Dear Editor,
Recently, human metapneumovirus (hMPV) has been identified as a major human viral pathogen and reported to be the etiologic agent of upper and lower respiratory tract infections in infants and young children as well as the elderly and the immunocompromised host (Boivin et al., 2002, Bastien et al., 2003, Falsey et al., 2003, Maggi et al., 2003). Virus has been mostly identified in clinical respiratory samples by reverse-transcription PCR (RT-PCR), but animal immune sera have also been used for hMPV identification both in nasopharyngeal aspirates (NPA) and cell cultures (Van den Hoogen et al., 2001, Percivalle et al., 2005).
More recently, immunological identification of hMPV strains was achieved by direct fluorescent antibody (DFA) staining of cells from NPA samples as well as from inoculated cell cultures using monoclonal antibodies (MAbs) (Ebihara et al., 2005, Percivalle et al., 2005, Landry et al., 2005). Although sensitivity and negative predictive value of MAbs were somewhat lower than those achieved by RT-PCR, rapidity of turnaround time and simplicity of test performance appeared as diagnostic parameters favouring the immunological approach (Percivalle et al., 2005).
All hMPV strains recovered till now in different countries of the five continents have been classified into two major clusters, referred to as types A and B, on the basis of sequencing and phylogenetic analysis of genes L, N, F, or P (Boivin et al., 2002, Boivin et al., 2004, Van den Hoogen et al., 2001, Van den Hoogen et al., 2004).
In the present study, type-specific monoclonal antibodies (MAbs) raised against type A and type B hMPV strains were developed, using virus strains recovered and propagated in LLC-MK2 cell cultures. These MAbs were shown to type all strains previously characterized by sequencing and phylogenetic analysis.
Our prototype A and B hMPV strains (I-PV 03/01 6621 and I-PV 03/04 4702, respectively) propagated in LLC-MK2 cell cultures (Gerna et al., 2005) were concentrated by ultracentrifugation and then inoculated into BALB/C mice according to a reported protocol (Percivalle et al., 2005). Following fusion of mouse spleen cell suspensions with Sp2/0Ag14 myeloma cells, hybridomas were tested for specific reactivity with hMPV by enzyme-linked immunosorbent assay and the indirect fluorescent antibody (IFA) assay. Following cloning and subcloning, MAbs previously selected for specific reactivity with hMPV were tested for type specificity by IFA using our type A and B prototype strains. Specific reactivity with viral proteins was tested by Skiadopoulos at NIH, NIAID (Bethesda, MD) by Western blot of sucrose-purified reference hMPV strains CAN83 (type A) and CAN75 (type B) which were isolated in Canada and represent known prototypes of each of the two major genetic lineages (Peret et al., 2002). In addition, MAbs were tested by IFA on LLC-MK2 cells, which were infected with recombinant human parainfluenza virus type 1 expressing the hMPV fusion (F), small hydrophobic (SH), and the attachment glycoprotein (G) of both Canadian prototypes (Newman et al., 2002, Skiadopoulos et al., 2004).
Two MAbs, including clones F4A1 (IgG1) and CB7F3 (IgG1), each reactive by both DFA and ELISA assays with either type A or type B hMPV strains, respectively, were selected and tested for cross-reactivities with conventional respiratory viruses (influenza viruses A and B, parainfluenza virus types 1–4, human respiratory syncytial virus, human adenovirus, human coronaviruses 229, OC43 and NL63, and rhinoviruses). No cross-reactivity with any of known respiratory viruses was detected for either one of the two selected MAbs. Both type-specific MAbs were found to react with the F protein of the homologous virus type by both IFA and Western blot.
A total of 67 NPA samples were tested by DFA using type-specific MAbs. On the whole, 24 hMPV strains were typed by MAbs on duplicate NPA slides (100% sensitivity). As a result, 16 strains were found to belong to type A, and 8 to type B (Table 1 ). These results exactly matched those obtained by sequencing and phylogenetic analysis (Gerna et al., 2005). In the meantime, type-specific MAbs were used to test 18 NPA samples positive for different respiratory viruses as reported in Table 1. No cross-reactivity with any of the other respiratory viruses tested was found (100% specificity). In addition, hMPV type-specific MAbs were tested against respiratory cells from 25 NPA samples negative for respiratory viruses. No non-specific reactivity with uninfected respiratory cells was detected (Table 1).
Table 1.
hMPV typing of 24 hMPV-positive NPA samples by DFA using type-specific MAbs compared to typing by phylogenetic analysis
| Respiratory virus | No. NPAs tested | Typing by hMPV MAbs |
|
|---|---|---|---|
| Type A | Type B | ||
| hMPV, type Aa | 16 | 16 | 0 |
| hMPV, type Ba | 8 | 0 | 8 |
| Influenzavirus A | 2 | 0 | 0 |
| Influenzavirus B | 2 | 0 | 0 |
| Parainfluenza virus 1–3 | 3 | 0 | 0 |
| Respiratory syncytial virus | 3 | 0 | 0 |
| Adenovirus | 2 | 0 | 0 |
| Human coronaviruses | 3 | 0 | 0 |
| Rhinoviruses | 3 | 0 | 0 |
| None (cells from NPA) | 25 | 0 | 0 |
In addition, 18 NPAs positive for different respiratory viruses and 25 NPA samples negative for respiratory viruses were tested as controls.
As typed by sequencing and phylogenetic analysis.
Furthermore, some NPA samples positive for hMPV were tested for typing following isolation in LLC-MK2 cell cultures. While 12/12 (100%) of samples inoculated as fresh NPAs were recovered in cell cultures and typed, only 8/25 (32%) samples thawed once or twice, could be typed. Thus, both hMPV recovery and typing are optimally achieved by inoculating fresh samples onto cell cultures.
Morphological patterns of the two type-specific MAbs in cell cultures infected with reference strains are reported in Fig. 1 . In addition, IFA patterns observed in respiratory tract cells from hMPV-infected NPA samples, as well as in LLC-MK2 cell cultures following hMPV isolation, are reported for both type A and type B hMPV strains in Fig. 2 A–D and E–H, respectively. The staining intensity ranged from 1+ to 4+ in different cells, while the staining pattern was similar to the granular pattern of one of three MAbs included in the pool for hMPV detection (Percivalle et al., 2005).
Fig. 1.
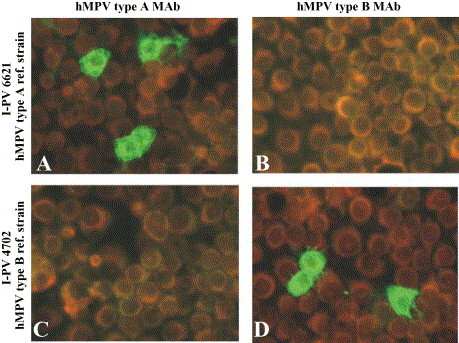
Typing of reference hMPV strains in LLC-MK2 cell cultures by type-specific MAbs and indirect immunofluorescence (IFA) 24 h p.i. (A and B) Type A (I-PV 03/01 6621); (C and D) type B (I-PV 03/04 4702) reference strains.
Fig. 2.
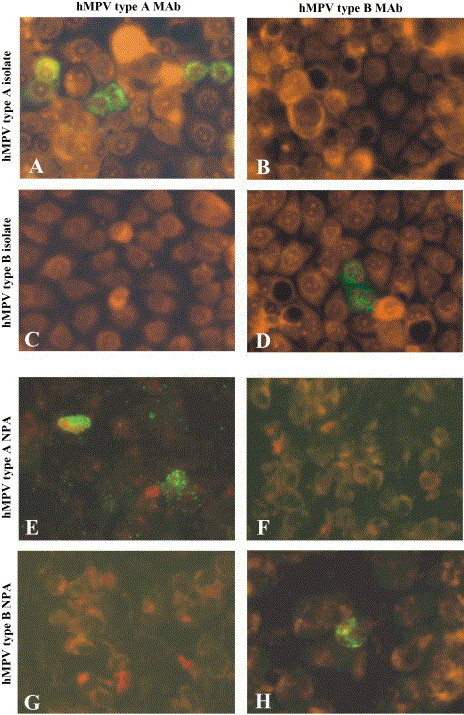
(A–D) Typing of two hMPV isolates recovered in LLC-MK2 cell cultures 48 h p.i. by IFA and MAbs. (A and B) Type A isolate; (C and D) type B isolate. (E–H) Typing of hMPV strains by IFA and type-specific MAbs on respiratory cells from NPA. (E and F) type A hMPV strain; (G and H) type B hMPV strain.
In the present study, we have succeeded in developing type-specific MAbs anti-hMPV, which have been shown to be able to classify all hMPV strains tested into types A or B, exactly matching results given by sequencing and phylogenetic analysis. Since the two hMPV types have been found to circulate at a much different rate in different years (Gerna et al., 2005), hMPV typing may be useful for epidemiological purposes. Typing by MAbs is highly preferable over typing by phylogenetic analysis in terms of practicality, rapidity and cost-effect benefits. Given the 100% sensitivity and specificity of type-specific MAbs with respect to hMPV detection by the MAb pool (Percivalle et al., 2005), detection and typing of new hMPV strains by MAbs may be performed simultaneously in viral diagnostic laboratories.
Acknowledgements
This work was partially supported by the Ministero della Salute, Ricerca Finalizzata IRCCS Policlinico San Matteo (convenzione no. 118 and grants 89282/A and 89288).
We thank Daniela Sartori for preparing the manuscript, Daniele Lilleri for help in picture processing, and Linda D’Arrigo for revision of the English. We are also indebted to Gabriella Garbagnoli and Viviana Landini for technical assistance.
References
- Bastien N., Ward D., Van Caeseele P., Brandt K., Lee S.H.S., McNabb G. Human metapneumovirus infection in the Canadian population. J. Clin Microbiol. 2003;41:4642–4646. doi: 10.1128/JCM.41.10.4642-4646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Abed Y., Pelletier G., Ruel L., Moisan D., Coté S. Virological features and clinical manifestations associated with human pneumovirus: A new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- Boivin G., Mackay I., Sloots T.P., Madhi S., Freymuth F., Wolf D. Global genetic diversity of human metapneumovirus fusion gene. Emerg Infect Dis. 2004;10:1154–1157. doi: 10.3201/eid1006.031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Ma X., Ishiguro N., Kikuta H. Detection of human metapneumovirus antigens in nasopharyngeal secretions by an immunofluorescent-antibody test. J Clin Microbiol. 2005;43:1138–1141. doi: 10.1128/JCM.43.3.1138-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Gerna G., Campanini G., Rovida F., Sarasini A., Lilleri D., Paolucci S. Changing circulation rate of human metapneumovirus strains and types among hospitalized pediatric patients during three consecutive winter-spring seasons. Arch Virol. 2005;150:2365–2375. doi: 10.1007/s00705-005-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M.L., Ferguson D., Cohen S., Peret T.C.T., Erdman D.D. Detection of human metapneumovirus in clinical samples by immunofluorescence staining of shell vial centrifugation cultures prepared from three different cell lines. J Clin Microbiol. 2005;43:1950–1952. doi: 10.1128/JCM.43.4.1950-1952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F., Pifferi M., Vatteroni M., Fornai C., Tempestini E., Anzilotti S.A. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–2991. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.T., Surman S.R., Riggs J.M., Hansen C.T., Collins P.L., Murphy B.R. Sequence analysis of the Washington/1964 strain of human parainfluenza virus type 1 (HPIV1) and recovery and characterization of wild-type recombinant HPIV1 produced by reverse genetics. Virus Genes. 2002;24:77–92. doi: 10.1023/a:1014042221888. [DOI] [PubMed] [Google Scholar]
- Percivalle E., Sarasini A., Visai L., Revello M.G., Gerna G. Rapid detection of human metapneumovirus strains in nasopharyngeal aspirates and shell vial cultures by monoclonal antibodies. J Clin Microbiol. 2005;43:3443–3446. doi: 10.1128/JCM.43.7.3443-3446.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret T.C., Boivin G., Li Y., Couillard M., Humphrey C., Osterhaus A.D. Characterization of human metapneumovirusesisolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos M.H., Biacchesi S., Buchholz U.J., Riggs J.M., Surman S.R., Amaro-Carambot E. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78:6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., De Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A.M. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., Herfst S., Sprong L., Cane P.A., Forleo-Neto E., de Swart R.L. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–665. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]


