Highlights
-
•
Rhinovirus infection unveils attenuation of the HPA-axis in infants exposed to prenatal distress.
-
•
The observed attenuation was more clear in boys than girls.
-
•
Studies are needed to test whether suppression of the HPA-axis associates with later health.
Abbreviations: AUC, area under the curve above/below baseline; EPDS, Edinburgh postnatal depression scale; CI, confidence interval; gwk, gestational week; HPA, hypothalamic-pituitary-adrenal; PCR, polymerase chain reaction; PD, psychological distress; PRAQ-R2, pregnancy-specific anxiety questionnaire-revised 2; SCL-90, symptom checklist-90, anxiety scale; SSRIs, selective serotonin reuptake inhibitors
Keywords: Cortisol, HPA axis, Infant, Prenatal distress, Programming, Rhinovirus
Abstract
Introduction
Prenatal exposure to maternal psychological distress (PD) may have programming effects on the fetus/infant hypothalamic-pituitary-adrenal (HPA) axis and subsequently on the development of the fetus’ immune function. Therefore, our aim was to study whether prenatal exposure to PD is related to early infant HPA axis reactivity in the context of a subclinical rhinovirus infection that challenges infants HPA axis postnatally.
Methods
This study included 336 10-week-old infants from the nested case control Focus Cohort of the FinnBrain Birth Cohort Study. The outcome was infant HPA axis reactivity in a stress test. The acute stressor comprised of pediatric examination with venipuncture and nasal swabs for virus assessment. Saliva cortisol samples were collected at 5 time points: baseline, 0, 15, 25 and 35 min after the stressor. HPA axis reactivity was defined by the cumulative post-stressor cortisol concentration.
Results
HPA axis reactivity was blunted in the PD/rhinovirus + group compared to the average of control/rhinovirus+, PD/rhinovirus-, and control/rhinovirus- groups (difference: 14.7 ln [nmol/L] × min, 95% confidence interval 3.8–25.6, p = .008). HPA axis reactivity was significantly blunted only in boys with rhinovirus detected when separately tested for boys and girls (p = .04).
Conclusion
Our finding of PD-exposed rhinovirus-positive infants having blunted cortisol secretion gives rise to a hypothesis that maternal PD during pregnancy influences infant HPA axis functioning and the functioning of the immune system. Future studies are needed to test whether this suppression of the HPA axis that co-occurs with rhinovirus infection associates with later disease development (e.g., asthma).
1. Introduction
Epidemiological studies suggest that during sensitive periods of fetal development, prenatal environmental events, including exposure to maternal psychological distress (PD), are important factors for shaping the risk for morbidity later in life (O’Donnell and Meaney, 2017; www.dohad.org). More specifically, recent evidence demonstrates an association between prenatal maternal PD exposure and the later development of immune-related disorders, such as recurrent respiratory infections (Korhonen et al., 2019) or asthma and atopic disorders (Andersson et al., 2016a).
Prenatal exposure to maternal PD may affect the development of the fetus/infant hypothalamic-pituitary-adrenal (HPA) axis (O’Donnell and Meaney, 2017). Lymphoid organs, and particularly the thymus, display a markedly elevated expression of glucocorticoid receptors during extra-uterine life (Merlot et al., 2008). In transgenic mice with impaired glucocorticoid function, the important role of glucocorticoids in the ontogeny of the immune system has been demonstrated (Sacedón et al., 1999). In contrast to the traditional view of glucocorticoids as immunosuppressant hormones, they are more accurately conceptualized as immunomodulatory hormones that can both stimulate as well as suppress immune function (McEwen, 2018). If the communication between the HPA axis and immune system is disrupted, for example by prenatal maternal PD, then important alterations in anti-viral immune responses may take place (Bailey et al., 2003; Webster and Sternberg, 2004). Bi-directional communication between the neuroendocrine and immune systems plays a significant regulatory role in response to a viral infection (Bailey et al., 2003). For example, in the general infant population, rhinovirus infections are common, often asymptomatic and do not cause long-term viral persistence in the respiratory tract, while in vulnerable subjects rhinovirus infection-induced wheezing during early life might be a marker for a susceptibility for the later development of asthma (Jackson et al., 2008; Lukkarinen et al.,2017).
In humans, the relationship between the exposure to maternal prenatal PD, the HPA axis functioning and anti-viral immune responses during early infancy have not been studied, so far. Here, we aimed at bridging and integrating previous data and to investigate whether maternal prenatal PD is associated with the early infant HPA axis reactivity, and whether this association is different in the presence of subclinical rhinovirus infection. We hypothesized that infant HPA axis reactivity is altered after exposure to prenatal PD, and the altered functioning related to this exposure emerges or is especially evident during a concurrent subclinical rhinovirus infection, which can be considered a natural stressor challenging the HPA axis functioning with relevance to the offspring’s later health
2. Methodology
2.1. Definition of maternal psychological distress during pregnancy
The FinnBrain Birth Cohort Study investigates the effects of prenatal and early-life stress exposure on child health (Karlsson et al., 2018). Within the main Cohort, a nested case-control study, called the Focus Cohort, was established to enable comparisons between subjects exposed to different types of prenatal PD with their non-exposed controls (Supplementary Material). To define maternal PD, the questionnaires for symptoms of depression (Edinburgh Postnatal Depression Scale [EPDS]), overall anxiety (Symptom Checklist-90, anxiety scale [SCL-90]) and pregnancy-related anxiety symptoms (Pregnancy-Related Anxiety Questionnaire-Revised 2 [PRAQ-R2]) were used at gwks 14, 24, and 34. Exploratory analyses establishing cut-points for the approximate highest and lowest 25th percentiles of maternal PD during pregnancy were performed. The total sum score cut-off points for the PD exposure and non-exposure were as follows: ≥12 and ≤ 6 for the EPDS, ≥10 and ≤ 4 for the SCL-90 anxiety subscale, and ≥ 34 and ≤ 25 points for PRAQ-R (Karlsson et al., 2018). The criteria to become identified as a case were: 1) scoring at least once above the selected threshold on two different questionnaires, 2) scoring at least twice above the selected threshold on the same instrument at any of the three prenatal time points, or 3) prenatal maternal use of serotonin reuptake inhibitors (SSRIs). The non-exposed controls needed to remain below the thresholds in all assessments (Karlsson et al., 2018).
2.2. Study design and population
The outcome of the current study was the infant HPA axis reactivity in response to an acute stressor in 10-week-old infants from the Focus Cohort (Flow chart, Fig. A.1). Due to project logistics (i.e., availability of assisting personnel) and factors not systematically related to any family characteristics, 792 families from the Focus Cohort target population (n = 1219) were attempted to be reached by phone for recruiting the infants for the current study. Out of those families who were reached (n = 586), a total of 418 (71%) agreed to participate in the study, and 168 (21%) declined. Eventually 374 infants attended the stress test, 38 infants were excluded from the data analyses (Fig.A.1). Thus, the final analyses of the current study comprised of 336 infants with adequate cortisol and virus samples. Data on maternal characteristics were collected from the self-report questionnaires at gestational weeks (gwks) 14, 24, and 34, while infant characteristics were obtained from the patient charts and Medical Birth Register of National Institute for Health and Welfare (www.thl.fi) (Karlsson et al., 2018). The study was approved by the Ethics Committee of the Hospital District of Southwest Finland and commenced only after obtaining written informed consent from the guardians.
2.3. The stress test of infant HPA axis reactivity
The stress test was carried out to investigate the outcome of infant HPA axis reactivity. The study visits were performed on October 2012 to February 2016 at 8:30 a.m.-6:00 p.m. in the research facilities. The study visit started with a peaceful period in order to standardize the baseline cortisol sampling. The stressor included a standardized pediatric examination with venipuncture and a nasal swabbing of each a source of mild physical discomfort. The infant HPA axis reactivity to the stressor was assessed measuring five saliva cortisol samples at: baseline, 0, 15, 25 and 35 min after a stressor. The saliva cortisol samples were collected using Salimetrics infant swabs (Stratech, Suffolk, UK) by a research nurse or a researcher. The polymer swab was held in an infant’s mouth for two minutes. Saliva was collected by centrifuging tubes (15 min, 1800 x g, 4 °C) and immediately frozen at −70 °C. The research nurse filled the protocol record form to keep track of timing, infant feeding, and possible deviations from the study protocol. The cortisol concentrations were measured with Cortisol Saliva Luminescence Immunoassay (IBL International, Hamburg, Germany).
2.4. Virus testing
The nasal swab specimen for virus assessment was taken from front nostril and stored at −80 °C before the analysis. Swabs were suspended in phosphate-buffered saline, and nucleic acids were extracted by NucliSense easyMag (BioMerieux, Boxtel, the Netherlands) or a MagnaPure 96 (Roche, Penzberg, Germany) automated extractor. PCR for adenovirus, bocavirus, coronaviruses, enteroviruses, metapneumovirus, influenza A and B viruses, respiratory syncytial virus A and B, rhinovirus, and parainfluenza virus types 1–4 was performed using a commercial multiplex test kit (Anyplex™RV16, Seegene, Seoul, Korea). The participating infants were without signs or symptoms of acute febrile respiratory infection as determined and systematically documented by a pediatrician during the study visit.
2.5. Statistical analyses
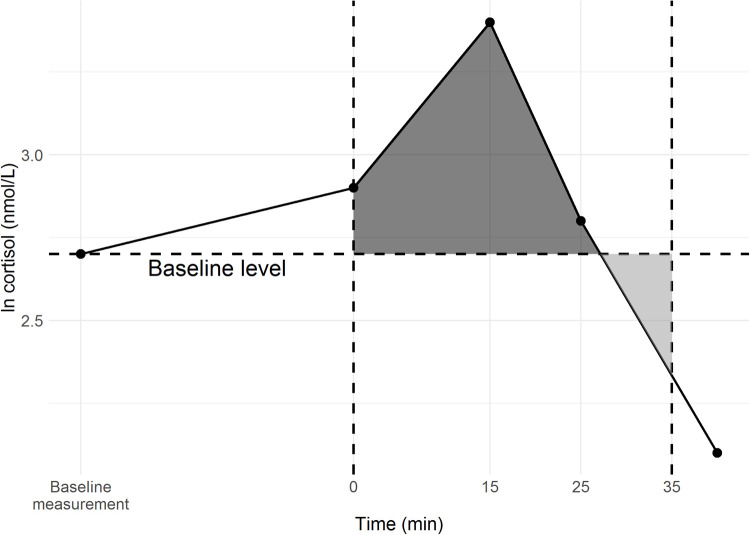
As the measure for the HPA axis reactivity, the area under the curve above/below the baseline (AUCi) was used (Fig. A.2.). Log-transformed (natural logarithm) cortisol values were used in the calculations to avoid outlying AUCi values. Only the infants with a baseline cortisol value and ≥2 measured/available cortisol values after the acute stressor were included in the analyses (Fig. A.1). The missing cortisol values for the infants included in the analyses were imputed using multiple imputation. The analyses of how AUCi associated with the PD exposure (PD vs. control), rhinovirus status (+ vs. -), and infant sex assessments were done using the t-tests. The main hypothesis being that the HPA axis reactivity is altered in the PD/rhinovirus + group was tested with an appropriate linear regression model, where infant sex was also controlled. Covariates for the adjusted model were selected based on statistical and clinical relevance (Table 1; Supplementary Material). Sensitivity analyses, excluding the mothers using SSRIs or corticosteroids, were performed to estimate the potential confounding effect of maternal medication. As the exclusion of these two groups did not alter the results, they were retained in the final analyses to maintain population representativity (Supplementary Material). All statistical analyses were performed in R 3.5.1 (R Core Team 2018).
3. Results
The study population included 336 10-week-old infants of whom 177 (53%) were boys, 148 (44%) were exposed to PD, and 76 (23%) were rhinovirus-positive (Table 1 ). Other viruses than the rhinovirus presented with only single positive findings in this general population-based study. No differences were found in the selected background characteristics between the PD (n = 148) and control (n = 188) groups with the exceptions of lower maternal education level (p = .004) and smoking during pregnancy (p < .001) associating with PD (Table 1).
Table 1.
Study characteristics.
| PD exposed (n = 148) | Controls (n = 188) | p | |
|---|---|---|---|
| Infant characteristics at birth | |||
| Gestational age, weeks (SD) | 39.4 (1.4) | 40.0 (1.6) | .30 |
| Birth weight, g (SD) / length, cm (SD) | 3595 (440)/ 51 (2) | 3581 (487) / 51 (2) | .69/.89 |
| Head circumference, cm (SD) | 35 (1.3) | 35 (1.5) | .90 |
| Umbilical artery, pH (SD) | 7.27 (0.8) | 7.28 (0.8) | .13 |
| Male sex, nr (%) | 80 (54) | 97 (52) | .65 |
| Maternal characteristics during pregnancy | |||
| Maternal age at birth, years (SD) | 30 (4.5) | 31 (4.3) | .05 |
| Maternal pre-pregnancy body-mass index, kg/m2 (SD) |
25.1 (5.1) | 24 (4.0) | .11 |
| Maternal education, nr (%) low (up to 12 years) middle (13-15 years) high (over 15 years) |
63 (44) 36 (24) 47 (32) |
48 (25) 56 (30) 84 (45) |
.004 |
| Maternal smoking, nr (%) | 24 (16) | 6 (3) | <.001 |
| Maternal use of inhaled or oral corticosteroids, nr (%) | 3 (2) | 7 (4) | .37 |
| Rhinovirus-positive infants at stress test, nr (%) | 31(21) | 45 (24) | .50 |
| Infant age at stress test, weeks (SD) | 10.8 (2.0) | 10.6 (2.0) | .21 |
p values are based on χ² and Mann-Whitney U tests for categorical and continuous variables, respectively. Bold values signify significance. PD: prenatal psychological stress, SD: standard deviation.
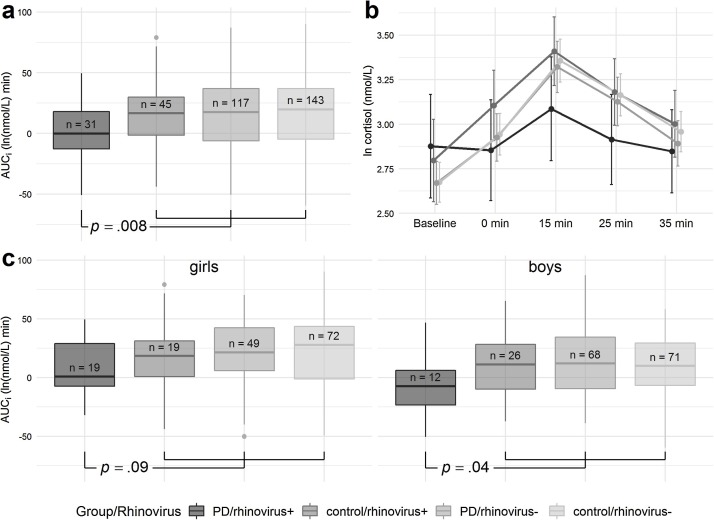
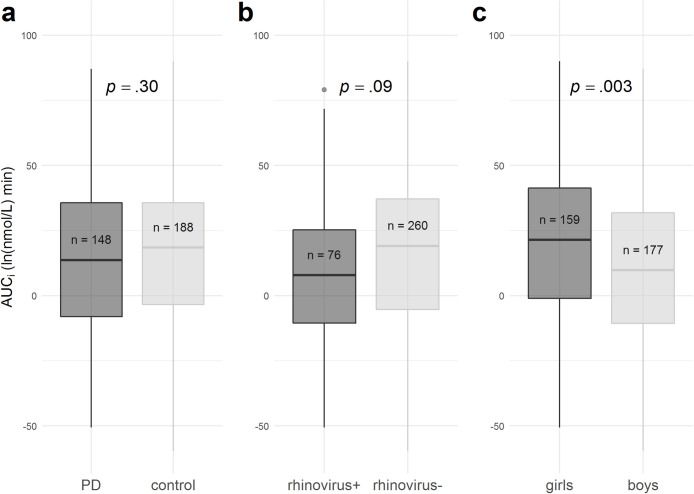
The infant HPA axis reactivity was not independently associated with the PD exposure (p = .30) or rhinovirus status (p = .09), but girls had higher AUCi than boys (p = .003) (Fig. A.3). Our hypothesis of HPA axis activity being different in the group of the composite phenotype of PD/rhinovirus+ was tested by comparing the PD/rhinovirus+ group with the average of control/rhinovirus+, PD/rhinovirus- and control/rhinovirus- groups, while controlling for infant sex. The AUCi was lower in PD/rhinovirus + than in the other groups (difference: 14.7 ln [nmol/L] × min; 95% confidence interval [CI] 3.8–25.6; p = .008) (Fig. 1 a-b). The same comparison was separately tested for boys and girls, and the AUCi difference was significant in boys (p = .04) but not in girls (p = .09) (Fig. 1c). Controlling for maternal education level and smoking did not alter results (15.3 ln [nmol/L] × min; 95% CI 4.1–26.4; p = .007). Finally, we tested the effect of the rhinovirus status within the PD group. The AUCi was lower in PD/rhinovirus + than in PD/rhinovirus- group (difference: 14.9 ln [nmol/L] × min; 95% CI 3.3–27.3; p = .01).
Fig. 1.
During the stress test: a) AUCi was compared between the PD/rhinovirus + group and the average of control/rhinovirus+, PD/rhinovirus-, and control/rhinovirus- groups; b) mean cortisol values at five time points: baseline, 0 min, 15 min, 25 min and 35 min after stressor; and c) AUCi was compared between the groups separately in girls and boys. The standard boxplots demonstrate the AUCi in the groups.
AUCi: area under the curve above/below baseline, PD: exposure to prenatal psychological distress.
4. Discussion
This is the first human-based study to investigate associations between prenatal PD exposure and early infant HPA axis reactivity, when a concurrent rhinovirus infection that challenged the immune system was present. Interestingly, we found that after the preceding prenatal PD exposure, the rhinovirus-positive infants had a blunted cortisol response in a stress test in comparison with their non-PD-exposed- and only PD-exposed counterparts. This association was more clearly seen in boys than in girls. Sex differences in HPA axis functioning have been reported previously (Gray et al., 2017; Giesbrecht et al., 2017). While human-based studies with a design similar to ours are lacking, so far, our results are in line with a mouse model study reporting that stress-exposed male mice had a blunted corticosterone response to influenza A virus compared to females (Avitsur et al., 2006).
Viral-induced inflammation activates the systemic stress response. Secreted glucocorticoids, the end-effectors of the HPA axis, protect peripheral organs from inflammation-induced tissue damage by suppressing an overshoot of the host immune reaction. In our study, the infants with prenatal PD exposure had suppressed HPA axis reactivity during rhinovirus infection. The consensus is that rhinovirus does not cause asthma in a straight-forward manner but rather brings up a child's underlying vulnerability trait of an altered immune response (Lukkarinen et al., 2017). The origins of these immunological alterations are not well understood, and PD exposure could be among the related factors.
The strengths of this study are the prospective design, relatively large sample size without major exclusion criteria and representing non-clinical populations, comprehensive data on maternal PD, a novel focus on the infant HPA axis reactivity during subclinical virus infection, a standardized baseline cortisol sampling and physical stressor, and good quality of the cortisol and virus measurements. As a limitation, some analyses of interest were precluded by the low number of infants in subpopulations due to the challenges in recruitment. Further, the inflammatory response to the infectious challenge was not measured. Regarding the generalizability of the results, it is noteworthy that the relation between prenatal PD and HPA axis responses may depend on infant age, sex, and/or the nature of the stressor (Giesbrecht et al., 2017; Tollenaar et al., 2011).
4.1. Conclusions
Our finding of the PD/rhinovirus + group having blunted cortisol secretion in comparison with control/rhinovirus+, PD/rhinovirus-, and control/rhinovirus- groups gives rise to a hypothesis that maternal prenatal PD during pregnancy is related to the early programming and development and interrelations of infant HPA axis and immune system placing our results in the context of fetal programming. Studies are needed to test whether the observed suppression of HPA axis in the context of rhinovirus infection associates with later disease development, such as atopic disorders and wheezing or asthma (Lukkarinen et al., 2017), and to further investigate child sex in its vulnerability to PD exposure.
Funding
This work was supported by the Academy of Finland (HK), the Foundation for Pediatric Research (LKo), the Signe and Ane Gyllenberg Foundation (LKo, LKa, SK, HK), the Yrjö Jahnsson Foundation (LKa), the Päivikki and Sakari Sohlberg Foundation (LKo, LKa, SK), Finnish State Grants for Clinical Research (LKo, ML, JJT, LKa, SK, HK), the Finnish Cultural Foundation (SK), the Juho Vainio Foundation (SK), the Finnish Brain Foundation (SK), the Maire Taponen Foundation (SK, LKo), and the Brain and Behavior Research Foundation YI (Grant #1956) (LKa), Helsinki, Finland. None of the funding sources had a role in the study design, data collection, analyses, interpretation of data, writing of the report, or in the decision to submit this manuscript for publication.
Disclosure of potential conflict of interest
None.
CRediT authorship contribution statement
Laura S. Korhonen: Methodology, Investigation, Formal analysis, Writing - original draft. Susanna Kortesluoma: Methodology, Investigation, Writing - review & editing. Minna Lukkarinen: Writing - original draft, Visualization. Ville Peltola: Supervision, Writing - review & editing. Henri Pesonen: Software, Formal analysis, Writing - review & editing. Juho Pelto: Software, Formal analysis, Writing - review & editing. Jetro J. Tuulari : Investigation, Writing - review & editing. Heikki Lukkarinen: Writing - review & editing. Tytti Vuorinen: Methodology, Writing - review & editing. Hasse Karlsson : Conceptualization, Project administration, Writing - review & editing. Linnea Karlsson: Conceptualization, Supervision, Project administration, Writing - review & editing.
Acknowledgements
Robert M. Badeau, M.Sc., Ph.D. of Aura Professional English Consulting, Ltd. (www.auraenglish.com) performed this manuscript’s English language checking service.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.psyneuen.2019.05.023.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Avitsur R., Hunzeker J., Sheridan J.F. Role of early stress in the individual differences in host response to viral infection. Brain Behav. Immun. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Andersson N.W., Hansen M.V., Larsen A.D., Hougaard K.S., Kolstad H.A., Schlunssen V. Prenatal maternal stress and atopic diseases in the child: a systematic review of observational human studies. Allergy. 2016;71(1):15–26. doi: 10.1111/all.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M., Engler H., Hunzeker J., Sheridan J.F. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16:141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- Gray J.D., Kogan J.F., Marrocco J., McEwen B.S. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 2017;13:661–673. doi: 10.1038/nrendo.2017.97. [DOI] [PubMed] [Google Scholar]
- Giesbrecht G.F., Letourneau N., Campbell T.S., Alberta Pregnancy Outcomes and Nutrition Study Team Sexually dimorphic and interactive effects of prenatal maternal cortisol and psychological distress on infant cortisol reactivity. Dev. Psychopathol. 2017;29:805–818. doi: 10.1017/S0954579416000493. [DOI] [PubMed] [Google Scholar]
- Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L., Tolvanen M., Scheinin N.M., Uusitupa H.-M., Korja R., Ekholm E. FinnBrain Birth Cohort Study Group Cohort Profile: The FinnBrain Birth Cohort Study (FinnBrain) Int. J. Epidemiol. 2018;47:15j–16j. doi: 10.1093/ije/dyx173. [DOI] [PubMed] [Google Scholar]
- Korhonen L.S., Karlsson L., Scheinin N.M., Korja R., Tolvanen M., Mertsola J., Peltola V., Karlsson H. Prenatal Maternal Psychological Distress and Offspring Risk for Recurrent Respiratory Infections. J. Pediatr. 2019 doi: 10.1016/j.jpeds.2018.12.050. [DOI] [PubMed] [Google Scholar]
- Lukkarinen M., Koistinen A., Turunen R., Lehtinen P., Vuorinen T., Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J. Allergy Clin. Immunol. 2017;140:988–995. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Redefining neuroendocrinology: epigenetics of brain-body communication over the life course. Front. Neuroendocrinol. 2018;49:8–30. doi: 10.1016/j.yfrne.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Merlot E., Couret D., Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav. Immun. 2008;22:42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- O’Donnell K.J., Meaney M.J. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am. J. Psychiatry. 2017;174:319–328. doi: 10.1176/appi.ajp.2016.16020138. [DOI] [PubMed] [Google Scholar]
- Sacedón R., Vicente A., Varas A., Morale M.C., Barden N., Marchetti B. Partial blockade of T-cell differentiation during ontogeny and marked alterations of the thymic microenvironment in transgenic mice with impaired glucocorticoid receptor function. J. Neuroimmunol. 1999;98:157–167. doi: 10.1016/s0165-5728(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Tollenaar M.S., Beijers R., Jansen J., Riksen-Walrave J.M.A., de Weerth C. Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress. 2011;14:53–65. doi: 10.3109/10253890.2010.499485. [DOI] [PubMed] [Google Scholar]
- Webster J.I., Sternberg E.M. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J. Endocrinol. 2004;181:207–221. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.