Summary
Viral respiratory tract infections are the most common infectious illnesses, though they are usually self-limiting and confined to the respiratory tract. The rapid identification of viruses and their effective elimination with minimal local and systemic inflammation is a testament to the efficiency of the innate immune response within the airways and lungs. A failure of this response appears to occur in those with asthma and chronic obstructive pulmonary disease, where viral infection is an important trigger for acute exacerbations.
The innate immune response to viruses requires their early detection through pathogen recognition receptors and the recruitment of the efficient antiviral response that is centred around the release of type 1 interferons. The airway epithelium provides both a barrier and an early detector for viruses, and interacts closely with cells of the innate immune response, especially macrophages and dendritic cells, to eliminate infection and trigger a specific adaptive immune response.
Keywords: respiratory viral infection, innate immune response, rhinovirus, influenza, Toll-like receptor, type 1 interferon
The problem of viral respiratory infections
Viral respiratory tract infections are amongst the most frequent infectious illnesses afflicting both adults and children, and result in a surprisingly diverse range of disease severity from the mild common cold to severe life-threatening lower respiratory tract infections. Whilst careful studies undertaken in the 1960s identified many of the viruses responsible for upper respiratory tract infections (URTIs),1 it was only with the advent of sensitive molecular diagnostic techniques that it was determined which viruses were responsible for the majority of URTIs and the viruses identified in population studies.2, 3 Human rhinoviruses (RVs) have consistently been found to be the most frequent cause of URTIs (30–50% of cases), followed by coronaviruses (10–15%), with parainfluenza virus, adenovirus and enteroviruses in 5% of cases or less.1, 2 Influenza virus causes 5–15% of viral respiratory infections and respiratory syncytial virus (RSV) 5–10%; these two viruses appear to have the capacity to cause more serious disease (see below).1 Recently, human metapneumovirus has been identified as a pathogen in humans and is thought to account for up to 6% of all URTIs in young children.4 Despite the use of polymerase chain reaction (PCR) in 20–30% of URTIs a pathogen cannot be identified. A more recent advance in viral diagnostics is the application of DNA microarray technology, which allows researchers to assess individuals with URTIs for all known viruses.5 Using this technology in a community study of adults, RVs were still found to predominate, but a much greater diversity in both RVs and coronaviruses was found to cause disease than had been anticipated.6
For the most part these viruses result in a syndrome of rhinorrhoea, nasal congestion, pharyngitis and cough.1 They affect children 6–8 times per year and adults 2–4 times per year.7 In temperate climates RV infection occurs throughout the year but peaks in autumn and spring, whilst RSV and influenza tend to cause disease clusters in winter.1 In an immunocompetent host, with the exception of influenza, these viruses lead to a disease confined to the respiratory tract, which may be complicated by otitis media and sinusitis, and rarely predispose to the development of bacterial pneumonia.1 Experimental studies have shown that RV infects airway epithelial cells of the upper respiratory tract but this leads to no discernable damage to the epithelium, though it does result in vasodilatation, increased vascular permeability and an influx of neutrophils.8 Symptom severity has also been found to correlate with the release of interleukin (IL)-8 and the influx of neutrophils.9
Influenza and RSV both cause a URTI syndrome but appear to have a greater capacity to infect the lower respiratory tract. RSV is the leading cause of bronchiolitis in infants,10 but is also increasingly being recognized as a disease of adults, especially the elderly and those with chronic lung disease.11 Infection targets the lower airway epithelial cells with resulting cytolysis of these cells due to direct viral effects and the resulting lymphocytic and neutrophilic inflammatory response.12 Influenza is similar in its propensity to infect the lower respiratory tract and, whilst it generally causes a self-limiting URTI, it occupies a unique place among respiratory tract viruses in its ability to cause a devastating respiratory illness and death, even in previously healthy individuals. This phenomenon occurs through a complex interaction of host susceptibility, including previous recognition by the adaptive immune response, and viral virulence.13 Even limited endobronchial infection with influenza leads to extensive damage to the epithelium.14 Of all the viruses, influenza appears the best able to avoid the innate immune response in the airways with the potential consequence of severe respiratory symptoms and systemic inflammation.
Viral respiratory tract infection and chronic airways disease
Viral respiratory infections have a major impact in individuals with chronic airways diseases such as asthma and chronic obstructive pulmonary disease (COPD). In these individuals the clinical response appears to be affected by an interaction that results from direct effects of the virus, the antiviral immune response and, presumably, the pre-existing chronic airway inflammation.
Viral respiratory infections, especially RV, are associated with the majority of acute asthma exacerbations in children15, 16 and adults.17, 18 A similar association is seen in COPD, where again RV is linked to the majority of exacerbations, including the most severe ones.19 Studies of in vitro 20, 21, 22, 23 and experimental RV infection in asthmatic subjects24, 25 show that RV can directly increase airway inflammation and worsen airflow obstruction.26 Whilst adults with asthma appear no more likely to develop colds, they do experience considerably more lower respiratory tract symptoms associated with them.27 In children, RV is more strongly associated with acute asthma exacerbations than other respiratory viruses.28 This is of particular interest as RV causes only limited epithelial damage and relatively less airway inflammation compared to infection with RSV or influenza. Viral triggers to acute exacerbation of asthma were also seen to have a direct impact on the airway inflammation in acute asthma, leading to a specific neutrophil infiltrate, with elevated levels of IL-8 which correlated with acute disease severity.18 These findings demonstrate that activation of the innate immune response to virus infection in asthma influences airway inflammation and clinical disease severity directly.
This susceptibility of individuals with asthma and COPD to virus infection is an intriguing finding, as despite the fact these individuals have pre-existing airway inflammation and a heightened airway immune response, they appear particularly incapable of efficiently eliminating the effect of viral infection. Given the important role of the early innate immune response to prevent infection and then eliminate it with minimal disruption to the host, a specific abnormality of the innate immune response in these individuals may be present.
Detecting and responding to viral infection
Pathogen recognition receptors
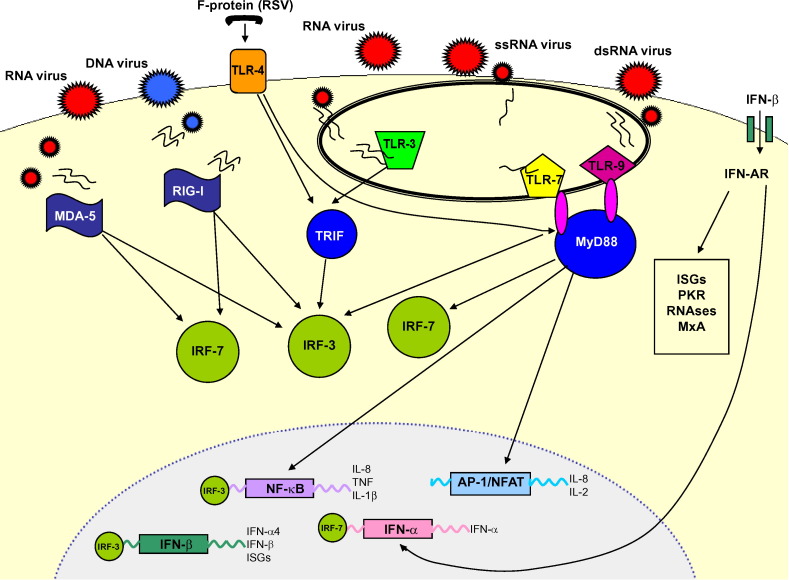
A vital feature of the innate immune response is the ability of host cells to survey their external and internal environment and to recognize foreign organisms. An evolutionally well conserved system of pathogen recognition receptors (PRRs) exists to detect the pathogen-associated molecular patterns (PAMPs) that characterize invading viruses. The best characterized of these PRRs are the family of Toll-like receptors (TLRs) and the RNA helicases, including retinoic-acid-inducible protein I (RIG-I) and melanoma-differentiation-associated gene 5 (MDA-5). Viral interaction with these PRRs is described in Table 1 and Fig. 1 .
Table 1.
Host pathogen recognition receptors (PRRs) and their interaction with respiratory viruses
| PRR | Viral PAMP | Viruses | PRR location | Signalling pathways triggered |
|---|---|---|---|---|
| TLR-4 | F protein | RSV | Cell surface | NF-κB, AP-1 |
| TLR-3 | dsRNA | RNA viruses | Endosome | IRF-3, NF-κB |
| TLR-7 | ssRNA | Influenza A | Endosome | IRF-7 |
| TLR-9 | Unmethylated DNA | Adenovirus | Endosome | IRF-7 |
| RIG-I | dsRNA | RSV and influenza | Cytosol | IRF-3, IRF-7 |
| MDA-5 | dsRNA | Picornaviruses | Cytosol | IRF-3, IRF-7 |
AP, activating protein; dsRNA, double-stranded RNA; IRF, interferon response factor; MDA-5, melanoma-differentiation-associated gene 5; PAMP, pathogen-associated molecular pattern; RIG-I, retinoic-acid-inducible protein I; ssRNA, single-stranded RNA; TLR, Toll-like receptor.
Figure 1.
RNA helicases, retinoic-acid-inducible protein (RIG)-I and melanoma-differentiation-associated gene (MDA)-5 detect viral replication in the cytosol. RIG-I detects negative-sense RNA from DNA viruses and MDA-5 detects positive-sense RNA from RNA viruses. RIG-I and MDA-5 signal through interferon response factor (IRF)-3 and IRF-7 to induce interferon (IFN)-β and NF-κB, leading to the transcription of pro-inflammatory mediators. TLR-3 is expressed in intracellular endosomes and responds to the presence of double-stranded RNA (dsRNA) which forms as a product of the replication of the majority of RNA viruses. TLR-3 is dependent on binding with the Toll/IL-1 receptor domain-containing adaptor (TRIF). This leads to phosphorylation of IRF-3 which forms a homodimer and translocates to the nucleus, resulting in the expression of IFN-β. Signalling can also occur that leads to the translocation of NF-κB. TLR-4 is expressed on the surface of cells and recognizes bacterial endotoxin, lipopolysaccharide and the F protein of respiratory syncytial virus (RSV). TLR-4 is able to signal via both MyD88-dependent and -independent pathways and is able to activate a response via IRF-3, NF-κB and activating protein (AP)-1. TLR-7 is found within endosomes and is activated by single-stranded RNA (ssRNA). TLR-9 is also found within endosomes and is activated by unmethylated CpG dsRNA. Both TLR-7 and -9 signal through a MyD88-dependent pathway, leading to the translocation of NF-κB, AP-1 and IRF-7, and the latter is responsible for release of IFN-α. The IFNs mediate their effects through the induction of hundreds of IFN stimulated genes (ISGs), the actions of many of which remain unknown. Induction of IFN-β and IFN-α4 occurs early after virus infection and is regulated by phosphorylation of IRF-3. The other IFN-α genes require synthesis of IRF-7 to lead to their activation and this occurs as a more delayed response requiring a positive feedback signal via the early release of IFN-β/α4 to lead to the full induction of ISGs. Release of type 1 IFNs can be recognized by infected and neighbouring cells and type 1 IFNs exert their actions through specific receptors (IFN receptor-α1/α2). Receptor engagement leads to activation of the IFN stimulated regulatory factor (ISGF)-3 comprised of members of the signal transducers and activators of transcription (STAT)-1 and -2, as well as IRF-9. This complex can directly bind in the nucleus to the IFN stimulated response element, leading to transcription of type 1 IFNs as well as ISGs, especially the antiviral proteins: protein kinase receptor (PKR), RNAse-I and 2′,5′′′ oligoadenylate synthetase, and myxovirus resistance proteins (MxA).
The TLRs are well expressed in dendritic cells (DCs) and macrophages, but can be found in nearly all human cells. TLR-3 is expressed in intracellular endosomes of bronchial epithelial cells (BECs), macrophages, DCs and CD8+ effector lymphocytes.29, 30, 31, 32, 33 TLR-3 responds to the presence of double-stranded (ds)RNA,34 which forms as a product of the replication of the majority of RNA viruses, including picornaviruses, RSV and influenza A.35 Signalling through TLR-3 is dependent on binding with the Toll/IL-1 receptor (TIR) domain-containing adaptor (TRIF).29 This leads to phosphorylation of the interferon response factor (IRF)-3, which forms a dimer and translocates to the nucleus, resulting in the expression of IFN-β (Fig. 1).36 Signalling can also occur that leads to the translocation of nuclear factor kappa-B (NF-κB) to the nucleus and results in the release of inflammatory cytokines.36 TLR-3 is known to play a crucial role in viral recognition by DCs and their release of antiviral type 1 interferons (IFNs).37
TLR-7/8 are also found within endosomes and their activation by single-stranded (ss)RNA signals through a MyD88-dependent pathway leads to the translocation of NF-κB, activating protein (AP)-1 and IRF-7; the latter is responsible for release of IFN-α (Fig. 1).38 Again, TLR-7 and IRF-7 are strongly expressed by DCs.37
TLR-4 is among the best characterized of the TLRs and, like TLR-2, is expressed on the cell surface. TLR-4 is best known for its ability to recognize bacterial endotoxin or lipopolysaccharide (LPS). TLR-4 has been found to interact with the F protein of RSV, and is able to signal via both MyD88-dependent and -independent pathways and to activate a response via IRF-3, NF-κB and AP-1.38
The RNA helicases, MDA-5 and RIG-I, are present in the cytosol and detect intracellular dsRNA and ssRNA downstream to initiate type 1 IFN and pro-inflammatory cytokine production.38 MDA-5 and RIG-I detect different groups of RNA viruses; MDA-5 detects picornavirus and is critical in regulating type 1 IFN and pro-inflammatory cytokine transcription,39 whilst RIG-I detects negative-sense ssRNA viruses, such as RSV and influenza virus, and appears to be very important in the antiviral response of non-myeloid cells to infection with these viruses.39 Interestingly, RIG-I appears less important in the antiviral response of plasmacytoid DCs.38 Apart from the responsiveness of these helicases, the differences in the structure and mechanism of their action are poorly understood. Binding of viral RNA is expected to occur at the helicase domains, but there are important differences as to how they are activated, e.g. RIG-I responds to the synthetic dsRNA analogue polyI:C, whereas MDA-5 does not, and the uniqueness of these domains and the resulting preferential actions of these RNA helicases remains to be determined.
Type 1 interferons and interferon stimulated genes
IFNs were among the first antiviral agents to be characterized and are still seen to be central in the early antiviral response, inducing in infected cells and their neighbours an antiviral state. In addition, it is now recognized that IFNs are important regulators of innate and adaptive immune responses, as well as regulating cell growth and viability.40
IFNs have been subdivided into groups according to the receptors with which they interact. Type 1 IFNs, which include IFN-α and -β, interfere with viral replication and spread. In BECs, fibroblasts, DCs and macrophages infected with viruses, the type 1 IFNs are transcribed upon signalling from TLRs and RNA helicases, as described above. The only type 2 IFN, IFN-γ, is an important cytokine which characterizes traditional Th-1 adaptive immune responses released by natural killer, CD4 and CD8 T cells.40
The IFNs mediate their effects through the induction of hundreds of IFN stimulated genes (ISGs), the actions of many of which remain unknown. Induction of IFN-β and select IFN-α subtypes (α2, 4, 5 and 6) occurs early after virus infection and is regulated by phosphorylation of IRF-3.41 The other IFN-α genes require synthesis of IRF-7 to be activated and this occurs as a more delayed response requiring a positive feedback signal via the early release of IFN-β/α to lead to the full induction of ISGs.41 Negative regulation of these responses occurs via suppressors of cytokine signalling (SOCS), especially SOCS-1, which is induced by IFN-α and IFN-γ.42
Type 1 IFNs exert their actions through specific receptors (IFN receptor-α1/α2). Receptor engagement leads to activation of the IFN stimulated regulatory factor (ISGF-3) comprised of members of the signal transducers and activators of transcription (STAT)-1 and -2, as well as IRF-9: this complex can directly bind in the nucleus to the IFN stimulated response element, leading to transcription of type 1 IFNs as well as ISGs.43
The effects of many of these ISGs are well characterized. Protein kinase receptor (PKR) is a negative regulator of cell proliferation and inhibits viral replication, though PKR is inhibited by many viruses including influenza.44 Infected cells also generate enzymes RNAse-I and 2′,5‴ oligoadenylate synthetase that degrade viral RNA and prevent replication.44 Myxovirus resistance proteins are induced by IFNs and are important in inhibiting replication of influenza viruses.44 IFNs also upregulate the expression of MHC class I and II on cells and act as a link to induce an adaptive immune response to infection.44 Viruses have also evolved to subvert the IFN response, such as influenza which has non-structural protein (NS)-1. NS-1 inhibits PKR and interacts directly with RIG-I to inhibit downstream IRF-3 translocation.45
Airway cells and their role in innate immune responses to respiratory viruses
Bronchial epithelial cells
BECs line the airways and provide a surface area for conduction, diffusion and exchange of gases. The epithelium provides a mechanical barrier to infection but also contributes as a sentinel for the innate immune response and acts as the trigger for a more specific adaptive immune response. The combined effect of these barriers to infection is to initiate an early effective immune response that minimizes damage and limits infection to the airways. Respiratory viruses, however, have evolved to exploit the BEC which is also their primary site of infection. The airway epithelium has evolved into a complex system of morphologically distinct cells; including ciliated epithelial cells, secretory cells as well as neurons, and migrated immune cells.46
BEC morphology itself appears to be important in determining susceptibility to infection with certain viruses. BECs cultured at the air−liquid interface as differentiated ciliated cells are markedly more resistant to infection with RV compared to the same cells grown in submerged culture as a basal phenotype, and infection results in a more subdued inflammatory response.47 BEC confluence also influences response to infection: RV infection of non-confluent cells leads to cytolysis, not seen with an intact monolayer.48 This suggests that a disrupted or repairing epithelium may be more susceptible to virus infection and its effect, a situation that has been proposed to occur in asthma.
The airway mucociliary escalator also provides a system to remove potential pathogens from the lower respiratory tract and an environment unfavourable for their exploitation. A specific antiviral effect of mucus has not so far been demonstrated, but within this layer BECs secrete antimicrobial peptides such as the β-defensins. These cationic peptides have a well characterized antibacterial role but also are active against enveloped viruses.49 Investigators have also shown that RV infection of cultured BECs induces the expression of human β2-defensin and that elevated levels are also seen in nasal lavage following experimental RV infection.50 However, this study also found that β2-defensin did not have an inhibiting effect on RV directly, though it is known to be chemotactic for DCs and T cells.51
The volatile gas nitric oxide (NO) is found in exhaled breath and elevated levels have been associated with asthma52 and viral URTIs,53 with the BEC implicated as the source of airway NO.54 NO has the ability directly to inhibit the growth of a number of viruses.55 In BEC culture models, NO has also been shown to reduce RV replication and reduce the release of IL-6, IL-8 and granulocyte-macrophage colony stimulating factor (GMCSF).56, 57 In addition, RV infection of BECs was shown to activate NO synthetases: this reduces the BEC’s ability to buffer acid which lowers airway pH and reduces RV replication.58 Unfortunately a lower airway pH has also been shown to worsen asthma.59
The immediate antiviral response of asthmatic BECs to infection with RV has also been shown to be impaired.60 In BECs cultured from healthy non-atopic individuals, infection with RV induces a robust release of IFN-β and this induces apoptosis or programmed cell death in the infected cell, effectively shutting it down and impairing viral replication. Asthmatic BECs respond to RV infection with a reduced IFN-β response and there is limited early apoptosis, but a marked increase in late cytolysis, resulting in enhanced viral replication. This indicates a specific defect in asthma BECs to recognize and respond to RV effectively.
Dendritic cells and alveolar macrophages
DCs are a complex immune cell with immediate innate responses and a sentinel responsibility in the adaptive immunity. Myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) are derived from progenitor cells produced by the bone marrow and mature into conventional DCs (cDCs). The mDCs and pDCs are phenotypically and functionally different, with pDCs migrating through small blood vessels, surveying for microbial presence,61 whilst mDCs migrate though the lymphatics and into lymph nodes where they act as antigen-presenting cells (APCs).62
Plasmacytoid DCs are prominent innate responders, strongly expressing TLR-7 and -9,63, 64 and are capable of quickly producing type 1 IFNs at levels 1000 times greater than any other leucocyte, and for this reason they are thought to have the potential greatly to influence the initial innate immune response to viral infection.65 These immature DCs also appear to have a high degree of plasticity. When pDCs harvested from the bone marrow of LMCV-infected mice were cultured for 4 days there was a transformation of pDCs into mDCs. This was dependent on viral replication and was seen by a downregulation of pDC markers and the development of the mDC phenotype; an increase in cell size and granularity, and myeloid marker expression.62 This transformed population of mDCs also switched functional states, achieving a heightened antigen-presenting capacity, an enhanced ability to stimulate naïve T cells and the ability to signal through TLR-4.62
In an important recent study using a knock-in mouse that was made to express green fluorescence protein (GFP) under the control of IFN-α6, the in vivo response to systemic and intranasal viral infection was assessed.66 Intranasal infection with Newcastle disease virus (NDV) led to GFP expression in alveolar macrophages (AMs) and cDCs as the first line of antiviral defence. This initial activation was mediated by RNA helicases with AM localized to the lungs and cDCs migrating to regional lymph nodes and producing IFNs. However, when AMs were depleted there was a marked defect in viral elimination evident and an IFN response. It was then seen that pDCs became GFP positive and localized to the lung interstitium, producing a delayed yet quantitatively similar IFN response via TLR-3 activation. Infection was then repeated with Sendai virus which has been used to model respiratory virus infection in mice and, like influenza virus, inhibits RNA helicases. A similar response was again seen with the activation of the alternate pDC response.
Both DCs and AMs occupy a unique position as cells of the innate immune response, reacting to viruses via PRR, and as potent releasers of type 1 IFNs. They also play a pivotal role as APCs in activating T lymphocytes and the recruitment of the more specific adaptive immune response.
Cytotoxic T lymphocytes
T lymphocytes influx to areas of acute inflammation surrounding viral infected cells where specific CD8+ lymphocytes mount an attack on targeted cells. Cytotoxic lymphocytes (CTL) are classically part of the adaptive immunity. This requires the development of a cohort of CD8 cells that recognize specific viral epitopes and infected cells, and selectively target them for elimination. Whilst this is a highly effective and specific system, it requires time to mount an effective adaptive response. Recent evidence now suggests that proliferating memory CD8+ lymphocytes activated for other reasons can provide an early source of IFN-γ acting in a non-specific bystander role.67, 68 This early source of IFN-γ comes from CD44highCD8+ lymphocytes through mechanisms which are independent of the T-cell receptor (TCR) and cannot be generated by naïve T cells.69, 70 It was shown that CD8 effector cells from mice could be activated by LPS via TLR-4 and by PolyI:C via TLR-3, leading to the release of IFN-γ without a specific TCR-mediated response.70 Evidence suggests this innate activation is highly influenced by cytokines secreted by APCs; DCs, macrophages and epithelial cells. When these cells release IL-12, IL-18 and IFN-α/β, an antiviral response is stimulated, whilst IL-15 assists in the development of memory phenotype of CD8+ lymphocytes.69, 70, 71, 72, 73 In fact, IL-15 enhances IL-12 and IL-18’s ability to induce IFN-γ and increases the sensitivity of CD8+ lymphocytes to stimulation with pro-inflammatory cytokines.73
This non-specific activation of CD8 also appears to be important in vivo. In a mouse deficient in IFN-γ, infection with Listeria monocytogenes results in significantly heightened mortality; however, if the mouse is sensitized to ovalbumin prior to infection, it can respond with an adequate IFN-γ response and this results in reduced organ bacterial counts.73 Therefore, bystander activated CD8 memory cells may not be redundant inflammatory cells when confronted by an unrecognized virus, but may provide important acute antiviral support as part of the innate immune response.
The other crucial effect that CD8 T lymphocytes have on the innate immune response is their ability to suppress tissue damage that may result as a consequence of an overzealous innate immune responses during acute infection.74 Mice without functional CD4+ and CD8+ lymphocytes (nude mice and Rag-1 knock-out mice) when infected with a sub-lethal dose of the RNA virus (MHV-A59) or exposed to PolyI:C died within 12–24 h post infection and suffered due to a cytokine storm with marked release of TNF-α.74 This effect was reversed when T cells activated by ovalbumin were replaced and they were found also to limit the inflammation associated with exposure to LPS and PolyI:C. Thus, T lymphocytes are both negative regulators of early innate responses to infection and non-specific IFN-γ releasing cells, and without these roles the adaptive components of immunity would never be able to be performed.
Conclusions
The human innate immune system has developed in parallel with a large number of viruses that attempt to subvert it and that are responsible for disease. The fact that most of these viruses are efficiently eliminated with minimal airway inflammation and damage reflects the effectiveness of a system that can quickly recognize invading viruses and initiate an appropriate response. The detection by PRR initiates an effective antiviral response characterized by type 1 interferons. This response begins with the epithelium acting both as a barrier and sentinel for the innate response and interacts with both macrophages and DCs. This response in itself can eliminate infection, but also leads to the recruitment of an effective adaptive immune response, whilst the presence of adaptive T cells acts as an important safety valve to limit inflammation associated with the innate response.
Important questions remain to be addressed, especially how viruses such as influenza can subvert this system. It is also unclear how chronic inflammatory airways diseases appear to impair the efficiency of this innate response and how this response appears to worsen these conditions both acutely and even in the long term.
Educational aims
-
•
To review the importance of respiratory viral infection in adults and children in healthy individuals, and to contrast this with subjects with asthma and other chronic inflammatory airways disease.
-
•
To describe the role of pathogen recognition receptors in detecting viral infection and how this initiates an antiviral and inflammatory immune response.
-
•
To assess the central role of type 1 interferons in the innate immune response to viral infection.
-
•
To determine the important role in the innate immune response of cells within the airways, especially the airway epithelium: macrophages, dendritic cells and CD8 lymphocytes.
References
- 1.Heikkinen T., Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monto A.S., Sullivan K.M. Acute respiratory illness in the community: frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elnifro E.M., Ashshi A.M., Cooper R.J., Klapper P.E. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 2000;13:559–570. doi: 10.1128/cmr.13.4.559-570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manoha C., Espinosa S., Aho S., Huet F., Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221–226. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Coscoy L., Zylberberg M. Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci U S A. 2002;99:15687–15692. doi: 10.1073/pnas.242579699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kistler A., Avila P.C., Rouskin S. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monto A.S. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winther B., Farr B., Turner R., Hendley J.O., Gwaltney J.M., Mygind N. Histopathological examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Oto Layrngol. 1984;413(Suppl):19–24. doi: 10.3109/00016488409128537. [DOI] [PubMed] [Google Scholar]
- 9.Turner R.B. The role of neutrophils in the pathogenesis of rhinovirus infections. Pediatr Infect Dis J. 1990;9:832–835. doi: 10.1097/00006454-199011000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Henderson F.W., Clyde W.A., Collier A.M. The etiologic and epidemeologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979;95:183–186. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- 11.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 12.Hacking D., Hull J. Respiratory syncytial virus − viral biology and the host response. J Infect. 2002;45:18–24. doi: 10.1053/jinf.2002.1015. [DOI] [PubMed] [Google Scholar]
- 13.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 14.Treanor J.J. Influenza A and B viruses. In: Lenfant C., editor. Viral Infections of the Respiratory Tract. Marcel Dekker; New York: 1999. pp. 105–160. [Google Scholar]
- 15.Johnston S.L., Pattemore P.K., Sanderson G. Community study of the role of viral infections in exacerbations of asthma in 9–11year old children. BMJ. 1995;310:1225–1228. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gern J.E. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112:19S–27S. doi: 10.1016/s0002-9343(01)01060-9. [DOI] [PubMed] [Google Scholar]
- 17.Grissell T.V., Powell H., Shaffren D.R. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 18.Wark P.A.B., Johnston S.L., Moric I., Simpson J.L., Hensley M.J., Gibson P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 19.Seemungal T., Harper-Owen R., Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 20.Schroth M.K., Grimm E., Frindt P. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 21.Subauste M.C., Jacoby D.B., Richards S.M., Proud D. Infection of human respiratory epithelial cell line with rhinovirus: induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staunton D.E., Merluzzi V.J., Rothlein R., Barton R., Marlin S.D., Springer T.A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 23.Gern J.E., Vrtis R., Grindle K.A., Swenson C.A., Busse W. Relationship of upper and lower airway cytokines to outcomes of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 24.Grunberg K., Sharon R.F., Sont J.K. Rhinovirus-induced airway inflammation in asthma. Effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med. 2001;164:1816–1822. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 25.Gern J.E., Galagan D.M., Jarjour N.N., Dick E.C., Busse W.W. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 26.Grunberg K., Timmers M.C., de Klerk E.P.A., Sterk P.J. Experimental rhinovirus 16 infection causes variable airflow obstruction in subjects with atopic asthma. Am J Respir Crit Care Med. 1999;160:1375–1380. doi: 10.1164/ajrccm.160.4.9810083. [DOI] [PubMed] [Google Scholar]
- 27.Corne J.M., Marshall C., Smith S. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 28.Khetsuriani N., Kazerouni N., Erdman D.D. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M., Sato S., Hemmi H. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 30.Edwards M.R., Slater L., Johnston S.L. Signalling pathways mediating type I interferon gene expression. Microbes Infect. 2007;9:1245–1251. doi: 10.1016/j.micinf.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Tabiasco J., Devevre E., Rufer N. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 32.Lore K., Betts M.R., Brenchley J.M. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 33.Sha Q., Truong-Tran A.Q., Plitt J.R., Beck L.A., Schleimer R.P. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 35.Bowie A.G., Haga I.R. The role of toll like receptors in the host response to viruses. Mol Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T., Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 37.Akira S., Uematsu S., Takeuchi K. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Thompson A.J.V., Locarnini S.A. Toll-like receptors. RIG-I-like helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;10:1–11. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- 39.Kato H., Takeuchi O., Sato S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 40.Katze M.G., He Y., Gale M., Jr., Viruses interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 41.Sato M., Suemori H., Hata N. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 42.Song M., Shuai K. The suppressor of cytokine signalling (SOCS) 1 and SOCS3 but not SOCS 2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 43.Darnell J.E., Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signalling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 44.Katze M., He Y., Gale M. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 45.Mibayashi M., Martinez-Sobrido L., Loo Y.-M., Cardenas W.B., Gale M., Jr., Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol. 2007;81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight D.A., Holgate S.T. The airway epithelium: Structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Souza N., Dolganov G., Dubin R. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004;286:L373–L381. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 48.Bossios A., Psarras S., Gourgiotis D. Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir Res. 2005;6:114–120. doi: 10.1186/1465-9921-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;7:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 50.Proud D., Sanders S.P., Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human (beta)-defensin 2 both in vitro and in vivo. J Immunol. 2004;172:4637–4645. doi: 10.4049/jimmunol.172.7.4637. [DOI] [PubMed] [Google Scholar]
- 51.Yang D., Chertov O., Bykovskaia S.N. B-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 52.Kharitonov S., Yates D.H., Robbins R.A., Logan-Sinclair R., Shinebourne E.A., Barnes P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 53.Kharitonov S., Yates D.H., Barnes P.J. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J. 1995;8:295–297. doi: 10.1183/09031936.95.08020295. [DOI] [PubMed] [Google Scholar]
- 54.Lane C., Knight D.A., Burgess S. Epithelial inducible nitric oxide synthetase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;196:340–344. doi: 10.1136/thx.2003.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiss C.S., Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders S.P., Siekierski E.S., Porter J.D., Richards S.M., Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanders S.P., Kim J., Connolly K.R., Porter J.D., Sierkierski E.S., Proud D. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony-stimulating factor production in bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;24:317–325. doi: 10.1165/ajrcmb.24.3.4131. [DOI] [PubMed] [Google Scholar]
- 58.Carraro S., Doherty J., Zaman K. S-nitrosothiols regulate cell-surface pH buffering by airway epithelial cells during the human immune response to rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2006;290:L827–832. doi: 10.1152/ajplung.00406.2005. [DOI] [PubMed] [Google Scholar]
- 59.Hunt J.F., Gaston B., Ricciardolo F. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113:610–619. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 60.Wark P.A.B., Johnston S.L., Bucchieri F. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y.J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 62.Zuniga E.I., McGavern D.B., Pruneda-Paz J.L., Teng C., Oldstone M.B. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kadowaki N., Ho S., Antonenko S. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarrossay D., Napolitani G., Colonna M., Sallusto F., Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 65.Siegal F.P., Kadowaki N., Shodell M. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 66.Kumagai Y., Takeuchi O., Kato H. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Tough D.F., Borrow P., Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 68.Berg R.E., Cordes C.J., Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 70.Kambayashi T., Assarsson E., Lukacher A.E., Ljunggren H.G., Jensen P.E. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol. 2003;170:2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- 71.Tough D.F., Sprent J. Bystander stimulation of T cells in vivo by cytokines. Vet Immunol Immunopathol. 1998;63:123–129. doi: 10.1016/s0165-2427(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 72.Yoon H., Legge K.L., Sung S.S., Braciale T.J. Sequential activation of CD8+ T cells in the draining lymph nodes in response to pulmonary virus infection. J Immunol. 2007;179:391–399. doi: 10.4049/jimmunol.179.1.391. [DOI] [PubMed] [Google Scholar]
- 73.Smeltz R.B. Profound enhancement of the IL-12/IL-18 pathway of IFN-gamma secretion in human CD8+ memory T cell subsets via IL-15. J Immunol. 2007;178:4786–4792. doi: 10.4049/jimmunol.178.8.4786. [DOI] [PubMed] [Google Scholar]
- 74.Dong Kim K., Zhao J., Auh S. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]