Graphical abstract
Keywords: Aminopeptidase N, APN, CD13, Probestin, Tumor angiogenesis
Abstract
Probestin is a potent aminopeptidase N (APN) inhibitor originally isolated from the bacterial culture broth. Here, we report probestin synthesis by solid phase peptide synthesis (SPPS) method and evaluated its activity to inhibit angiogenesis using a chicken embryo chorioallantoic membrane (CAM) assay and a CAM tumor xenograft model. Results from these studies demonstrate that probestin inhibits the angiogenic activity and tumor growth.
Aminopeptidase N (APN, also known as aminopeptidase M, alanine aminopeptidase, CD13, EC 3.4.11.2) is a zinc-dependent membrane-bound exopeptidase that catalyzes the removal of N-terminal amino acids from peptides.1, 2 In addition to peptide metabolism, it plays an important roles in many physiological processes, including cell motility and adhesion, and serves as a receptor for various mammalian coronaviruses.1, 2, 3, 4, 5 APN is predominantly expressed in monocytic cells and epithelial cells of the liver, intestine, and kidney.1, 2, 6 In addition, high levels of APN expression have been detected in various solid tumors.7, 8, 9, 10, 11, 12 Several studies have shown the important role of APN in angiogenesis, tumor cell invasion, and metastasis.5, 10, 13, 14, 15, 16, 17, 18, 19, 20 These reports indicate that APN plays a critical role in angiogenesis. Thus, APN is considered an important therapeutic target for tumor angiogenesis and metastasis.
Probestin is a potent APN inhibitor (K i value of 19 nM) originally isolated from the culture broth of Streptomyces azureus MH663-2F6 two decades ago by Aoyagi et al.21, 22, 23 Later two methods were developed for the chemical synthesis of probestin from α-keto amide and α,β-epoxy ester precursors.24, 25, 26 In the present study, we investigated solid phase peptide synthesis (SPPS) method to synthesize probestin using commercially available bestatin (Acros Organics). The –NH2 group of bestatin was protected by N-tert-butoxycarbonyl (Boc) group using previously reported procedure.27 Since probestin is not commercially available, the SPPS method would allow synthesizing it in a short time in milligram quantities required for preclinical in vitro and in vivo studies. It is important to note that probestin is the most potent APN inhibitor based on a natural product.21, 28 Additionally, we investigated the effect of probestin as an angiogenesis inhibitor using a chicken embryo chorioallantoic membrane (CAM) assay and a CAM breast tumor xenograft model. To our best knowledge, this is the first report to investigate the anti-angiogenesis effects of probestin.
We synthesized probestin by the SPPS method (Fig. 1 a) manually in 0.1 mmol scale using our previously reported conditions.29, 30 We selected 2-chlorotrityl chloride (2-Cl Trt) resin after failing to get a product by using Wang resin. The reason was that the synthesis of a peptide containing a proline residue or its derivative at the first or second position from the C-terminal using Wang resin is prone to undergo diketopiperazine (DKP) formation during the Fmoc deprotection step of the second residue.31 In our case, a cyclic diproline (a DKP) shown in Figure 1b is expected to be formed during the Fmoc deprotection of the second proline residue by 20% piperidine. Thus, we have chosen the sterically hindered 2-Cl Trt resin. The use of sterically bulky chlorotrityl group has been shown to prevent the cyclization and the premature peptide cleavage due to formation of DKP.31 The probestin was obtained in an overall yield of 31–33% (three batches) after HPLC purification. The HPLC purification was carried out using our previously reported conditions.30 Mass spectral analyses was consistent with the calculated value (ES-MS calcd m/z for C26H38N4O6: 502.3; found: 503.4 ([M+H]+) and ESI HRMS calculated: 501.2713 ([M−H]−); found: 501.2736). The 1H NMR and IR spectroscopy data is consistent with the reported values.23, 25, 26
Figure 1.
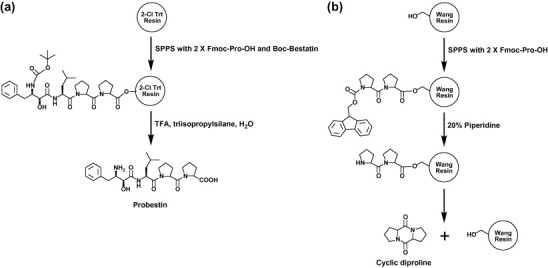
(a) Solid phase synthesis of probestin and (b) diketopiperizine formation during probestin synthesis using Wang resin.
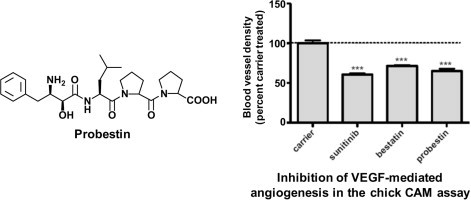
To assess whether probestin was able to exert an anti-angiogenic activity in vivo, we tested probestin in the chicken embryo CAM assay using vascular endothelial growth factor (VEGF) as an angiogenic stimulus.32 In this assay, 10 days old chicken embryos were used to induce angiogenesis in the CAM using filter discs soaked with VEGF. About 8–10 separate embryos were used for each compound. After 8 h, either PBS or sunitinib (25 μM), bestatin (10 μM), or probestin (10 μM) were applied topically to the surface of the filter discs. We included sunitinib as a reference to compare the antiangiogenic activity of probestin since sunitinib is a well-known FDA-approved angiogenesis inhibitor. Sunitinib is a standard VEGF receptor 2 (VEGFR-2)/PDGFR receptor β (PDGFRβ) kinase inhibitor. The VEGFR-2 is an important receptor in VEGF-induced mitogenesis.33 Similarly bestatin was also included for comparison as it is a well known APN inhibitor and it is an approved anticancer drug (Ubenimex) in Japan.34 After 48 h, the CAMs were removed, fixed and the representative areas were photographed (Fig. 2 a). The blood vessel branch points present within the area defined by the filter disc were counted in a blinded fashion using a high power stereo microscope. As shown in Figure 2b, probestin was effective in blocking VEGF-induced angiogenesis similar to sunitinib. Interestingly, probestin had very little effect on preexisting blood vessels of the CAM, indicating that the compound exerts a specific action on proliferating microvessels (Fig. 2a).
Figure 2.
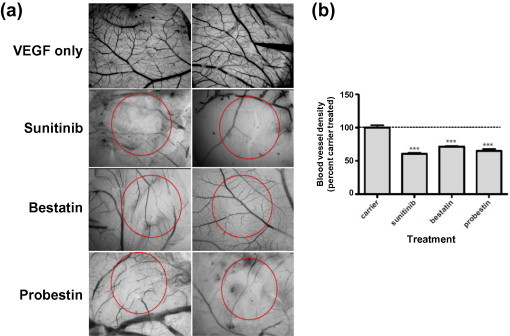
Inhibition of VEGF-mediated angiogenesis in the chicken embryo CAM assay by sunitinib, bestatin, and probestin. (a) Chicken embryo CAMs 10 days of age were exposed to filter discs soaked with VEGF (200 ng/embryo). After 24 h, either PBS or sunitinib (25 μM), bestatin (10 μM), or probestin (10 μM) were applied topically to the surface of the filter discs. After 48 h, CAMs were dissected out and the representative areas were photographed and (b) the blood vessel branch points present within the area defined by the filter disc were counted in a blinded fashion using a high-power stereo microscope. Results were analyzed by Graph Pad Prism 5.0 software. ∗P <0.05, ∗∗P <0.01 and ∗∗∗P <0.001 by one-way ANOVA with Neuman–Keuls post-test. Sample size = 8–10 separate CAMs.
Next we examined whether probestin would affect tumor growth and vascularity in a chicken embryo CAM human tumor xenograft model.35 For the CAM xenograft model, 500,000 human breast carcinoma MDA-MB-435 cells were implanted under the vascularized CAM of 10 days old chicken embryos and then the embryos were treated systemically with 20 mg/kg probestin, bestatin, or sunitinib on days one and two; and CAMs were fixed, excised, and imaged. It was found that probestin reduced both the vascularity and the size of resulting tumors significantly more than bestatin at an equivalent concentration (Fig. 3 ). It is important to note that MDA-MB-435 cells are APN negative,20 the tumor growth inhibition by probestin thus appeared to be through a direct targeting of APN expressed on the tumor angiogenesis. These results are consistent with those previously observed growth inhibition of mouse xenografts derived from MDA-MB-435 cells by two rat antimouse APN antibodies.20 The effect of sunitinib on the reduction of the blood vessel density and the tumor volume was significantly more than both bestatin and probestin. The reasons for this lower effect exhibited by probestin or bestatin compared to sunitinib are currently unknown. It is important to note that probestin/bestatin and sunitinib binds to different targets and the expression levels of those targets could be different. However, these preliminary results indicate the antitumor and anti-angiogenic activity of probestin and warrants further investigation of probestin as an angiogenesis inhibitor.
Figure 3.
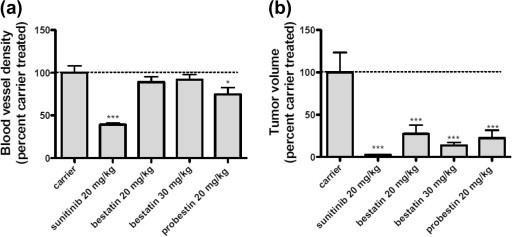
Inhibition of (a) tumor blood vessels and (b) tumor volume in the chicken embryo CAM human tumor MDA-MB-435 xenograft by sunitinib, bestatin, and probestin. Results were analyzed by Graph Pad Prism 5.0 software. ∗P <0.05, ∗∗P <0.01 and ∗∗∗P <0.001 by one-way ANOVA with Neuman–Keuls post-test. Sample size = 8–10 separate CAMs.
To determine the maximal tolerated dose (MTD) of probestin, groups of four 10 weeks old healthy female BalbC mice were intraperitoneally injected with 50, 100, 200, and 300 mg/kg of probestin three times weekly. Weights were taken over a 6 week period and animals were observed for acute distress during the first 96 h after injection. Our main criteria for MTD was significant (P <0.05, one-way ANOVA with Neuman–Keuls post test) weight loss over the 6 week period. No apparent toxicity and no significant weight loss (P >0.05; one-way ANOVA with Neuman–Keuls post-test) was observed throughout a 6 week period after drug administration for 50, 100, and 200 mg/kg dosage groups. However, the animals displayed signs of weight loss for 300 mg/kg dosage group and thus this study indicates 300 mg/kg as the MTD. Previously probestin isolated from the bacterial culture broth displayed a LD50 value >250 mg/kg when intravenously administered in mice.22
In conclusion, probestin can be conveniently synthesized by SPPS method. Furthermore, our data demonstrate that probestin inhibits the angiogenic activity and support the idea that probestin could be potentially evaluated as a therapeutic drug for prevention and treatment of cancers and other angiogenesis-related diseases.
Acknowledgment
This work was funded by the University of Oklahoma College of Pharmacy Startup Grant.
References and notes
- 1.Look A.T., Ashmun R.A., Shapiro L.H., Peiper S.C. J. Clin. Invest. 1989;83:1299. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjostrom H., Noren O., Olsen J. Adv. Exp. Med. Biol. 2000;477:25. doi: 10.1007/0-306-46826-3_2. [DOI] [PubMed] [Google Scholar]
- 3.Mina-Osorio P., Winnicka B., O’Conor C., Grant C.L., Vogel L.K., Rodriguez-Pinto D., Holmes K.V., Ortega E., Shapiro L.H. J. Leukocyte Biol. 2008;84:448. doi: 10.1189/jlb.1107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Nature. 1992;357:420. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashida H., Takabayashi A., Kanai M., Adachi M., Kondo K., Kohno N., Yamaoka Y., Miyake M. Gastroenterology. 2002;122:376. doi: 10.1053/gast.2002.31095. [DOI] [PubMed] [Google Scholar]
- 6.Lendeckel U., Arndt M., Frank K., Wex T., Ansorge S. Int. J. Mol. Med. 1999;4:17. [PubMed] [Google Scholar]
- 7.Dixon J., Kaklamanis L., Turley H., Hickson I.D., Leek R.D., Harris A.L., Gatter K.C. J. Clin. Pathol. 1994;47:43. doi: 10.1136/jcp.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tokuhara T., Hattori N., Ishida H., Hirai T., Higashiyama M., Kodama K., Miyake M. Clin. Cancer Res. 2006;12:3971. doi: 10.1158/1078-0432.CCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 9.Hashida H., Takabayashi A., Kanai M., Adachi M., Kondo K., Kohno N., Yamaoka Y., Miyake M. Gastroenterology. 2002;122:376. doi: 10.1053/gast.2002.31095. [DOI] [PubMed] [Google Scholar]
- 10.Ishii K., Usui S., Sugimura Y., Yoshida S., Hioki T., Tatematsu M., Yamamoto H., Hirano K. Int. J. Cancer. 2001;92:49. [PubMed] [Google Scholar]
- 11.Wickström M., Larsson R., Nygren P., Gullbo J. Cancer Sci. 2011;102:501. doi: 10.1111/j.1349-7006.2010.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menrad A., Speicher D., Wacker J., Herlyn M. Cancer Res. 1993;53:1450. [PubMed] [Google Scholar]
- 13.Bhagwat S.V., Lahdenranta J., Giordano R., Arap W., Pasqualini R., Shapiro L.H. Blood. 2001;97:652. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagwat S.V., Petrovic N., Okamoto Y., Shapiro L.H. Blood. 2003;101:1818. doi: 10.1182/blood-2002-05-1422. [DOI] [PubMed] [Google Scholar]
- 15.Guzman-Rojas L., Rangel R., Salameh A., Edwards J.K., Dondossola E., Kim Y.G., Saghatelian A., Giordano R.J., Kolonin M.G., Staquicini F.I., Koivunen E., Sidman R.L., Arap W., Pasqualini R. Proc. Natl. Acad. Sci. U.S.A. 2012;109:1637. doi: 10.1073/pnas.1120790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukasawa K., Fujii H., Saitoh Y., Koizumi K., Aozuka Y., Sekine K., Yamada M., Saiki I., Nishikawa K. Cancer Lett. 2006;243:135. doi: 10.1016/j.canlet.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 17.Fujii H., Nakajima M., Saiki I., Yoneda J., Azuma I., Tsuruo T. Clin. Exp. Metastasis. 1995;13:337. doi: 10.1007/BF00121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saiki I., Yoneda J., Azuma I., Fujii H., Abe F., Nakajima M., Tsuruo T. Int. J. Cancer. 1993;54:137. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovic, N.; Schacke, W.; Shapiro, L. H. In Aminopeptidases in Biology and Disease; Hooper, N. M., Lendeckel, U., Eds.; Kluwer Academic/Plenum Publishers; New York, 2004; Vol. 2, p. 179.
- 20.Pasqualini R., Koivunen E., Kain R., Lahdenranta J., Sakamoto M., Stryhn A., Ashmun R.A., Shapiro L.H., Arap W., Ruoslahti E. Cancer Res. 2000;60:722. [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyagi T., Yoshida S., Nakamura Y., Shigihara Y., Hamada M., Takeuchi T. J. Antibiot. (Tokyo) 1990;43:143. doi: 10.7164/antibiotics.43.143. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi, T.; Aoyagi, T.; Hamada, M.; Naganawa, H.; Yoshida, S. 5,021,549, 1991. [DOI] [PubMed]
- 23.Yoshida S., Nakamura Y., Naganawa H., Aoyagi T., Takeuchi T. J. Antibiot. (Tokyo) 1990;43:149. doi: 10.7164/antibiotics.43.149. [DOI] [PubMed] [Google Scholar]
- 24.Wasserman H.H., Xia M.D., Petersen A.K., Jorgensen M.R., Curtis E.A. Tetrahedron Lett. 1999;40:6163. [Google Scholar]
- 25.Righi G., D’Achille C., Pescatore G., Bonini C. Tetrahedron Lett. 2003;44:6999. [Google Scholar]
- 26.Wasserman H.H., Petersen A.K., Xia M.D. Tetrahedron. 2003;59:6771. [Google Scholar]
- 27.Luan Y.P., Wang X.J., Zhu H.W., Qu X.J., Fang H., Xu W.F. Lett. Drug Des. Discov. 2009;6:420. [Google Scholar]
- 28.Bauvois B., Dauzonne D. Med. Res. Rev. 2006;26:88. doi: 10.1002/med.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathuri G., Hedrick A.F., Disch B.C., Doan J.T., Ihnat M.A., Awasthi V., Gali H. Bioconjugate Chem. 2012;23:115. doi: 10.1021/bc200546b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathuri G., Hedrick A.F., Disch B.C., Ihnat M.A., Awasthi V., Gali H. Bioorg. Med. Chem. Lett. 2012;22:4567. doi: 10.1016/j.bmcl.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 31.Stewart, A. S. J. U.S. Patent 7,645,858, 2010.
- 32.Marks M.G., Shi J., Fry M.O., Xiao Z., Trzyna M., Pokala V., Ihnat M.A., Li P.K. Biol. Pharm. Bull. 2002;25:597. doi: 10.1248/bpb.25.597. [DOI] [PubMed] [Google Scholar]
- 33.Holmes K., Roberts O.L., Thomas A.M., Cross M.J. Cell. Signal. 2007;19:2003. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Ota K. Biomed. Pharmacother. 1991;45:55. doi: 10.1016/0753-3322(91)90123-b. [DOI] [PubMed] [Google Scholar]
- 35.Lin L., Hutzen B., Zuo M., Ball S., Deangelis S., Foust E., Pandit B., Ihnat M.A., Shenoy S.S., Kulp S., Li P.K., Li C., Fuchs J., Lin J. Cancer Res. 2010;70:2445. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]


