Abstract
Objective:
To better understand the prevalence and impact of gastroparesis in the T1D Exchange clinic registry database.
Methods:
The analysis included 7107 adult participants with T1D across 45 sites (median age 46 years. and median duration 24 years). Linear and logistic regression models were used to assess the association of gastroparesis vs. no gastroparesis (obtained from medical record) with demographic characteristics, glycemic control and diabetes complications.
Results:
Among 7107 registry participants, 340 (4.8%) had a clinical diagnosis of gastroparesis. Females were more likely to have gastroparesis compared with males (5.8% vs. 3.5%, P < 0.001). Participants with gastroparesis compared with those without gastroparesis were older (median age 49.4 vs. 45.3 years, P b 0.001), had a longer duration of T1D (median duration 32 vs. 23 years, P < 0.001), higher mean HbA1c (8.1% vs. 7.7% [65 vs. 61 mmol/mol], P < 0.001), more frequent severe hypoglycemia (25% vs. 11% with ≥ 1 event in the past 12 months, P < 0.001), lower socio-economic status, less likely to be using CGM and insulin pump and greater prevalence of microvascular and neuropathic complications than participants without gastroparesis.
Conclusion:
Gastroparesis is associated with higher risk of severe hypoglycemia despite higher HbA1c levels than in T1D patients without gastroparesis. The increased presence of multiple long-term complications and overall poor glycemic control in these subjects emphasizes the need to establish diagnostic protocols for earlier diagnosis, achieve tighter glycemic control with more extensive use of insulin pumps and continuous glucose monitoring, and the need for wider availability of medical therapies for treatment of diabetic gastroparesis.
Keywords: Type 1 diabetes, Gastroparesis, Glycemic control, Diabetes management, Complications, Adult
1. Introduction
Gastroparesis is a syndrome characterized by delayed gastric emptying in the absence of mechanical obstruction.1 It is a relatively rare disease in the general population with an age—adjusted annual incidence of 2.4/100,000 for men and 9.8/100,000 for women as reported in a US population in 2007.2 However, individuals with type 1 diabetes (T1D), have a 30-fold increased risk of developing gastroparesis.3 The pathophysiology of gastroparesis includes functional factors and histologic/biochemical factors. The delay of solid food transit in T1D is multi- factorial and attributed to impaired phasic antral contractions, impaired fundic contraction/accommodation as well as pylorospasm. Moreover, vagal neuropathy is more severe in diabetic gastroparesis and histologic examination of vagus nerves reveals variable degrees of myelin degeneration. Additional histopathologic factors include loss or dysfunction of myenteric neurons, depletion of interstitial cells of Cajal and smooth muscle fibrosis with eosinophilic inclusions.4 Gastroparesis’ cardinal symptoms include early satiety, postprandial fullness, nausea, vomiting, abdominal pain, and weight loss.5 This complication of diabetes causes serious problems with glycemic control and its presentation can range from intermittent symptoms to total disability and frequent hospitalizations. Patients with severe gastroparesis may have difficulty maintain- ing hydration and nutritional status and are at risk for malnutrition with vitamin and mineral deficiency.6,7
The prevalence of gastroparesis in individuals with T1D and impact of diabetic gastroparesis on glycemic control and diabetes complications is not known; many studies report all causes of gastroparesis, without specific focus on gastroparesis in persons with diabetes. Several additional factors affecting a reliable quantification of gastroparesis prevalence in T1D have been identified.8 Among these, poor correlation between symptoms and gastric emptying, reports based on symptoms survey only, uncertainty of T1D versus type 2 diabetes diagnosis, and lack of glycemic control targets prior to gastroparesis diagnostic tests contribute to the difficulty in achieving a reliable diagnosis. For example, the rate of gastric emptying in T1D has been shown to be delayed in the setting of hyperglycemia (blood glucose N 180 mg/dL).9 Similarly, smoking and other medications may affect gastric emptying during diagnostic testing.5
Most importantly, the studies reported so far have been performed on small selected cohorts and with significant differences in prevalence/incidence of gastroparesis in diabetes.3
Risk factors for diabetic gastroparesis in T1D are poorly defined. Several small studies have reported a variable degree of correlation among duration of diabetes, microvascular complications and increased risk or presence of gastroparesis in these individuals.3,5,10
To better understand the prevalence and characteristics of gastroparesis in adults with T1D, we investigated the clinical diagnosis of gastroparesis and related risk factors in the large T1D Exchange clinic registry database.
1.1. Subjects, materials, and methods
This analysis utilizes data from the T1D Exchange clinic registry which at the time of this analysis includes N 25,000 individuals with T1D enrolled across 67 U.S. based pediatric and adult endocrinology practices. The registry participants represent about one-fourth of the atients with T1D who are followed at one of the 67 clinics. The majority of participants were enrolled between September 2010 and August 2012.11,12 To be enrolled in the clinic registry, an individual must have a clinical diagnosis of presumed autoimmune T1D and either islet cell antibodies present or if antibodies were negative or unknown, then insulin must have been started at or shortly after diagnosis and used continually thereafter (except in the case of a pancreas or islet cell transplant). Each clinic received approval from an institutional review board (IRB). Informed consent was obtained according to IRB requirements from adult participants. Data were collected for the registry’s central database from the participant’s medical record and by having the participant or parent complete a comprehensive questionnaire, as previously described.11
The analysis cohort included all registry participants who were ≥ 26 years old with T1D duration of at least 2 years at the time of analysis for a total N of 7107 across 45 clinics. Presence or absence of gastroparesis and diabetic complications (retinopathy, nephropathy, and neuropathy) were obtained from medical record. Most recent HbA1c within 6 months of registry enrollment was obtained from clinic medical record. Demographic characteristics, insulin delivery and diabetes management, and the occurrence of severe hypoglycemia and diabetic ketoacidosis were obtained from the registry participant questionnaire.
1.2. Statistical methods
Univariate logistic regression models were used to assess associations between participant characteristics and gastroparesis status. A multivariate logistic or linear regression model was fit to assess the association between gastroparesis and continuous glucose monitor (CGM) use, insulin pump use, and frequency of self-monitoring of blood glucose (SMBG) controlling for age and duration of diabetes. Adjusting for age, duration of diabetes, frequency of SMBG measurements per day, insulin delivery method, and CGM use, a multivariate logistic or linear regression model was used to assess the association between gastroparesis status and most recent HbA1c, frequency of one or more severe hypoglycemia (SH) events (episode resulting in seizure or loss of consciousness) in the past 12 months, treatment of retinopathy, nephropathy, neuropathy, charcot joint, orthostatic hypo- tension and tachycardia. All P-values are 2-sided and all statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). In lieu of the large sample size and multiple comparisons only P values < 0.01 were considered statistically significant.
2. Results
In this cohort of 7107 participants, a clinical diagnosis of gastroparesis was present in 4.8%: 4% age 26–49, 6% age 50–64, 5% age ≥ 65 years old (Table 1). Females were more likely to have gastroparesis compared with males (6% vs. 4%, P < 0.001). No differences between those with and without gastroparesis were noted for race and ethnicity (P = 0.83). The individuals with a clinical diagnosis of gastroparesis were older (median age 49.4 vs. 45.3 years old, P < 0.001) and had a longer duration of diabetes (median T1D duration 32 vs. 23 years, P < 0.001) compared with participants without gastroparesis. Participants with gastroparesis also typically had a lower household income, lower education level, and non-private health insurance compared with those without gastroparesis (P < 0.001, Table 1).
Table 1.
Patient characteristics and their association with gastroparesis.
| Gastroparesis | No Gastroparesis | P-valuea | |
|---|---|---|---|
| Overall | 340 (4.8%) | 6767 (95.2%) | − |
| Ageb | <0.001 | ||
| Median (Q1, Q3) | 49.4 (40.0, 58.8) | 45.3 (35.0, 56.3) | |
| 26–49 years old. | 172 (4%) | 4136 (96%) | |
| 50–64 years old. | 134 (6%) | 1972 (94%) | |
| ≥ 65 years old. | 34 (5%) | 659 (95%) | |
| T1D Durationb | <0.001 | ||
| Median (Q1, Q3) | 32 (23, 42) | 23 (14, 33) | |
| 1–9 years | 10 (1%) | 923 (99%) | |
| 10–19 years | 41 (2%) | 1735 (98%) | |
| 20–49 years | 259 (6%) | 3879 (94%) | |
| ≥ 50 years | 30 (12%) | 230 (88%) | |
| Gender | <0.001 | ||
| Female | 225 (6%) | 3635 (94%) | |
| Male | 114 (4%) | 3131 (96%) | |
| Race/Ethnicity | 0.83 | ||
| White non-Hispanic | 309 (5%) | 6144 (95%) | |
| Black non-Hispanic | 14 (6%) | 229 (94%) | |
| Hispanic or Latino | 9 (4%) | 198 (96%) | |
| Other | 7 (4%) | 172 (96%) | |
| Household incomeb | <0.001 | ||
| <$25,000 | 72 (13%) | 491 (87%) | |
| $25,000–<$35,000 | 15 (4%) | 367 (96%) | |
| $35,000–<$50,000 | 34 (5%) | 613 (95%) | |
| $50,000–<$75,000 | 41 (4%) | 1028 (96%) | |
| $75,000–<$100,000 | 32 (3%) | 926 (97%) | |
| ≥$100,000 | 44 (2%) | 1826 (98%) | |
| Education levelb | <0.001 | ||
| Less than high school diploma | 11 (6%) | 164 (94%) | |
| High school diploma/GED | 140 (7%) | 1824 (93%) | |
| Associate degree | 39 (6%) | 642 (94%) | |
| Bachelor degree | 77 (3%) | 2133 (97%) | |
| Master degree | 31 (3%) | 1098 (97%) | |
| Professional or doctorate degree | 14 (3%) | 437 (97%) | |
| Health insurance | <0.001 | ||
| Private | 194 (4%) | 5135 (96%) | |
| Other | 115 (10%) | 1070 (90%) | |
| No | 9 (7%) | 127 (93%) | |
| SMBG per day | |||
| Median (Q1, Q3) | 5 (4, 7) | 5 (4, 7) | 0.14 |
| BMI | |||
| Median (Q1, Q3) | 26.3 (23.0, 30.7) | 26.8 (24.0, 30.3) | 0.36 |
P-value from a univariate logistic regression with gastroparesis status as the outcome.
Variable treated as continuous or ordinal in logistic model. Categories are shown for display purposes.
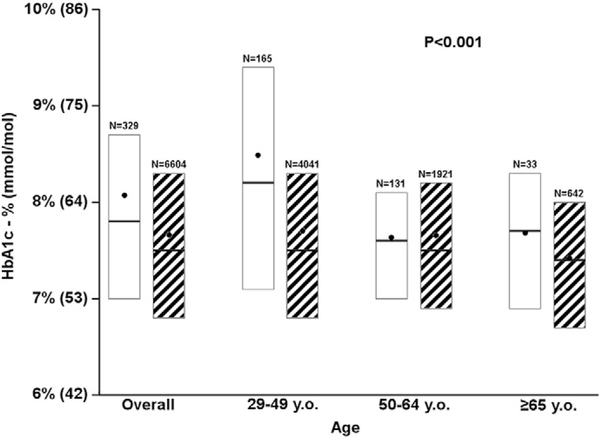
Mean HbA1c was 0.4% (4.4 mmol/mol) higher for participants with gastroparesis vs. those without gastroparesis (8.1% [65 mmol/mol] vs. 7.7% [61 mmol/mol], P < 0.001, Fig. 1); this difference in HbA1c was largest for individuals aged 26–49 years old.
Fig. 1.
Association of HbA1c and gastroparesis. Solid white bar represents gastroparesis. Black and white striped bar represents no gastroparesis. In each box, the black dot represents the mean, the horizontal line inside each box represents the median, and the bottom and top of each box represents the 25th and 75th percentiles, respectively.
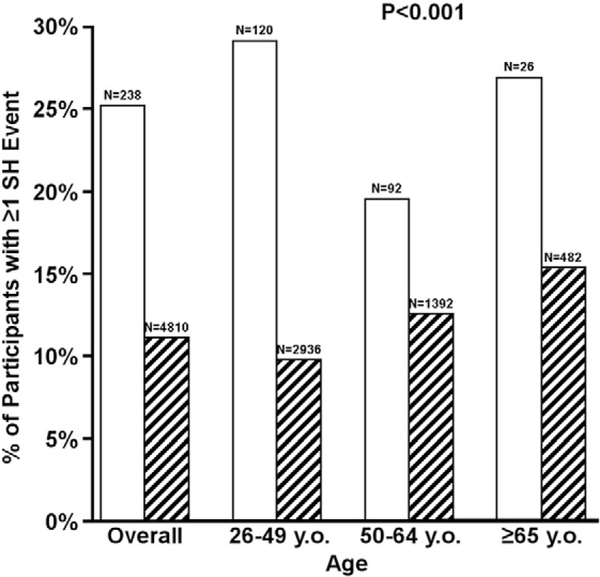
Participants with gastroparesis also reported increased frequency of severe hypoglycemia with one or more events in the previous 12 months compared with those without gastroparesis (Fig. 2). The overall frequency of severe hypoglycemia was 25% in the gastroparesis group vs. 11% in the no gastroparesis group, and the frequency of severe hypoglycemia was higher in the gastroparesis group irrespective of age (29% vs. 10% age 26–49 years, 20% vs. 13% age 50–64 years and 27% vs. 15% age ≥ 65 years, respectively, P < 0.001).
Fig. 2.
Association of severe hypoglycemia (SH) and gastroparesis. Solid white bar represents gastroparesis. Black and white striped bar represents no gastroparesis.
Insulin pump (57% vs. 61%, P = 0.02) and CGM (14% vs. 22%, P < 0.001) use was lower among participants with gastroparesis compared with participants without gastroparesis after controlling for age and duration of diabetes. The frequency of SMBG per day was similar between those with and without gastroparesis (median for both 5 finger-sticks per day, P = 0.14).
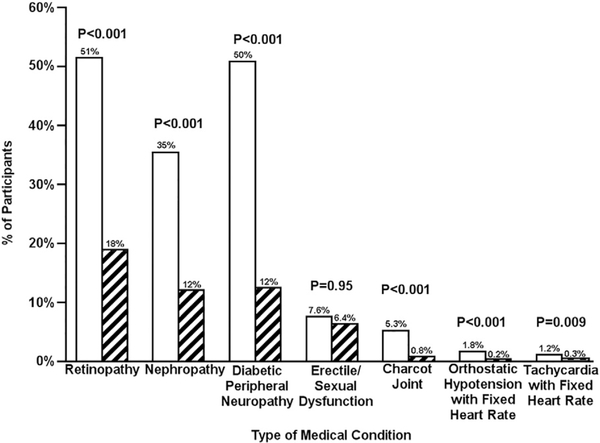
After controlling for age, diabetes duration, SMBG frequency, insulin pump use, and CGM use, there was an increased presence of long-term complications of diabetes in the gastroparesis group vs. the no gastroparesis group including retinopathy requiring treatment (51% vs. 19%, P < 0.001), nephropathy (36% vs. 12%, P b 0.001), peripheral neuropathy (51% vs. 12%, P < 0.001), and Charcot joint (5.3% vs. 0.8%, P < 0.001) (Fig. 3). The symptoms of autonomic neuropathy also were higher among participants with gastroparesis compared with participants without gastroparesis including orthostatic hypotension (1.8% vs. 0.2%, P < 0.001) and tachycardia with fixed heart rate (1.2% vs. 0.3%, P = 0.009) (Fig. 3).
Fig. 3.
Association between diabetes-related complications and gastroparesis in T1D. Solid white bar represents gastroparesis. Black and white striped bar represents no gastroparesis.
In the gastroparesis group, the proportion of participants taking medication for gastroparesis at the time of data collection for the registry varied according to age, with 30% on a medication for gastroparesis in the 26–49 years old group versus 23% in the ≥ 50 year old group. The most frequently used medications were metoclopramide in 17% of the participants, followed by ondansetron in 6%, domperidone in 4%, and erythromycin in 2%. Ten (3%) participants with gastroparesis were currently using two medications.
3. Discussion
The epidemiology of diabetic gastroparesis, especially its incidence, is very difficult to evaluate due to the paucity of community based studies. In the only community-based study available thus far (Olmsted County, MN), the cumulative incidence over 10 years for symptomatic gastroparesis was reported at 5% for T1D.2 Much wider variability has been reported regarding the prevalence of gastroparesis in the diabetic population. Early studies in patients with diabetes, predominantly T1D and small cohorts (70–100 patients), showed delayed gastric emptying frequency of 30–60%.10,13 Newer studies have documented delayed gas- tric emptying or gastroparesis between 10% (n = 1028) and 33% (n =72)of patients with T1D.14,15 Very recently, data from the DCCT-EDIC trial reported the presence of delayed gastric emptying in 47% of 74 pa- tients with long standing T1D when assessed with gastric emptying breath test.16 The conflicting results in the literature indicate that the prevalence of diabetic gastroparesis remains largely unknown. In the present analysis, the reported presence of gastroparesis among individuals with T1D was lower than that which has been reported elsewhere. This might be due to the differences in the assessment methods for the presence of delayed gastric emptying in gastroparesis from study to study, making strict comparisons impossible.3,9,10,15,17The methods used ranged from esophageal manometry and esophageal scintigraphy to gastric emptying assessed by scintigraphy to acid breath test (13C–OBT) and 13C–Spirulina gastric emptying breath test. Additionally, more recent studies have shown how the presence of dysmotility in patients with T1D can be found not only in the proximal gut, but also in the distal gut, with even pan-enteric prolongation of gastrointestinal transit times in some cases, thus making the diagnosis of gastroparesis only a portion of digestive tract dysmotility in patients with Diabetes and its prevalence and incidence even more challenging to determine.18 In this regard, a new technique has been recently adopted for quantifica- tion of transit in all gut regions with a single test. Wireless motility capsule (WMC) testing, has uncovered a significant number of patients with diabetic gastroparesis who also exhibited abnormal small intestinal and/or colonic transit.19 Gastroparesis status for the present work was extracted from the medical chart and therefore some cases may have been missed due to under-reporting.
Risk factors for diabetic gastroparesis in T1D remain poorly defined. A distinct female predominance of gastroparesis in T1D has been previously reported, with an incidence and prevalence up to four times higher than in men.2,3,10,17 Several studies have also reported a variable degree of correlation among duration of diabetes, glycemic control, mi- crovascular complications, and increased risk or presence of gastroparesis in these individuals.3,5,10 Cardiac autonomic neuropathy, including postural hypotension and loss of vagotonic cardiac reflexes, are prominent in patients with diabetic gastroparesis.4 In our cohort, prevalence of gastroparesis was slightly higher for females, but was associated with older age and longer duration of diabetes, consistent with previous reports.3 Participants with gastroparesis had lower educational and economical status; however, we found no differences in gastroparesis diagnosis by race and ethnicity.
In the present cohort, not surprisingly, the HbA1c levels were higher in the participants with gastroparesis compared with the ones without gastroparesis, thus supporting the hypothesis that suboptimal glycemic control increases the risk of developing this complication. However, the higher HbA1c could as well represent a consequence of gastroparesis, from a mismatch between insulin kinetics and nutrient absorption.14 The direction of this relationship is not possible to tease out given the cross-sectional data collection. Most interestingly, even though the HbA1c was higher in these individuals, there was an associated significant increase in the occurrence of severe hypoglycemia in participants with gastroparesis vs. those without gastroparesis across all age groups, this difference was most prominent in the 26–49 age group (29% vs. 10% respectively). Finally, gastroparesis was associated with multiple diabetic complications in our study, as reported in other studies.3,5,10 This finding should be interpreted with caution, though, since both gastroparesis and diabetic complications are associated with longer du- ration of disease and poor glycemic control.
Several patients reported the use of medications for the treatment of gastroparesis. Treatment of gastroparesis has been only partially successful, likely due to a limited number of available pharmacologic agents.5,20 In this cohort, younger participants were taking medications to treat gastroparesis in a higher frequency than older participants. Theoretically, this may be attributed either to more severe gastroparesis symptoms or earlier detection of gastroparesis in this age group. Metoclopramide was the most commonly used medication (17%) followed by ondansetron (6%) and domperidone (4%), although the latter is not FDA approved in the US for this clinical use.
The present study results are clinically important because the presence of diabetes gastroparesis can result in poor glycemic control along with increased hypoglycemia due to a mismatch between food transit/absorption and insulin action. Lower education level and socio-economic status explain why fewer individuals with gastroparesis use an insulin pump and/or CGM in the present study. This is potentially problematic because the insulin pump offers various features such as programmable advanced boluses with delayed insulin delivery that may help patients with gastroparesis to improve postprandial glucose control. Similarly, CGM may be of great assistance to patients with gastroparesis in minimizing hypoglycemia.21,22
This study has several strengths: it is based on a very large sample size of individuals with T1D, the largest to our knowledge; it has the ad- vantage of data collected from a multicenter national registry, with wide diabetes duration and age distribution. Additionally, each report of gastroparesis was confirmed by the diagnosis of gastroparesis in electronic medical records systems. The lack of specific diagnostic methods of gastroparesis in our cohort, however, constitutes a limitation of this study, a challenge similar to what reported previously, likely due to the lack of unified diagnostic protocols for gastroparesis in clinical practice. We do not have information on the method for diagnosing gastroparesis used by each of the participating clinics.
In conclusion, our study showed a lower than previously reported diagnosis of gastroparesis among adults with T1D in the T1D Exchange clinic registry, with few individuals receiving medical treatment and few individuals receiving insulin pump or CGM therapy. The small percentage of individuals taking medications for gastroparesis emphasizes not only the paucity of available pharmacologic treatments, but also the need to develop safe and effective treatment modalities for this condition. Future studies should evaluate whether insulin pump and/or CGM therapy improves glycemic control while reducing hypoglycemia among individuals with gastroparesis.
Acknowledgements
GA wrote and edited the manuscript. PC and NCF performed statistical analyses and wrote/edited the manuscript. DMM, VNS, and KMM contributed to data interpretation and reviewed/edited the manuscript.
Funding
This work was supported through the Leona M. and Harry B. Helmsley Charitable Trust (2016PG-T1D053).
Footnotes
Conflicts of Interest and Financial Disclosures: The authors do not have any conflicts of interest or financial disclosures.
References
- 1.Hornbuckle K, Barnett JL. The diagnosis and work-up of the patient with gastroparesis. J Clin Gastroenterol. 2000;30:117–24. [DOI] [PubMed] [Google Scholar]
- 2.Jung HK, Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Szarka LA, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choung RS, Locke III GR, Schleck CD, Zinsmeister AR, Melton III LJ, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasler WL. Type 1 diabetes and gastroparesis: diagnosis and treatment. Curr Gastroenterol Rep. 2007;9:261–9. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–9. [DOI] [PubMed] [Google Scholar]
- 6.Parkman HP, Yates KP, Hasler WL, Nguyan L, Pasricha PJ, Snape WJ, et al. Dietary in- take and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141:486–98. [98 e1–7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf). 2009;70:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to un- learn in the past 5 years. Gut. 2010;59:1716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–80. [DOI] [PubMed] [Google Scholar]
- 10.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of de- layed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–9. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, Dubose SN, Hall CA. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97:4383–9. [DOI] [PubMed] [Google Scholar]
- 12.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971–8. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz M, Jones KL, Rayner CK, Read NW. ‘Gastric’ hypoglycaemia—an important concept in diabetes management. Neurogastroenterol Motil. 2006;18:405–7. [DOI] [PubMed] [Google Scholar]
- 14.Kofod-Andersen K, Tarnow L. Prevalence of gastroparesis-related symptoms in an unselected cohort of patients with type 1 diabetes. J Diabetes Complications. 2012;26:89–93. [DOI] [PubMed] [Google Scholar]
- 15.Sfarti C, Trifan A, Hutanasu C, Cojocariu C, Singeap AM, Stanciu C. Prevalence of gastroparesis in type 1 diabetes mellitus and its relationship to dyspeptic symptoms. J Gastrointestin Liver Dis. 2010;19:279–84. [PubMed] [Google Scholar]
- 16.Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, et al. De- layed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology. 2015;149:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastro- intestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–96. [DOI] [PubMed] [Google Scholar]
- 18.Farmer AD, Pedersen AG, Brock B, Jakobsen PE, Karmisholt J, Mohammed SD, et al. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia. 2017;60:709–18. [DOI] [PubMed] [Google Scholar]
- 19.Coleski R, Wilding GE, Semler JR, Hasler WL. Blunting of colon contractions in dia- betics with gastroparesis quantified by wireless motility capsule methods. PLoS One. 2015;10:e0141183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis-backwards and for- wards. J Gastroenterol Hepatol. 2011;26:46–57. [DOI] [PubMed] [Google Scholar]
- 21.Ramzan Z, Duffy F, Gomez J, Fisher RS, Parkman HP. Continuous glucose monitoring in gastroparesis. Dig Dis Sci. 2011;56:2646–55. [DOI] [PubMed] [Google Scholar]
- 22.Tanenberg RJ, Pfeifer MA. Continuous glucose monitoring system: a new approach to the diagnosis of diabetic gastroparesis. Diabetes Technol Ther. 2000;2:S73–80. [DOI] [PubMed] [Google Scholar]