Abstract
In nearly 50% of patients with drug-induced liver injury, the bile flow is impaired known as cholestasis. Intrahepatic cholestasis of pregnancy (ICP) is the most common liver disease that happens in pregnancy. Some of the clinical symptoms include pruritus, dark urine, and abnormal liver function tests. A rise of serum bile acids is the most accurate diagnostic evidence. ICP may lead to premature birth, fetal distress, and even postpartum hemorrhage or stillbirth in some severe cases. Higher bile acid levels (>40μmol/L) are associated with higher rates of adverse fetal outcomes. Due to the multifactorial nature of ICP, its etiology is still not fully understood. Therefore, the current treatments of ICP are limited to control symptoms and protect fetuses. Among various causing factors, drug exposure during pregnancy is one common factor, and it can be prevented if we know drugs with increasing risk of cholestasis. Here we analyzed over 9.5 million FDA adverse effect reports to identify drugs with increasing risks of cholestasis as an adverse effect. Patients treated for cholestasis or liver diseases were removed. The odds ratio analysis reveals that lansoprazole (LSPZ), omeprazole (OMPZ) and amoxicillin (AMXC) are associated with an increased risk of cholestasis. LSPZ is associated with increased reported cholestasis by a factor of 2.32 (OR with 95% confidence interval [2.21, 2.43]). OMPZ is associated with increased reported cholestasis by a factor of 2.61 [2.54, 2.69]. AMXC is associated with increased reported cholestasis adverse effect by a factor of 6.79 [6.49, 7.11]. The risk of cholestasis associated with these three drugs is further increased in pregnant women. These findings justify careful reassessment of the safety of the identified three drugs.
Keywords: Adverse effect, drug induced cholestasis, intrahepatic cholestasis of pregnancy, odds ratio
1. Instruction
In nearly 50% of patients with drug-induced liver injury, the bile flow is impaired known as cholestasis[1]. Intrahepatic cholestasis of pregnancy (ICP), also known as obstetric cholestasis, is the most common liver disease in pregnant women. ICP is a condition where bile cannot flow from the liver. The typical clinical symptoms of ICP are intense pruritus without a skin rash, dark urine, and abnormal liver function tests[2–5]. Besides, ICP sometimes is associated with increased risk of postpartum hemorrhage due to disruption of bile acid metabolism, which may also lead to disturbance of absorption of lipophilic vitamins, especially vitamin K[6]. Even more severely, in some cases the ICP can lead to prematurity, the passage of meconium, fetal distress, increasing caesarean section rate, and stillbirth[2, 3, 7–9].
There are no effective therapeutic interventions for ICP. The established treatments focus on controlling the symptoms and protect fetuses. For example, ursodeoxycholic acid is used to decrease the concentration of bile acid to relieve pruritus and hepatic impairment[10–15], but it is not recommended during pregnancy. In addition, some drugs may be used to protect the fetus and increase its maturity, e.g. dexamethasone is used in to speed up the maturity of the baby’s lung[16, 17]. Those treatments provide an opportunity for the pregnant woman to get an elective delivery between gestation weeks 37–38 to pre-empt potential stillbirth[12].
One possible reason for lacking effective therapeutic interventions for ICP is that the detailed etiology of ICP is still not clear. Currently, cholestasis is considered to be caused by several potential factors: 1) mechanical blockage of duct; 2) metabolism disruption due to the genetic defects[18–23] or hormone fluctuation (especially for pregnant women)[24, 25]; 3) adverse effects of drug exposure[1, 26–29] or cumulative hazardous effects of environmental chemicals or metabolites[30–32]. As medications are known to be one leading contributor to ICP, and there is no effective treatment for ICP except for withdrawal of the drug[33], so risk factor mitigation is key to prevent negative outcomes. Then to succeed for any prevention efforts, clinicians should have a good understanding of medications associated with cholestasis in all clinical settings.
In the EASL Clinical Practice Guidelines, Management of cholestatic liver diseases[34], some drugs, including sex hormones, medicinal herbs, and NSAIDs, are known to cause hepatocellular or ductular/ductal cholestasis as adverse effects. More drugs need to be assessed. The United States Food and Drug Administration Adverse Event Reporting System (FAERS) is the most comprehensive database of drug-associated adverse event records. Though there exist the prevalence of ICP and the demand for recognizing hepatotoxicity medications in pregnant women, till now, despite several small scale clinical studies about drug-induced cholestasis[35], no study has determined which drugs are most frequently reported with cholestasis or ICP as adverse events in the FAERS database. A systematic evaluation of cholestasis risks of drugs hasn’t been conducted. In this paper, we report the first cohort study with over 9.5 million FAERS reports to identify drugs that are associated with high risks of cholestasis as an adverse effect. According to the odds ratio (OR) and usage frequency of each drug, we identified three drugs, lansoprazole, omeprazole, and amoxicillin that have higher risks of cholestasis, especially for pregnant women. Our analysis reveals and quantifies the potential risks of these three drugs, justifying that more assessments are needed for clinical usage of these drugs for pregnant women.
2. Materials and methods
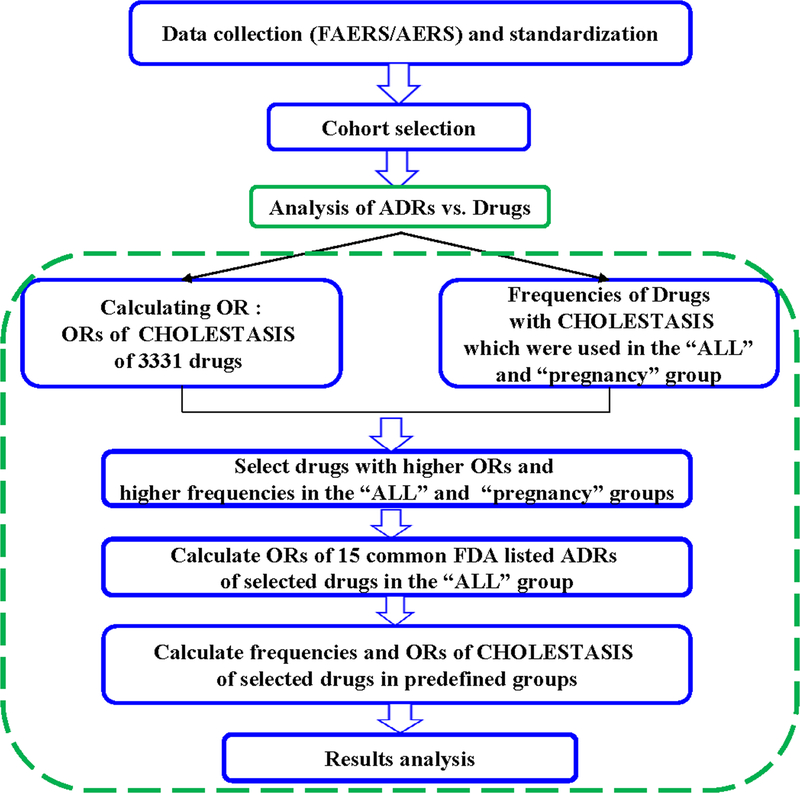
The whole procedures of the cohort study were shown in the flow chart in Figure 1. This study includes four steps: data collection, data normalization and standardization, cohort selection and statistical analysis.
Figure 1.
Flow chart of the procedures of the cohort study.
2.1. Data collection
The study used over 9.5 million reports available from the FDA’s Adverse Event Reporting System (FAERS) and Adverse Event Reporting System (AERS) data sets. At the time of the study, the FAERS set contained data from September 2012 to September 2017, and its older AERS set contained data from January 2004 to August 2012. The reports were used to conduct a cohort study for drugs and cholestasis. Both data sets are available online at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm.
2.2. Data normalization and standardization
The FAERS and AERS data sets have adverse event reports collected and shown quarterly, but those quarterly reports not homogenous throughout the whole data sets[36, 37]. For our study, each quarterly data set was downloaded and modified into a text table with consistent table field structure. The column names were homogenized and missing columns in older data sets were added with no values. Even though most of the reports in FAERS and AERS were submitted from the Unites States, there are still many reports submitted from other countries. Therefore, some field contents, such as drug names, date formats, and weight units were translated into consistent names/values. The various forms of drug names were converted to generic drug names corresponding to therapeutic ingredients. All identified misspellings of the drugs were homogenized into consistent values. The final version of the data set contained over 9.5 million reports from the first quarter of 2004 to the third quarter of 2017.
2.3. Cohort selection
A total number of 9,586,673 individual records were collected. Reports with cholestasis or liver disease indications were removed from the cohort to avoid confounding factors affecting the frequencies of cholestasis related adverse effects (See the full list of excluded indications in Table S1, S1 Appendix). This data set was named as “ALL”, containing 9,425,513 records. Then the “ALL” group was split into two cohorts, patients with cholestasis related effects (named as CHOLESTASIS) and patient without cholestasis related effects (named as non-CHOLESTASIS). The CHOLESTASIS cohort contained all records whose “adverse effect” field contained at least one of the following items: cholestasis, cholestasis of pregnancy, jaundice cholestatic, hepatitis cholestatic, bile duct obstruction, cholestatic pruritus, cholestatic liver injury, and mixed hepatocellular cholestatic injury.
The CHOLESTASIS cohort contained 17,385 records and the non-CHOLESTASIS cohort contained 9,408,128 records. Usage frequencies of each reported drug in both cohorts were calculated[36–38]. Odds ratios (ORs) and natural log odds ratios (LnORs, see definition below) for each drug were calculated. A positive value of LnOR with 95% confidence interval above zero value indicates an increasing risk of CHOLESTASIS.
The “ALL” group was also divided into patients who are pregnant women (“pregnancy” group), male patients (“male” group), female patients (“female” group), and female patients whose ages are from 12 to 55 years old (“female 12–55” group). Each group was split into CHOLESTASIS cohort and non-CHOLESTASIS cohort. The frequencies of each drug in both cohorts were calculated to generate the OR and LnOR values for each drug in each group. For drugs that may be used together, usage frequencies and ORs of several drug combinations were also calculated for all groups. The drugs were ranked by LnOR values of CHOLESTASIS. To assess the potential risks of the selected drugs, ORs and LnORs for common adverse effects of the selected drug were also calculated in the “ALL” group for comparison.
2.4. Data analysis
2.4.1. Descriptive statistics
The common adverse effects of selected drugs were taken from www.drugs.com. Frequencies for common adverse effects and CHOLESTASIS of selected drug were calculated using Equation 1 in all groups[36–38].
| (Equation 1) |
2.4.1. Comparative statistics
Drug usage report rates were compared via the natural log Odds Ratio (LnOR) by Equation 2:
| (Equation 2) |
a = Number in an adverse effect group with drug-exposed
b = Number in an adverse effect group with no drug-exposed
c = Number in control group with drug-exposed
d = Number in control group with no drug-exposed
Standard Error (SE) of the LnOR value was calculated using Equation 3:
| (Equation 3) |
where a, b, c, and d were defined in Equation 2.
Error bars were given in 95% confidence interval by Equation 4:
| Limit = LnOR ± 1.96 SE | (Equation 4) |
3. Results and discussions
3.1. Drugs with high LnORs for cholestasis
After data collection, normalization and standardization, cohort selection and statistical analysis based on FAERS/AERS database, LnORs of CHOLESTASIS with 95% confidence intervals and usage frequencies of all 3,331 drugs reported in the “ALL” group were calculated and ones of 200 drugs whose frequencies were bigger than 0.20% were shown in Table S2 (see in S2 Appendix). Drugs with high risks of CHOLESTASIS were selected based on the following criteria: 1) the 95% confidence interval of LnOR of CHOLESTASIS of the drug is larger than 0.69, corresponding to OR larger than two; 2) the number of records containing the drug in CHOLESTASIS cohort is larger than 300; 3) frequency of causing CHOLESTASIS of the drug in “pregnancy” group is larger than 4%; 4) the number of the drug associated CHOLESTASIS records in “pregnancy” group is larger than 20.
Three drugs, amoxicillin (AMXC), omeprazole (OMPZ), and lansoprazole (LSPZ), were selected based on these criteria. The detailed statistics of each drug and some external information (the pregnancy category by the US FDA and the ATC code) were shown in Table 1. These three drugs showed high LnORs for cholestasis.
Table 1.
Frequencies and ORs of CHOLESTASIS information of selected drugs.
| Drug name | NC+all | Nall | FreqC/all | lnOR | Error | NP | NC+P | FreqC/P | Pregnancy Category | ATC code |
|---|---|---|---|---|---|---|---|---|---|---|
| OMPZ | 1197 | 260202 | 0.46% | 0.96 | 0.03 | 621 | 28 | 4.51% | C | A02BC01, A02BD01, A02BD05 |
| AMXC | 498 | 41168 | 1.21% | 1.92 | 0.05 | 445 | 27 | 6.07% | B | A02BD01, A02BD03, A02BD04, A02BD05, A02BD06, A02BD07, A02BD10, A02BD11, J01CA04, J01CR02. |
| LSPZ | 431 | 102591 | 0.42% | 0.84 | 0.05 | 192 | 24 | 12.50% | B | A02BC03, A02BC53, A02BD02, A02BD03, A02BD07, A02BD09, A02BD10 |
NC+all, Number of records with CHOLESTASIS and the drug in the “ALL” group; Nall, Number of records containing the drug in the “ALL” group; FreqC/all, Frequency of CHOLESTASIS after taking the drug in the “ALL” group; NP, Number of drug in pregnancy group; NC+P, Number of records with CHOLESTASIS and the drug in the “pregnancy” group; FreqC/P, Frequency of CHOLESTASIS after taking the drug in the “pregnancy” group.
3.2. Adverse drug reactions (ADRs) comparison
The selected drugs, OMPZ, LSPZ, and AMXC are frequently used, and abundantly present in the FAERS and AERS reports (OMPZ: 260,202; LSPZ: 102,591; AMXC: 66,448). To assess the potential risks of those three drugs, cholestasis as an ADR was compared with other frequently reported adverse events for each drug. The OR values of their common ADRs were also calculated in the “ALL” group. The LnOR values of common ADRs of these drugs, including CHOLESTASIS for comparison, are shown in Figure 2.
Figure 2.
Ln odds ratios (LnORs) of 15 common FDA-listed ADRs as well as CHOLESTASIS of OMPZ (2a), LSPZ (2b), and AMXC (2c). Odds ratios were calculated by comparing records which contain selected drugs and records containing other medications. URI: Upper respiratory infection.
Figure 2a shows the LnORs of various ADRs calculated for OMPZ. Among various common ADRs of OMPZ, CHOLESTASIS has the second largest LnOR value (0.96±0.03). The top LnOR value is the “regurgitation” term that is associated with the main indication for OMPZ. LnOR values for CHOLESTASIS are among the top five ADRs for LSPZ (0.84±0.05, Figure 2b) and the highest compared to other ADRs for AMXC (LnOR =1.92±0.05, Figure 2c).
3.3. Results of drug-induced cholestasis during Pregnancy
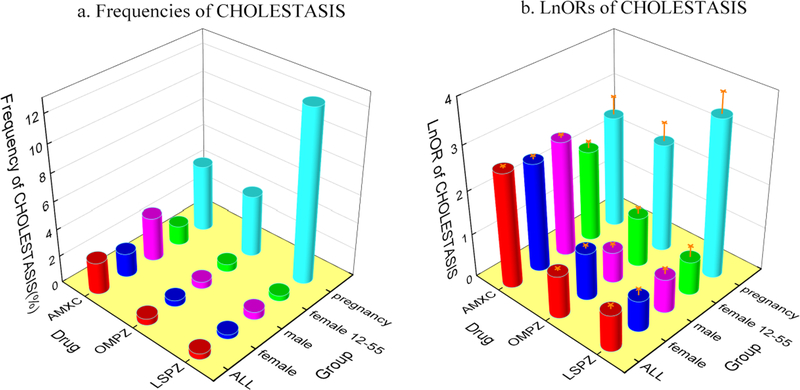
To explore whether pregnancy, gender, and age correlate with drug-induced cholestasis, we calculated the CHOLESTASIS occurrence in five predefined groups (See Cohort selection in Methods and in Figure 3). The “pregnancy” group is associated with significantly higher frequencies of CHOLESTASIS than other groups for all three drugs, while the gender and age don’t make significant differences. On the drug side, except for the “pregnancy” group, AMXC is associated with significantly higher frequencies of CHOLESTASIS than OMPZ and LSPZ. This is consistent with the higher OR of CHOLESTASIS for AMXC than OMPZ and LSPZ (Figure 3). To further dissect the risks of drug-induced cholestasis, we analyzed the frequencies of cholestasis occurrence in each of the following age ranges, (12, 20), [20, 25), [25, 30), [30, 35), [35, 40), [40, 45), [45, 50), and [50, 55). Shown in Table S3, the [35, 40) group has a higher occurrence frequency of cholestasis for AMXC, OMPZ, and LSPZ. However, the record sizes of those age groups are relatively small, which may result to relatively high uncertainties.
Figure 3.
Frequencies (3a) and Ln odds ratios (LnORs) (3b) of CHOLESTASIS of selected drugs in the five predefined groups. The 95% confidence intervals are shown in orange.
3.4. Drug combinations
We found that out of the 24 records in which LSPZ is associated with CHOLESTASIS in the “pregnancy” group, 23 records also contain OMPZ and AMXC. Therefore, the high CHOLESTASIS frequency of LSPZ results from the combination with OMPZ and AMXC. We calculated the CHOLESTASIS occurrences and ORs of various combinations of the three drugs, shown in Table 2. The combination of OMPZ, LSPZ, and AMXC showed a high OR of CHOLESTASIS (LnOR: 3.70±0.37) in the “ALL” group, with the CHOLESTASIS occurrence frequency being 100% (23/23) in the “pregnancy” group and 6.97% in the “ALL” group (3¼45). Other drug combinations are also associated with high odds ratios of CHOLESTASIS in the “ALL” group. But the number of reports with drug combinations in the “pregnancy” group is relatively small (only 23 reports) that increased the 95% confidence range. However, the “ALL” group was sufficiently large, and the combinations were excluded from the OR calculations. Based on the single drug OR values we concluded that the cholestasis causing effects of drug combinations are not dominated by a single drug.
Table 2.
Frequencies and ORs of CHOLESTASIS information if three drugs were used in different combinations in ALL group.
| Drug combination | NC+all | Nall | FreqC/all | OR | lnOR | Error | NP | NC+P | FreqC/P |
|---|---|---|---|---|---|---|---|---|---|
| AMXC+OMPZ+LSPZ | 31 | 445 | 6.97% | 40.59 | 3.70 | 0.37 | 23 | 23 | 100.00% |
| AMXC+OMPZ-LSPZ | 170 | 6793 | 2.50% | 14.02 | 2.64 | 0.15 | 21 | 2 | 9.52% |
| AMXC+LSPZ-OMPZ | 31 | 2864 | 1.08% | 5.93 | 1.78 | 0.35 | 5 | 0 | 0.00% |
| LSPZ+OMPZ-AMXC | 27 | 4731 | 0.57% | 3.11 | 1.13 | 0.38 | 6 | 0 | 0.00% |
| AMXC-LSPZ-OMPZ | 1279 | 56346 | 2.27% | 13.49 | 2.60 | 0.06 | 542 | 4 | 0.74% |
| OMPZ-LSPZ-AMXC | 974 | 250340 | 0.39% | 2.18 | 0.78 | 0.06 | 574 | 3 | 0.52% |
| LSPZ-OMPZ-AMXC | 342 | 94558 | 0.36% | 1.98 | 0.69 | 0.11 | 158 | 1 | 0.63% |
NC+all, Number of records with CHOLESTASIS and the drug combination in the “ALL” group; Nall, Number of records containing the drug combination in the “ALL” group; FreqC/all, Frequency of CHOLESTASIS after taking the drug combination in the “ALL” group; NP, Number of drug combination in the “pregnancy” group; NC+P, Number of records with CHOLESTASIS and the drug combination in the “pregnancy” group; FreqC/P, Frequency of CHOLESTASIS after taking the drug combination in the “pregnancy” group.
3.5. Discussion
Our study based on the 9.5 more million FAERS/AERS records suggested that AMXC, LSPZ, and OMPZ are associated with increased risks of cholestasis, especially for pregnant women. Since cholestasis may lead to further complications in women and fetuses, the usage of these three drugs for pregnant women should be carefully considered.
Our results are further supported by the EASL Clinical Practice Guidelines. The EASL Clinical Practice Guidelines: Management of cholestatic liver diseases[34] includes a list of drugs that are known to be associated with cholestasis, containing AMXC, but not OMPZ and LSPZ. Amoxicillin-clavulanic acid which is used frequently is known to be associated with hepatocellular or ductular/ductal cholestasis. From our results, the high OR of CHOLESTASIS of AMXC (LnOR: 1.92±0.05) further confirms the risk of AMXC. The ORs of OMPZ (LnOR: 0.84±0.05) and LSPZ (LnOR: 0.96±0.03), also illustrated the elevated risks of cholestasis of these two drugs.
In addition, AMXC and OMPZ association with cholestasis, hepatocellular injury or cholestatic injury of hepatotoxicity were also noticed in several case studies[27, 39–42], with AMXC being the most prominent [43–48]. Cholestasis risk of OMPZ has also been reported as some cases[14, 49–51]. However, no report about the cholestasis risk of LSPZ has been published to our knowledge.
AMXC, OMPZ, and LSPZ are relatively common in daily life, so their using safety should be more considered during pregnancy. AMXC is one of the most widely used antibiotics in many countries[42–48]. Although it is considered to be relatively safe, AMXC may cause liver injury in some cases, even after AMXC was treated with the patient only 5–12 days[52]. The cholestasis risk of AMXC is relatively well-known, as many cases have been reported. Our cohort analysis in this paper further quantified the risk of AMXC with large population data. OMPZ and LSPZ are broadly used proton pump inhibitors (PPIs). Even though the embryonic and fetal toxicity of OMPZ has been pointed out in the American Gastroenterological Association Institute Medical Position Statement[14], cholestasis risks of them haven’t been systematically studied, other than some case reports in which OMPZ might induce cholestatic hepatitis or liver injury[50, 53, 54]. Our results demonstrated their risks of causing cholestasis and quantify the risks based on the populational scale retrospective study. So their risks should be considered when giving them to pregnant women.
Where the FDA Pregnancy Categories of these three drugs were shown in Table 1, their pregnancy categories should be re-assigned carefully. For clinical practice, the FDA Pregnancy Category is the main and most straightforward resource when physicians rely on to treat pregnant patients. Currently, OMPZ is a Category C drug, which means that the drug showed adverse effects on the fetus in an animal study but there are no adequate and well-controlled studies in humans. AMXC and LSPZ are Category B drugs, meaning that animal studies failed to show adverse effects on the fetus and there are no adequate studies in humans. However, our finding based on over 9.5 million clinical records provide some evidence of potential risks of cholestasis with OMPZ, LSPZ and AMXC. Exposure to them during pregnancy is associated with higher risks for cholestasis that may increase the likelihood of premature birth, fetal distress, and even postpartum hemorrhage or stillbirth[6,7]. These findings call for new consideration and possibly reassignment of Pregnancy Categories for OMPZ, LSPZ and AMXC.
However, this study also has some limitations. The records in the FAERS/AERS system only represent a subset of actual cases because of the voluntary submission nature of FAERS and AERS system. Therefore, data in FAERS/AERS may over-represent patients with adverse effects, and overlook patients who didn’t experience adverse effects after taking the drugs.
4. Conclusions
There is some information about drug exposure during pregnancy causing adverse events in kinds of clinical databases. The OR and frequency of cholestasis as an adverse effect of drugs were analyzed based on over 9.5 million FAERS/AERS records. The drugs with both high OR values and frequencies in the “ALL” group and in the “pregnancy” group were picked out. The database OR analysis revealed that LSPZ was associated with increased reported cholestasis by a factor of 2.32 (OR with 95% confidence interval [2.21, 2.43]). OMPZ was 2.61 [2.54, 2.69]. AMXC was 6.79 [6.49, 7.11]. The risk of cholestasis associated with these three drugs is further increased in pregnant women. Even though with some inherent limitation, our cohort study which is based on over 9.5 million records can still provide some evidence with clinical values. Of course, further studies are needed to experimentally assess the risk of these drugs (AMXC, OMPZ, and LSPZ), and even to elucidate the mechanisms. Ultimately, their potential risks, after experimentally confirmed, should be clearly pointed out and considered in clinical practices. This approach is an effective tool for mining drug-induced cholestasis from FAERS databases and it is transferable to other diseases.
Supplementary Material
Acknowledgement
Financial Support: National Natural Science Foundation of China for Young Scholars (Grant No. 21707012). Science and technology project of Chongqing Municipal Education Commission of China (Grant No. KJ1500205). The open project of Key Laboratory of Natural Medicine Research of Chongqing Education Commission (1456032).
The authors thank Chris Edwards (UC San Diego) for processing the FAERS data files and supporting the computing environment. We’re also thankful to Dr. Brookie M. Best (PharmD, M.A.S. Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pediatrics, School of Medicine-Rady Children’s Hospital San Diego, UC San Diego), Dr. Yong Shao and Dr. Wenwu Gui (the First Affiliated Hospital of Chongqing Medical University) for useful discussions. Thanks to the China Scholarship Council and the Chongqing Municipal Education Commission for funding Yong-Hong Zhang to study abroad.
Footnotes
Appendices
There are three supplementary tables (Table S1, S2, and S3).
References
- 1.Sundaram V, Bjornsson ES. Drug-induced cholestasis. Hepatol Commun 2017,1:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes H. The Enigma of Intrahepatic Cholestasis of Pregnancy: Lessons from Chile. Hepatology 1982,2:87S–96S. [DOI] [PubMed] [Google Scholar]
- 3.Gabzdyl EM, Schlaeger JM. Intrahepatic cholestasis of pregnancy: a critical clinical review. J Perinat Neonatal Nurs 2015,29:41–50. [DOI] [PubMed] [Google Scholar]
- 4.Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology 2014,59:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pongcharoen P, Fleischer AB Jr. An evidence-based review of systemic treatments for itch. Eur J Pain 2016,20:24–31. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon VK, Lachlan NJ, Hoy AM, Hadoke PW, Semple SI, Patel D, et al. Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Med 2017,14:e1002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puljic A, Kim E, Page J, Esakoff T, Shaffer B, LaCoursiere DY, et al. The risk of infant and fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol 2015,212:667 e661–665. [DOI] [PubMed] [Google Scholar]
- 8.Wensink MJ. The risk of infant and fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age: various objections. Am J Obstet Gynecol 2016,215:807–808. [DOI] [PubMed] [Google Scholar]
- 9.Kawakita T, Parikh LI, Ramsey PS, Huang CC, Zeymo A, Fernandez M, et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol 2015,213:570 e571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain R, Suri V, Chopra S, Chawla YK, Kohli KK. Obstetric cholestasis: outcome with active management. J Obstet Gynaecol Res 2013,39:953–959. [DOI] [PubMed] [Google Scholar]
- 11.Chappell LC, Chambers J, Thornton JG, Williamson C. Does ursodeoxycholic acid improve perinatal outcomes in women with intrahepatic cholestasis of pregnancy? BMJ 2018,360:k104. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez M, Sprohnle A, Munoz J, Cadena C, Corvalan R, Sepulveda-Martinez A. Treatment of intrahepatic cholestasis of pregnancy with ursodeoxycholic acid associated with improvement of fetal first-degree atrioventricular block. Ultrasound Obstet Gynecol 2018. [DOI] [PubMed] [Google Scholar]
- 13.Williamson C. Gastrointestinal disease. Best Practice & Research Clinical Obstetrics & Gynaecology 2001,15:937–952. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan U, Kane S. American gastroenterological association institute medical position statement on the use of gastrointestinal medications in pregnancy. Gastroenterology 2006,131:278–282. [DOI] [PubMed] [Google Scholar]
- 15.Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology 2012,143:1492–1501. [DOI] [PubMed] [Google Scholar]
- 16.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017,3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran TT, Ahn J, Reau NS. ACG Clinical Guideline: Liver Disease and Pregnancy. Am J Gastroenterol 2016,111:176–194; quiz 196. [DOI] [PubMed] [Google Scholar]
- 18.Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology 2000,118:422–430. [DOI] [PubMed] [Google Scholar]
- 19.Mullenbach R, Linton KJ, Wiltshire S, Weerasekera N, Chambers J, Elias E, et al. ABCB4 gene sequence variation in women with intrahepatic cholestasis of pregnancy. J Med Genet 2003,40:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasmuth HE, Glantz A, Keppeler H, Simon E, Bartz C, Rath W, et al. Intrahepatic cholestasis of pregnancy: the severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut 2007,56:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon PH, van Mil SW, Chambers J, Strautnieks S, Thompson RJ, Lammert F, et al. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut 2009,58:537–544. [DOI] [PubMed] [Google Scholar]
- 22.Shagrani M, Burkholder J, Broering D, Abouelhoda M, Faquih T, El-Kalioby M, et al. Genetic profiling of children with advanced cholestatic liver disease. Clin Genet 2017,92:52–61. [DOI] [PubMed] [Google Scholar]
- 23.Figge A, Lammert F, Paigen B, Henkel A, Matern S, Korstanje R, et al. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J Biol Chem 2004,279:2790–2799. [DOI] [PubMed] [Google Scholar]
- 24.Parizek A, Duskova M, Vitek L, Sramkova M, Hill M, Adamcova K, et al. The role of steroid hormones in the development of intrahepatic cholestasis of pregnancy. Physiol Res 2015,64 Suppl 2:S203–209. [DOI] [PubMed] [Google Scholar]
- 25.Rao ZZ, Zhang XW, Ding YL, Yang MY. miR-148a-mediated estrogen-induced cholestasis in intrahepatic cholestasis of pregnancy: Role of PXR/MRP3. PLoS One 2017,12:e0178702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naschitz JE, Khamessi R, Elias N, Yeshurun D. Ticlopidine-Induced Prolonged Cholestasis. Journal of Toxicology: Clinical Toxicology 1995,33:379–380. [DOI] [PubMed] [Google Scholar]
- 27.Erlinger S. Drug-induced cholestasis. Journal of Hepatology 1997,26:1–4. [DOI] [PubMed] [Google Scholar]
- 28.Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology 2011,53:1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 2017,65:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muchova L, Vanova K, Suk J, Micuda S, Dolezelova E, Fuksa L, et al. Protective effect of heme oxygenase induction in ethinylestradiol-induced cholestasis. J Cell Mol Med 2015,19:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JS, Li YF, Li YY, Dai Y, Li WK, Zheng M, et al. Huangqi Decoction Alleviates Alpha-Naphthylisothiocyanate Induced Intrahepatic Cholestasis by Reversing Disordered Bile Acid and Glutathione Homeostasis in Mice. Front Pharmacol 2017,8:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Liu R, Luo L, Yu L, Chen X, Sun L, et al. Role of AMP-activated protein kinase alpha1 in 17alpha-ethinylestradiol-induced cholestasis in rats. Arch Toxicol 2017,91:481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006,354:731–739. [DOI] [PubMed] [Google Scholar]
- 34.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009,51:237–267. [DOI] [PubMed] [Google Scholar]
- 35.Hamoir C, Dano H, Komuta M, Druez P, Negrin Dastis S. Cholestatic hepatitis after diagnostic ajmaline challenge. Acta Gastroenterol Belg 2017,80:425–426. [PubMed] [Google Scholar]
- 36.Cohen IV, Makunts T, Atayee R, Abagyan R. Population scale data reveals the antidepressant effects of ketamine and other therapeutics approved for non-psychiatric indications. Sci Rep 2017,7:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makunts T, Cohen IV, Lee KC, Abagyan R. Population scale retrospective analysis reveals distinctive antidepressant and anxiolytic effects of diclofenac, ketoprofen and naproxen in patients with pain. PLoS One 2018,13:e0195521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balasubramanian H, Ananthan A, Rao S, Patole S. Odds ratio vs risk ratio in randomized controlled trials. Postgrad Med 2015,127:359–367. [DOI] [PubMed] [Google Scholar]
- 39.Pedro-Botet J, Supervia A, Barranco C, Sola R, Bruguera M. Intrahepatic cholestasis without hepatitis induced by amoxycillin/clavulanic acid. J Clin Gastroenterol 1996,23:137–138. [DOI] [PubMed] [Google Scholar]
- 40.Keller J, Frederking D, Layer P. The spectrum and treatment of gastrointestinal disorders during pregnancy. Nat Clin Pract Gastroenterol Hepatol 2008,5:430–443. [DOI] [PubMed] [Google Scholar]
- 41.Bhamidimarri KR, Schiff E. Drug-induced cholestasis. Clin Liver Dis 2013,17:519–531, vii. [DOI] [PubMed] [Google Scholar]
- 42.deLemos AS, Ghabril M, Rockey DC, Gu J, Barnhart HX, Fontana RJ, et al. Amoxicillin-Clavulanate-Induced Liver Injury. Dig Dis Sci 2016,61:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Broek JW, Buennemeyer BL, Stricker BH. Cholestatic hepatitis caused by a combination of amoxicillin and clavulanic acid (Augmentin). Ned Tijdschr Geneeskd 1988,132:1495–1497. [PubMed] [Google Scholar]
- 44.Bolzan H, Spatola J, Castelletto R, Curciarello J. Intrahepatic cholestasis induced by amoxicillin alone. Gastroenterol Hepatol 2000,23:237–239. [PubMed] [Google Scholar]
- 45.Gresser U. Amoxicillin-clavulanic acid therapy may be associated with severe side effects -- review of the literature. Eur J Med Res 2001,6:139–149. [PubMed] [Google Scholar]
- 46.Daghfous R, El Aidli S, Ben Mami N, Amrani R, Loueslati MH, Lakhal M, et al. Cholestasis soon after administration of amoxicillin-clavulanic acid. Therapie 2004,59:658–660. [DOI] [PubMed] [Google Scholar]
- 47.Dominguez Jimenez JL, Marin Moreno M, Bernal Blanco E, Puente Gutierrez JJ, Guiote Malpartida S, de la Mata Garcia M. Acute cholestatic hepatitis induced by amoxicillin-clavulanic acid. Gastroenterol Hepatol 2008,31:46. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz Rebollo ML, Aller De La Fuente R, Macho Conesa A, Salado Valdivieso I, Sainz Gil M, Carvajal A, et al. Amoxicillin-induced cholestatic hepatitis. Gastroenterol Hepatol 2011,34:474–477. [DOI] [PubMed] [Google Scholar]
- 49.Hitzl M, Klein K, Zanger UM, Fritz P, Nussler AK, Neuhaus P, et al. Influence of omeprazole on multidrug resistance protein 3 expression in human liver. J Pharmacol Exp Ther 2003,304:524–530. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez Garrido A. Omeprazole-induced acute cholestatic hepatitis. Gastroenterol Hepatol 2007,30:54. [DOI] [PubMed] [Google Scholar]
- 51.Kanth R, Shah MS, Flores RM. Statin-associated polymyositis following omeprazole treatment. Clin Med Res 2013,11:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Ruiz-Granados E, Bejarano-Garcia A, Uceda-Torres E. Recurrent cholestasis by amoxicillin-clavulanic acid: the importance of a correct diagnosis of hepatotoxicity. Rev Esp Enferm Dig 2012,104:616–617. [DOI] [PubMed] [Google Scholar]
- 53.Namba S. Inhibitory effects of omeprazole and cimetidine on the formation of gastric ulcer in rats with obstructive jaundice and acute renal failure. Nihon Shokakibyo Gakkai Zasshi 1988,85:1223–1232. [PubMed] [Google Scholar]
- 54.Riedmaier S, Klein K, Winter S, Hofmann U, Schwab M, Zanger U. Paraoxonase (PON1 and PON3) Polymorphisms: Impact on Liver Expression and Atorvastatin-Lactone Hydrolysis. Frontiers in Pharmacology 2011,2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.