the mitochondria has been described (discussed below).
In mice, FtH is located in region B of chromosome 19 and has an exon count of 5.12 FtL is located on chromosome 7 and has an exon count of 4.13 In humans, the 21 kDa FtH subunit is encoded by the FtH gene, located on chromosome 11q12.3 and has an exon count of four. The 19-kDa FtL subunit is encoded by the FtL gene, located on chromosome 19q13.1 and has an exon count of five.14, 15 While both the FtH and FtL subunits share almost 55% of their sequence, and have similar structures, they have distinctly different functions.16, 17 The FtH subunit has enzymatic activity that rapidly oxidizes Fe2+ into the ferric form (Fe3+) and incorporates iron into the shell.11, 18 However, unlike FtH, FtL lacks enzymatic activity and cannot independently contribute to iron uptake and oxidation. While FtL can only incorporate iron in the presence of FtH, FtL has a stronger ability to induce iron core nucleation.19 It has also been shown that FtL contains a salt bridge within its structural fold that plays a large role in the stability of the ferritin protein.11 Together, these FtH and FtL subunits are bound to form ferritin, the iron binding protein that plays a key role in iron detoxification, storage and recycling.2
Tissue Distribution – FtH:FtL
Ferritin is ubiquitously expressed but is expressed at higher levels at sites of high iron storage and recycling such as the liver and spleen. FtH and FtL subunits assemble in specific ratios to form the ferritin shell. This ratio can differ among cell types and can affect cellular function, such as iron uptake, proliferation and reduce the impact of cytokine and oxidative stress.20–24 It has been shown that FtL is higher in iron storage tissues such as the liver and spleen, while FtH is higher in the heart, brain and kidney.25 This may be attributed to the fact that ferritins with high FtL:FtH ratios are the most effective at incorporating iron into highly stable molecules.20, 26 Higher FtH levels are found in metabolically active organs, such as the heart, brain and kidney resulting in high ferroxidase activity, allowing these tissues to rapidly oxidize and regulate iron metabolism.26 In the kidney, both FtH and FtL are predominantly expressed in the proximal tubules (PT).27, 28 Indeed, deletion of FtH specifically in the PTs alone led to a significant reduction (~80%) in expression of FtH in whole kidney lysates (Figure. 1). It has been shown that FtL is mainly expressed in the cytoplasm whereas FtH is expressed in the cytoplasm and nucleus, suggesting an anti-oxidant or gene regulatory role for FtH in the nucleus.29 Notably, while FtL stabilizes the iron core, FtL homopolymers are incapable of iron storage, suggesting that FtH is essential for iron uptake and processing. These data underscore the lack of functional redundancy between these subunits. This is further emphasized in the embryonic lethality of transgenic mice with global FtH deletion but not with FtL deletion (Table 1).30–32 The difference in expression ratios of FtH and FtL in target tissues is thought to be mediated via multiple regulatory mechanisms that include requirement for iron utilization and storage.
Figure 1. Ferritin expression in the kidney.
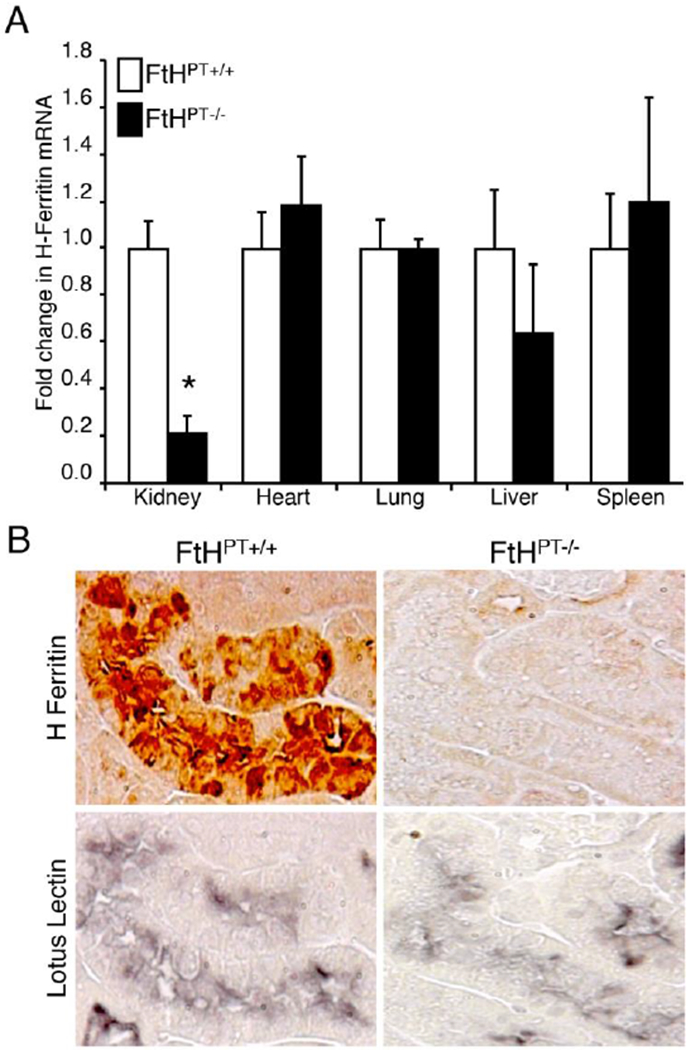
(A) Real-time PCR was used to analyze FtH mRNA expression in the organs of wild-type (FtHPT+/+) and proximal tubule specific FtH deletion mice (FtHPT−/−). Results were normalized to GAPDH and expressed as fold change compared with FtHPT+/+ Data are mean ± SEM. *P < 0.001 vs. FtHPT+/+. (B) Immunohistochemical staining on serial kidney sections from FtHPT+/+ and FtHPT−/− mice for FtH and the proximal tubule marker, lotus lectin. Reproduced with permission.28
Table 1.
Characteristics of ferritin subunits
| Properties | FtH | FtL | FtMt |
|---|---|---|---|
| Cellular Localization | Nucleus and cytoplasm | Cytoplasm | Mitochondria |
| Chromosomal Localization | 11q12.3 | 19q13.1 | 5q23.1 |
| Molecular Weight | 21 kDa | 19 kDa | 22 kDa |
| Ferroxidase Activity | Yes | No | Yes |
| Tissue Distribution | Higher in the heart, brain and kidneys | Higher in the liver and spleen | Higher in the testis, spermatocytes, neurons and cardiomyocytes |
| Regulation | Cytosolic iron levels, oxidative stress, inflammation and others | Cytosolic iron levels, oxidative stress, inflammation and others | Mitochondrial iron levels |
| Signaling Mechanisms | JNK, ERK and others | JNK, ERK, NFkB, γ-secretase activity and others | Not known |
| Global Genetic Deletion in Mice | Embryonic lethal | No effect | No effect |
Abbreviations: ERK, Extracellular signal-regulated kinases; FtH, ferritin heavy chain; FtL, ferritin light chain; FtMt, mitochondrial ferritin; JNK, c-Jun N-terminal kinases; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells.
Mitochondrial Ferritin
Human mitochondrial ferritin (FtMt) was recently discovered and described as an intronless gene, found on chromosome 5q23.1, responsible for encoding a 242-amino acid precursor FtH-like protein.33 FtMt is a 30-kDa protein, targeted to mitochondria and processed to a 22-kDa subunit that has ferroxidase activity similar to FtH. This is the first discovery of a mammalian ferritin that is specifically targeted to an organelle. Unlike FtH and FtL which are ubiquitously expressed, FtMt has limited tissue distribution. High levels of FtMt have been found within the testis, an organ that is not very rich in mitochondria. Additionally, FtMt levels do not correlate with iron content, as seen by the low levels of FtMt in iron rich organs such as the liver and spleen.33, 34 FtMt is associated with cells that have elevated oxygen consumption and high metabolic activity, such as spermatocytes, neurons and cardiomyoctes.35, 36 The high expression of FtMt in neurons has led to the study of FtMt in neurodegenerative diseases (Reviewed in 37). FtMt levels are elevated in the cerebral cortex of patients with Alzheimer’s disease and substantia nigra of patients with Parkinson’s disease and restless legs syndrome.38–40 These studies suggest that FtMt plays a neuroprotective role by regulating apoptotic signaling and by limiting the toxicity of iron overload and oxidative stress by sequestering excess iron.40, 41 Li et al overexpressed FtMt in mice and determined that FtMt does not control systemic iron metabolism.36 Another study identified that mice with global deletion of FtMt were associated with smaller litters due to decreased spermatozoa, implicating a role for FtMt in male fertility.42 While it has been shown that levels of FtMt increase with mitochondrial iron loading, and may protect the mitochondria from oxidative damage, the physiological functions of FtMt in other organs merits further investigation.
Regulation of ferritins
Induction
Ferritin is the only known protein complex that is capable of storing large quantities of iron. Therefore, in order to maintain iron homeostasis, ferritin expression is tightly regulated. Post-transcriptional iron dependent regulation is based upon the interaction of iron regulatory proteins 1 and 2 (IRP1, IRP2), as well as the iron responsive elements (IRE) on ferritin mRNAs. The IRE is a region of the 5’ untranslated region of both FtH and FtL mRNA that has a stem-loop secondary structure. IRPs are RNA binding proteins that bind to the IRE stem-loop structure and inhibit mRNA translation. This IRP-IRE system is sensitive to intracellular iron content as well as oxidative stress. When cellular iron levels are high, there is an increase in ferritin protein expression. As cellular iron increases, iron-sulfur clusters are incorporated into IRP1, preventing its binding to IREs. Similarly, as cytosolic iron levels increase, IRP2 is degraded. Without the IRPs binding to IREs, translation of ferritin occurs. Therefore, both IRPs and the IRE have an inhibitory effect on the synthesis of ferritin by inhibiting its translation.2, 43, 44 While the amount of iron incorporation into ferritin is directly correlated to the amount of synthesized ferritin, multiple mechanisms control ferritin iron storage. The iron chaperone, poly rC-binding protein 1 (PCBP1) binds iron in the cytosol and transfers it to ferritin via a direct protein-to-protein interaction.45 It has also been shown that nuclear coactivator 4 (NCOA4), an autophagic cargo receptor mediates degradation of ferritin via lysosomes (see below).46 Collectively, the actions of PCBP1 and NCOA4 on ferritin enable cells to adapt to fluctuations in iron availability. As cellular iron levels increase, free Fe2+mediates the binding activity of PCBP1 to ferritin. As excess Fe2+ ions increase, iron loading of ferritin increases until the iron binding sites for both ferritin and PCBP1 become saturated and ferritin loading becomes less efficient, simultaneously inhibiting ferritin turnover. Excess iron is shuttled out of the cells via ferroportin.44
Cytokines, such as IL-1β, IL-6 and TNFα also transcriptionally regulate ferritin, specifically FtH.47 Cytokines regulate FtH through distinctive GC-rich regions of the mRNA that are unrelated to IRE.48 Pro-inflammatory cytokines may also indirectly regulate FtH translation by inducing nitric oxide synthase (iNOS).49, 50 Other reactive oxygen species (ROS) such as hydrogen peroxide also regulate expression of FtH and FtL.51 It has also been shown that FtH has a functional crosstalk with the inflammatory kinase, c-Jun N-terminal kinase (JNK), where FtH prevents JNK activation and conversely, JNK inhibits FtH expression.52 Together, transcription and translation of ferritins are dependent on multiple factors, that include intracellular free iron levels and binding of iron regulatory proteins to conserved elements within the ferritin genes.
Degradation: Ferritinophagy
In the intracellular compartment, heteropolymeric ferritin shell comprising of both FtH and FtL can store large quantities of iron. Intracellular ferritin is degraded by two mechanisms: lysosomes and proteasome.46, 53, 54 While it was known that autophagy promotes degradation of ferritin, the exact mechanism underlying this process was recently described. Mancias and colleagues coined the term “ferritinophagy” which refers to the selective autophagic turnover of ferritin by the lysosomes. Two independent studies led by Mancias and Dowdle identified nuclear receptor coactivator 4 (NCOA4) as the specific cargo receptor for ferritin.46, 55 Under iron depleted conditions, NCOA4 binds and delivers the iron-rich ferritin to the lysosome for iron release. They also identified that NCOA4-deficient cells fail to activate ferritinophagy and were associated with decreased bioavailable iron. This underscores the role of ferritinophagy in maintaining cellular iron homeostasis. In subsequent studies, it was determined that interaction of NCOA4 with FtH required a surface arginine (R23) on FtH. Site-directed mutagenesis of this arginine to alanine prevented interaction of NCOA4 with FtH and inhibited ferritinophagy.56 Of note, NCOA4 does not interact with FtL and FtL homopolymers are incapable of iron storage, suggesting that NCOA4 regulates ferritin expression to maintain cellular iron homeostasis.
Expression of NCOA4 is regulated by autophagy and the ubiquitin proteasome degradation systems. Under conditions of iron excess, NCOA4 is ubiquitinated by ubiquitin ligase, HERC2 and degraded.56 Therefore, cellular iron levels are tightly regulated by ferritinophagy via multiple mechanisms that ensure iron availability for cellular processes while preventing excess iron from participating in free radical generation. Additionally, transgenic mice with global deletion of NCOA4 recapitulate in vitro findings with increased accumulation of iron-rich ferritin and reduced ferritinophagy. These mice also displayed hypochromic microcytic anemia, suggesting a role for ferritinophagy in systemic iron homeostasis. Importantly, NCOA4 deficient mice experienced severe liver injury and succumbed to death when placed on an iron-enriched diet. On the other hand, iron-deficient diet caused ineffective erythropoiesis and exacerbated anemia in these transgenic mice.57 Pioneering work by Mancias and colleagues further delineated the temporal and erythroid-specific role of NCOA4 in regulating systemic iron homeostasis and erythropoiesis.58 While much of the work on ferritinophagy has been limited to erythropoiesis, it is still unclear whether ferritinophagy plays a role in mediating disease pathogenesis, especially in conditions where iron overload or accumulation has shown to promote kidney injury.
Ferroptosis: iron-mediated cell death in kidney health and disease
Ferroptosis is an iron-dependent form of cell death that is characterized by increased lipid peroxidation.59 Cytosolic iron can participate in the Fenton reaction, leading to increased generation of ROS. These reactive species enable peroxidation of lipids and accentuate oxidative stress culminating in ferroptotic cell death. Therefore, limiting the amounts of free iron and increasing the antioxidant potential may prevent cell death. In this context, iron chelating agents such as DFO have already shown great promise in preventing ferroptosis.60 Increased iron levels induce synthesis of ferritins in several cell types, which can then sequester iron and prevent its participation in ROS generation. Indeed, manipulation of ferritin levels via NCOA4 mediated ferritinophagy has shown to regulate sensitivity to ferroptosis. For instance, deletion of NCOA4 prevented ferritinophagy, leading to a rapid accumulation of ferritin and prevented ferroptosis.61
Under physiological conditions, glutathione peroxidase 4 (gpx4) rapidly detoxifies lipid peroxides and prevents cell death. The significance of gpx4 expression and ferroptosis in kidney health is substantiated by transgenic mice with inducible deletion of gpx4. Following deletion of gpx4, mice succumb to AKI and mortality. Importantly, these pathological events were partly impeded by the use of liproxstatin, a potent inhibitor of ferroptosis.62 Interestingly, both gpx4 and ferritin are highly expressed in the proximal tubules of the kidney. Deletion of FtH specifically in this segment led to exacerbated AKI and mortality in rodent models.28 While this study did not examine ferroptosis, it is tempting to speculate that the increased susceptibility to AKI may be mediated by ferroptosis. Future studies will need to examine whether the use of ferroptotic agents in these mice can reduce AKI severity and prevent death. A recent study demonstrated that ferroptosis participates in the pathogenesis of renal ischemia reperfusion (IR) injury. They further demonstrate that inhibition of ferroptosis using ferrostatin-1 provided substantial protection during IR.63 Another study highlighted the role of curcumin in mediating protection against rhabdomyolysis in a rodent model via inhibition of ferroptosis.64 Together, these studies suggest that sensitivity to ferroptosis can be achieved by altering the expression of ferritins and antioxidants in rodent models. Nevertheless, translational studies determining the use of ferroptosis inhibitors in humans is lacking.
Ferritins in AKI
Under quiescent and injured state, FtH is maximally expressed in the proximal tubules of the nephron. Nath and colleagues demonstrated that FtH and FtL were co-induced with HO-1 (heme oxygenase-1) in the injured kidney during rhabdomyolysis.65 This seminal study sparked interest in HO-1 research in nephrology that led to identification of HO-1 as a potent renoprotective agent with anti-apoptotic and anti-oxidant properties.66, 67 However, the role of ferritin during AKI was not recognized until recently. To determine whether FtH is renoprotective, we generated transgenic mice with targeted deletion of FtH specifically in the proximal tubules. In two different models of AKI, cisplatin nephrotoxicity and glycerol-induced rhabdomyolysis, mice with FtH deletion displayed worse structural and functional kidney damage compared to their wild-type littermates (Figure. 2). Intriguingly, mice with FtH deletion expressed significantly higher levels of HO-1 but experienced worse renal injury, suggesting a possible dependence of HO-1 on ferritin for its cytoprotective effects.28 In a subsequent study, it was demonstrated that tin protoporphyrin conferred protection against renal ischemia reperfusion injury by upregulating FtH expression. Of note, tin protoporphyrin is a potent inhibitor of HO activity. These findings suggest that expression of ferritin is imperative for reno-protection.68 Following renal ischemia reperfusion, mice with targeted FtH overexpression in the renal cortex were protected against loss of kidney function, lipid peroxidation and cell death.69 Using a pharmacological approach, Scindia and colleagues identified that administration of hepcidin (a hepatic hormone that targets iron exporter, ferroportin for degradation and thereby prevents iron egress into circulation) to mice reduced renal ischemia reperfusion injury-induced kidney dysfunction by increasing the expression of FtH in kidneys and spleen.70 These findings were also recapitulated during hemoglobin mediated kidney injury.71, 72 More recently, using a mouse model of intravascular hemolysis, Rubio-Navvaro et al demonstrated that activation of nuclear factor erythroid 2-related factor 2 (Nrf2) conferred protection against hemolysis-induced kidney injury via upregulation of HO-1 and FtL.73 Another study demonstrated that targeted deletion of renal tubular ferroportin expression led to marked induction in FtH and prevented renal ischemic AKI.74 These studies underscore the protective attributes of renal ferritin expression in iron sequestration and inhibition of oxidative stress and subsequent protection against AKI. Indeed, a detrimental role for free iron has been implicated in the pathogenesis of AKI in both rodents and humans.75–78
Figure 2. Renal FtH regulates heme-induced AKI.
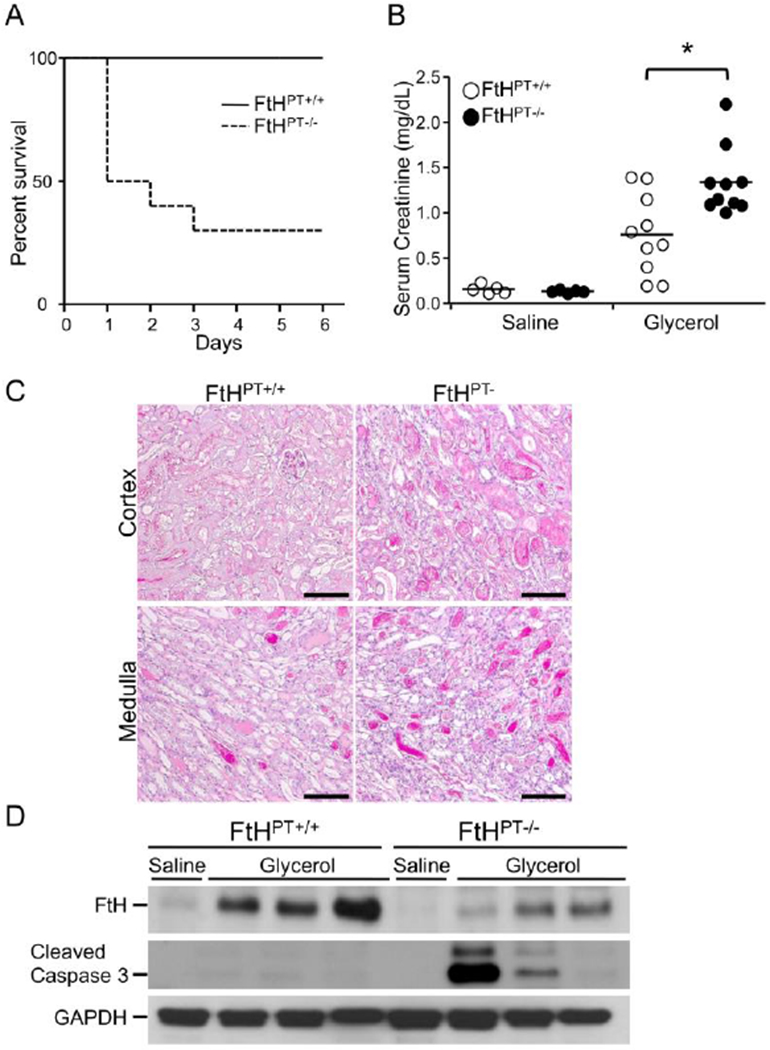
(A) Glycerol was administered to wild-type (FtHPT+/+) and proximal tubule specific FtH deletion mice (FtHPT−/−), and survival was monitored up to 6 days. (B) Blood was collected 24 hours post saline or glycerol administration and serum creatinine was measured 24 hours after. Data are mean ± SEM. *P < 0.01 vs. glycerol-treated FtHPT+/+ (C) Representative PAS staining of cortex and medulla of glycerol-treated FtHPT+/+and FtHPT−/−mice. Scale bar: 100 μm (D) Western blot analysis verified FtH, cleaved caspase-3 GAPDH expression. Reproduced with permission.28
On the contrary, while deletion of renal FtH aggravated fibrosis, we demonstrated that myeloid FtH deletion led to a marked reduction in obstructive nephropathy-induced fibrosis (Figure. 3).10 Following unilateral ureteral obstruction, macrophages rapidly infiltrate into the injured kidney and regulate the reparative responses. While FtH deletion did not alter the accumulation (or polarization) of macrophages within the kidney, it was associated with increased arginase expression. Emerging evidence in multiple rodent models of kidney injury identify arginase as a marker of reparative macrophages (also known as M2 macrophages), which are essential for recovery from AKI.79, 80 Seminal work by Lee and colleagues first identified the dynamic and multi-faceted functions of macrophages in renal ischemic injury.81 They showed that pro-inflammatory (M1) macrophages augment the initial injury response but anti-inflammatory (M2) macrophages mediate repair. These M2 macrophages were characterized by increased expression of arginase and mannose receptor. Following this study, several groups recapitulated these specific functions for macrophages during nephrotoxic and obstructive nephropathy.80, 82, 83 Recent work by Zhang et al further identified renal tubule-derived colony stimulating factor (CSF-1) as a mediator of M2 macrophage polarization after AKI.84, 85 In a recent study, they examined the mechanism of IL-4/IL-13 cytokine-mediated M2 macrophage polarization in regulating renal injury responses.86 Furthermore, mice with myeloid specific arginase deletion were associated with increased inflammation and exacerbated fibrosis following injury.87 These studies highlight an essential role for arginase in preventing injury associated fibrosis.
Figure 3. Role of myeloid-and proximal tubule-specific Ferritin heavy chain deletion on fibrosis during reversible obstructive nephropathy.
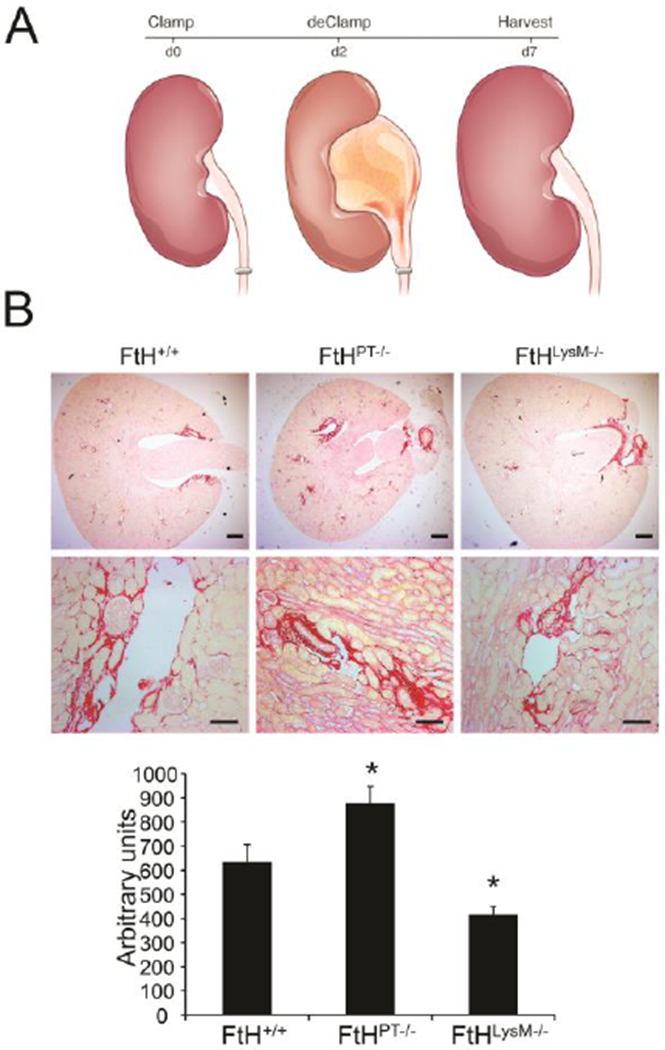
(A) Illustration of experimental design. Reversible obstructive nephropathy was induced by clamping one of the ureters for two days, following which the clamp was removed and animals were allowed to recover for five days. (B) Fibrosis following injury was determined by picrosirius staining on the obstructed kidney sections from wild-type (FtH+/+), proximal tubule specific FtH deletion mice (FtHPT−/−) and myeloid specific FtH deletion mice (ftHLysM−/−). Representative images of the stained kidney sections are shown in the upper panel (scale bar – 400 μm) and middle panel (scale bar – 100 μm). Lower panel: graphical representation of the collagen deposition in the kidneys. *p<0.05 vs FtH+/+ mice; n=5–6 per group. Reproduced with permission.10
Of note, deletion of FtH in mice led to a compensatory increase in serum FtL levels. An intriguing study demonstrated that renal allograft recipients with elevated serum ferritin levels at the time of transplant were associated with more positive allograft outcomes.88 A recent study also highlighted the usefulness of serum FtL as a reliable predictor of renal function recovery in patients with AKI.89 Taken together, expression of ferritin regulates the injury response following AKI. Future studies will need to further elucidate ferritin-mediated cellular responses during AKI.
Ferritin and CKD
Chronic kidney disease (CKD) is defined by sustained and impaired renal function that may result from a loss of functional nephrons. CKD is often associated with complications that increase mortality, such as iron deficiency anemia and cardiovascular disease.90–92 Kidneys are the main source of erythropoietin (EPO), a hormone that regulates red blood cell production. During CKD, reduced EPO levels and functional iron deficiency contribute to anemia. In fact, supplementation of EPO and iron have shown some beneficial effects. Serum ferritin, a measure of body iron stores, is reduced during iron deficiency and patients with low serum ferritin levels benefit from iron supplementation. However, anemic CKD patients exhibit a contradictory elevation in serum ferritin levels which confounds iron treatment strategies. This may be attributed to inflammation and oxidative stress, both of which occur in the majority of patients diagnosed with CKD. Inflammation associated with CKD increases ferritin and hepcidin independent of the body’s iron composition. Hepcidin prevents iron egress from cells and increases intracellular ferritin expression. As both of these iron regulatory molecules increase, total iron availability for red blood cell synthesis decreases, leading to a functional iron deficiency. Thus, the discrepancies in serum ferritin levels could result in a misleading diagnosis of iron stores during CKD.93, 94 Implications of elevated serum ferritin in patients with CKD is not well defined and needs to be analyzed in greater detail to effectively manage iron therapy during CKD.
Ferritin and Renal Cell Carcinoma
Changes in ferritin expression, independent of iron metabolism is associated with many types of cancers. These malignances include but are not limited to renal cell carcinoma (RCC), Hodgkin’s lymphoma, breast cancer, non-small-cell lung cancer and hepatocellular carcinoma (reviewed in 1, 95). RCC is the sixth most prevalent tumor worldwide and has the eighth highest death rate. Due to its high prevalence and death rate, detecting RCC at an early stage is critical for the survival of the patients. Renal FtH expression was shown to be an effective biomarker for RCC, where increased FtH correlated with worse outcomes.96 FtH may increase the antioxidant potential and thereby promote survival of cancerous cells. In contrast, emerging data demonstrate that FtH may inhibit tumor growth by interacting with survivin.97 Survivin is a regulatory protein that controls apoptosis, cell division and metastasis, and is often overexpressed in cancer cells. It has been shown that exposing cancer cells to a recombinant peptide that contains the survivin-interacting domain of FtH, leads to a decrease in growth and viability of the tumor cells. While this finding suggests additional functions of FtH, further studies must be conducted to fully understand the implications of FtH and FtL during tumorigenesis. Serum ferritin measurement has also been used as a tumor marker in RCC and exhibits a strong correlation with the stage of RCC and the kidney tumor volume.98, 99 Using ferritin as a marker for RCC may ensure an accurate diagnosis and enable development of a successful treatment regimen.
Ferritins in infection-associated kidney injury
FtH expression is required for a robust Hepatitis C virus infection, an effect that could be reversed by downregulating FtH.100 In this context, we identified that targeted deletion of macrophage FtH expression was associated with a survival advantage during a severe model of polymicrobial sepsis (cecal ligation and puncture) that was independent of gender.101 Specifically, we identified loss of FtH prevented the cytokine storm and AKI following sepsis induction. Importantly, we further determined that FtH deficiency did not alter the profile of lymphoid and myeloid populations or the bacterial killing activity of the immune cells. Instead, these mice were associated with a blunted, but not abated cytokine response following sepsis. Upon further analysis, it was determined that loss of FtH led to a compensatory increase in FtL levels. Infusion of recombinant FtL protein to wild-type mice prior to sepsis induction recapitulated the protective effects observed in myeloid FtH deficient mice (Table 2). These findings highlight the subunit specific functions of this evolutionarily conserved protein. More importantly, it is well-established that FtL levels in circulation increase during inflammation and yet the physiological significance of this elevation is not understood. It was presumed that circulating ferritin sequesters iron and contributes to the hypoferremic response during infection. However, our data provides evidence to suggest alternate functions for FtL. We identified that FtL prevents activation of infection-induced pro-inflammatory signaling via ERK and JNK activation. Indeed, another study demonstrated similar findings in an in vitro model of inflammation.102 Treatment of mice with hepcidin conferred similar protection during sepsis, which was associated with increased ferritin expression.103 Meyron-Holtz et al. demonstrated that FtL homopolymers are incapable of iron storage and that circulating serum ferritin is derived from myeloid cells and is predominantly comprised of FtL.27 Therefore, the protective response in transgenic mice deficient in myeloid FtH or following infusion of FtL are most likely independent of the hypoferremic response. In support of this theory, it was demonstrated that infusion of tissue ferritin (iron-rich heteropolymers) protected mice from severe infection (E.coli) but apoferritin (iron-devoid ferritin heteropolymers) failed to confer similar protection.104 Another recent study reported that apoferritin promoted tolerance against cecal ligation and puncture-induced sepsis and that this effect required ferroxidase activity of FtH. The latter study determined that FtH promoted tolerance but did not reduce bacteremia.105 Interestingly, an imbalance in the ratio of FtL and FtH is commonly found in patients with Parkinson’s disease, suggesting that a reduction in FtL may be pathogenic.106 In another infectious model, it was identified that Hepatitis E virus macro domain protein inhibits secretion of FtL into circulation.107 Cumulatively, we speculate that FtL mediates immunoregulation while FtH may be essential for the hypoferremic and anti-oxidant effects. These studies underscore the under-appreciated role of FtL in regulating inflammation and preventing AKI following infections.
Table 2.
Serum cytokine profile during sepsis
| Cytokine (pg/ml) | Vehicle sham | Vehicle CLP | FtH−/− CLP | FtL CLP |
|---|---|---|---|---|
| IFNγ | 0.23±0.03 | 4.58±0.84 | 0.58±0.09 | 0.56±0.16 |
| IL-1β | 1.15±0.08 | 34.35±7.37 | 2.31±0.31 | 3.098±1.1 |
| IL-2 | 0.54±0.09 | 27.18±5.07 | 1.09±0.33 | 0.47±0.11 |
| IL-4 | undet | 13.16±4.09 | 0.89±0.37 | 0.44±0.17 |
| IL-5 | 6.3±1.76 | 12.9±1.78 | 6.48±2.8 | 4.3±1.67 |
| IL-6 | 152±23.9 | 28905±3191 | 2045±300 | 1701±514 |
| IL-10 | 17.28±2.5 | 3075±711 | 49.43 ±5.32 | 292±125 |
| IL-12 | undet | 1229±330 | 88.53±14.3 | 49.4±11.4 |
| CXCL1 | 278.4±53 | 5466±238 | 2790±472 | 1306±376 |
| TNFα | 16.15±2.9 | 517±83 | 453±174 | 60.87±24 |
Abbreviations: CLP, cecal ligation and puncture; FtH−/−, ferritin heavy chain knockout; FtL, ferritin light chain; undet, undetectable levels. Adapted with permission.92
While resistance to infections is essential for host survival, emerging data identify tolerance as an equally important strategy to preserve host metabolism and promote survival. In this context, it was demonstrated that expression of FtH in hepatocytes prevented oxidative stress and tissue damage via inhibition of JNK signaling during Plasmodium chabaudi chabaudi (Pcc) infection in mice. While hepatic FtH expression did not influence parasite burden, this study provided the first evidence for a tolerogenic function of tissue FtH expression during infectious diseases.52 Indeed, similar findings were reported during polymicrobial sepsis. 105 In another seminal study, it was shown that FtH expression in proximal tubules of the kidney was essential to establish tolerance against malaria-induced AKI, which significantly impacts mortality.108 During malarial infection, hemolysis leads to increased circulating heme that burdens the kidney, ultimately leading to AKI. In this context, proximal tubular HO and FtH expression detoxifies heme and preserves kidney function.28, 108, 109 Loss of FtH was also shown to impair energy metabolism and worsen Mycobacterium tuberculosis infection in mice.110 Together, these studies highlight the role of FtH in mediating tolerance against infections.
Mechanisms of action
Ferritins are traditionally associated with iron sequestration and storage. In fact, each holosphere is capable of storing 4500 iron atoms. Therefore, the salutary effects of ferritin are presumed to be dependent on its ability to oxidize ferrous iron and prevent iron-mediated oxidative stress. It was also suggested that FtH may serve as an iron carrier protein and enable intercellular exchange.111 FtH has shown to increase p53 expression during oxidative stress.112 Ferritin subunits also regulate angiogenesis.113, 114 In an elegant study, Torti and colleagues demonstrated that both ferritin heavy and light chains interact with kininogen and prevent it from inducing endothelial apoptosis. They further confirmed that ferritin chains restored MAPK signaling, promoted endothelial cell survival and angiogenesis. On the other hand, it was demonstrated that FtH specifically interacts with CXC chemokine receptor 4 (CXCR4) and following activation with CXCL12, FtH translocates to the nucleus and regulates ERK signaling.115, 116 Ferritin localization in the nucleus has been reported in multiple cell types, including hepatocytes, neurons and epithelial cells (reviewed in 117). The functional significance of nuclear translocation in these various cell types is not completely understood and requires further study. In hepatocytes, FtH activates pro-inflammatory signaling and induces expression of iNOS.10, 118 FtH has also shown to inhibit JNK activation.52 In contrast, FtH has shown to prevent vascular calcification.119 Recent work also highlights the role of ferritin in homeostatic regulation of heat and energy production.120 While majority of ferritin related research is focused on the heavy chain due to its ferroxidase activity, few studies delineated a role for FtL underscore in regulating cellular function. FtL is catalytically inactive and cannot store iron in the absence of FtH. Using an in vitro system to specifically delete FtL, Cozzi et al identified that FtL induces cellular proliferation that is independent of iron metabolism.121 Work by us and others elucidated that macrophage FtL prevents lipopolysaccharide-induced activation of MAPK pathways and inflammatory signaling.101, 102
Implications of ferritin in body fluids
Seminal work by Addison and Jacobs in the 1970s demonstrated the usefulness of ferritin measurement in the serum to assess body iron stores. 122, 123 They demonstrated that serum ferritin levels were lower in patients with iron deficiency and conversely increased in patients with iron overload. These remarkable findings revolutionized the field and provided clinicians with a convenient and reliable tool to evaluate and treat iron related disorders. Serum ferritin has since remained mainstay for evaluation of systemic iron stores despite evidence suggesting that ferritin is elevated during infection and malignancies. These underlying co-morbid conditions often confound the interpretation of serum ferritin levels (reviewed in 94, 124). Until recently, it was assumed that serum ferritin is a leakage product, derived from damaged cells and studies demonstrate that serum levels correlate with disease severity.125–127 However, the converse association was also demonstrated indicating that ferritin may serve as a protective strategy.88, 89, 101, 128 While it is possible that damaged cells contribute to an increase in ferritin during disease states, emerging evidence support that ferritin is actively secreted by uninjured cells as a normal physiological process.27, 129 It is predominantly secreted by macrophages (and to a smaller extent, by renal proximal tubules) via non-classical vesicular pathways. More importantly, they identified that serum ferritin is mainly comprised of FtL homopolymers. The ferroxidase activity of FtH is essential for iron storage and therefore FtL homopolymers are devoid of iron. Serum also contains a small proportion of heteropolymeric ferritin which is capable of iron sequestration. The presence of these iron-rich ferritins may contribute to the low but detectable iron content in serum ferritin. It is proposed that iron-rich ferritin is rapidly removed from circulation by binding to FtH receptors and may serve as a mechanism of iron redistribution.111 There are currently several receptors that recognize FtH such as TFR and TIM-2 that are widely expressed on multiple cell types.130, 131 However, the only known receptor for FtL is SCARA5.132, 133 Earlier studies that demonstrated that iron saturation of serum ferritin is 5% in healthy volunteers and did not significantly increase in patients with iron overload.134–136 These findings suggest that the elevated ferritin in the serum is mainly comprised of iron-devoid FtL homopolymers. In a recent article, Lan and Zenobi utilized mass spectrometry (MALDI-TOF-MS) to accurately quantitate the iron content of ferritin from multiple sources.137 Thus, measurement of ferritin per se may not reliably inform total iron content, especially under inflammatory conditions. These studies raise several unanswered questions that require further investigation such as the purpose of FtL in circulation, its effector cells and its clearance from circulation.
Ferritin is also detected in the urine (or urinary exosomes) of humans and correlates well with serum ferritin levels and body iron stores in healthy individuals.136, 138–140 These studies provide a novel, non-invasive method to quantitate ferritin. This is particularly relevant to the neonatal population, specifically pre-term babies, where phlebotomy for serum measurements deplete nearly one-tenth of their total blood volume. In fact, a recent study compared serum ferritin to urinary ferritin levels and provided evidence for its clinical utility.136 It should also be noted that the iron content of urinary ferritin was low and similar to that of serum ferritin. Given this evidence, we predict that urinary ferritin levels will increase during inflammation, mirroring the serum levels. Urinary ferritin levels were also found to be high in patients with hemolytic disorders. It was demonstrated that patients with chronic hemolytic anemia exhibit a disproportionately higher ratio of urine to serum ferritin compared to healthy volunteers.138 In these patients, increased delivery of hemoglobin to the renal tubular epithelium leads to an induction of ferritin, which may subsequently be excreted into the urinary compartment. Therefore, future studies will need to examine the levels of urinary ferritin in the context of kidney disease.
Ferritin in renal iron trafficking
In the kidney, ferritin heavy and light chains are predominantly expressed in the proximal tubules.27, 28 Importantly, both ferritin and ferroportin are localized near the apical membrane, suggesting a role for these proteins in iron uptake from the filtrate. This may explain the lack of iron in urine in healthy subjects. In this context, deletion of FtH led to aggravated kidney injury following cisplatin nephrotoxicity and rhabdomyolysis and was associated with significantly increased iron excretion. We also showed that deletion of FtH from proximal tubules led to a reduction in ferroportin expression in these cells. We further identified that the apical localization of ferroportin enables iron uptake in vitro and in vivo. These findings propose a role for FtH in mediating iron trafficking and regulating ferroportin expression.28 Following this study, several reports published conflicting data that demonstrate both apical and basolateral localization of ferroportin, confounding earlier observations.141–144 The exact role of ferroportin in renal iron trafficking is still unclear.
During development, transferrin delivers iron to the uretic bud whereas heteropolymeric ferritin delivers iron to the stroma and capsule in a transferrin-independent manner. Uptake of ferritin is mediated by Scavenger Receptor Class A Member 5 (SCARA5), which binds FtL.133 Another study further confirmed the role for SCARA5 in FtL trafficking in human and rodent retinas and demonstrated that a reduction in this receptor was associated with retinopathy.132 Recently, it was shown that ferritin mediates intra-cellular and inter-cellular iron transport in the testis.145 The same research group identified that dietary induced iron overload led to an increase in iron content of proximal tubules and was associated with redistribution of ferritin from the apical to basolateral compartment. They suggested that such relocation of ferritin may promote its secretion from the basolateral side. In contrast, they identified that intra-peritoneal administration of iron to mice led to a marked increase in iron accumulation and ferritin expression in the interstitium, specifically in the macrophages.146 These intriguing data underscore our limited understanding of ferritin in renal iron trafficking and homeostasis.
Conclusion
In this review, we highlighted the salient features of ferritin during physiology and pathophysiology. While it is known that ferritins are expressed ubiquitously in most tissues, we intentionally focused on the role of ferritins in kidney health and disease. Previously, it was presumed that ferritin only functioned as an iron sequestering protein. However, recent studies provide evidence to support additional roles for the ferritin subunits that may be unrelated to iron sequestration. It is clear that our understanding of this evolutionarily conserved protein is still in its infancy. With the advent of gene editing, we are now able to specifically target (delete or overexpress) individual subunits of ferritin in a tissue-specific manner. The use of these novel transgenic mice may shed more light on the role of ferritins in cellular function and systemic homeostasis. This review not only provides a brief overview of the functions of ferritins in health and disease but also discusses the gaps in knowledge that warrant further investigation.
Acknowledgments
Financial Support: This work was supported in part by a NIH grant (DK103931, to SB) and an ASN grant (Carl W. Gottschalk award to SB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work is from The University of Alabama at Birmingham.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Arosio P, and Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33:457–63. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PM, and Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. [DOI] [PubMed] [Google Scholar]
- 3.Crichton RR. Structure and function of ferritin. Angew Chem Int Ed Engl. 1973;12:57–65. [DOI] [PubMed] [Google Scholar]
- 4.Lawson DM, Artymiuk PJ, Yewdall SJ, Smith JM, Livingstone JC, Treffry A, et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991;349:541–4. [DOI] [PubMed] [Google Scholar]
- 5.Ford GC, Harrison PM, Rice DW, Smith JM, Treffry A, White JL, et al. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984;304:551–65. [DOI] [PubMed] [Google Scholar]
- 6.Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Bio chem. 1987;56:289–315. [DOI] [PubMed] [Google Scholar]
- 7.St Pierre TG, Bell SH, Dickson DP, Mann S, Webb J, Moore GR, et al. Mossbauer spectroscopic studies of the cores of human, limpet and bacterial ferritins. Biochim Biophys Acta. 1986;870:127–34. [DOI] [PubMed] [Google Scholar]
- 8.Ketola-Pirie CA. Characterization of an insect ferritin subunit synthesized in a cell-free system. Biochem Cell Biol. 1990;68:1005–11. [DOI] [PubMed] [Google Scholar]
- 9.Andrews SC, Arosio P, Bottke W, Briat JF, von Darl M, Harrison PM, et al. Structure, function, and evolution of ferritins. J Inorg Biochem. 1992;47:161–74. [DOI] [PubMed] [Google Scholar]
- 10.Bolisetty S, Zarjou A, Hull TD, Traylor AM, Perianayagam A, Joseph R, et al. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int. 2015;88:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santambrogio P, Levi S, Arosio P, Palagi L, Vecchio G, Lawson DM, et al. Evidence that a salt bridge in the light chain contributes to the physical stability difference between heavy and light human ferritins. J Biol Chem. 1992;267:14077–83. [PubMed] [Google Scholar]
- 12.Yachou AK, Renaudie F, Guenet JL, Simon-Chazottes D, Jones R, Grandchamp B, et al. Mouse ferritin H multigene family is polymorphic and contains a single multiallelic functional gene located on chromosome 19. Genomics. 1991;10:531–8. [DOI] [PubMed] [Google Scholar]
- 13.Filie JD, Buckler CE, and Kozak CA. Genetic mapping of the mouse ferritin light chain gene and 11 pseudogenes on 11 mouse chromosomes. Mamm Genome. 1998;9:111–3. [DOI] [PubMed] [Google Scholar]
- 14.Worwood M, Brook JD, Cragg SJ, Hellkuhl B, Jones BM, Perera P, et al. Assignment of human ferritin genes to chromosomes 11 and 19q13.3----19qter. Hum Genet. 1985;69:371–4. [DOI] [PubMed] [Google Scholar]
- 15.Caskey JH, Jones C, Miller YE, and Seligman PA. Human ferritin gene is assigned to chromosome 19. Proc Natl Acad Sci U S A. 1983;80:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi S, Luzzago A, Cesareni G, Cozzi A, Franceschinelli F, Albertini A, et al. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. 1988;263:18086–92. [PubMed] [Google Scholar]
- 17.Boyd D, Vecoli C, Belcher DM, Jain SK, and Drysdale JW. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985;260:11755–61. [PubMed] [Google Scholar]
- 18.Levi S, Santambrogio P, Albertini A, and Arosio P. Human ferritin H-chains can be obtained in non-assembled stable forms which have ferroxidase activity. FEBS Lett. 1993;336:309–12. [DOI] [PubMed] [Google Scholar]
- 19.Levi S, Yewdall SJ, Harrison PM, Santambrogio P, Cozzi A, Rovida E, et al. Evidence of H-and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem J. 1992;288 ( Pt 2):591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi S, Santambrogio P, Cozzi A, Rovida E, Corsi B, Tamborini E, et al. The role of the L-chain in ferritin iron incorporation. Studies of homo and heteropolymers. J Mol Biol. 1994;238:649–54. [DOI] [PubMed] [Google Scholar]
- 21.Kwak EL, Larochelle DA, Beaumont C, Torti SV, and Torti FM. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem. 1995;270:15285–93. [DOI] [PubMed] [Google Scholar]
- 22.Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, and Arosio P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J Biol Chem. 2000;275:25122–9. [DOI] [PubMed] [Google Scholar]
- 23.Sammarco MC, Ditch S, Banerjee A, and Grabczyk E. Ferritin L and H subunits are differentially regulated on a post-transcriptional level. J Biol Chem. 2008;283:4578–87. [DOI] [PubMed] [Google Scholar]
- 24.Theil EC. Ferritin: at the crossroads of iron and oxygen metabolism. J Nutr. 2003;133:1549S–53S. [DOI] [PubMed] [Google Scholar]
- 25.Arosio P, Yokota M, and Drysdale JW. Structural and immunological relationships of isoferritins in normal and malignant cells. Cancer Res. 1976;36:1735–9. [PubMed] [Google Scholar]
- 26.Arosio P, Ingrassia R, and Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–99. [DOI] [PubMed] [Google Scholar]
- 27.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–84. [DOI] [PubMed] [Google Scholar]
- 28.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest. 2013;123:4423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S, Moriconi F, Naz N, Sultan S, Sheikh N, Ramadori G, et al. Ferritin L and Ferritin H are differentially located within hepatic and extra hepatic organs under physiological and acute phase conditions. Int J Clin Exp Pathol. 2013;6:622–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Garringer HJ, Goodwin CB, Richine B, Acton A, VanDuyn N, et al. Systemic and cerebral iron homeostasis in ferritin knock-out mice. PLoS One. 2015;10:e0117435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, et al. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275:3021–4. [DOI] [PubMed] [Google Scholar]
- 32.Darshan D, Vanoaica L, Richman L, Beermann F, and Kuhn LC. Conditional deletion of ferritin H in mice induces loss of iron storage and liver damage. Hepatology. 2009;50:852–60. [DOI] [PubMed] [Google Scholar]
- 33.Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, et al. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem. 2001;276:24437–40. [DOI] [PubMed] [Google Scholar]
- 34.Drysdale J, Arosio P, Invernizzi R, Cazzola M, Volz A, Corsi B, et al. Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol Dis. 2002;29:376–83. [DOI] [PubMed] [Google Scholar]
- 35.Yang M, Yang H, Guan H, Bellier JP, Zhao S, and Tooyama I. Mapping of mitochondrial ferritin in the brainstem of Macaca fascicularis. Neuroscience. 2016;328:92–106. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Wang P, Wu Q, Xie L, Cui Y, Li H, et al. The Construction and Characterization of Mitochondrial Ferritin Overexpressing Mice. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Yang M, Guan H, Liu Z, Zhao S, Takeuchi S, et al. Mitochondrial ferritin in neurodegenerative diseases. Neurosci Res. 2013;77:1–7. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Yang H, Zhao S, Sato H, Konishi Y, Beach TG, et al. Expression and localization of mitochondrial ferritin mRNA in Alzheimer’s disease cerebral cortex. PLoS One. 2011;6:e22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder AM, Wang X, Patton SM, Arosio P, Levi S, Earley CJ, et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 2009;68:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi ZH, Nie G, Duan XL, Rouault T, Wu WS, Ning B, et al. Neuroprotective mechanism of mitochondrial ferritin on 6-hydroxydopamine-induced dopaminergic cell damage: implication for neuroprotection in Parkinson’s disease. Antioxid Redox Signal. 2010;13:783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenirri S, Santambrogio P, Setaccioli M, Erba BG, Pia Manitto M, Rovida E, et al. Study of FTMT and ABCA4 genes in a patient affected by age-related macular degeneration: identification and analysis of new mutations. Clin Chem Lab Med. 2012;50:1021–9. [DOI] [PubMed] [Google Scholar]
- 42.Maccarinelli F, Regoni M, Carmona F, Poli M, Meyron-Holtz EG, and Arosio P. Mitochondrial ferritin deficiency reduces male fertility in mice. Reprod Fertil Dev. 2017;29:2005–10. [DOI] [PubMed] [Google Scholar]
- 43.Torti FM, and Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–16. [DOI] [PubMed] [Google Scholar]
- 44.Ryu MS, Duck KA, and Philpott CC. Ferritin iron regulators, PCBP1 and NCOA4, respond to cellular iron status in developing red cells. Blood Cells Mol Dis. 2018;69:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi H, Bencze KZ, Stemmler TL, and Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mancias JD, Wang X, Gygi SP, Harper JW, and Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torti SV, Kwak EL, Miller SC, Miller LL, Ringold GM, Myambo KB, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638–44. [PubMed] [Google Scholar]
- 48.Hirayama M, Kohgo Y, Kondo H, Shintani N, Fujikawa K, Sasaki K, et al. Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology. 1993;18:874–80. [DOI] [PubMed] [Google Scholar]
- 49.Drapier JC, Hirling H, Wietzerbin J, Kaldy P, and Kuhn LC. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993;12:3643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domachowske JB, Rafferty SP, Singhania N, Mardiney M 3rd, and Malech HL. Nitric oxide alters the expression of gamma-globin, H-ferritin, and transferrin receptor in human K562 cells at the posttranscriptional level. Blood. 1996;88:2980–8. [PubMed] [Google Scholar]
- 51.Tsuji Y, Ayaki H, Whitman SP, Morrow CS, Torti SV, and Torti FM. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol Cell Biol. 2000;20:5818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gozzelino R, Andrade BB, Larsen R, Luz NF, Vanoaica L, Seixas E, et al. Metabolic adaptation to tissue iron overload confers tolerance to malaria. Cell Host Microbe. 2012;12:693–704. [DOI] [PubMed] [Google Scholar]
- 53.Kidane TZ, Sauble E, and Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol. 2006;291:C445–55. [DOI] [PubMed] [Google Scholar]
- 54.De Domenico I, Vaughn MB, Li L, Bagley D, Musci G, Ward DM, et al. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25:5396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–79. [DOI] [PubMed] [Google Scholar]
- 56.Mancias JD, Pontano Vaites L, Nissim S, Biancur DE, Kim AJ, Wang X, et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellelli R, Federico G, Matte A, Colecchia D, Iolascon A, Chiariello M, et al. NCOA4 Deficiency Impairs Systemic Iron Homeostasis. Cell Rep. 2016;14:411–21. [DOI] [PubMed] [Google Scholar]
- 58.Santana-Codina N, Gableske S, Rey MQD, Malachowska B, Jedrychowski MP, Biancur DE, et al. NCOA4 maintains murine erythropoiesis via cell autonomous and non-autonomous mechanisms. Haematologica. 2019;104:1342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adedoyin O, Boddu R, Traylor A, Lever JM, Bolisetty S, George JF, et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol. 2018;314:F702–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111:16836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guerrero-Hue M, Garcia-Caballero C, Palomino-Antolin A, Rubio-Navarro A, Vazquez-Carballo C, Herencia C, et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019;33:8961–75. [DOI] [PubMed] [Google Scholar]
- 65.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolisetty S, Zarjou A, and Agarwal A. Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury. Am J Kidney Dis. 2017;69:531–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal A, and Bolisetty S. Adaptive responses to tissue injury: role of heme oxygenase-1. Trans Am Clin Climatol Assoc. 2013;124:111–22. [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson ACM, Delrow JJ, and Zager RA. Tin protoporphyrin activates the oxidant-dependent NRF2-cytoprotective pathway and mitigates acute kidney injury. Transl Res. 2017;186:1–18. [DOI] [PubMed] [Google Scholar]
- 69.Hatcher HC, Tesfay L, Torti SV, and Torti FM. Cytoprotective Effect of Ferritin H in Renal Ischemia Reperfusion Injury. PLoS One. 2015;10:e0138505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, et al. Hepcidin Mitigates Renal Ischemia-Reperfusion Injury by Modulating Systemic Iron Homeostasis. J Am Soc Nephrol. 2015;26:2800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Swelm RPL, Vos M, Verhoeven F, Thevenod F, and Swinkels DW. Endogenous hepcidin synthesis protects the distal nephron against hemin and hemoglobin mediated necroptosis. Cell Death Dis. 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Swelm RP, Wetzels JF, Verweij VG, Laarakkers CM, Pertijs JC, van der Wijst J, et al. Renal Handling of Circulating and Renal-Synthesized Hepcidin and Its Protective Effects against Hemoglobin-Mediated Kidney Injury. J Am Soc Nephrol. 2016;27:2720–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubio-Navarro A, Vazquez-Carballo C, Guerrero-Hue M, Garcia-Caballero C, Herencia C, Gutierrez E, et al. Nrf2 Plays a Protective Role Against Intravascular Hemolysis-Mediated Acute Kidney Injury. Front Pharmacol. 2019;10:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Zheng X, Zhang J, Zhao S, Wang Z, Wang F, et al. Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury. Am J Physiol Renal Physiol. 2018;315:F1042–F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker VJ, and Agarwal A. Targeting Iron Homeostasis in Acute Kidney Injury. Semin Nephrol. 2016;36:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scindia Y, Leeds J, and Swaminathan S. Iron Homeostasis in Healthy Kidney and its Role in Acute Kidney Injury. Semin Nephrol. 2019;39:76–84. [DOI] [PubMed] [Google Scholar]
- 77.Leaf DE, and Swinkels DW. Catalytic iron and acute kidney injury. Am J Physiol Renal Physiol. 2016;311:F871–F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baliga R, Ueda N, Walker PD, and Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–97. [DOI] [PubMed] [Google Scholar]
- 79.Cao Q, Harris DC, and Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda). 2015;30:183–94. [DOI] [PubMed] [Google Scholar]
- 80.Huen SC, and Cantley LG. Macrophages in Renal Injury and Repair. Annu Rev Physiol. 2017;79:449–69. [DOI] [PubMed] [Google Scholar]
- 81.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, et al. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol. 2015;26:1363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, and Cantley LG. GM-CSF Promotes Macrophage Alternative Activation after Renal Ischemia/Reperfusion Injury. J Am Soc Nephrol. 2015;26:1334–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, et al. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int. 2015;88:1274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang MZ, Wang X, Wang Y, Niu A, Wang S, Zou C, et al. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017;91:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaugier C, Amano MT, Chemouny JM, Dussiot M, Berrou C, Matignon M, et al. Serum Iron Protects from Renal Postischemic Injury. J Am Soc Nephrol. 2017;28:3605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dimitrijevic ZM, Salinger-Martinovic SS, Jankovic RJ, and Mitic BP. Elevated Serum Ferritin Levels Are Predictive of Renal Function Recovery among Patients with Acute Kidney Injury. Tohoku J Exp Med. 2019;248:63–71. [DOI] [PubMed] [Google Scholar]
- 90.Babitt JL, and Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zumbrennen-Bullough K, and Babitt JL. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant. 2014;29:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Webster AC, Nagler EV, Morton RL, and Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–52. [DOI] [PubMed] [Google Scholar]
- 93.Ueda N, and Takasawa K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalantar-Zadeh K, Kalantar-Zadeh K, and Lee GH. The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol. 2006;1 Suppl 1:S9–18. [DOI] [PubMed] [Google Scholar]
- 95.Alkhateeb AA, and Connor JR. The significance of ferritin in cancer: antioxidation, inflammation and tumorigenesis. Biochim Biophys Acta. 2013;1836:245–54. [DOI] [PubMed] [Google Scholar]
- 96.Huang H, Qiu Y, Huang G, Zhou X, Zhou X, and Luo W. Value of Ferritin Heavy Chain (FTH1) Expression in Diagnosis and Prognosis of Renal Cell Carcinoma. Med Sci Monit. 2019;25:3700–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weiss A, Brill B, Borghouts C, Delis N, Mack L, and Groner B. Survivin inhibition by an interacting recombinant peptide, derived from the human ferritin heavy chain, impedes tumor cell growth. J Cancer Res Clin Oncol. 2012;138:1205–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh KJ, Singh SK, Suri A, Vijjan V, Goswami AK, and Khullar M. Serum ferritin in renal cell carcinoma: effect of tumor size, volume grade, and stage. Indian J Cancer. 2005;42:197–200. [PubMed] [Google Scholar]
- 99.Essen A, Ozen H, Ayhan A, Ergen A, Tasar C, and Remzi F. Serum ferritin: a tumor marker for renal cell carcinoma. J Urol. 1991;145:1134–7. [DOI] [PubMed] [Google Scholar]
- 100.Mancone C, Montaldo C, Santangelo L, Di Giacomo C, Costa V, Amicone L, et al. Ferritin heavy chain is the host factor responsible for HCV-induced inhibition of apoB-100 production and is required for efficient viral infection. J Proteome Res. 2012;11:2786–97. [DOI] [PubMed] [Google Scholar]
- 101.Zarjou A, Black LM, McCullough KR, Hull TD, Esman SK, Boddu R, et al. Ferritin Light Chain Confers Protection Against Sepsis-Induced Inflammation and Organ Injury. Front Immunol. 2019;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan Y, Zhang J, Cai L, Wang S, Liu C, Zhang Y, et al. The effect of anti-inflammatory properties of ferritin light chain on lipopolysaccharide-induced inflammatory response in murine macrophages. Biochim Biophys Acta. 2014;1843:2775–83. [DOI] [PubMed] [Google Scholar]
- 103.Scindia Y, Wlazlo E, Leeds J, Loi V, Ledesma J, Cechova S, et al. Protective Role of Hepcidin in Polymicrobial Sepsis and Acute Kidney Injury. Front Pharmacol. 2019;10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lipinski P, Jarzabek Z, Broniek S, and Zagulski T. Protective effect of tissue ferritins in experimental Escherichia coli infection of mice in vivo. Int J Exp Pathol. 1991;72:623–30. [PMC free article] [PubMed] [Google Scholar]
- 105.Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, et al. Metabolic Adaptation Establishes Disease Tolerance to Sepsis. Cell. 2017;169:1263–75 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koziorowski D, Friedman A, Arosio P, Santambrogio P, and Dziewulska D. ELISA reveals a difference in the structure of substantia nigra ferritin in Parkinson’s disease and incidental Lewy body compared to control. Parkinsonism Relat Disord. 2007;13:214–8. [DOI] [PubMed] [Google Scholar]
- 107.Ojha NK, and Lole KS. Hepatitis E virus ORF1 encoded macro domain protein interacts with light chain subunit of human ferritin and inhibits its secretion. Mol Cell Biochem. 2016;417:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramos S, Carlos AR, Sundaram B, Jeney V, Ribeiro A, Gozzelino R, et al. Renal control of disease tolerance to malaria. Proc Natl Acad Sci U S A. 2019;116:5681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim J, Zarjou A, Traylor AM, Bolisetty S, Jaimes EA, Hull TD, et al. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int. 2012;82:278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reddy VP, Chinta KC, Saini V, Glasgow JN, Hull TD, Traylor A, et al. Ferritin H Deficiency in Myeloid Compartments Dysregulates Host Energy Metabolism and Increases Susceptibility to Mycobacterium tuberculosis Infection. Front Immunol. 2018;9:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyron-Holtz EG, Moshe-Belizowski S, and Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm (Vienna). 2011;118:337–47. [DOI] [PubMed] [Google Scholar]
- 112.Lee JH, Jang H, Cho EJ, and Youn HD. Ferritin binds and activates p53 under oxidative stress. Biochem Biophys Res Commun. 2009;389:399–404. [DOI] [PubMed] [Google Scholar]
- 113.Torti SV, and Torti FM. Human H-kininogen is a ferritin-binding protein. J Biol Chem. 1998;273:13630–5. [DOI] [PubMed] [Google Scholar]
- 114.Coffman LG, Parsonage D, D’Agostino R Jr., Torti FM, and Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci U S A. 2009;106:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, Rubin JB, et al. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29:2534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li R, Luo C, Mines M, Zhang J, and Fan GH. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281:37616–27. [DOI] [PubMed] [Google Scholar]
- 117.Alkhateeb AA, and Connor JR. Nuclear ferritin: A new role for ferritin in cell biology. Biochim Biophys Acta. 2010;1800:793–7. [DOI] [PubMed] [Google Scholar]
- 118.Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology. 2009;49:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balla J, Balla G, and Zarjou A. Ferritin in Kidney and Vascular Related Diseases: Novel Roles for an Old Player. Pharmaceuticals (Basel). 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blankenhaus B, Braza F, Martins R, Bastos-Amador P, Gonzalez-Garcia I, Carlos AR, et al. Ferritin regulates organismal energy balance and thermogenesis. Mol Metab. 2019;24:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, and Arosio P. Analysis of the biologic functions of H-and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103:2377–83. [DOI] [PubMed] [Google Scholar]
- 122.Jacobs A, Miller F, Worwood M, Beamish MR, and Wardrop CA. Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. Br Med J. 1972;4:206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Addison GM, Beamish MR, Hales CN, Hodgkins M, Jacobs A, and Llewellin P. An immunoradiometric assay for ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. J Clin Pathol. 1972;25:326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang W, Knovich MA, Coffman LG, Torti FM, and Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010;1800:760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim T, Streja E, Soohoo M, Rhee CM, Eriguchi R, Kim TW, et al. Serum Ferritin Variations and Mortality in Incident Hemodialysis Patients. Am J Nephrol. 2017;46:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kell DB, and Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6:748–73. [DOI] [PubMed] [Google Scholar]
- 127.Garcia PC, Longhi F, Branco RG, Piva JP, Lacks D, and Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007;96:1829–31. [DOI] [PubMed] [Google Scholar]
- 128.Shoji T, Niihata K, Fukuma S, Fukuhara S, Akizawa T, and Inaba M. Both low and high serum ferritin levels predict mortality risk in hemodialysis patients without inflammation. Clin Exp Nephrol. 2017;21:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood. 2018;131:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010;107:3505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen TT, Li L, Chung DH, Allen CD, Torti SV, Torti FM, et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med. 2005;202:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mendes-Jorge L, Ramos D, Valenca A, Lopez-Luppo M, Pires VM, Catita J, et al. L-ferritin binding to scara5: a new iron traffic pathway potentially implicated in retinopathy. PLoS One. 2014;9:e106974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Worwood M, Aherne W, Dawkins S, and Jacobs A. The characteristics of ferritin from human tissues, serum and blood cells. Clin Sci Mol Med. 1975;48:441–51. [DOI] [PubMed] [Google Scholar]
- 135.Nielsen P, Gunther U, Durken M, Fischer R, and Dullmann J. Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. J Lab Clin Med. 2000;135:413–8. [DOI] [PubMed] [Google Scholar]
- 136.Bahr TM, Christensen RD, Ward DM, Meng F, Jackson LK, Doyle K, et al. Ferritin in serum and urine: A pilot study. Blood Cells Mol Dis. 2019;76:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lan J, and Zenobi R. Characterizing the iron loading pattern of ferritin using high-mass MALDI-MS. Rapid Commun Mass Spectrom. 2019. [DOI] [PubMed] [Google Scholar]
- 138.Lipschitz DA, Allegre A, and Cook JD. The clinical significance of ferritinuria. Blood. 1980;55:260–4. [PubMed] [Google Scholar]
- 139.Ishikawa K, Narita O, Saito H, and Kato K. Determination of ferritin in urine and in serum of normal adults with a sensitive enzyme immunoassay. Clin Chim Acta. 1982;123:73–81. [DOI] [PubMed] [Google Scholar]
- 140.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolff NA, Liu W, Fenton RA, Lee WK, Thevenod F, and Smith CP. Ferroportin 1 is expressed basolaterally in rat kidney proximal tubule cells and iron excess increases its membrane trafficking. J Cell Mol Med. 2011;15:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Veuthey T, D’Anna MC, and Roque ME. Role of the kidney in iron homeostasis: renal expression of Prohepcidin, Ferroportin, and DMT1 in anemic mice. Am J Physiol Renal Physiol. 2008;295:F1213–21. [DOI] [PubMed] [Google Scholar]
- 143.van Raaij S, van Swelm R, Bouman K, Cliteur M, van den Heuvel MC, Pertijs J, et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci Rep. 2018;8:9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Starzynski RR, Canonne-Hergaux F, Lenartowicz M, Krzeptowski W, Willemetz A, Stys A, et al. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem J. 2013;449:69–78. [DOI] [PubMed] [Google Scholar]
- 145.Leichtmann-Bardoogo Y, Cohen LA, Weiss A, Marohn B, Schubert S, Meinhardt A, et al. Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am J Physiol Endocrinol Metab. 2012;302:E1519–30. [DOI] [PubMed] [Google Scholar]
- 146.Weiss A, Spektor L, L AC, Lifshitz L, Magid Gold I, Zhang DL, et al. Orchestrated regulation of iron trafficking proteins in the kidney during iron overload facilitates systemic iron retention. PLoS One. 2018;13:e0204471. [DOI] [PMC free article] [PubMed] [Google Scholar]


