Abstract
BACKGROUND.
Early accounts of forced thought were reported at the onset of a focal seizure, and characterized as vague, repetitive and involuntary intellectual auras distinct from perceptual or psychic hallucinations or illusions. Here we examine the neural underpinnings involved in conceptual thought by presenting a series of 3 epilepsy patients reporting intrusive thoughts during electrical stimulation of the left lateral prefrontal cortex (PFC) during invasive surgical evaluation. We illustrate the widespread networks involved through two independent brain imaging modalities: resting state fMRI (rs-fMRI) and task-based meta-analytic connectivity modeling (MACM).
METHODS.
We report the clinical and stimulation characteristics of three patients with left hemispheric language dominance who demonstrate forced thought with functional mapping. To examine the brain networks underlying this phenomenon, we used the regions of interest (ROI) centered at the active electrode pairs. We modeled functional networks using two approaches: (1) resting-state fMRI (rs-fMRI) functional connectivity analysis, representing 81 healthy controls and (2) meta-analytic connectivity modeling (MACM), representing 8260 healthy subjects. We also determined the overlapping regions between these three subjects’ rs-fMRI and MACM networks through a conjunction analysis.
RESULTS.
We identified that left PFC was associated with a large-scale functional network including frontal, temporal and parietal regions, a network that has been associated with multiple cognitive functions including semantics, speech, attention, working memory, and explicit memory.
CONCLUSIONS.
We illustrate the neural networks involved in conceptual thought through a unique patient population and argue that PFC supports this function through activation of a widespread network.
Keywords: Thought, Prefrontal Cortex, Electric Stimulation, Functional Neuroimaging, Networks
1. INTRODUCTION
Forced thinking is a phenomenon of recurrent, intrusive conceptual thoughts. Early descriptions involved patients who experienced forced thinking as an initial symptom of a focal-onset seizure (Allen, 1952; Mendez, Cherrier, & Perryman, 1996; Penfield, 1946). Penfield characterized the phenomenon as an intellectual aura, a vague and ill-defined crowding of thoughts, often stereotyped, that were distinct from a sensory hallucination (Penfield, 1946). More recent cases of patients with left frontal lesions described repeated, involuntary urges to verbalize short phrases. Paradoxically, these patients were unable to communicate during their seizure (Mendez et al., 1996).
Patients with refractory focal onset epilepsy arising from the dominant hemisphere (left hemisphere in most right handed patients) may undergo intracranial EEG monitoring to precisely localize the seizure focus and to guide surgical resection. When there is potential for overlap of the seizure-onset zone with functional cortex (supporting language, motor, or sensory function), electrocortical stimulation mapping (ESM) is performed to determine the “safe” margins of resection. In primary motor and sensory cortex, ESM often elicits elemental responses such as a clonic limb movement, focal paresthesias or phosphenes. In language cortex, ESM can lead to disruptions in speech, naming or comprehension tasks. In association areas of the brain, ESM may elicit complex experiential or behavioral phenomena which can inform our understanding of the structural correlates of complex cognitive functions(K. C. R. Fox et al., 2018; Parvizi, Rangarajan, Shirer, Desai, & Greicius, 2013; Rangarajan et al., 2014). These behavioral distinctions reflect the brain’s underlying functional anatomy, however the networks involved in complex forced thinking during ESM have not been previously described.
Here we present three patients who reported a set of conceptual thoughts, which were repeatedly and spontaneously induced by ESM in left lateral prefrontal cortex (PFC) not involved in the seizure onset zone. Here we define a conceptual thought as a general precept based on the cross-modal and cross-temporal association of information or experiences (Tanji, Shima, & Mushiake, 2007), and use the term synonymously with categorical thought. While the phenomenon of forced thought has been previously described during seizures and during neurostimulation(Popa et al., 2016), we explore the neurobiology of this complex cognitive phenomenon using two complementary methods of network analyses, resting state functional magnetic resonance imaging (rs-fMRI) connectivity and coordinate-based meta-analytic connectivity modeling (MACM). Rs-fMRI functional networks are defined by correlated spontaneous fluctuations in the BOLD signal in the resting brain. MACM functional networks are defined by co-activations across task-based functional neuroimaging studies databased within BrainMap. These two functional neuroimaging methods have repeatedly demonstrated common neural networks supporting both rest and activity (Laird et al., 2013; Smith et al., 2009). Furthermore, we reference the BrainMap behavioral database to describe the tasks that often engage these shared regions. We hypothesize that regions capable of producing forced thoughts possess widespread functional connections, thus supporting their role in conceptual thinking.
2. METHODS
2.1. Participants.
This study was an observational study. Informed consent was obtained from these patients with the NYU Institutional Review Board. Epilepsy patients undergoing invasive EEG monitoring for surgical evaluation underwent ESM as part of routine clinical care. From July 2006 to January 2018, there were 76 patients who had bedside ESM for language mapping performed in English.
2.2. Electrocorticography.
Brain activity was recorded from implanted subdural stainless steel electrodes embedded in silastic sheets (Ad-Tech Medical Instrument, Racine, WI). Patients 1 and 2 had a combination of a standard subdural grid (2.3 mm diameter, 10 mm center-center interelectrode distance), pediatric grid (2.3 mm diameter, 5 mm center-center interelectrode distance), and strips (2.3 mm diameter, 10 mm center-center interelectrode distance). Patient 3 had a combination of a standard grid and strips. The pediatric grids in patients 1 and 2 were placed over the lateral temporal neocortex and provided additional coverage of receptive language areas. The decision to implant, the electrode targets, and the duration of invasive monitoring were determined solely on clinical grounds and without reference to this study.
Common clinical practice at our center is to perform ESM after an adequate number of seizures have been recorded. ESM occurs after the patient has been restarted on their anti-epileptic medication regimen to reduce the risk of provoking seizures with stimulation. By mapping after ictal data has been captured, stimulation can be targeted to the planned region of resection. The approach of stimulation through the grid is guided by clinician’s knowledge of the identified seizure focus, planned resection and known functional neuroanatomy.
Electrical stimulation was delivered using a biphasic square wave pulses between 2 adjacent electrode contacts. Stimulation occurred between 1-15 mA using a 300-500 μs width pulse at a frequency of 50 Hz, with a maximum train duration of 5 seconds. The stimulating current was manually controlled during the stimulation, starting at 1 mA and gradually increasing in increments of 1-4 mA until a minimum of 10 mA was achieved (with a maximum threshold of 15 mA), a functional response (i.e. loss or gain of function) was observed, or prolonged afterdischarges were detected. Interstimulus interval ranged between 5-20 seconds, depending on whether afterdischarges were observed. The EEG was simultaneously monitored during stimulation for the presence of seizures or afterdischarges. Patients were asked to describe any cognitive, perceptual, sensory, or motor phenomena they experienced during or after each stimulation trial. Language evaluation was performed by testing continuous spontaneous speech, visual naming, auditory naming and auditory comprehension tasks with language disruption noted as a positive finding. Observed and reported clinical responses were recorded, as well as the stimulation parameters used to elicit these responses. Patients were not explicitly prompted for a possible occurrence of forced thought. These spontaneous responses were reproduced and confirmed by repeated stimulation between 2 to 4 trials per patient. While the epilepsy physician (PD, DF) and neuropsychologist were conducting the stimulation and testing, patients were unaware as to the exact timing of stimulation Afterdischarges at the positive stimulation sites were not seen after stimulation. (Additional details about electrode localization are included in Supplemental Materials.) Further details about neuropsychological testing at our center have been published elsewhere(Morrison, 2015).
To calculate the cortical surface area affected by our stimulation parameters, we referenced a previous report of ESM delivered to visual cortex, which measured cortical surface area affected as a function of charge delivered per trial (Winawer & Parvizi, 2016) (Figure 4B). Then, based on an extrapolation of these published measurements, we estimated the cortical surface area affected by the minima and maxima of charge delivered per trial.
Figure 4. Difference between L and R hemispheric fMRI connectivity.
Paired t-test demonstrates that left seeds have greater connectivity to regions in the left hemisphere compared to their equivalent seeds in the right hemisphere to left hemisphere (p<0.05, FWE corrected)
2.3. Incidence of Forced Thinking Phenomenon.
To determine the incidence of the forced thinking phenomenon among our epilepsy surgical population who had stimulation in the same left frontal region as the index 3 patients, we performed a retrospective query of the NYU functional mapping database. We first determined the number of patients who had bedside ESM for language mapping from July 2006 to January 2018. We then determined the subset of patients who (1) consented for research, (2) had electrodes located in either the combined ROI for Patients 1 and 2 or ROI for Patient 3 (i.e. similar MNI coordinates), and (3) were stimulated in at least one of the electrodes within the ROI. We retrospectively examined their mapping reports to see which patients with stimulated electrodes within a target ROI had a functional “hit.”
2.4. Ellipsoid Definition.
We used patient-specific ellipsoid seed regions of interest (ROI) encompassing the positive stimulation sites for Patient 1 (GA3: −55,37,23 and GA4: −55,32,31); Patient 2 (GA3: −55,34,29 and GA4: −55,27,36); and Patient 3 (G25: −57,32,−13 and G26: −60,24,−7, G17: −61,39,−5 and G18:−62,32,1). An ellipsoid ROI was created to closely capture the field produced through bipolar stimulation of two adjacent electrodes, with an outer border of 5mm around the outer edges of the electrodes and including the inter-electrode space, with a longitudinal axis of 20 mm, and short axis of 10 mm.(Nathan, Sinha, Gordon, Lesser, & Thakor, 1993). For Patient 3, two ellipsoid ROIs were created for the analyses. All positive stimulation sites were in the left hemisphere, so equivalent ellipsoids were created in the right hemisphere by reversing the x-coordinates of the ellipsoid’s image volume, thus allowing across-hemisphere comparisons of functional connectivity as described in Section 2.3.
2.5. Functional Connectivity Analysis.
The mean time series of the seed was obtained by applying the seed ROI to each of the 81 healthy subject’s 4-D time-series warped to MNI 3mm template space and averaging across the rs-fMRI time series of each voxel within the ROI. These healthy subjects have been previously described (17 female, age range 20-66 years, mean 36.7 years, SD 12.6 years)(McGill et al., 2014; Reyes et al., 2016; Thesen et al., 2011). Within-patient, left hemisphere resting state functional connectivity maps of all voxels were generated by correlating each voxel’s time-series with the seed’s mean time series. Correlation coefficients were normalized using Fisher’s Z transformation for further statistical analysis. One-sample t-test was employed to examine whether the mean functional connectivity of normal controls was significantly different than a hypothesized correlation of zero (p<0.05, FWE corrected). The FWE corrected t-stats maps for each ROI were then binarized, and added together. An across-patients, left hemisphere rs-fMRI conjunction analysis was performed by thresholding the summed t-stats map with the number of ROIs. Within-patient, hemispheric differences in rs-fMRI connectivity were compared using a two-tailed paired t-test. Conjunction analyses is based on the minimum statistic (Friston, Holmes, Price, Buchel, & Worsley, 1999; Nichols, Brett, Andersson, Wager, & Poline, 2005)Here we calculate the intersection of the connectivity clusters thresholded at p=0.05 with TFCE method (Smith & Nichols, 2009) which requires that all comparisons are individually significant at the usual level instead of testing against the global null. For imaging protocol and preprocessing steps, please see supplemental methods.
2.6. Meta-Analytic Connectivity Modeling and BrainMap Behavioral Analysis
The BrainMap database manually curates x-y-z location foci and meta-data from ~17,000 previously-published functional neuroimaging experiments (Barron, 2015). Meta-analytic connectivity models (MACM) have been validated as a measure of functional brain connectivity (defined as x-y-z focus co-activation) by reference to resting-state(Cauda et al., 2011; Cieslik et al., 2013; Rottschy et al., 2013), diffusion tractography(Cauda et al., 2011; Eickhoff et al., 2010; Robinson et al., 2012), electrophysiology(Narayana et al., 2012), and non-human primate tracer studies(Robinson, Laird, Glahn, Lovallo, & Fox, 2010). For each patient’s left hemisphere ellipsoid, the BrainMap database was searched for studies reporting foci. This search returned: for P1, 2479 foci from 150 experiments representing 132 papers; P2, 846 foci from 102 experiments representing 84 papers; P3, 3028 foci from 239 experiments from 195 papers. Activation likelihood estimation (ALE) algorithm was used to compute which coordinates were most consistently co-activated, thus producing a MACM for each patient’s left hemisphere ellipsoid.(Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012) Within-subject, hemispheric differences in MACM connectivity was computed by performing with the contrast analysis function found on the GingerAle 2.3.6 (brainmap.org) software platform, using methods previously described(Eickhoff et al., 2011).
A behavioral profile for each patient’s ellipsoid was defined by referencing the BrainMap database’s experimental metadata.(P. T. Fox et al., 2005) Because behavioral meta-data is associated with x,y,z coordinates, a behavioral profile can be computed within the ellipsoid as a z-score that represents the number of behavior-coordinate pairings found within the ellipsoid compared to the number of behavior-coordinate pairings expected if they were uniformly distributed throughout the brain. A high z-score indicates a high specificity of a particular behavior for that ellipsoid.
3. RESULTS
3.1. Case descriptions.
Three patients with refractory focal epilepsy undergoing evaluation for resective surgery who spontaneously reported forced thinking during cortical mapping were included in this observational study. None of the patients reported this cognitive behavior during their habitual seizures. To ensure that we captured all cases of forced thought in our surgical database, we performed a retrospective query and did not find any additional cases.
3.1.1. Patient 1 was a 40-year-old left-handed woman who sustained a left frontotemporal brain injury during a motor vehicle accident at age 16 and developed refractory focal epilepsy. Her typical seizures were characterized by “a feeling of something overcoming her,” finger numbness, altered breathing patterns, fear, speech disruption, facial grimacing, and motor automatisms. She was determined to have left hemisphere language dominance by Wada testing, and therefore underwent invasive monitoring with extensive coverage of the left hemisphere involving subdural grids, strips, and depths electrodes. The majority of her seizures arose from the left anterior temporal neocortex. She subsequently underwent a left anteromedial temporal lobectomy. After 5 years of follow-up, she suffers from rare non-disabling sensory seizures since surgery (Engel Class 1B outcome).
3.1.2. Patient 2 as a 42-year-old, left-handed man with a history of refractory seizures secondary to head trauma at age 31. His seizures were characterized by a feeling of “someone setting up sound equipment, and the humming getting louder, like a power surge,” which progressed to staring, slurred speech, altered awareness, and motor automatisms. He had left hemisphere language dominance by Wada testing and therefore underwent invasive monitoring with extensive coverage of the left hemisphere involving subdural grids, strips, and depth electrodes targeting the left frontotemporal cortex. His typical seizures had left mesial temporal lobe onset. He underwent a left anteromedial temporal resection. After 5 years of follow-up, he suffers from rare non-disabling sensory seizures since surgery (Engel Class 1B outcome).
3.1.3. Patient 3 was a 35-year-old right-handed man with a history of left temporal hemorrhage of unknown etiology at age 33 resulting in refractory focal epilepsy. His seizures began with a "rolling" feeling in his brain, described as "everything coming into his brain at once," followed by speech arrest with retention of awareness. These events would sometimes progress to impaired awareness or bilateral tonic-clonic seizures. Implanted grid, strips and depth electrodes revealed that the seizures arose from temporal neocortex around his lesion and he underwent a tailored lateral temporal cortical resection. After 5 years of follow up, he had a single disabling seizure after surgery, but has been free of disabling seizures for at least 2 years (Engel Class 1C outcome).
3.2. Stimulation
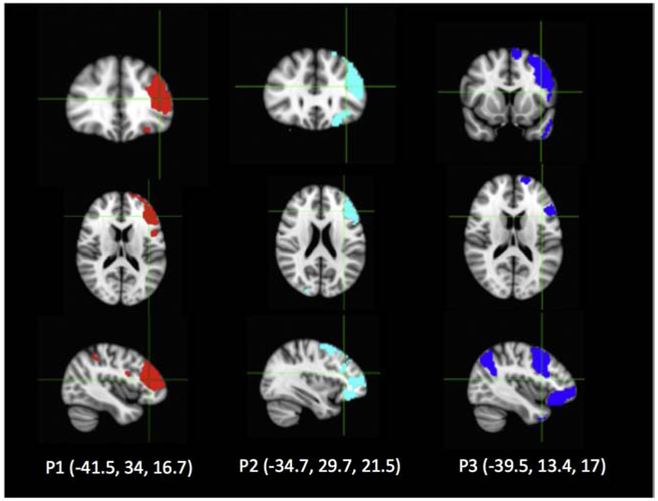
3.2.1. Patient 1 described forced thoughts about “a game show I used to watch on TV but I haven’t seen in years” when stimulated over electrodes GA3-4 (Figure 1, Table 1). When questioned, she could not provide any other details about this game show except to clarify that this was a thought or concept, and not an elicited visual perception or memory of anything she had seen or experienced. The MNI coordinates of electrodes GA 3 (−55,37,23) and GA 4 (−55,32,31) correspond to the left dorsolateral prefrontal cortex and rostral middle frontal gyrus, which includes Brodmann areas 9 and 46 (Figure 1, Table 1). The forced thought was solicited by stimulation of GA3-4 at 11.9 mA, 50 Hz, 500 μs, for trains between 1.5 to 3.9 seconds. The charge delivered per trial was 446.3-1160 μC (Table 2). Stimulation did not result in any afterdischarges. The estimated cortical surface area affected ranged from 96 mm2 to 128 mm2 (Table 2). In addition, a visual naming task was interrupted with stimulation of the adjacent electrodes at GA 1-2. Other nearby electrodes demonstrated disruption of visual naming (GA 9-10).
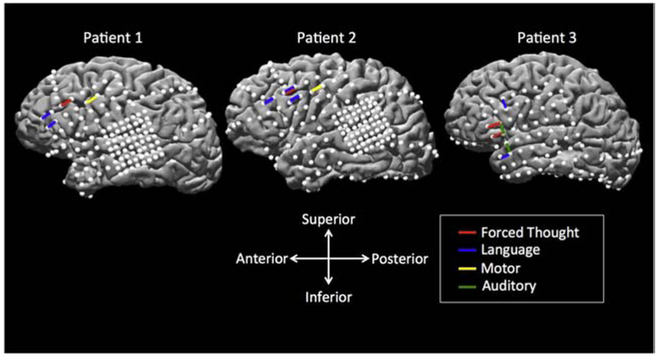
Figure 1. Electrode localization on the reconstructed brain surface from each individual patient’s MRI, with left hemispheric coverage.
Surface electrodes are represented by white dots. Bipolar stimulation across electrode pairs which elicited forced thoughts are shown as red bars and are located in the prefrontal cortex. Adjacent electrode pairs which elicited a functional response during electrocortical stimulation mapping (ESM) are shown in blue (language), yellow (motor), and green (auditory). Patients 1 and 2 had a combination of a standard subdural grid (2.3 mm diameter, 10 mm center-center interelectrode distance), pediatric grid (2.3 mm diameter, 5 mm center-center interelectrode distance), and strips (2.3 mm diameter, 10 mm center-center interelectrode distance. Patient 3 has a combination of a standard subdural grid and strips.
Table 1. Details of Electrical Stimulation Procedure and Subjective Reports.
Maximum stimulation settings, with patient responses of electrode pairs which induced a forced thought reponses, and adjacent electrode pairs. Electric stimulation was delivered using biphasic square wave pulses between 2 adjacent electrode contacts. Stimulation occurred between 1-15 mA using a 500 μs width pulse at a frequency of 50 Hz, with a maximum train duration of 2-5 seconds. The stimulating current was manually controlled during the stimulation, starting at 1 mA and gradually increasing in increments of 1-4 mA until a minimum of 10 mA was achieved (with a maximum threshold of 15 mA), a functional response (i.e. loss or gain of function) was observed, or prolonged afterdischarges were detected. Patients were asked to describe any cognitive, perceptual, sensory, or motor phenomena they experienced during or after each stimulation trial.
| Stimulation Location (Brodmann Area) |
Electrode 1 | Electrode 2 | Stim Intensity (mA) |
Stim Duration (sec) |
Patient Response |
|---|---|---|---|---|---|
| Patient 1 | |||||
| Left dorsolateral prefrontal cortex/ Rostral middle frontal gyrus (BA 9, 46) | GA1 | GA2 | 11.7 | 1-3.3 | Visual naming impairment |
| GA3 | −GA4 | 11.9 | 1.5-3.9 | Forced thought about a game show “she used to watch on TV but had not seen in years.” | |
| GA5 | GA6 | 6.0 | 1.7 | No response | |
| GA9 | GA10 | 12.7 | 1.7 | Visual naming impairment | |
| GA11 | GA12 | 11.9 | 1.6-2.7 | No response | |
| GA13 | GA14 | 6.0 | 2.6 | Motor response (rightward tongue deviation) | |
| Patient 2 | |||||
| Left dorsolateral prefrontal cortex/ Rostral middle frontal gyrus (BA 9, 46) | GA1 | GA2 | 11.6 | 1.0-4.9 | Visual naming impairment |
| GA3 | GA 4 | 11.7 | 1.0-3.7 |
Forced thought about an [unfamiliar] individual, unable to report name, physical characteristics, or relationship. Visual naming impairment |
|
| GA5 | GA6 | 5.0 | 1.0 | No response | |
| GA9 | GA10 | 11.7 | 1.0-4.9 | No response | |
| GA11 | GA12 | 6.8 | 1.0 | Speech arrest | |
| GA13 | GA14 | 5.8 | 1.6 | Motor response (rightward tongue deviation) | |
| Patient 3 | |||||
| Pars triangularis (BA 45) | G17 | G18 | 14.4 | 1.5-5.0 | “Memory of something that I can’t describe.” |
| Pars orbitalis (BA 47) | G25 | G26 | 11.6 | 1.0-5.0 | Memory of a child’s game. “I had a thought about a game that kids play in the summer, I can’t think of the exact game.” |
| G3 | G11 | 12.5 | 1.0-5.0 | Visual naming impairment | |
| G17 | G25 | 11.5 | 1.0-4.9 | Complex auditory phenomena | |
| G18 | G26 | 14.6 | 1.0-4.5 | Hears unfamiliar voice as an echo | |
| G19 | G27 | 5.8 | 2.2 | Ipsilateral pain | |
| G34 | G42 | 12.1 | 1.0-4.3 | Auditory hallucination (hears a familiar song playing) | |
| G41 | G42 | 11.6 | 1.0-4.9 | Language (comprehension) | |
Table 2. Calculation of Charge Delivered and Estimate of Cortical Area Affected to Elicit Forced Thinking.
To calculate the cortical surface area affected by our stimulation parameters, we referenced a previous report of ESM delivered to visual cortex, which measured cortical surface area affected as a function of charge delivered per trial (Winawer and Parvizi 2016, Figure 4B). Maximum charge delivery was generated for each patient with stimulation of longer duration, and was reproduced with stimulation of shorter duration to ensure that the cognitive phenomenon was due to direct stimulation of the region and not to spread. We calculated charge per pulse delivered and the charge per trial. We note that the highest charge deliveries for our three patients exceeded the maximum charge deliveries delivered in Winawer and Parvizi (Neuron 2016), which were generally ≤500 μC per trial. Estimates of cortical area affected are based on extrapolations of their published data on Figure 4B. As there was significant variance in their cortical area measured even within the visual cortex, we took the median cortical area for each charge delivered per trial.
| Patient | Current (mA) | Frequency (Hz) | Pulse Width (μS) |
Duration (s) | Charge Per Pulse (μC) |
Charge per Trial (μC) |
Cortical Area (mm2) |
| 1 | 11.9 | 50 | 500 | 1.5 - 3.9 | 5.95 | 446.3 – 1160 | 96 mm2-128 mm2 |
| 2 | 11.7 | 50 | 500 | 1 - 3.7 | 5.85 | 292.5 – 1082.2 | 90 mm2-124 mm2 |
| 3 | 11.6 | 50 | 500 | 1.0 – 5.0 | 5.80 | 290 – 1450 | 88 mm2-137 mm2 |
3.2.2. Patient 2 described forced thoughts about “a person” when stimulated over electrode pair GA 3-4 (Figure 1, Supplementary Table 1). When probed, the patient reported that this individual was unfamiliar and could not report their name, describe their physical characteristics or their relationship to the individual. The patient clarified that their experience was not a visual phenomenon or a specific person they knew. MNI coordinates of electrodes GA 3 (−55,34,29) and GA 4 (−55,27,36) correspond to the left dorsolateral prefrontal cortex and rostral middle frontal gyrus, which includes Brodmann areas 9 and 46 (Figure 1, Table 1). The forced thought was solicited by stimulation of GA3-4 at 11.7 mA, 50 Hz, 500 μs, for trains between 1.0 to 3.7 seconds. The charge delivered per trial was 292.5 – 1082.2 μC (Table 2). Again, stimulation did not result in any afterdischarges. The estimated cortical surface area affected ranged from 90 mm2 to 124 mm2 (Table 2). Disruption of visual naming was also observed with stimulation of the same electrodes (at 11.7 mA). Adjacent electrode stimulation disrupted visual naming (GA 1-2, GB 11-12, GB 15-16), auditory naming (GB 15-16), and caused speech arrest (GA 11-12, 17-20, 25-28).
3.2.3. Patient 3 reported, “I had a thought about a game that kids play in the summer, I can’t think of the exact game.” This forced thought was reproducible at contacts G25-26 (11.6 mA), which correspond to Brodmann area 47 (pars orbitalis). The forced thought was solicited by stimulation of at 11.6 mA, 50 Hz, 500 μs, for trains between 1.0 to 5.0 seconds. The charge delivered per trial was 290 – 1450 μC. The estimated cortical surface area affected ranged from 88 mm2 to 137 mm2 (Table 2). At G17-18, stimulation produced a memory of something that he could not describe (14.4 mA), which corresponds to the Brodmann area 45 (pars triangularis, Figure 1, Table 1). None of the positive stimulation sites were associated with afterdischarges. The latter sites had clear auditory features whereas the positive site was more abstract, which may illustrate that these thoughts were not bound to specific sensory modalities or features as the other sites were. The sites were distinct from language areas (including naming) and did not overlap with epileptogenic cortex.
These patients, on further questioning, stated that elicited thoughts were spontaneous, out-of-context, and involuntary. The object/person was not familiar, and they could not volunteer further sensory (including visual) detail, suggesting an abstract nature of the thought. The patients described these forced thoughts only during stimulation, although they were unaware of the timing of delivery. The thoughts stopped with the cessation of stimulation, and in all cases, were reproduced between two to four times.
3.3. Functional connectivity
The ROI centered at the positive electrode pair for P1 [GA3 (−55, 37, 23) and GA4 (−55, 32, 31)] demonstrated functional resting state connectivity between the inferior and middle frontal gyri, pars triangularis and opercularis, superior parietal lobule, supramarginal gyrus, angular gyrus, and the inferior/middle temporal gyri (Table 3a and Figure 2, top row). The ROI centered at the positive electrode pair for P2 [GA3 (−55, 34, 29) and GA4 (−55, 27, 36)] similarly demonstrated functional resting state connectivity between the inferior and middle frontal gyri, pars triangularis and opercularis, the superior parietal, angular gyrus, and the inferior/middle temporal gyri (Table 3b and Figure 2, middle row). The ROIs centered at the positive electrode pair for P3 [G25 (−57, 32, −13) and G26 (−60, 24, −7); and G17 (−61, 39, −5) and G18 (−62, 32, 1)] were located slightly inferiorly to the positive stimulation sites for P1 and P2 and demonstrated functional resting state connectivity between the frontal and central operculum, superior frontal gyrus, insula, planum polare, and temporal pole (Table 3c and Figure 2, bottom row).
Table 3. Functional MRI Connectivity Conjunction Analysis.
The fMRI resting state (rs-fMRI) conjunction analysis demonstrates a widespread network.
| A. Patient 1 | ||||||
|---|---|---|---|---|---|---|
| Cluster # | Volume (mm^3) | Weighted Center MNI (x,y,z) | Harvard-Oxford Cortical Label | Talairach Label | ||
| 1 | 61998 | −40.1 | 26.6 | 20.7 | Inferior Frontal Gyrus, pars triangularis. Middle Frontal Gyrus. Inferior Frontal Gyrus, pars opercularis | Left Cerebrum. Frontal Lobe. Middle Frontal Gyrus. White Matter. Brodmann area 48 |
| 2 | 45520 | 42.8 | 30.9 | 16.6 | Inferior Frontal Gyrus, pars triangularis. Middle Frontal Gyrus. Frontal Pole. Inferior Frontal Gyrus, pars opercularis | Right Cerebrum. Frontal Lobe. Sub-Gyral. White Matter. Brodmann area 48 |
| 3 | 23337 | −36.3 | −53.9 | 44.6 | Angular Gyrus. Superior Parietal Lobule. Supramarginal Gyrus, posterior division. Lateral Occipital Cortex, superior division. Supramarginal Gyrus, anterior division | Left Cerebrum. Parietal Lobe. Inferior Parietal Lobule. White Matter. Brodmann area 7 |
| 4 | 12842 | 42 | −49.2 | 46 | Angular Gyrus. Superior Parietal Lobule. Supramarginal Gyrus, posterior division | Right Cerebrum. Parietal Lobe. Inferior Parietal Lobule. White Matter. Brodmann area 40 |
| 5 | 5489 | −55.5 | −51.9 | −12.2 | Inferior Temporal Gyrus, temporooccipital and posterior part Middle Temporal Gyrus, temporooccipital and posterior part | Left Cerebrum. Temporal Lobe. Inferior Temporal Gyrus. White Matter.Brodmann area 37 |
| 6 | 3879 | 59.8 | −46 | −14 | Inferior Temporal Gyrus, temporooccipital and posterior part Middle Temporal Gyrus, temporooccipital part | Right Cerebrum. Temporal Lobe. Middle Temporal Gyrus. Brodmann area 20 |
| 7 | 3786 | −1.32 | 23.4 | 44.7 | Paracingulate Gyrus. Superior Frontal Gyrus | Left Cerebrum. Frontal Lobe. Medial Frontal Gyrus. Brodmann area 32 |
| 8 | 1122 | −14 | 4.9 | 10.3 | Left Cerebral White Matter. Left Caudate | Left Cerebrum. Sub-lobar. Extra-Nuclear. White Matter |
| B. Patient 2 | ||||||
| Cluster # | Volume (mm^3) | Weighted Center MNI (x,y,z) | Harvard-Oxford Cortical Label | Tailarach Label | ||
| 1 | 74305 | −35.6 | 27.2 | 24.5 | Middle Frontal Gyrus. Inferior Frontal Gyrus, pars triangularis. Inferior Frontal Gyrus, pars opercularis | Left Cerebrum. Frontal Lobe. Middle Frontal Gyrus. White Matter. Brodmann area 48 |
| 2 | 44752 | 40.9 | 31.8 | 21.7 | Middle Frontal Gyrus. Inferior Frontal Gyrus, pars triangularis Frontal Pole | Right Cerebrum. Frontal Lobe. Sub-Gyral. White Matter. Brodmann area 48 |
| 3 | 22768 | −35.2 | −57.7 | 45 | Superior Parietal Lobule. Lateral Occipital Cortex, superior division. Angular Gyrus. Supramarginal Gyrus, posterior division | Left Cerebrum. Parietal Lobe. Inferior Parietal Lobule. White Matter. Brodmann area 7 |
| 4 | 12818 | 41.9 | −54.4 | 46.1 | Angular Gyrus. Superior Parietal Lobule. Lateral Occipital Cortex, superior division | Right Cerebrum. Parietal Lobe. Inferior Parietal Lobule. White Matter. Brodman area 40 |
| 5 | 4644 | −57.6 | −48.3 | −12.6 | Inferior Temporal Gyrus, temporooccipital and posterior part Middle Temporal Gyrus, temporooccipital and posterior part | Left Cerebrum. Temporal Lobe. Inferior Temporal Gyrus. Gray Matter. Brodmann area 20 |
| 6 | 2719 | 61.4 | −43.8 | −15 | Inferior Temporal Gyrus, temporooccipital and posterior part Middle Temporal Gyrus, temporooccipital and posterior part | Right Cerebrum. Temporal Lobe. Middle Temporal Gyrus. Gray Matter. Brodmann area 20 |
| c. Patient 3 | ||||||
| Cluster # | Volume (mm^3) | Weighted Center MNI (x,y,z) | Harvard-Oxford Cortical Label | Tailarach Label | ||
| 1 | 101880 | −46.5 | 3.76 | 3.56 | Central Opercular Cortex. Frontal Operculum Cortex. Precentral Gyrus. Inferior Frontal Gyrus, pars opercularis | Left Cerebrum. Frontal Lobe. Precentral Gyrus. White Matter. Brodmann area 48 |
| 2 | 61431 | 51.3 | 5.65 | −3.14 | Planum Polare. Temporal Pole. Central Opercular Cortex. Frontal Operculum Cortex. Precentral Gyrus. Inferior Frontal Gyrus, pars opercularis | Right Cerebrum. Temporal Lobe. Superior Temporal Gyrus. Brodmann area 38 |
| 3 | 39020 | −2.76 | 37.4 | 44.4 | Superior Frontal Gyrus. Paracingulate Gyrus | Left Cerebrum. Frontal Lobe. Superior Frontal Gyrus. Gray Matter. Brodmann area 8 |
| D. Conjunction Analysis for patients 1-3 | ||||||
| Cluster # | Volume (mm^3) | Weighted Center MNI (x,y,z) | Harvard-Oxford Cortical Label | Tailarach Label | ||
| 1 | 53283 | −41.4 | 25.4 | 17.4 | Inferior Frontal Gyrus, pars triangularis and pars opercularis. Middle Frontal Gyrus | Left Cerebrum. Frontal Lobe. Sub-Gyral. White Matter. Brodmann area 45 |
| 2 | 27481 | 46.7 | 29.3 | 12.6 | Inferior Frontal Gyrus, pars triangularis and pars opercularis. Middle Frontal Gyrus. Frontal Pole | Right Cerebrum. Frontal Lobe. Sub-Gyral. White Matter. Brodmann area 45 |
| 3 | 7414 | −47.6 | −53.1 | 44.8 | Angular Gyrus. Supramarginal Gyrus, posterior division. Lateral Occipital Cortex, superior division | Left Cerebrum. Parietal Lobe. Inferior Parietal Lobule. White Matter. Brodmann area 40 |
| 4 | 5127 | −2.5 | 25.4 | 46.1 | Superior Frontal Gyrus. Paracingulate Gyrus | Left Cerebrum. Frontal Lobe. Medial Frontal Gyrus. Gray Matter. Brodmann area 8 |
| 5 | 4323 | −60.2 | −45.1 | −9.56 | Middle Temporal Gyrus, temporooccipital part and posterior division Inferior Temporal Gyrus, temporooccipital part and posterior division | Left Cerebrum. Temporal Lobe. Middle Temporal Gyrus. White Matter. Brodmann area 20 |
| 6 | 3550 | 64.2 | −40 | −11.6 | Middle Temporal Gyrus, temporooccipital part and posterior division Inferior Temporal Gyrus, temporooccipital part and posterior division | Right Cerebrum. Temporal Lobe. Middle Temporal Gyrus. White Matter. Brodmann area 20 |
| 7 | 3119 | −14 | 5.08 | 11.7 | Left Cerebral White Matter. Left Caudate | Left Cerebrum. Sub-lobar. Caudate. Gray Matter. Caudate Body |
| 8 | 630 | 14.7 | 7.32 | 10.4 | Right Cerebral White Matte. Right Caudate | Right Cerebrum. Sub-lobar. Caudate. Gray Matter. Caudate Body |
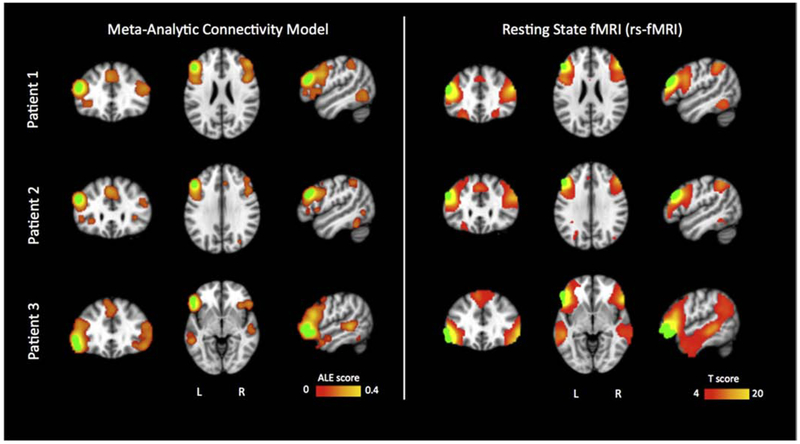
Figure 2.
Comparison of co-activated networks between Meta-Analytic Connectivity Model (MACM) and rs-fMRI. Subject elliptical ROIs were marked in green color. Rs-fMRI connectivity one sample t-test showed significant effect in bilateral frontal gyri and temporal gyri, t(80)=3.67, p<0.001. Meta-Analytic Connecitivity Models (MACM) reported Activation Likelihood Estimation (ALE) scores significant at cluster-level (<0.001) and False Discovery Rate (p<0.05) thresholds. These MACM-defined areas mirror those reported by rs-fMRI. Patient 1 is shown at MNI152 x,y,z slices (−50,32,24); Patient 2 is shown at (−49,28,30); Patient 3 at (−53,21,−5).
A subsequent conjunction analysis for the 3 patients’ ROIs (2 ROIs for patient 3) revealed that the shared rs-fMRI regions include the middle and inferior frontal regions, pars triangularis and opercularis, angular and supramarginal gyrus, superior parietal regions, and middle temporal gyrus, among other regions (Table 2d, Figure 3, bottom row).
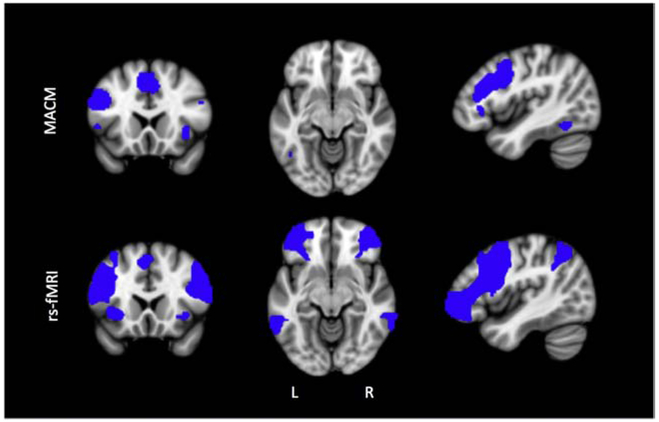
Figure 3. Conjunction analysis of MACM and rs-fMRI connectivity.
Both analyses showed left PFC connectivity that is consistent across subjects and imaging modality. Slices shown are x,y,z (−43,19,−10) in MNI space.
3.4. Behavioral Analysis and Meta-Analytic Connectivity Modeling
The ROI centered at the positive electrode pair for P1 [GA3 (−55, 37, 23) and GA4 (−55, 32, 31)] demonstrated MACM co-activation with the middle and medial frontal gyri, inferior and superior parietal lobule, and inferior temporal gyrus, among other regions (Table 4a and Figure 2, top row). The ROI centered at the positive electrode pair for P2 [GA3 (−55, 34, 29) and GA4 (−55, 27, 36)] demonstrated MACM coactivation with middle frontal gyrus, inferior parietal lobule, cingulate gyrus, and occipital lobe, among others (Table 4b and Figure 2, middle row). The ROI centered at the positive electrode pair for P3 [G25 (−57, 32, −13) and G26 (−60, 24, −7); and G17 (−61, 39, −5) and G18 (−62, 32, 1)] demonstrated MACM co-activation with the inferior frontal gyrus, superior frontal gyrus, middle temporal gyrus, and parahippocampal gyrus, among other regions (Table 4c and Figure 2, bottom row). A conjunction analysis of the 3 patients’ ROIs (2 ROIs for patient 3) showed a shared MACM network that included the middle frontal gyrus, paracingulate and cingulate gyrus, parietal lobe, and insula (Table 4d, Figure 3, top row).
Table 4. MACM Summary Table.
Individual MACM analysis shows brain-wide functional co-activation clusters. Clusters represent regions of statistically significant convergence of co-activation foci as reported in the BrainMap database. Statistical significance is reported as ALE score (Eickhoff, et al., 2012).
| a. Patient 1 | ||||||
|---|---|---|---|---|---|---|
| Cluster # | Volume (mm^3) | ALE Score (x10) | Weighted Center MNI152 (x,y,z) |
Talairach Label | ||
| 1 | 32384 | 3.6972487 | −48 | 32 | 22 | Left Cerebrum.Frontal Lobe.Middle Frontal Gyrus.Gray Matter.Brodmann area 46 |
| 2 | 14560 | 0.9527298 | 48 | 38 | 24 | Right Cerebrum.Frontal Lobe.Middle Frontal Gyrus.Gray Matter.Brodmann area 9 |
| 3 | 11616 | 0.85477084 | 2 | 26 | 42 | Left Cerebrum.Frontal Lobe.Medial Frontal Gyrus.Gray Matter.Brodmann area 8 |
| 4 | 10872 | 0.95113054 | −42 | −44 | 44 | Left Cerebrum.Parietal Lobe.Inferior Parietal Lobule.Gray Matter.Brodmann area 40 |
| 5 | 5056 | 0.70201054 | 34 | −62 | 50 | Right Cerebrum.Parietal Lobe.Superior Parietal Lobule.Gray Matter.Brodmann area 7 |
| 6 | 3960 | 0.5746817 | −50 | −62 | −2 | Left Cerebrum.Temporal Lobe.Inferior Temporal Gyrus.Gray Matter.Brodmann area 19 |
| 7 | 2536 | 0.8662599 | 34 | 22 | 0 | Right Cerebrum.Sub-lobar.Claustrum.Gray Matter. |
| 8 | 2200 | 0.4513255 | −28 | 8 | 56 | Left Cerebrum.Frontal Lobe.Sub-Gyral.Gray Matter.Brodmann area 6 |
| 9 | 2080 | 0.43759383 | −12 | −12 | 6 | Left Cerebrum.Sub-lobar.Thalamus.Gray Matter.Ventral Lateral Nucleus |
| 10 | 2056 | 0.5204129 | 30 | 2 | 48 | Right Cerebrum.Frontal Lobe.Middle Frontal Gyrus.Gray Matter.Brodmann area 6 |
| 11 | 888 | 0.4587746 | 10 | −10 | 8 | Right Cerebrum.Sub-lobar.Thalamus.Gray Matter. |
| 12 | 432 | 0.47315214 | 50 | 20 | −6 | Right Cerebrum.Frontal Lobe.Inferior Frontal Gyrus.Gray Matter.Brodmann area 47 |
| 13 | 320 | 0.36938224 | 58 | −56 | −12 | Right Cerebrum.Temporal Lobe.Inferior Temporal Gyrus.Gray Matter.Brodmann area 20 |
| 14 | 320 | 0.45044336 | −20 | −100 | −2 | Left Cerebrum.Occipital Lobe.Lingual Gyrus.Gray Matter.Brodmann area 18 |
| b. Patient 2 | ||||||
| Cluster # | Volume (mm^3) | ALE Score (x10) | Weighted Center MNI152 (x,y,z) |
Talairach Label | ||
| 1 | 15712 | 3.1472412 | −48 | 26 | 30 | Left Cerebrum.Frontal Lobe.Middle Frontal Gyrus.Gray Matter.Brodmann area 9 |
| 2 | 7880 | 0.6158217 | −34 | −52 | 46 | Left Cerebrum.Parietal Lobe.Inferior Parietal Lobule.Gray Matter.Brodmann area 40 |
| 3 | 6840 | 0.77475175 | −4 | 26 | 42 | Left Cerebrum.Limbic Lobe.Cingulate Gyrus.Gray Matter.Brodmann area 32 |
| 4 | 5856 | 0.4794898 | 48 | 18 | 30 | Right Cerebrum.Frontal Lobe.Middle Frontal Gyrus.Gray Matter.Brodmann area 9 |
| 5 | 2720 | 0.7074343 | −30 | 22 | −2 | Left Cerebrum.Sub-lobar.Claustrum.Gray Matter. |
| 6 | 1976 | 0.49369562 | −46 | −52 | −22 | Left Cerebellum.Anterior Lobe.Culmen.Gray Matter. |
| 7 | 1944 | 0.47308784 | 30 | −92 | −6 | Right Cerebrum.Occipital Lobe.Inferior Occipital Gyrus.Gray Matter.Brodmann area 18 |
| 8 | 1880 | 0.59526917 | −18 | −96 | −8 | Left Cerebrum.Occipital Lobe.Inferior Occipital Gyrus.Gray Matter.Brodmann area 17 |
| 9 | 1728 | 0.63747704 | 34 | 22 | 0 | Right Cerebrum.Sub-lobar.Claustrum.Gray Matter. |
| 10 | 1512 | 0.53964928 | −28 | 6 | 58 | Left Cerebrum.Frontal Lobe.Sub-Gyral.Gray Matter.Brodmann area 6 |
| 11 | 1376 | 0.37447874 | 30 | −66 | 42 | Right Cerebrum.Parietal Lobe.Precuneus.Gray Matter.Brodmann area 7 |
| 12 | 640 | 0.40365443 | −40 | 48 | 4 | Left Cerebrum.Frontal Lobe.Middle Frontal Gyrus.Gray Matter.Brodmann area 10 |
| 13 | 408 | 0.34802593 | −46 | 30 | 0 | Left Cerebrum.Frontal Lobe.Inferior Frontal Gyrus.Gray Matter.Brodmann area 13 |
| 14 | 400 | 0.40031504 | 10 | −10 | 10 | Right Cerebrum.Sub-lobar.Thalamus.Gray Matter. |
| 15 | 280 | 0.33685982 | −20 | 0 | 6 | Left Cerebrum.Sub-lobar.Lentiform Nucleus.Gray Matter.Putamen |
| 16 | 272 | 0.4005287 | 6 | 12 | 0 | Right Cerebrum.Sub-lobar.Caudate.Gray Matter.Caudate Head |
| 17 | 272 | 0.36310226 | −50 | 14 | 2 | Left Cerebrum.Sub-lobar.Insula.Gray Matter.Brodmann area 13 |
| 18 | 232 | 0.37760735 | −50 | −64 | 0 | Left Cerebrum.Temporal Lobe.Middle Temporal Gyrus.Gray Matter.Brodmann area 37 |
| 19 | 224 | 0.32760523 | 54 | 10 | 18 | Right Cerebrum.Frontal Lobe.Inferior Frontal Gyrus.Gray Matter.Brodmann area 44 |
| c. Patient 3 | ||||||
| Cluster # | Volume (mm^3) | ALE Score (x10) | Weighted Center MNI152 (x,y,z) |
Talairach Label | ||
| 1 | 39384 | 3.9066708 | −50 | 28 | −8 | Left Cerebrum.Frontal Lobe.Inferior Frontal Gyrus.Gray Matter.Brodmann area 47 |
| 2 | 10104 | 0.8000214 | 48 | 28 | −10 | Right Cerebrum.Frontal Lobe.Inferior Frontal Gyrus.Gray Matter.Brodmann area 47 |
| 3 | 9824 | 0.9233824 | −2 | 14 | 54 | Left Cerebrum.Frontal Lobe.Superior Frontal Gyrus.Gray Matter.Brodmann area 6 |
| 4 | 6736 | 1.1416699 | −56 | −42 | 0 | Left Cerebrum.Temporal Lobe.Middle Temporal Gyrus.Gray Matter.Brodmann area 22 |
| 5 | 2072 | 0.55852685 | −44 | −52 | −20 | Left Cerebellum.Anterior Lobe.Culmen.Gray Matter.* |
| 6 | 2032 | 0.673141 | −20 | −6 | −16 | Left Cerebrum.Limbic Lobe.Parahippocampal Gyrus.Gray Matter.Amygdala |
| 7 | 1480 | 0.6643619 | −8 | 60 | 24 | Left Cerebrum.Frontal Lobe.Superior Frontal Gyrus.Gray Matter.Brodmann area 9 |
| 8 | 1376 | 0.5034907 | 54 | −20 | −6 | Right Cerebrum.Temporal Lobe.Superior Temporal Gyrus.Gray Matter.Brodmann area 22 |
| 9 | 1016 | 0.5651355 | −8 | 48 | 40 | Left Cerebrum.Frontal Lobe.Superior Frontal Gyrus.Gray Matter.Brodmann area 8 |
| 10 | 880 | 0.48787095 | −32 | −52 | 46 | Left Cerebrum.Parietal Lobe.Inferior Parietal Lobule.Gray Matter.Brodmann area 40 |
| 11 | 408 | 0.47318645 | −2 | −48 | 30 | Left Cerebrum.Limbic Lobe.Cingulate Gyrus.Gray Matter.Brodmann area 31 |
| 12 | 384 | 0.4897453 | 24 | −2 | −18 | Right Cerebrum.Limbic Lobe.Parahippocampal Gyrus.Gray Matter.Amygdala |
| d. Conjunction Analysis for P1, P2, P3 | ||||||
| Cluster # | Volume (mm^3) | ALE Score (x10) | Weighted Center MNI152 (x,y,z) |
Talairach Label | ||
| 1 | 2198 | 0.124 | −48 | 19 | 27 | Left Cerebrum.Frontal Lobe.Middle Frontal Gyrus.White Matter.* |
| 2 | 1113 | 0.0705 | 1 | 22 | 44 | Paracingulate Gyrus, Cingulate Gyrus |
| 3 | 205 | 0.0463 | −29 | −56 | 48 | Left Cerebrum.Parietal Lobe.Superior Parietal Lobule.White Matter.* |
| 4 | 180 | 0.047 | 41 | 23 | −3 | Right Cerebrum.Sub-lobar.Insula.Gray Matter.Brodmann area 13 |
| 5 | 172 | 0.0394 | 57 | 27 | 21 | Right Cerebrum.Frontal Lobe.Inferior Frontal Gyrus.Gray Matter.Brodmann area 9 |
| 6 | 124 | 0.0409 | −46 | −56 | −13 | Left Cerebrum.Temporal Lobe.Fusiform Gyrus.White Matter.* |
| 7 | 64 | 0.0315 | −52 | 15 | 3 | Left Cerebrum.Frontal Lobe.Precentral Gyrus.Gray Matter.Brodmann area 44 |
| 8 | 8 | 0.0177 | 54 | 40 | 14 | Right Cerebrum.Frontal Lobe.Middle Frontal Gyrus.White Matter.* |
Behavioral analysis reported that the ROIs for patients 1 and 2 were most likely activated in cognitive tasks including attention, working memory, semantics, speech and explicit memory (Table 5). Patient 3’s ROIs demonstrated greatest activation of cognitive domains including semantics, speech, explicit memory, working memory, and phonology. In a conjunction analysis between the 3 patients, the cognitive tasks which were significantly co-activated included semantics, working memory, speech, attention, and explicit memory (Table 5).
Table 5. Behavioral analysis of each patient’s MACM model, as defined by the BrainMap Database behavioral ontology.
Effect size z-scores summarize the probability that a particular behavioral domain and subdomain is likely to activate within the respective MACM network than throughout the brain in a random fashion (significance at z ≥ 3.0, Lancaster 2013).
| Patient 1 | ||
|---|---|---|
| Category | Domain | Z-score |
| Attention | Cognition | 22.16090286 |
| Memory (Working) | Cognition | 22.0914981 |
| Language (Semantics) | Cognition | 21.80608259 |
| Language (Speech) | Cognition | 18.81479238 |
| Memory (Explicit) | Cognition | 17.1458282 |
| Other | Emotion | 16.48603099 |
| Other | Cognition | 15.68727071 |
| Language (Phonology) | Cognition | 13.26915092 |
| Reasoning | Cognition | 12.45225558 |
| Inhibition | Action | 10.81008545 |
| Space | Cognition | 8.576015811 |
| Audition | Perception | 8.349167826 |
| Language (Orthography) | Cognition | 8.097118785 |
| Observation | Action | 7.981455977 |
| Somesthesis (Pain) | Perception | 7.268937123 |
| Execution (Other) | Action | 7.138425222 |
| Language (Syntax) | Cognition | 7.109279227 |
| Vision (Motion) | Perception | 6.520057019 |
| Social Cognition | Cognition | 6.474688325 |
| Music | Cognition | 6.474561941 |
| Imagination | Action | 5.328575209 |
| Vision (Other) | Perception | 4.993948243 |
| Somesthesis (Other) | Perception | 4.992566341 |
| Language (Other) | Cognition | 4.507240488 |
| Execution (Speech) | Action | 4.334635428 |
| Patient 2 | ||
| Category | Domain | Z-score |
| Attention | Cognition | 18.62161892 |
| Language (Semantics) | Cognition | 18.05798834 |
| Memory (Working) | Cognition | 17.44047883 |
| Language (Speech) | Cognition | 15.13939812 |
| Memory (Explicit) | Cognition | 15.1283065 |
| Other | Emotion | 13.96135327 |
| Other | Cognition | 13.76267012 |
| Language (Phonology) | Cognition | 11.97769481 |
| Reasoning | Cognition | 11.21371322 |
| Inhibition | Action | 8.550866002 |
| Vision (Shape) | Perception | 8.112930642 |
| Language (Orthography) | Cognition | 8.092832135 |
| Space | Cognition | 7.292688254 |
| Observation | Action | 6.957447555 |
| Vision (Other) | Perception | 6.302011341 |
| Audition | Perception | 5.864866562 |
| Language (Syntax) | Cognition | 5.741064694 |
| Social Cognition | Cognition | 4.973273906 |
| Imagination | Action | 4.827810101 |
| Vision (Motion) | Perception | 4.747242528 |
| Language (Other) | Cognition | 4.616027603 |
| Music | Cognition | 3.958923718 |
| Somesthesis (Pain) | Perception | 3.632459965 |
| Somesthesis (Other) | Perception | 3.593796226 |
| Soma | Cognition | 3.40871942 |
| Execution (Other) | Action | 3.404878536 |
| Execution (Speech) | Action | 3.334963615 |
| Patient 3 | ||
| Category | Domain | Z-score |
| Language (Semantics) | Cognition | 21.92277246 |
| Patient 1 Language (Speech) |
Cognition | 15.50573373 |
| Memory (Explicit) | Cognition | 13.67789917 |
| Memory (Working) | Cognition | 10.99684742 |
| Language (Phonology) | Cognition | 10.94778568 |
| Other | Emotion | 10.09642648 |
| Attention | Cognition | 8.053486892 |
| Audition | Perception | 7.079277695 |
| Language (Syntax) | Cognition | 6.84405347 |
| Language (Orthography) | Cognition | 6.257114254 |
| Reasoning | Cognition | 6.176250133 |
| Social Cognition | Cognition | 5.777944231 |
| Language (Other) | Cognition | 5.387445324 |
| Other | Cognition | 4.204017252 |
| Observation | Action | 4.191039849 |
| Disgust | Emotion | 4.183472731 |
| Sadness | Emotion | 3.883785231 |
| Fear | Emotion | 3.525733137 |
| Music | Cognition | 3.391329101 |
| Execution (Speech) | Action | 3.200798739 |
| Conjunction Analysis (Patients 1–3) | ||
| Category | Domain | Z-score |
| Language (Semantics) | Cognition | 15.75563985 |
| Memory (Working) | Cognition | 12.18419465 |
| Language (Speech) | Cognition | 12.10744032 |
| Attention | Cognition | 12.04995326 |
| Memory (Explicit) | Cognition | 11.61198732 |
| Language (Phonology) | Cognition | 9.967595958 |
| Other | Emotion | 9.094676748 |
| Other | Cognition | 8.116608217 |
| Reasoning | Cognition | 6.755383442 |
| Audition | Perception | 6.030549321 |
| Language (Orthography) | Cognition | 5.877503052 |
| Language (Syntax) | Cognition | 4.89137995 |
| Observation | Action | 4.395229772 |
| Music | Cognition | 4.135933261 |
| Vision (Shape) | Perception | 4.025768045 |
| Vision (Other) | Perception | 3.795259385 |
| Imagination | Action | 3.788025485 |
| Inhibition | Action | 3.681078329 |
| Social Cognition | Cognition | 3.579592828 |
| Language (Other) | Cognition | 3.552842659 |
| Somesthesis (Other) | Perception | 3.229401654 |
| Space | Cognition | 3.005289483 |
3.5. Incidence of Forced Thought Phenomenon.
Between 2006 to 2018, there were 76 epilepsy surgical patients underwent ESM for language mapping in English at our center. Forty-four (44) patients consented for research. Thirty-six (36) patients had at least 1 electrode in the target ROI. Fourteen (14) patients had stimulation performed in at least 1 electrode in a target ROI. These 14 patients were all left language dominant. Therefore, we estimate that the incidence of the forced thought phenomenon in our epilepsy population to be 3/14, or 21%. The most frequent other positive responses in this area were related to language function, such as disruption in auditory or visual naming, spontaneous speech.
4. DISCUSSION
We present three cases of forced conceptual thought induced by electrocortical stimulation mapping (ESM) in the left prefrontal cortex. These thoughts were intrusive and conceptual, and lacked perceptual, psychic, and emotional features that have characterized positive stimulation behaviors from other regions, and make them distinct from episodic memories. To better define the brain-wide functional networks involved in this phenomenon, we performed functional connectivity (rs-fMRI) and functional co-activation (MACM) analyses that implicate networks associated with multiple cognitive functions. We argue that this behavioral phenomenon is less a result of the stimulation of the specific cortical region (as demonstrated by the distance between the P1/P2 and P3 stimulation sites), than the activation of a widespread functional network that supports multiple cognitive functions.
While the notion that prefrontal cortex supports conceptual thought through integration of widespread neural network and cognitive domains is understood, our case series illustrates this idea in a unique patient population and clinical setting. Furthermore, to our knowledge, previous studies have demonstrated the role of prefrontal cortex in categorical thought through a “bottom up” behavioral approach in monkeys through single unit recordings (Freedman & Miller, 2008; Freedman, Riesenhuber, Poggio, & Miller, 2001) and in humans using fMRI (Gillebert, Op de Beeck, Panis, & Wagemans, 2009; Gotts, Milleville, Bellgowan, & Martin, 2011; Jiang et al., 2007). Our findings provide a complementary insight into conceptual thought by demonstration of this behavior elicited via a “top-down” approach using suprathreshold stimulation. We claim that conceptual thought represents less the activation of a specific gyrus, or even region, and more the activation of a widespread network, by demonstrating its functional connectivity to widespread regions through complementary resting state fMRI and task-based MACM analysis, which is also a novel approach. To the best of our knowledge, neither of these approaches have been applied to this patient group or to describe the neural underpinnings of conceptual thought.
Prior reports of forced thought during stimulation and seizures
Recently, there has been a report of intrusive thinking induced by electrical stimulation of the dorsolateral prefrontal cortex and prefrontal white matter seen in 3 patients (Popa et al., 2016). In this report, connectivity was probed by analyzing the cortico-cortical potentials elicited by single pulse electrical stimulation in one patient, which revealed a network including the ventro-medial prefrontal cortex (VMPFC), DLPFC, DMPFC, PMC, preSMA, and the dorsal-anterior insula. Our report extends these findings, by demonstrating that the same behavior is elicited by different neocortical areas in the left frontal region, strongly suggesting that this cognitive phenomenon engages a widespread network. We further define this functional network and provide a behavioral analysis supporting its involvement in diverse functions such as speech, language, attention, and memory by referencing the BrainMap database. However, our findings do not imply that stimulation at any hub in the network would necessarily produce the same behavioral phenomenon. As our experience of this phenomemon has been observed only with stimulation of the prefrontal cortex, and historical cases have also only been reported with ESM or seizures to the prefrontal or frontal region, a directionality to activation of the network is implied.
Other reports of forced thinking have described intellectual auras of frontal lobe seizures (Allen, 1952; Mendez et al., 1996; Penfield, 1946). Similar to these reports, our patient’s forced thoughts were spontaneous and compulsive. Penfield described cases of specific forced thoughts: an individual from a patient’s hometown; a piece of bread on the table that a patient felt compelled to move; a delusion that the consciousness of individuals a patient had recently talked to were continuing to talk to him. In a previous case series (Allen, 1952), forced thoughts were always out of context. A number of the cases were associated with an inability to recall details, such as “something in the past” or “queer thoughts”, confirming conceptual nature of the thought.
As suggested from these prior ESM and clinical seizure reports, these evoked concepts are repetitive and stereotyped. This may an observed phenomenon of a top-down suprathreshold activation of these circuits, in contrast to the more dynamic and flexible conceptual representations of lateral prefrontal cortex seen during bottom-up behavioral and fMRI paradigms (Gotts et al., 2011).
Notably, the semiology of forced thinking involved in frontal seizures differed from the experiences reported from mesial temporal lobe seizures, which have been described as déjà vu, and involve more psychic and affective features (Cho et al., 2011; Penfield, 1946). Likewise, forced thought differs from the examples of hearing one’s voice repeated as particular phrases or words, without overt speech induced by electrical stimulation of the white matter tracts in the perislyvian anterior arcuate fascicle, which represents a more complex auditory hallucination (Koubeissi, Fernandez-Baca Vaca, Maciunas, & Stephani, 2016). For example, Mendez’s case series included three individuals with left frontal lobe lesions and resulting seizures characterized by repetitive phrases (i.e. “tell me yes,” “why don’t you have a seizure,” “I need to grab something”).
Conceptual thought is elicited through ESM and involves widespread neural networks.
Forced thought from electrical stimulation of the PFC has been proposed as a positive complex behavior elicited from stimulation during cortical mapping (SU Schule, 2008). Positive effects are also observed with stimulation of primary motor, supplementary sensorimotor areas, primary sensory areas, secondary sensory areas, auditory and visual cortex. In contrast, negative effects interfere with underlying cortical function, and are elicited when stimulating language areas (producing speech arrest, alexia, agraphia, anomia, paraphasia), and in the primary and supplementary negative motor areas (producing negative motor symptoms in the contralateral or less commonly, ipsilateral muscle groups). Patients are often unaware for negative symptoms until they are asked to perform the specific function. The observation that our patients spontaneously describe the forced thought is consistent with a positive elicited phenomenon(SU Schule, 2008).
fMRI-based connectivity and MACM co-activation techniques are complementary approaches to describing functionally interconnected regions. While fMRI connectivity approaches describe resting-state networks and MACM is based on task-related networks, the two approaches have consistently yielded similar findings (Laird et al., 2013; Smith et al., 2009; Toro, Fox, & Paus, 2008). Likewise, our report reveals that fMRI and MACM methods yield highly overlapping, although not identical, functional networks (Figs 2 and 3). Given the independent types and sources of data, the similarity reveals a core network that robustly interacts with the ROI, independent of mental state. Similar to the limited number of studies which have utilized these two analytic methods, we show that resting state correlations demonstrate slightly more extensive networks compared to task-based networks when thresholded at similar levels of significance(Laird et al., 2013).
Forced thought relates to language
The left PFC and associated functional network likely contribute to the phenomenology of forced thought. While our patients had invasive coverage restricted to the left hemisphere, thereby precluding stimulation of homologous cortices, 14 of the 16 historical cases of ictal forced thought had evidence of left frontal seizure onset as determined by semiology, EEG, or radiology (Allen, 1952; Mendez et al., 1996; Penfield, 1946). Indeed, disruption of motor language function, spontaneous speech, and visual naming was coincidentally observed with stimulation in Patients 1 and 2. In our historical cohort of 14 patients who had electrodes located in any of the ROIs, the most common observation during stimulation was disruption of language function (either spontaneous speech, auditory, or visual naming). In previous clinical series, speech arrest or stuttering was a commonly reported feature of the seizure semiology. Mendez has suggested that forced thought may be a rare manifestation of seizures arising from the motor language areas. While difficult to prove with electrical stimulation, other non-invasive modalities such as transcranial magnetic stimulation (TMS) may be used to probe whether forced thought can be elicited by activation of homologous non-dominant (right) prefrontal cortex. Furthermore, the strong functional and anatomical connectivity between PFC and multiple cognitive domains including semantic and speech-related aspects of language, attention, working memory, and explicit memory may support the integration of diverse experiences across time.
The positive stimulation sites activated in the three patients included left dorsolateral prefrontal cortex and rostral middle frontal gyrus (BA 9 and 46, Patients 1 and 2), and the pars triangularis and orbitalis (BA 45 and 47, Patient 3). Brodmann areas 9 and 46 comprise the dorsolateral prefrontal cortex, and are involved in working memory, reasoning, attention, executive function, and verbal fluency, among other numerous functions.(Abrahams et al., 2003; Fincham, Carter, van Veen, Stenger, & Anderson, 2002; Kubler, Dixon, & Garavan, 2006; Ranganath, Johnson, & D'Esposito, 2003; Shallice, Stuss, Alexander, Picton, & Derkzen, 2008) Inferior to BA 9 and 46 are the pars triangularis (BA 45) and orbitalis (BA 47). BA 45 comprises the triangular portion and BA 47 the orbital portion of the inferior frontal gyrus. Together, BA 45 and 47 form “Broca’s complex,” supporting language production, including semantic decision making and word generation(Ardila, Bernal, & Rosselli, 2016).
Forced thought and categorical knowledge
It is possible that the left DLPFC thoughts elicitied in patients 1 and 2 represent broader categories (i.e. “a game show” and “a person”), and the thoughts elicited from Broca’s area in patient 3 represents a narrower category (i.e. “a game that kids play in the summer.”). This would be consistent with findings in monkeys which suggest that there is a hierarchically organized representation of broader and narrower concepts supported by distinct locations within prefrontal cortex. One fMRI activation study in humans demonstrated similar findings, with “conceptual-broad areas” supported by the inferior frontal sulcus (among other widespread regions including occipital and parietal lobes, fusiform gyrus, and dorsomedial thalamus); and “conceptual narrow regions” located more anteriorly with bilateral activations in inferior frontal gyrus, anterior insular, and anterior cingulate (Gotts et al., 2011), this possibility would need to be substantiated by TMS studies or further reports in epilepsy patients with ESM.
Limitations
The major limitation of this report is the small number of patients included, with epilepsy patients with lesions which may have resulted in some pathological reorganization of functional networks. However, while all 3 patients possessed lesions, these injuries were acquired during adulthood through trauma or hemorrhage. Late insults are less likely to result in significant functional reorganization of frontal functions such as speech and motor control. Furthermore, our understanding of the quality of the expressed precepts was strongly constrained by the patients’ ability to express themselves through linguistic concepts. The patients’ descriptions of the forced thoughts were limited, despite being probed by the clinicians for more details. These minimalist reports were not a function of underreporting or word-finding difficulty, because the patients were able to verbally express that there was no further detail they could offer despite leading questions. However, we note that these minimalist descriptions are consistent with prior reports elicited by ESM or seizures, and lack sensory detail. By their very nature, the lack of sensory detail suggest that these forced thoughts were distinct from episodic memories.
Finally, another caveat to interpretation is that stimulation at suprathreshold intensities may permit current spread to nearby and distant brain regions through mono or polysynaptic mechanisms (SU Schule, 2008), thereby engaging regions outside our ellipsoid seed region.
Forced thought, while a rarely reported phenomenon associated with frontal onset seizures, and here reported in three cases resulting from electrical stimulation of the left PFC, offers a fascinating insight into the nature of conceptual knowledge. As suggested by complementary neuro-imaging approaches, abstract thought may represent an emergent network property of multiple cognitive functions, including language, working memory, and attention.
SUPPLEMENTAL METHODS
Electrodes were arranged as grid arrays (8 X 8 contacts, 10 or 5 mm center-to-center spacing), linear strips (1 X 8/12 contacts), or depth electrodes (1 X 8/12 contacts), or some combination thereof. Subdural electrodes covered extensive portions of lateral and medial frontal, parietal, occipital, and temporal cortex of the left and/or right hemisphere. Recordings from grid, strip and depth electrode arrays were made using a NicoletOne C64 clinical amplifier (Natus Neurologic, Middleton, WI), bandpass filtered from 0.16-250 Hz and digitized at 512 Hz. ECoG signals were referenced to a two-contact subdural strip facing towards the skull near the craniotomy site. A similar 2 contact strip screwed to the skull was used for the instrument ground.
Anatomical localization.
Electrodes were localized in relation to each patient’s anatomy using the methods described in (Yang et al., 2012). Briefly, before electrode implantation, each patient underwent high-resolution T1-weighted MRI. Subsequent to electrode implantation, the patients underwent postoperative MRI. Electrode coordinates obtained from postoperative scans were coregistered with preoperative MRI and overlaid onto the patient’s reconstructed cortical surface using Freesurfer (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999). A spatial optimization algorithm was used to integrate additional information from the known array geometry and intraoperative photos to achieve high spatial accuracy of the electrode locations in relation to the cortical MRI surface created during FreeSurfer reconstruction. The cortex was automatically parcellated into 36 regions using FreeSurfer methods (Desikan et al., 2006) to aid in anatomical identification of sulcal and gyral structures and to obtain MNI coordinates for each electrode. implanted electrodes were localized first in subject individual space, then warped to MNI space with DARTEL (Ashburner, 2007), using the toolbox developed by our group (Yang et al., 2012) The DARTEL nonlinear warping is a toolbox which has been demonstrated to yield the most accurate results (Klein et al., 2009). Likewise, BrainMap coordinates are databased within a standardized template space, individual patient regions of interest (ROIs) were transformed into MNI=152 space prior to meta-analyses.
fMRI.
Functional MRI data in 81 healthy controls (39 males and 42 females, age range from 18-66, mean 34.2 years, SD 12.6 years) were acquired on a Siemens Allegra 3.0 T scanner. We collected 197 contiguous echo planar imaging functional volumes for each subject (TR = 2000 ms; TE = 25 ms; flip angle = 90, 39 slices, matrix = 64 × 64; FOV = 192 mm; acquisition voxel size = 3 × 3 × 3 mm). All participants were instructed to lie as still as possible with their eyes closed for the duration of the 6-min, 38-second scan. A T1-weighted anatomical image was also acquired for spatial normalization using a magnetization prepared gradient echo sequence (TR = 2530 ms; TE = 3.25 ms; T1 = 1100 ms; flip angle = 7; 128 slices; FOV = 256 mm).
Resting state fMRI Data Preprocessing.
The Resting-State fMRI Data Analysis Toolkit, or REST (Song et al., 2011) was used to perform slice timing correction, motion correction, and detection and reduction of extreme time series outliers. The first 10 time points of each subject’s scan were discarded. To control for the effects of motion, as well as normal physiologic processes such as cardiac and respiratory rhythms, each participant's 4-dimensional (4-D) volume was regressed on 9 predictors that modeled nuisance signals from white matter, cerebrospinal fluid and the global signal and 6 motion parameters. Further processing included temporal bandpass filtering (0.01–0.1 Hz), normalization to standard space using linear registration, spatial smoothing with FWHM 8mm Gaussian kernel, and detrending.
Neuropsychological Testing.
The patients’ post-implant performance level is established before stimulation is started. A set of 8-10 items for each modality is utilized during baseline testing then repeated throughout the mapping procedure to determine whether stimulation produces a functional lesion. For expressive speech, patients may be asked to deliver a speech monologue (e.g. the Pledge of Allegiance) or the months of the year in a continuous and repeating manner. To test visual naming, patients are shown pictures of items that are easily, rapidly, and consistently identified. Auditory naming yields distinct and clinically meaningful information (e.g. “What do you use on a rainy day to stay dry?”). For verbal comprehension, patients may be asked to follow simple commands (e.g. “touch your nose”) or provide the last word in an incomplete sentence (e.g. “The ball fell to the ___.”). To test reading, the patient may be given a passage or a series of words on flashcards to read aloud, with stimulation periodically introduced during the task. Finally, writing single words to command or spontaneous writing may be utilized during mapping, although writing involves both cognitive and motor components that are difficult to isolate from each other. Motor and sensory responses during stimulation are typically voluntary and spontaneous, and may not be tested by an overt maneuver. For every functional lesion, the response is repeated. Additional details can be found in Morrison and Carlson(Morrison, 2015).
Supplementary Material
HIGHLIGHTS.
Forced thoughts have been reported at the onset of a focal seizure, and have been characterized as involuntary and stereotyped intellectual auras.
We present a series of 3 epilepsy patients reporting intrusive thoughts during electrical stimulation of the left lateral prefrontal cortex (PFC) during invasive surgical evaluation.
We illustrate the widespread neural networks involved through two independent brain imaging modalities: resting state functional MRI (rs-fMRI) and task-based meta-analytic connectivity modeling (MACM).
We find that left PFC is associated with a large-scale functional network including frontal, temporal, and parietal regions, which support cognitive functions such as semantics, speech, attention, working memory and explicit memory.
These findings present an original insight into the nature of conceptual thought, elicited via a “top down” activation of a widespread neural network in a unique patient population.
Acknowledgments
Funding: Dr. Liu is supported by K23-NS104252.
ABBREVIATIONS
- PFC
Prefrontal cortex
- ESM
Electrocortical Stimulation Mapping
- MACM
meta-analytic connectivity model
- ROI
region of interest
- MNI
Montreal Neurological Institute
- fMRI
functional magnetic resonance imaging
Footnotes
Author Disclosures: The authors have no competing financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anli Liu, NYU Langone Medical Center, Department of Neurology.
Daniel Friedman, NYU Langone Medical Center, Department of Neurology.
Daniel S. Barron, Yale University, Department of Psychiatry.
Xiuyuan Wang, NYU Langone Medical Center, Department of Neurology and Radiology.
Thomas Thesen, NYU Langone Medical Center, Department of Neurology.
Patricia Dugan, NYU Langone Medical Center, Department of Neurology.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, … Leigh PN (2003). Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp, 20(1), 29–40. doi: 10.1002/hbm.10126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IM (1952). Forced Thinking as part of the epileptic attack. New Zealand Medical Journal, 51(282), 86–95. [PubMed] [Google Scholar]
- Ardila A, Bernal B, & Rosselli M (2016). How Localized are Language Brain Areas? A Review of Brodmann Areas Involvement in Oral Language. Arch Clin Neuropsychol, 31(1), 112–122. doi: 10.1093/arclin/acv081 [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. doi: 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Barron DS, Fox PT (2015). BrainMap Database as a Resource for Computational Modeling In Toga AW (Ed.), Brain Mapping: An Encyclopedic Reference (Vol. 1, pp. 675–683): Academic Press. [Google Scholar]
- Cauda F, Cavanna AE, D'Agata F, Sacco K, Duca S, & Geminiani GC (2011). Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci, 23(10), 2864–2877. doi: 10.1162/jocn.2011.21624 [DOI] [PubMed] [Google Scholar]
- Cho YJ, Song SK, Jang SH, Chang JW, Lee BI, & Heo K (2011). Simple Partial Status of Forced Thinking Originated in the Mesial Temporal Region: Intracranial Foramen Ovale Electrode Recording and Ictal PET. J Epilepsy Res, 1(2), 77–80. doi: 10.14581/jer.11015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, … Eickhoff SB (2013). Is there "one" DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex, 23(11), 2677–2689. doi: 10.1093/cercor/bhs256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006).An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, & Fox PT (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage, 59(3), 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, & Fox PT (2011). Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage, 57(3), 938–949. doi: 10.1016/j.neuroimage.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, & Behrens TE (2010). Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci, 30(18), 6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, van Veen V, Stenger VA, & Anderson JR (2002). Neural mechanisms of planning: a computational analysis using event-related fMRI. Proc Natl Acad Sci U S A, 99(5), 3346–3351. doi: 10.1073/pnas.052703399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. doi: 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Fox KCR, Yih J, Raccah O, Pendekanti SL, Limbach LE, Maydan DD, & Parvizi J (2018). Changes in subjective experience elicited by direct stimulation of the human orbitofrontal cortex. Neurology, 91(16), e1519–e1527. doi: 10.1212/WNL.0000000000006358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, … Lancaster JL (2005). BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp, 25(1), 185–198. doi: 10.1002/hbm.20141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, & Miller EK (2008). Neural mechanisms of visual categorization: insights from neurophysiology. Neurosci Biobehav Rev, 32(2), 311–329. doi: 10.1016/j.neubiorev.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, & Miller EK (2001). Categorical representation of visual stimuli in the primate prefrontal cortex. Science, 291(5502), 312–316. doi: 10.1126/science.291.5502.312 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, & Worsley KJ (1999). Multisubject fMRI studies and conjunction analyses. Neuroimage, 10(4), 385–396. doi: 10.1006/nimg.1999.0484 [DOI] [PubMed] [Google Scholar]
- Gillebert CR, Op de Beeck HP, Panis S, & Wagemans J (2009). Subordinate categorization enhances the neural selectivity in human object-selective cortex for fine shape differences. J Cogn Neurosci, 21(6), 1054–1064. doi: 10.1162/jocn.2009.21089 [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Milleville SC, Bellgowan PS, & Martin A (2011). Broad and narrow conceptual tuning in the human frontal lobes. Cereb Cortex, 21(2), 477–491. doi: 10.1093/cercor/bhq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Bradley E, Rini RA, Zeffiro T, Vanmeter J, & Riesenhuber M (2007). Categorization training results in shape- and category-selective human neural plasticity. Neuron, 53(6), 891–903. doi: 10.1016/j.neuron.2007.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, … Parsey RV (2009). Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage, 46(3), 786–802. doi: 10.1016/j.neuroimage.2008.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubeissi MZ, Fernandez-Baca Vaca G, Maciunas R, & Stephani C (2016). A white matter tract mediating awareness of speech. Neurology, 86(2), 177–179. doi: 10.1212/WNL.0000000000002246 [DOI] [PubMed] [Google Scholar]
- Kubler A, Dixon V, & Garavan H (2006). Automaticity and reestablishment of executive control-an fMRI study. J Cogn Neurosci, 18(8), 1331–1342. doi: 10.1162/jocn.2006.18.8.1331 [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, & Fox PT (2013). Networks of task co-activations. Neuroimage, 80, 505–514. doi: 10.1016/j.neuroimage.2013.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill ML, Devinsky O, Wang X, Quinn BT, Pardoe H, Carlson C, … Thesen T (2014). Functional neuroimaging abnormalities in idiopathic generalized epilepsy. Neuroimage Clin, 6, 455–462. doi: 10.1016/j.nicl.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Cherrier MM, & Perryman KM (1996). Epileptic forced thinking from left frontal lesions. Neurology, 47(1), 79–83. [DOI] [PubMed] [Google Scholar]
- Morrison C a. C. C (2015). Electrocortical Mapping of Language In Barr WB, and Morrison C (Ed.), Handbook on the Neuropsychology of Epilepsy (pp. 139–154): Springer. [Google Scholar]
- Narayana S, Laird AR, Tandon N, Franklin C, Lancaster JL, & Fox PT (2012). Electrophysiological and functional connectivity of the human supplementary motor area. Neuroimage, 62(1), 250–265. doi: 10.1016/j.neuroimage.2012.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan SS, Sinha SR, Gordon B, Lesser RP, & Thakor NV (1993). Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalogr Clin Neurophysiol, 86(3), 183–192. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, & Poline JB (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25(3), 653–660. doi: 10.1016/j.neuroimage.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, & Greicius MD (2013). The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron, 80(6), 1359–1367. doi: 10.1016/j.neuron.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W (1946). Psychical Seizures. British Medical Journal, 2(4478), 639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa I, Donos C, Barborica A, Opris I, Maliia MD, Ene M, … Mindruta I (2016). Intrusive Thoughts Elicited by Direct Electrical Stimulation during Stereo-Electroencephalography. Front Neurol, 7, 114. doi: 10.3389/fneur.2016.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, & D'Esposito M (2003). Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia, 41(3), 378–389. [DOI] [PubMed] [Google Scholar]
- Rangarajan V, Hermes D, Foster BL, Weiner KS, Jacques C, Grill-Spector K, & Parvizi J (2014). Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J Neurosci, 34(38), 12828–12836. doi: 10.1523/JNEUROSCI.0527-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Thesen T, Wang X, Hahn D, Yoo D, Kuzniecky R, … Blackmon K (2016). Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia, 57(9), 1475–1484. doi: 10.1111/epi.13456 [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, … Fox PT (2012). The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage, 60(1), 117–129. doi: 10.1016/j.neuroimage.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, & Fox PT (2010). Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp, 31(2), 173–184. doi: 10.1002/hbm.20854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, … Eickhoff SB (2013).Differentiated parietal connectivity of frontal regions for "what" and "where" memory. Brain Struct Funct, 218(6), 1551–1567. doi: 10.1007/s00429-012-0476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Alexander MP, Picton TW, & Derkzen D (2008). The multiple dimensions of sustained attention. Cortex, 44(7), 794–805. doi: 10.1016/j.cortex.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF (2009). Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A, 106(31), 13040–13045. doi: 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage, 44(1), 83–98. doi: 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, … Zang YF (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6(9), e25031. doi: 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU Schule CM, Luders HO (2008). General principles of cortical mapping by electrical stimulation In Luders H (Ed.), Textbook of Epilepsy Surgery (pp. 963–977). United Kingdom: Informa Healthcare. [Google Scholar]
- Tanji J, Shima K, & Mushiake H (2007). Concept-based behavioral planning and the lateral prefrontal cortex. Trends Cogn Sci, 11(12), 528–534. doi: 10.1016/j.tics.2007.09.007 [DOI] [PubMed] [Google Scholar]
- Thesen T, Quinn BT, Carlson C, Devinsky O, DuBois J, McDonald CR, … Kuzniecky R (2011). Detection of epileptogenic cortical malformations with surface-based MRI morphometry. PLoS One, 6(2), e16430. doi: 10.1371/journal.pone.0016430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, & Paus T (2008). Functional coactivation map of the human brain. Cereb Cortex, 18(11), 2553–2559. doi: 10.1093/cercor/bhn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer J, & Parvizi J (2016). Linking Electrical Stimulation of Human Primary Visual Cortex, Size of Affected Cortical Area, Neuronal Responses, and Subjective Experience. Neuron, 92(6), 1213–1219. doi: 10.1016/j.neuron.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AI, Wang X, Doyle WK, Halgren E, Carlson C, Belcher TL, … Thesen T (2012). Localization of dense intracranial electrode arrays using magnetic resonance imaging. Neuroimage 63(1), 157–165. doi: 10.1016/j.neuroimage.2012.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.