Abstract
Acute kidney injury (AKI) portends a poor clinical prognosis and increases the risk for the development of chronic kidney disease (CKD). Currently, there are no therapies to treat AKI or prevent its progression to CKD. Wnt/β-catenin is a critical regulator of kidney development that is up-regulated after injury. Most of the literature support a beneficial role for Wnt/β-catenin in AKI but suggest that this pathway promotes the progression of tubulointerstitial fibrosis, the hallmark of CKD progression. We review Wnt/β-catenin’s role in renal injury with a focus on its potential as a therapeutic target in AKI and AKI to CKD transition.
Keywords: acute kidney injury, beta-catenin, chronic kidney disease
Introduction
Acute kidney injury (AKI) is increasing in incidence and is associated with longer hospitalizations and reduced survival1. AKI is also an independent predictor for the development of chronic kidney disease (CKD), end-stage renal disease (ESRD), and a risk factor for the progression of pre-existing CKD2–4. According to the United States Renal Data System, CKD affects 14.8% of Americans, greatly raises the risk of cardiovascular disease and stroke, and places a huge economic burden on the healthcare system once it progresses to ESRD5. Thus, treatment of AKI and the prevention of AKI progression to CKD would have an enormous benefit on morbidity, mortality, and the cost of healthcare. Unfortunately, there are no drugs that can either prevent AKI or prevent the progression of AKI to CKD.
AKI injures many cells but primarily targets the tubule epithelium given this compartment’s high energetic demands. After injury, the interplay of injured tubules with inflammatory cells, the microvasculature, and fibroblasts is important in the response to AKI and its transition to CKD. Growth factors mediate many of the cellular responses, both reparative and injurious, following AKI. Many of these growth factors, such as Wnt/β-catenin, play a critical role in renal development, have little activity in the uninjured adult kidney, but become re-expressed after injury. This review focuses on the role of Wnt/β-catenin in AKI and AKI to CKD and its potential as a therapeutic target.
Wnt/β-catenin Signaling
The protein β-catenin has dual functions, serving as both a structural protein and a transcription factor, and the specific role depends upon its cellular location and the presence of Wnt ligands. Membrane-bound β-catenin plays a structural role as part of the adherens junction complex that, together with α-catenin and cadherins, mediate cell-cell interactions. In the uninjured kidney, cytosolic β-catenin is usually targeted for degradation by the destruction complex consisting of axin, adenomatous polyposis coli (APC), glycogen synthase kinase-3β (GSK-3β), and casein kinase 16. β-catenin is phosphorylated by GSK-3β, which then targets it for ubiquitination and proteasomal degradation7. However, ligands from the Wnt family (Wnt is a portmanteau named for the Drosophila homologue Wingless and the Int-1 integration site in murine breast tumors) can rescue cytosolic β-catenin from degradation (Figure 1). There are 19 mammalian Wnt ligands, and these bind to a frizzled (Fzd)/low density lipoprotein receptor-related protein (LRP) receptor complex8. This binding recruits the protein Dishevelled to facilitate removal of the destruction complex away from β-catenin, thus allowing β-catenin to accumulate in the nucleus and act as a transcription factor to affect gene expression. It is important to note that Wnt ligands can also affect proximal/distal epithelial polarity, or planar cell polarity, but this signaling occurs independent of the β-catenin-dependent (i.e. canonical Wnt signaling) pathway. This review focuses specifically on the canonical Wnt/ β-catenin signaling pathway in the context of renal injury and repair.
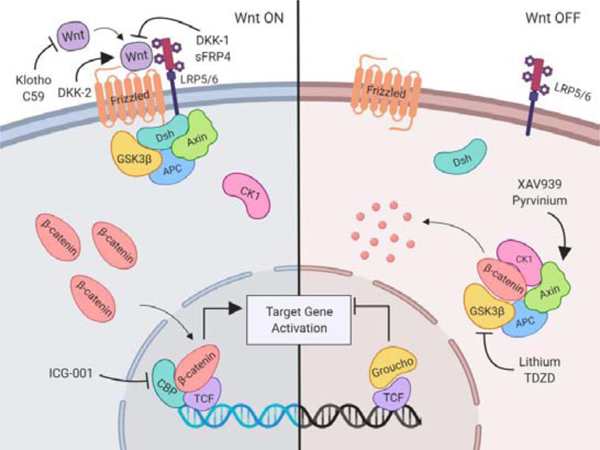
Figure 1. Canonical Wnt/β-catenin signaling pathway and pharmacologic pathway mediators.
Wnt ligands bind online but to Frizzled and LRP5/6 leading to Dsh recruitment of the β-catenin destruction complex, including GSK3-β, Axin, and APC. This prevents the degradation of β-catenin, allowing for cytosolic accumulation and nuclear translocation. In the nucleus, β-catenin and CBP form a complex to displace Groucho and bind to TCF, activating transcription of TCF family target genes. Early pathway inhibitors include C59 which prevents secretion of Wnt ligands; Klotho binds to and sequesters Wnt ligands86; DKK-1 binds to LRP5/6 to prevent Wnt binding; sFRP4 binds Wnt and Frizzled to disrupt signaling; and DKK-2 binds to LRP5/6 to enhance binding of Wnt. Several pharmacologic agents alter the activity of components of the destruction complex, including lithium and TDZD-8, which inhibit the activity of GSK3-β and GSK-3, respectively, and prevents β-catenin degradation. Pyrvinium prevents Axin degradation and XAV939, a tankyrase inhibitor, stabilizes cytosolic Axin with both compounds leading to enhanced β-catenin degradation87, 88. ICG–001 binds to CBP and reduces β-catenin interactions with TCF, thus blocking β-catenin/TCF-dependent target gene expression. This figure was created with Biorender.com. Abbreviations: LRP5/6, low density lipoprotein (LDL) receptor related protein 5/6; Dsh, disheveled; GSK3-β, glycogen synthase kinase 3-β; APC, adenomatosis polyposis coli; CBP, cAMP response element-binding (CREB) binding protein; TCF, T-cell factor; DKK-1, Dickkopf-1; sFRP4, secreted Frizzled-related protein 4; DKK2, Dickkopf-2; TDZD-8, thiadiazolidinone 8; CK1, casein kinase 1.
Once liberated from the destruction complex, β-catenin accumulates in the nucleus and interacts with other transcription factors in the T cell factor/lymphoid enhancer factor (TCF/LEF) family (Figure 1). TCF/LEF transcription factors act as transcriptional repressors while bound to Groucho in the absence of β-catenin. Nuclear p-catenin displaces Groucho and leads to activation of transcriptional responses9. One classic transcriptional target of β-catenin/TCF/LEF is axin2 which serves as a feedback repressor given that axin is a component of the destruction complex10. Axin2 transcripts are frequently used as a measure of β-catenin/TCF/LEF activity. Indeed, some reporter mice for β-catenin activity utilize the axin2 promoter to express LacZ11, 12. The downstream transcriptional responses of β-catenin/TCF/LEF are important in modulating cell proliferation and differentiation, key biological responses in injury. However, the exact target genes affected vary depending upon the target cell type.
The microenvironment is also critical in determining the targets downstream of β-catenin. During oxidative stress, nuclear β-catenin has been shown to preferentially bind FoxO transcription factors which outcompete TCF/LEF binding13. FoxO/β-catenin signaling promotes different transcriptional responses including cell cycle arrest, antioxidant production, and cell survival, all biological functions associated with the FoxO family. Investigators have shown that this switch from TCF/LEF- to FoxO-mediated β-catenin-dependent transcription is relevant to bone formation and liver metabolism14–16. However, the role of β-catenin/FoxO-dependent signaling in vivo has not been well investigated in the context of kidney injury with the notable exception of T cell differentiation as will be discussed later. A recent publication shows a protective effect of FoxO1 in the context of TGF-β treatment in murine tubular epithelial cells in vitro, and future studies should explore β-catenin/FoxO interactions in vivo17.
Wnt/β-catenin and Renal Development
An extensive discussion of Wnt/ β-catenin in renal development is beyond the scope of this manuscript, and interested readers are directed to prior reviews18, 19. Wnt is one of many growth factors involved in development (e.g. Notch, hedgehog) that subsequently become re-expressed after renal injury20. An understanding of their biological actions in development may provide insights into their function in the injured kidney. The developing kidney requires finely controlled cellular communication between the epithelial cells of the invading ureteric bud (UB) and the undifferentiated metanephric mesenchyme (MM). The UB rapidly branches and forms the ureter, papilla, and collecting ducts, while the MM condenses around the invading UB to form the remaining nephron (glomerulus, proximal tubule down to collecting duct). Expression of Wnt ligands as well as a TCF/LEF reporter (i.e. β-catenin) localizes Wnt/β-catenin activity to the distal UB tips and surrounding MM21, suggesting that Wnt/β-catenin signaling plays an important role in this epithelial/mesenchymal crosstalk.
Murine genetic studies indicate that Wnt/β-catenin signaling controls renal tubular differentiation. Deleting β-catenin in the UB using the homeobox B7 (Hoxb7)-Cre caused a spectrum of malformed kidneys from renal agenesis to cystic/dysplastic kidneys with the severity depending upon the degree of β-catenin suppression22, 23. Cells lacking β-catenin had premature expression of differentiated collecting ducts as noted by aquaporin-3 and zona occludens-1α expression. In a model in which β-catenin was stabilized (i.e. activated) in the UB, aquaporin-3 expression was reduced23. These data suggest that UB-derived β-catenin signaling maintains epithelial cells in a de-differentiated precursor state. In the MM, β-catenin signaling is necessary for the formation of tubules as deletion of β-catenin in the MM (Six2-Cre) led to fewer nephrons and these remaining nephrons retained β-catenin expression24. This persistent β-catenin was thought to be in the earliest formed renal vesicles prior to induction of Cre recombinase24. Conversely, overexpression of β-catenin in the MM caused ectopic tubule induction without proper epithelialization as defined by lack of differentiation and mesenchymal-to-epithelial transition18, 24. In both the UB and MM, suppression and overactivation of Wnt/β-catenin signaling led to aberrant renal development. Thus, there may be a critical dose-dependent effect as well as spatial and temporal control of this pathway to ensure proper tubule development and differentiation. It is likely that these variables are also important in determining the role of Wnt/β-catenin in the injured kidney.
Wnt/β-catenin and Kidney Injury
Wnt/β-catenin signaling is expressed at low levels in the uninjured adult kidney, primarily in the papilla, but this pathway is upregulated in rodent models of acute and chronic renal injury12, 25, 26. Wnts and β-catenin activity have been localized to the proximal tubule after the ischemia-reperfusion injury (IRI) model12, 27, a common rodent model of AKI involving temporary clamping of the renal pedicle. This signaling pathway is also present in the interstitium after IRI and unilateral ureteral obstruction (UUO), the classical chronic injury model of tubulointerstitial fibrosis28. There is also evidence that the Wnt/β-catenin pathway is altered in human kidney disease. Transcriptomic data from human kidneys showed increased Wnt signaling in diabetic nephropathy29. In addition, renal biopsy tissue from proteinuric patients revealed alterations in Wnt/β-catenin signaling, particularly in the proximal tubules30. More recently, urinary levels of Dickkopf-3 (Dkk3), a modulator of Wnt/β-catenin signaling, predicted preoperatively the risk of AKI after cardiac surgery and was a biomarker for renal decline in patients with CKD31, 32. Although these data support a link between Wnt/β-catenin signaling and kidney injury, the critical question is whether Wnt/β-catenin signaling promotes repair and recovery or exacerbates the response to injury.
Wnt/β-catenin and Acute Kidney Injury
AKI is a rapid decline in renal function caused by many different injuries such as drugs, ischemia from cardiopulmonary bypass, sepsis, and toxins. The initial target of AKI is thought to be the renal tubules, particularly the proximal tubule and thick ascending limb, both of which are very metabolically active segments with reduced oxygen tensions33. Injured tubular epithelial cells undergo cell death through a variety of processes (e.g. apoptosis, necrosis, necroptosis), and even viable cells may slough off the tubular basement membrane due to alterations in integrin expression. The surrounding viable cells then de-differentiate, migrate to cover the denuded basement membrane, proliferate to replace the lost tubule cells, and ultimately re-differentiate to restore tubular function34. Although the tubular epithelial cells play a key role in AKI, several other cells types (e.g. inflammatory cells, microvasculature, pericytes/fibroblasts) also modulate the response to AKI and influence AKI progression to CKD.
Abundant data suggest that Wnt/β-catenin signaling is protective in the context of AKI, and the beneficial effects are likely mediated by the proximal tubule. Studies that systemically block the activity of GSK-3β, the kinase that targets β-catenin for degradation, improved renal recovery after AKI induced by mercuric chloride, cisplatin, or IRI35, 36. Genetic deletion of GSK-3β in proximal tubules reduced apoptosis and mortality after mercuric chloride-induced AKI, suggesting that GSK-3β activity specifically within the proximal tubule plays an important role in the outcome of AKI 35. One study used lithium, a known inhibitor of GSK-3β, and showed histologic improvement in proximal tubules after injury by the cisplatin or IRI models36. One cautionary note regarding these studies is that GSK-3β can affect signaling proteins other than β-catenin, so the beneficial effects in the proximal tubules might not be solely due to augmented β-catenin signaling. Addressing this concern, tubule-specific β-catenin deletion (using the Ksp-Cre) worsened the response to two different AKI rodent models: IRI and folic acid nephropathy indicating that β-catenin per se plays a protective role in proximal tubules37. β-catenin deletion also can affect the adherens junctions, though the authors showed that γ-catenin (aka plakoglobulin) compensated at the adherens junctions in these conditional knockout mice. However, it is possible that subtle differences in cell/cell interactions also contributed to the phenotype. Another study found that Wnt7b produced by macrophages had a protective effect on kidney tubular epithelial cells after IRI12. This same group demonstrated that Dickkopf-2 (Dkk2), a soluble protein that binds to LRP5/6 on the cell surface and enhances canonical Wnt signaling, reduced the number of TUNEL-positive apoptotic tubule cells after injury. Thus, both genetic and pharmacologic approaches show that Wnt/β -catenin signaling is protective to renal tubules in AKI (Table 1).
Table 1.
Pharmacologic and genetic mediators of Wnt/β-catenin signaling in acute kidney injury.
| Pharmacologic | Mechanism | Effect | Ref |
| DKK-2 | Binds to LRP5/6 to enhance binding of Wnt and pathway activation | Reduced number of apoptotic tubular epithelial cells in the IRI model of AKI | 12 |
| Lithium | nhibits GSK3β activity, preventing β-catenin degradation | Improved renal morphology, especially in proximal tubules, in cisplatin and IRI models of AKI | 35 |
| TDZD-8 | Specifically inhibits GSK3β activity, preventing β-catenin degradation | Reduced tubular epithelial cell damage and death in the IRI model of AKI | 39 |
| Genetic | Mechanism | Effect | Ref |
| γ-GT-Cre GSK3β Deletion | GSK3β ablated specifically in proximal tubular epithelium | Reduced apoptosis and mortality in toxin-induced and IRI models of AKI | 34 |
| Ksp-Cre β-catenin Deletion | β-catenin selectively deleted in tubular epithelium | More severe injury and worse mortality in IRI and folic acid models of AKI | 36 |
| Gli1-Cre β-catenin Deletion | β-catenin selectively deleted in Gli1+fibroblasts | Attenuated inflammation and tubular injury in IRI model of AKI | 56 |
Listed in this table are the various compounds and genetic models used to study the role of Wnt/β-catenin signaling in acute kidney injury. In addition, the mechanism of action, the model of injury and the effect on injury are represented. Abbreviations: DKK2, Dickkopf-2; LRP5/6, low density lipoprotein (LDL) receptor related protein 5/6; IRI, ischemia- reperfusion injury; AKI, acute kidney injury; GSK3-β, glycogen synthase kinase 3-β; TDZD-8, thiadiazolidinone 8; γ-GT, gamma-glutamyl transferase; Ksp, kidney-specific cadherin.
Mechanisms whereby Wnt/β-catenin is protective in AKI
There may be several mechanisms by which Wnt/β-catenin signaling protects renal tubules including modulation of apoptosis and survival pathways (Figure 2). Mice containing a tubule-specific deletion of β-catenin sustained greater tubular apoptosis after IRI or folic acid administration37. This increased apoptosis was associated with greater expression of Bcl-2 associated X protein (Bax), a pro-apoptotic protein in the Bcl-2 family that induces mitochondrial injury. In addition, the conditional knockout mice had greater expression of p53, a protein that may promote apoptosis through upregulation of Bax38, and reduced phosphorylated protein kinase B (Akt), which can suppress Bax expression, after folic acid injection37. Consistent with these in vivo findings, β-catenin induced tubular cells’ Akt phosphorylation and reduction of Bax activation, mitochondrial membrane injury, and apoptosis following ATP depletion in vitro39. In addition, the GSK-3 inhibitor TDZD-8 reduced tubule damage in rats after IRI associated with decreased Bax and cell death40. In addition to suppressing pro-apoptotic proteins, Wnt/β-catenin signaling has also been shown to increase expression of survivin, a member of the inhibitor of apoptosis protein (IAP) family41. In the IRI model, levels of survivin were decreased in two separate mouse models with reduced β-catenin signaling37, 42. Thus, Wnt/β-catenin signaling likely protects tubular epithelial cells in AKI by modulating the expression of pro/anti-apoptotic proteins to favor tubule survival.
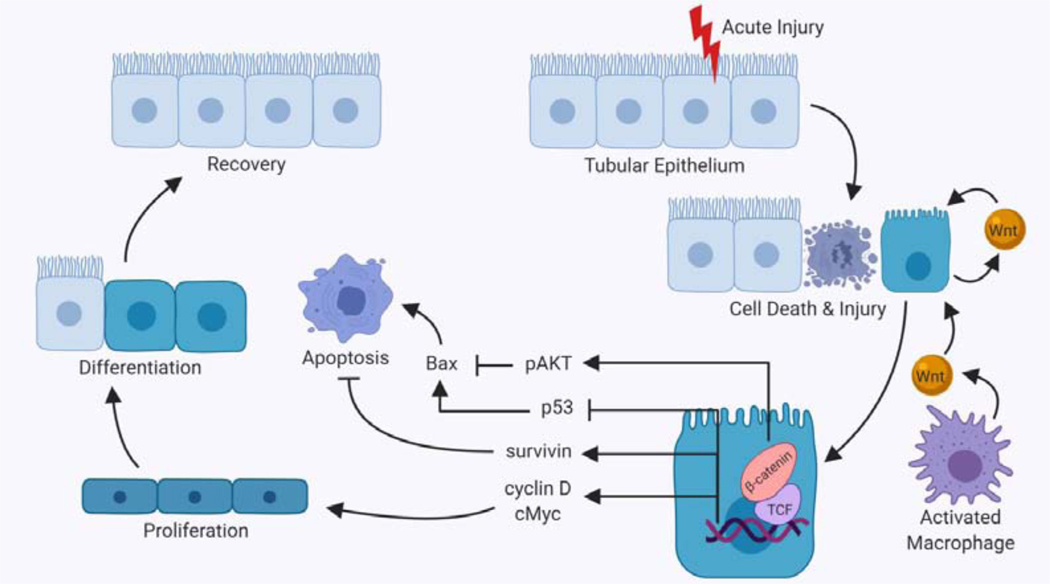
Figure 2. Effect of Wnt signaling in acute kidney injury.
Acute injury to the kidneys results in death and injury to tubular epithelial cells. These damaged cells secrete both Wnts and pro-inflammatory cytokines, resulting in recruitment of macrophages, which further contribute to Wnt pathway activation. Activation of Wnt/β-catenin signaling in tubular epithelium has many downstream effects which primarily result in proliferation or prevention of apoptosis. Apoptosis is prevented through the inhibition of pro-apoptotic Bax, mediated by β-catenin-dependent phosphorylation of Akt and/or inhibition of p53. Wnt/ β-catenin signaling also promotes cell survival through increased expression of survivin. The target genes activated by Wnt/β-catenin signaling include cyclin D and cMyc, both of which are pro-proliferative proteins. Proliferation helps to replace cells killed during the injury and differentiation of these injured and replacement cells leads to recovery. This figure was created with Biorender.com. Abbreviations: Bax, B-cell lymphoma-2 (Bcl-2) associated X protein; Akt, protein kinase B; TCF, T-cell factor.
Wnt/β-catenin signaling may also improve repair and regeneration after AKI by promoting proliferation of the surviving renal tubule cells. One of β-catenin’s downstream target genes is cyclin D1 which binds with cyclin dependent kinases (CDK) 4 and 6 to promote cell cycle progression43. After the IRI model, proximal tubule cells with Wnt4 protein expression also co-stained for proliferating cell nuclear antigen (PCNA), suggesting that Wnt/β-catenin activity was present in proliferating proximal tubule cells27. In addition, overexpression of Wnt4 and β-catenin increased the protein expression of cyclin D1 and promoted cell cycle progression in LLC-PK1 cells27. A single dose of lithium given on day 3 after cisplatin-induced AKI resulted in greater expression of cyclin D1 and c-Myc, another pro-proliferative protein, in renal tubules and improved recovery from AKI36. However, it is possible that this increase in cyclin D1 is mediated by GSK-3β independent of β-catenin. Another group showed that selective deletion of Wnt7b in macrophages caused increased G2/M arrest in tubule cells after IRI, indicating that macrophage-derived Wnt7b promotes epithelial progression through the cell cycle12.
Several lineage tracing studies have shown that the proliferating renal tubule cell population after AKI has increased Wnt/β-catenin activity. Cells with augmented β-catenin activity were followed using a tamoxifen-inducible Cre under the axin2 promoter that was crossed with the mT/mG (“tomato”) reporter mouse44 in which β-catenin activity (i.e. active Axin2 promoter) was marked with GFP45. After AKI induced by intramuscular injection of glycerol, large clones of cells with active β-catenin (i.e. GFP+) proliferated to repair the injured tubules45. Another group used the β-catenin/TCF/LacZ reporter mouse and confirmed that limited β-galactosidase (β-gal) staining, indicative of β-catenin signaling, was present in the uninjured mouse kidney but widespread staining was present in tubules 3 days after glycerol-induced AKI46. In addition, many of these p-gal positive cells also expressed CD24, a marker of renal progenitor cells46. Infusing CD24+ cells from embryonic mice into injured mouse showed integration of these exogenous CD24+ cells into the proximal tubule, a response that was inhibited by pre-treatment with a Wnt/β-catenin inhibitor46. Given the role of Wnt/β-catenin signaling in the developing kidney, it is tempting to speculate that this signaling pathway is important for maintenance of a unipotent renal progenitor cell that is responsible for repair after AKI. It remains unclear whether there is a certain subset of renal tubule cells with the ability to activate Wnt/β-catenin signaling after injury or if all tubules can potentially turn on this signaling in response to injury. It is also uncertain which biological functions mediated by Wnt/β-catenin signaling (e.g. maintenance of “sternness”, proliferation, anti-apoptosis) regulate this repair process.
Most of the literature attribute Wnt/β-catenin’s protective effects on acutely injured tubules to either modulation of cell death or augmented proliferation. However, there are other β-catenin-dependent cellular effects that may also promote repair after AKI. Wnt/β-catenin promotes epithelial de-differentiation and migration, key responses of surviving tubule cells that then proliferate to cover the denuded basement membrane34. The β-catenin-dependent de-differentiation is likely mediated, in part, by the transcription factor Snail which is known to suppress E-cadherin expression47. Although this β-catenin/Snail signaling has been shown to induce de-differentiation in cancer and other organs, β-catenin’s effects on epithelial differentiation and migration have not been well-defined in the context of AKI48. In summary, there is consensus that Wnt/β-catenin signaling protects renal tubules in AKI though the exact mechanisms may still be debatable.
Acute Kidney Injury to Chronic Kidney Disease Progression and Wnt/β-catenin
Recent data suggests that there is a continuum between AKI and CKD. Although some patients have restoration of their serum creatinine following AKI, it is well-recognized that patients with severe AKI are at increased risk for the development of CKD. In addition, patients with CKD due to diabetes, hypertension or other insults are at increased risk for AKI. The mechanisms of AKI to CKD progression are not completely understood, but most of the attention has focused on persistent tubule injury and/or microvascular impairment.
There is strong preclinical data to suggest that the persistently injured proximal tubule may promote development of CKD through the production of profibrotic growth factors and inflammatory cytokines49. These tubule cells remain de-differentiated, and the growth factors they produce can have detrimental effects on the surrounding vasculature and interstitial cells (e.g. fibroblasts/pericytes, inflammatory cells). There is also evidence that these chronically injured tubules are cell cycle arrested, likely in G2/M which is associated with a phenotype that secretes large amounts of fibrotic growth factors like TGF-β and CTGF50–52. Several groups have targeted the proximal tubules specifically using the diphtheria toxin murine model. This model takes advantage of the fact that mice are not susceptible to diphtheria unless they express the human diphtheria receptor. When this human receptor is expressed in a cell-specific manner, the administration of diphtheria results in cell death limited to those cells expressing the receptor. Targeting the proximal tubules with the diphtheria toxin was sufficient to cause AKI to CKD progression if the severity was high or if repeated injury occurred53, 54.
Given the importance of the renal tubules in AKI to CKD progression and the vital role of Wnt/β-catenin signaling in this cellular compartment, a logical question is how Wnt/β-catenin signaling modulates AKI to CKD progression. One group addressed this and demonstrated that overexpression of Wnt1 by hydrodynamic-based gene transfer 5 days after IRI worsened fibrosis 6 days later55. Although this exogenous Wnt1 was expressed primarily in tubules, Wnt expression led to greater fibroblast activation and matrix production. The same group then showed that the inhibitor ICG-001 reduced fibrosis 11 days after IRI55. This is the first paper to really look at the role of Wnt/β-catenin signaling in AKI to CKD which is sorely needed. There are a few caveats worth mentioning. The overexpression of Wnt1 appeared effective based upon the staining, but it is unclear how much β-catenin signaling occurred as a result and how these levels compare to Wnt/β-catenin signaling in pathophysiological states. As illustrated by renal development, too little and too much signaling is detrimental, suggesting that the effect may vary with the level of β-catenin signaling. Additionally, the ICG-001 inhibitor, widely used as a β-catenin inhibitor, is a small molecule that binds to cyclic AMP response element-binding protein (CBP), a transcriptional co-activator of β-catenin56. This was shown to inhibit β-catenin/TCF-mediated responses rather than blocking all of β-catenin-dependent responses and may augment β-catenin/FoxO-dependent responses57. Additionally, inhibition of CBP-dependent responses that are β-catenin-independent cannot be ruled out and have been reported by others58. This study took a valuable first step towards defining the role of Wnt/β-catenin signaling in AKI to CKD. However, given the caveats with ICG-001 and remaining questions about the amount and location of β-catenin signaling with the Wnt1 transgene, additional studies are warranted.
Addressing the question about which cells may be mediating Wnt/β-catenin’s deleterious effects in AKI to CKD, β-catenin was selectively deleted in a subset of fibroblasts (Gli1-Cre). These conditional knockout mice had attenuated inflammation and tubular injury after IRI-induced AKI59. This effect was thought to be due to enhanced hepatocyte growth factor (HGF) in the mice lacking β-catenin in Gli1+ fibroblasts. Although this study looked at AKI at 1 day after IRI rather than AKI to CKD, it shows that, despite the beneficial effect of systemically augmenting Wnt/β-catenin in AKI, this signaling pathway may be deleterious in certain fibroblast populations.
The microvasculature is recognized as an important mediator of AKI to CKD progression. Early studies showed that IRI injury damaged peritubular capillaries with a 30% reduction in capillary density by microfil analysis from 4–40 weeks after injury60. There is not much data on Wnt/β-catenin signaling in the renal microvasculature in the context of AKI to CKD. However, several studies suggest that β-catenin signaling may play an important role in the injured renal peritubular capillaries. The Wnt/β-catenin agonist Dkk3 was present in the conditioned media of renal microvascular endothelial cells in vitro and induced a mesenchymal transition in these endothelial cells61. Dkk3 was also detected in the tubular and endothelial compartments after adriamycin-induced nephropathy, a model of glomerular injury. In a rat model of kidney transplant chronic allograft nephropathy, increased nuclear β-catenin staining in endothelial cells suggest that this pathway is activated62. Rats with streptozotocin-induced diabetes had increased expression of β-catenin and decreased expression of CD31, an endothelial marker, in glomerular endothelial cells63. These data suggest that β-catenin signaling may impair endothelial function and promote de-differentiation, but none of them genetically manipulated the Wnt/β-catenin pathway specifically in the microvasculature. However, other studies using human umbilical vein endothelial cells (HUVECs) showed that β-catenin signaling stimulated endothelial cell proliferation and survival, possibly through the angiogenic factor interleukin-864. The Wnt/β-catenin pathway has been described as pro-angiogenic, potentially through downstream vascular endothelial growth factor (VEGF) and/or matrix metalloproteinase (MMP)7, but most of these studies were either done with in vitro model systems or in the context of cancer metastasis rather than response to injury65. Thus, more research is needed to determine the role of endothelial Wnt/β-catenin signaling in AKI to CKD progression.
Wnt/β-catenin Signaling in Chronic Kidney Injury
There is much more data on the role of Wnt/β-catenin signaling in AKI than in AKI to CKD transition. As renal injury exists on a continuum between acute and chronic, an understanding of how this signaling pathway affects CKD has important treatment implications. Furthermore, growth factor pathways that are beneficial in AKI (e.g. epidermal growth factor) may be deleterious in CKD66, 67. Many systemic inhibitors of Wnt/β-catenin ameliorate fibrosis in rodent models of CKD, implying that this pathway promotes fibrosis (Table 2)68–71. Some studies used the ICG-001 inhibitor and concerns about this approach were discussed above. However, others used either a secreted frizzled-related protein that competes with Wnt ligands for receptor binding or inhibited porcupine, which promotes Wnt secretion and receptor binding, and both approaches showed a protective effect70, 71. These pre-clinical studies suggest that Wnt/β-catenin signaling promotes fibrosis in CKD models, but do not inform about the cell-specific effects. Future studies that look at cell-autonomous β-catenin signaling may provide more information about the cell-specific roles of this signaling pathway in CKD.
Table 2.
Pharmacologic and genetic mediators of Wnt/β-catenin signaling in chronic kidney disease.
| Pharmacologic | Mechanism | Effect | Ref |
| ICG-001 | Competitively binds CBP to prevent β-catenin/CBP binding to TCF | Reduced fibrosis in models of IRI, UUO, and in vitro models of CKD | 52, 65 |
| DKK-1 | Binds to LRP5/6 to prevent dimerization with Frizzled and Wnt binding | Reduced fibrosis and pericyte activation in UUO models of CKD | 25, 74 |
| Klotho | Binds to and sequesters Wnt ligands | Reduced fibrosis in UUO models of CKD | 83 |
| XAV939 | Stabilizes Axin to activate destruction complex and stimulate β-catenin degradation | Reduced myofibroblast activation in vitro models of CKD | 69, 84 |
| sFRP4 | Binds Wnt and Frizzled to disrupt signaling functions | Reduced fibrosis in UUO models of CKD | 69 |
| C59 | Binds Porcupine to prevent acylation and secretion of all Wnts | Reduced fibrosis in UUO models of CKD | 67 |
| Pyrvinium | Inhibits axin degradation, which stimulates p-catenin degradation | Reduced fibrosis and myofibroblast markers in Ang II injury in vitro | 85 |
| Genetic | Mechanism | Effect | Ref |
| SLC34a1-Cre Wnt1 Overexpression | Inducible and sustained Wnt1 production only in proximal tubular epithelium | Caused interstitial fibrosis by activation of myofibroblasts without induction by injury | 69 |
| Ksp-Cre β-catenin Stabilization | β-catenin stabilized (i.e. activated) in tubular epithelium by removing phosphorylation sites that target b-catenin for degradation | Caused increased inflammatory infiltration in protein overload injury model | 73 |
| Wnt9a Overexpression | Overexpression of Wnt9a by delivery of DNA expression plasmids | Increased tubular cell senescence and worsened fibrosis in IRI model of CKD | 70 |
| Ksp-Cre Wntless Deletion | Wntless ablated in tubular epithelium, preventing secretion of Wnts | Reduced fibrosis and fibroblast activation in UUO and UIRI models of CKD | 71 |
| Gli1-Cre β-catenin Stabilization | β-catenin stabilized (i.e. activated) selectively in Gli1+ fibroblasts | Activation of myofibroblasts in uninjured kidney | 27 |
Both the pharmacologic and genetic approaches to modulate Wnt/β-catenin signaling in chronic kidney disease are listed as well as mechanism of action, effect on renal injury, and relevant reference. Abbreviations: CBP, cAMP response element-binding (CREB) binding protein; IRI, ischemia-reperfusion injury; UUO, unilateral ureteric obstruction; CKD, chronic kidney disease; DKK-1, Dickkopf-1; LRP5/6, low density lipoprotein (LDL) receptor related protein 5/6; sFRP4, secreted Frizzled-related protein 4; Ang II, angiotensin II; SLC34a1, solute carrier family 34 member 1; Ksp, kidney-specific cadherin.
Tubular Epithelial Wnt/β-catenin Signaling and CKD
Tubular epithelial β-catenin signaling is particularly important because of this cellular compartment’s role in AKI to CKD progression. Several groups have generated transgenic mice in which the epithelial cells overexpress Wnt ligands. Overexpression of Wnt1 by proximal tubules was sufficient to cause tubulointerstitial fibrosis by paracrine signaling in fibroblasts/pericytes and without epithelial injury72. Similarly, Wnt9a overexpression by hydrodynamic tail injection after IRI increased tubular senescence and worsened fibrosis73. Both of these studies demonstrate the potential for Wnt signaling to cause tubulointerstitial fibrosis, the hallmark of CKD. However, given that Wnt is a soluble factor, it is difficult to attribute all the effects to epithelial signaling. The Wnt1 overexpressing mice had interstitial fibrosis and increased signaling in this compartment without epithelial injury. In addition, the levels of Wnt in the transgenic models may have exceeded those even in injury states which may be relevant if there is a dose-dependent effect. It is likely that injured tubules are important producers of Wnt ligands, but many of their deleterious effects may be mediated by paracrine signaling on fibrobasts/pericytes. Consistent with this, tubule-specific but not fibroblast-specific ablation of Wntless, a transmembrane protein that is required for the secretion of Wnt proteins, reduced fibroblast activation and fibrosis74.
A more direct approach to target epithelial Wnt/β-catenin signaling is to alter theβ-catenin activity specifically in epithelial cells. Tubule-specific deletion of β-catenin did not affect tubulointerstitial fibrosis development after the UUO model of injury75. These conditional knockout mice did have enhanced fibroblast survival due to reduced MMP7-mediated Fas ligand expression in fibroblasts. This data suggests that epithelial β-catenin does not play a major role in fibrosis induced by UUO. This is not too surprising as this model is an excellent fibrosis rodent model but is not ideal for epithelial injury/repair as it progressively destroys the renal parenchyma. Another group stabilized β-catenin in tubule epithelial cells and, in a model of protein overload induced by uninephrectomy and bovine serum albumin (BSA) injections, noted increased macrophage infiltration and blood urea nitrogen (BUN) levels but no fibrosis76. These data suggest that augmented epithelial β-catenin signaling may induce macrophage infiltration through increased tubular cytokine expression76. More studies are warranted to assess how epithelial β-catenin affects other models of injury including AKI to CKD.
Wnt/β-catenin Signaling in Renal Fibroblasts/Pericytes and CKD
Tubulointerstitial fibrosis consists of extracellular matrix (ECM) proteins like collagen and fibronectin, and myofibroblasts are the main producers of ECM. Although there is some debate about the origin of myofibroblasts, most agree that resident fibroblasts and pericytes are stimulated by growth factors to transform into myofibroblasts. Wnt1 and Wnt4 have both been shown to promote myofibroblast transformation from fibroblast and pericyte precursors in vitro28, 72. As mentioned above, overexpression of tubule-derived Wnt1 had a profound effect on interstitial cells driving myofibroblast activation and proliferation72. Consistent with this, stabilization of β-catenin (i.e. activation) specifically in Gli1+ interstitial fibroblasts led to myofibroblast differentiation in the uninjured murine kidney28. Many other studies that systemically inhibit Wnt/β-catenin and reduce fibrosis are associated with reduced numbers of myofibroblasts26, 70, 77. These studies taken together with those that directly modulate β-catenin signaling in myofibroblast precursors provide strong evidence for a fibroblast/pericyte-dependent profibrotic effect of Wnt/β-catenin in CKD.
Wnt/β-catenin Signaling in Inflammatory Cells and CKD
Inflammatory cells are another important component and modulator of both AKI and CKD. Most of the attention has focused on macrophages, which are subtyped into many different subsets with different functions. Broadly speaking, they have been divided into the proinflammatory M1 (classically activated) and profibrotic M2 (alternatively activated)78. In AKI, the M1 macrophage phenotype initially predominates and clodronate depletion of macrophages at this stage is beneficial. By contrast, the M2 phenotype is considered reparative in the context of AKI and targeting this population at a later stage after AKI worsened renal recovery12, 79. In CKD, the M2 phenotype has been implicated in promoting fibrosis in CKD through the production of TGF-β, PDGF, and galectin-3, and human biopsy samples with persistent macrophage infiltration portend a worse prognosis80–82. Wnt3a, given with TGF-β or IL-4, potentiated the M2 differentiation of macrophages in vitro83. Furthermore, mice with inducible deletion of β-catenin in macrophages had fewer M2 macrophages as well as reduced fibrosis after the UUO model of injury83. Thus, Wnt/β-catenin signaling in macrophages promotes M2 differentiation, an effect that may be beneficial in AKI but detrimental in CKD.
The role of T cells in CKD is not as well-studied, but regulatory T cells (Tregs), which suppress the activity of reactive effector T cells, may play a role in chronic kidney disease84. A group recently showed that using ICG-001, a blocker of β-catenin/TCF/LEF interactions, re-directs β-catenin signaling to FoxO-mediated transcription. In the context of injury (UUO and IRI), recombinant TGF-β1 plus ICG-001 increased β-catenin/FoxO signaling leading to Treg-mediated anti-inflammation and renoprotection57. Upon Treg depletion, the benefit of ICG-001 and TGF-β1 was abrogated. This immunosuppressive effect of β-catenin/FoxO required TGF-β1, but other effects of β-catenin/FoxO in the injured kidney have yet to be explored. In summary, β-catenin had divergent effects on macrophages and Tregs in the context of chronic injury. However, it may be that the transcriptional signaling partners (e.g. TCF/LEF versus FoxO) determine whether β-catenin signaling in inflammatory cells is beneficial or detrimental in CKD.
Modulating Wnt/β-catenin in AKI: Challenges and Opportunities
The preclinical data overwhelmingly suggest a strong protective role for Wnt/β-catenin signaling in AKI. Although one study did show a deleterious effect of β-catenin signaling in fibroblasts, this probably does not outweigh the beneficial effects in renal tubules in AKI based on the studies which used systemic inhibitors. Furthermore, there is data to suggest that augmenting Wnt/β-catenin signaling is beneficial when present both at the time of injury and when activated after the initial injury. The putative mechanisms whereby Wnt/β-catenin signaling protects injured renal tubules focus on either cell survival/anti-apoptosis or its proliferative effects on epithelial cells. It is possible that the protection against apoptosis accounts for the attenuated severity of injury when the Wnt/β-catenin pathway is activated at or before injury, and the pro-proliferative effects mediate the improved renal recovery when the pathway is modulated after injury.
There are a few challenges to Wnt/β-catenin activation in AKI related both to the pharmacologic strategy as well as issues regarding prevention versus treatment and the target population in AKI. The best approach to activate Wnt/β-catenin signaling in AKI is not clear. Both lithium and GSK-3 inhibitors, commonly used in preclinical trials, have β-catenin-independent effects. There may be long-term concerns about using GSK-3 inhibitors, though their use in treatment or prevention of AKI would presumably be of relatively short duration. The bigger challenge is knowing which patient population is most likely to benefit: should it be for prevention of AKI or treatment of AKI? Although the patient population targeted for prevention of AKI is more homogeneous (e.g. cardiopulmonary bypass patients), most AKI is not preventable, so treatment has the potential to offer more benefit. Improved phenotyping of AKI, which has diverse etiologies and therefore is unlikely to respond to a one-drug-fits-all approach, should help guide more targeted subpopulations likely to benefit. More studies are warranted to answer these questions as well as the ideal duration and dose of treatment.
Modulating Wnt/β-catenin in AKI to CKD Progression: Challenges and Opportunities
As most human AKI is difficult to prevent, treatment of severe AKI to prevent progression to CKD/ESRD is an attractive approach85. Although AKI causes a huge burden acutely in terms of mortality and hospitalization duration, there is also a huge effect on health and healthcare costs once AKI progresses to CKD. The clear concern with augmenting Wnt/β-catenin in AKI to CKD progression is the detrimental effects of this pathway that have been demonstrated in rodent CKD models. The general paradigm with Wnt/β-catenin signaling is that it is beneficial in AKI but detrimental in CKD. However, it may be that β-catenin signaling has more to do with location than duration. As recently shown, deleting β-catenin in fibroblasts was beneficial in AKI, suggesting that β-catenin mediates different effects in AKI based upon the target cell. In AKI, β-catenin’s salutary effects on epithelial cells likely outweigh its adverse effects on fibroblasts. It is likely that β-catenin signaling always mediates detrimental effects on fibroblasts as this data is strong in CKD models. However, β-catenin signaling in fibroblasts likely contributes more to the effects of systemic Wnt/β-catenin modulation in CKD than it does in AKI. Thus, the beneficial effect of increasing β-catenin activity in AKI may be mediated by epithelial cells whereas in CKD, the detrimental effect may be driven by fibroblasts. However, there are legitimate concerns about chronic stimulation of β-catenin in epithelial cells as well. In summary, preclinical data support a protective role for Wnt/β-catenin agonists in AKI. However, more studies are needed to determine if this pathway should be augmented in AKI to CKD. If so, the optimal treatment duration as well as dose that can be both safe and effective will also need defining. Although more work is needed, this unmet medical need of treating AKI to CKD progression merits the investment of resources.
Acknowledgments
Financial support: This work was supported by NHLBI HL 135790 (WDM), VA Merit 1I01BX003425-01A21 (LG) and NIDDK R01DK108968-01 (LG).
Footnotes
Financial disclosure and conflict of interest statement:Dr. Gewin is a consultant for Surrozen which is interested in Wnt pathway activation to promote regeneration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84(3):457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and metaanalysis. Kidney Int. 2012;81(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–233. [DOI] [PubMed] [Google Scholar]
- 4.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.System USRD. USRDS annual data report: Epidemiology of kidney disease in the United States: National Institutes of Health, NIDDK; 2016. [Google Scholar]
- 6.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. [DOI] [PubMed] [Google Scholar]
- 7.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–1677. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12(4):364–371. [DOI] [PubMed] [Google Scholar]
- 10.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132(8):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107(9):4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308(5725):1181–1184. [DOI] [PubMed] [Google Scholar]
- 14.Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282(37):27298–27305. [DOI] [PubMed] [Google Scholar]
- 15.Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013;123(8):3409–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4(158):ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao P, Pang M, Qiao X, Yu H, Wang H, Yang Y, et al. Promotion of beta-catenin/Foxo1 signaling ameliorates renal interstitial fibrosis. Laboratory investigation; a journal of technical methods and pathology. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Ott KM, Barasch J. WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int. 2008;74(8):1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merkel CE, Karner CM, Carroll TJ. Molecular regulation of kidney development: is the answer blowing in the Wnt? Pediatr Nephrol. 2007;22(11):1825–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edeling M, Ragi G, Huang S, Pavenstadt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nature reviews. Nephrology. 2016;12(7):426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, et al. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol. 2007;293(2):F494–500. [DOI] [PubMed] [Google Scholar]
- 22.Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, et al. Canonical WNT/beta-catenin signaling is required for ureteric branching. Developmental biology. 2008;317(1):83–94. [DOI] [PubMed] [Google Scholar]
- 23.Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Developmental biology. 2008;314(1):112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134(13):2533–2539. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. The Journal of pathology. 2013;229(2):221–231. [DOI] [PubMed] [Google Scholar]
- 26.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20(4):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14(5):1223–1233. [DOI] [PubMed] [Google Scholar]
- 28.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24(9):1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen CD, Lindenmeyer MT, Eichinger F, Hahn A, Seifert M, Moll AG, et al. Improved elucidation of biological processes linked to diabetic nephropathy by single probe-based microarray data analysis. PloS one. 2008;3(8):e2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudnicki M, Eder S, Perco P, Enrich J, Scheiber K, Koppelstatter C, et al. Gene expression profiles of human proximal tubular epithelial cells in proteinuric nephropathies. Kidney Int. 2007;71(4):325–335. [DOI] [PubMed] [Google Scholar]
- 31.Schunk SJ, Zarbock A, Meersch M, Kullmar M, Kellum JA, Schmit D, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394(10197):488–496. [DOI] [PubMed] [Google Scholar]
- 32.Zewinger S, Rauen T, Rudnicki M, Federico G, Wagner M, Triem S, et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J Am Soc Nephrol. 2018;29(11):2722–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishibe S, Cantley LG. Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr Opin Nephrol Hypertens. 2008;17(4):379–385. [DOI] [PubMed] [Google Scholar]
- 35.Howard C, Tao S, Yang HC, Fogo AB, Woodgett JR, Harris RC, et al. Specific deletion of glycogen synthase kinase-3beta in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int. 2012;82(9):1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao H, Ge Y, Wang Z, Zhuang S, Dworkin L, Peng A, et al. Delayed administration of a single dose of lithium promotes recovery from AKI. J Am Soc Nephrol. 2014;25(3):488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82(5):537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima K, Shimanuki M, Shikami M, Andreeff M, Nakakuma H. Cyclin-dependent kinase 1 inhibitor R0–3306 enhances p53-mediated Bax activation and mitochondrial apoptosis in AML. Cancer Sci. 2009;100(6):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009;20(9):1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, et al. GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol. 2010;21(2):284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci U S A. 2006;103(41):15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Bataineh MM, Kinlough CL, Poland PA, Pastor-Soler NM, Sutton TA, Mang HE, et al. Muc1 enhances the beta-catenin protective pathway during ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2016;310(6):F569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T, et al. S0X6 suppresses cyclin D1 promoter activity by interacting with beta-catenin and histone deacetylase 1, and its down-regulation induces pancreatic beta-cell proliferation. J Biol Chem. 2007;282(26):19052–19061. [DOI] [PubMed] [Google Scholar]
- 44.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 45.Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014;7(4):1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Iglesias DM, Corsini R, Chu L, Goodyer P. WNT/beta-Catenin Signaling Is Required for Integration of CD24+Renal Progenitor Cells into Glycerol-Damaged Adult Renal Tubules. Stem Cells Int. 2015;2015:391043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20(9):1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnemmi V, Bouillez A, Gaudelot K, Hemon B, Ringot B, Pottier N, et al. MUC1 drives epithelial-mesenchymal transition in renal carcinoma through Wnt/beta-catenin pathway and interaction with SNAIL promoter. Cancer Lett. 2014;346(2):225–236. [DOI] [PubMed] [Google Scholar]
- 49.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26(8):1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, et al. PTEN loss defines a TGF-beta-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol. 2012;302(9):F1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonventre JV. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int Suppl (2011). 2014;4(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine. 2010;16(5):535–543, 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, et al. Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis. J Am Soc Nephrol. 2016;27(8):2393–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, et al. Sustained Activation of Wnt/beta-Catenin Signaling Drives AKI to CKD Progression. J Am Soc Nephrol. 2016;27(6):1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A. 2004;101(34):12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiao X, Rao P, Zhang Y, Liu L, Pang M, Wang H, et al. Redirecting TGF-beta Signaling through the beta-Catenin/Foxo Complex Prevents Kidney Fibrosis. J Am Soc Nephrol. 2018;29(2):557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiese M, Walther N, Diederichs C, Schill F, Monecke S, Salinas G, et al. The beta-catenin/CBP-antagonist ICG-001 inhibits pediatric glioma tumorigenicity in a Wnt-independent manner. Oncotarget. 2017;8(16):27300–27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou D, Fu H, Xiao L, Mo H, Zhuo H, Tian X, et al. Fibroblast-Specific beta-Catenin Signaling Dictates the Outcome of AKI. J Am Soc Nephrol. 2018;29(4):1257–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–899. [DOI] [PubMed] [Google Scholar]
- 61.Lipphardt M, Dihazi H, Jeon NL, Dadafarin S, Ratliff BB, Rowe DW, et al. Dickkopf-3 in aberrant endothelial secretome triggers renal fibroblast activation and endothelial-mesenchymal transition. Nephrol Dial Transplant. 2019;34(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Toerne C, Schmidt C, Adams J, Kiss E, Bedke J, Porubsky S, et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9(10):2223–2239. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Chen L, Zang J, Tang X, Liu Y, Zhang J, et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64(5):597–610. [DOI] [PubMed] [Google Scholar]
- 64.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8(1):43–51. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Wu Z, Tian J, Mi Y, Ren X, Kang J, et al. Intermedin protects HUVECs from ischemia reperfusion injury via Wnt/beta-catenin signaling pathway. Ren Fail. 2019;41(1):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, et al. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol. 2012;23(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, et al. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol. 2011;22(9):1642–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2015;26(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madan B, Patel MB, Zhang J, Bunte RM, Rudemiller NP, Griffiths R, et al. Experimental inhibition of porcupine-mediated Wnt O-acylation attenuates kidney fibrosis. Kidney Int. 2016;89(5):1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16(8):2373–2384. [DOI] [PubMed] [Google Scholar]
- 72.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, et al. Paracrine Wnt1 Drives Interstitial Fibrosis without Inflammation by Tubulointerstitial Cross-Talk. J Am Soc Nephrol. 2016;27(3):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo C, Zhou S, Zhou Z, Liu Y, Yang L, Liu J, et al. Wnt9a Promotes Renal Fibrosis by Accelerating Cellular Senescence in Tubular Epithelial Cells. J Am Soc Nephrol. 2018;29(4):1238–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou D, Fu H, Zhang L, Zhang K, Min Y, Xiao L, et al. Tubule-Derived Wnts Are Required for Fibroblast Activation and Kidney Fibrosis. J Am Soc Nephrol. 2017;28(8):2322–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou D, Tan RJ, Zhou L, Li Y, Liu Y. Kidney tubular beta-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep. 2013;3:1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong DWL, Yiu WH, Chan KW, Li Y, Li B, Lok SWY, et al. Activated renal tubular Wnt/beta-catenin signaling triggers renal inflammation during overload proteinuria. Kidney Int. 2018;93(6):1367–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin SL, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci U S A. 2013;110(4):1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nature reviews. Nephrology. 2014;10(11):625–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vinuesa E, Hotter G, Jung M, Herrero-Fresneda I, Torras J, Sola A. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. The Journal of pathology. 2008;214(1):104–113. [DOI] [PubMed] [Google Scholar]
- 80.Sung SA, Jo SK, Cho WY, Won NH, Kim HK. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron. Experimental nephrology. 2007;105(1):e1–9. [DOI] [PubMed] [Google Scholar]
- 81.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172(2):288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ninichuk V, Khandoga AG, Segerer S, Loetscher P, Schlapbach A, Revesz L, et al. The role of interstitial macrophages in nephropathy of type 2 diabetic db/db mice. Am J Pathol. 2007;170(4):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng Y, Ren J, Gui Y, Wei W, Shu B, Lu Q, et al. Wnt/beta-Catenin-Promoted Macrophage Alternative Activation Contributes to Kidney Fibrosis. J Am Soc Nephrol. 2018;29(1):182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60(11):2954–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fiorentino M, Kellum JA. Improving Translation from Preclinical Studies to Clinical Trials in Acute Kidney Injury. Nephron. 2018;140(2):81–85. [DOI] [PubMed] [Google Scholar]
- 86.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24(5):771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. [DOI] [PubMed] [Google Scholar]
- 88.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the beta-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol. 2015;308(4):F358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]