Abstract
Over a period of almost two years, broilers chickens on several hundred Italian farms, were monitored for infectious bronchitis virus. Detections were genotyped using a hypervariable region of the gene coding for the S1 segment of the spike protein. A range of genotypes were detected which comprised QX, Q1, Mass, D274 and 793B. Sequences of 793B viruses detected in chickens, vaccinated with either of the two commonly used 793B type vaccines were almost identical to sequences of one or other of these vaccines. This strong indication of vaccine association led to the withdrawal of live 793B vaccine use on all of the farms of the study. Except for one sample collected soon after 793B vaccination ceased, it was no longer possible to detect 793B vaccine on these farms. It appears that field 793B strains have disappeared from the region of Italy tested thus obviating any need for current vaccine protection against 793B.
Keywords: Infectious bronchitis virus, Broiler farm survey, S1 sequence, 793B vaccination discontinued, 793B genotype disappeared
1. Introduction
Infectious bronchitis virus (IBV) is highly contagious and causes serious economic losses for the worldwide chicken industry. It replicates in the respiratory, urogenital and gastroenteric tracts and can cause lesions in these and other regions. As for other coronaviruses, IBV evolves via high mutation and recombination rates [1], [2], [3], [4]. Infection with one IBV protectotype generally only confers protection again that same protectotype. Other IBVs breaking through that protection are considered to have a different protectotype. Sequencing of the IBV RNA genome has shown these protectotypes roughly correlate to sequence differences between their spike surface proteins, in particular hyper-variable regions of S1 which have also been implicated in virus attachment and neutralization epitopes [5], [6], [7]. Numerous genotypes have been detected, some of which have quickly disappeared while others have caused major worldwide disease with economic relevance [8], [9], [10].
Vaccination is generally considered essential for the protection of commercial chickens [11]. The limited cross-protection between genotypes poses a great challenge in the control of this disease, so knowledge of field genotypes can be invaluable in any effective vaccination strategy. However, the presence of live vaccines confuses most such epidemiological studies due to the impossibility of differentiating vaccine from field strains. Our study aimed to investigate the circulation in broilers of different genotypes before and after the discontinuing of live 793B-based vaccination.
2. Materials and methods
2.1. Samples
Between November 2012 and June 2014 on 513 farms located mainly in Northern Italy, pools of tracheal swabs were collected from broilers in response to clinical episodes thought potentially attributable to IBV. This was part of systematic diagnostic activity.
On the day of collection, IBV real time RT-PCR was performed on tracheal swabs.
At the time of sampling, age, clinical status and geographical location of chickens were recorded. All were vaccinated with live H120 vaccine at the hatchery and, until late spring 2013, a live 793B booster (A and B) via drinking water. The 793B vaccination was introduced in Italy in the late '90 after the detection of this genotype in the field.
2.2. IBV detection by real time RT-PCR
Pools of ten tracheal swabs from the same flock were vortexed in 1 ml of PBS. The solution was used for RNA extraction using NucleoSpin® 8/96 RNA (Macherey-Nagel, Düren, Germany). Samples were tested for IBV using a real time RT-PCR commercial kit (Quantification of Avian Infectious Bronchitis Virus-IBV-kit; Genesig, Southampton, UK) following manufacturer instruction.
2.3. Sequencing of partial S1 gene
RT-PCRs covering a hypervariable region of the S1 gene were carried out on all real time RT-PCR positive samples using primer pairs SX3+ (5′-TAATACTG GC/T AATTTTTCAGA-3′) and SX2− (5′-TCCACCTCTATAAACACC C/T TT-3′). Nested PCRs were performed where results were weak or negative [12]. Amplicons were sequenced in both directions with the same primers used for PCR. Chromatograms were evaluated with FinchTV (http://www.geospiza.com) and consensus sequences were obtained using CromasPro (CromasPro Version 1.5)
2.4. IBV strain genotyping
IBV strains were initially genotyped at first performing a BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Samples were more rigorously genotyped by comparison with a collection of reference sequences. For 793B the full range of database available S1 sequences were used. Briefly sequences were aligned using the MUSCLE method implemented in MEGA6 [13] and phylogenetic analysis was performed using the same software. To clarify any relationship to 793/B vaccines, the number of mismatches between samples and 793B vaccine in the sequenced region were calculated.
3. Results
3.1. IBV genotypes and distribution
One hundred and twenty-nine samples were positive to IBV by real time RT-PCR. Partial S1 gene sequences were obtained from 100 IBV strains.
Five genotypes were identified. The genotype with the highest prevalence was QX (72%), followed by 793/B (16%), Mass (8%), Q1 (3%) and D274 (1%). Genotype distribution from November 2012 is given in Fig. 1 where it can be seen that QX, Mass, D274 and Q1 were detected throughout, whereas 793/B was no longer detected after July 2013. 793B viruses were numbered according to chronological detection and classified as N (north) or C (central) according to the region where viruses were detected. Two 793/B clusters were identified, with the larger of 14 sequences being related to vaccine A and the smaller being related to vaccine B (Fig. 2 ).
Fig. 1.

Distribution over time of detected genotypes in Italian broiler farms.
Fig. 2.
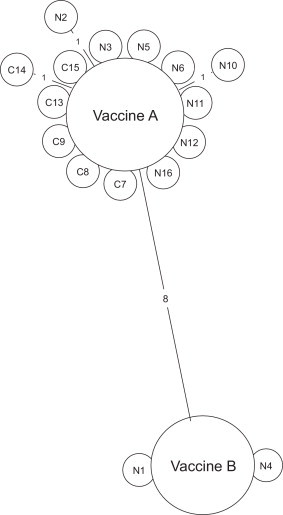
Identities between 793B viruses detected on Italian broiler farms. Touching circles indicate identical sequences. Numbers indicate nucleotide differences between viruses in the tested region. Strains are named progressively according to their collection date and geographical locations are codified as N (North Italy) or C (Central Italy).
4. Discussion
For about half a century, countries with developed poultry industries have applied one or more live vaccines to all broiler, layer and breeder chickens to control disease caused by infectious bronchitis virus. More recently the availability of molecular diagnostics able to distinguish between genotypes has allowed those IBVs actually present on farms to be readily identified, and this has allowed the vaccine genotype to match that of any current field threat. Furthermore the technique has allowed the detection of new genotypes of which some might cause disease. Of these a proportion can become largely undetectable within a short period while others can become a dominant disease causing genotype persisting for a considerable period. Italy 02 would be a recent example of the former in most countries while 793B would be generally considered to fall into the latter category and present a threat over the longer term. This has either led to the development of new live vaccines of matching genotype or the deployment of established vaccines, sometimes in specific combination, to counter that disease threat. In general such an approach has allowed the poultry industry to control the effects of infection from both established and more recently detected IBV genotypes.
A weakness of the system has been the inability to unequivocally distinguish between field and vaccine viruses. For almost two decades 793B detection has been associated with disease and for most of that period one or more live 793B vaccines have been in use. In that time many 793B detections have been made. Currently our study as well as another study [14] following a large number of unrelated farms in Italy, has found consistent detections of 793B. However it has not been possible to know whether encountered disease has been caused by inadequately controlled 793B field viruses or persisting, and possibly unstable, vaccine viruses. This is in contrast with, for example, AMPV, another common virus affecting poultry worldwide, where the deduction of genetic vaccine markers has shown that live vaccine derived virus can persist on farms and cause disease [15], [16], [17], [18]. While it should be possible to similarly identify markers for IBV vaccines, this has not to date been reported, probably due to the unavailability of the IBV vaccine progenitor viruses needed in the critical vaccine-progenitor sequence comparisons.
Results very strongly suggest that 793B detected on the farms currently studied, derived from the two 793B vaccines in use. This conclusion rests on four pieces of evidence of ascending importance. Firstly, in the Massi study [14] where chickens were given more doses of vaccine, a greater proportion of 793B detections were made. Secondly, all 793B detections in the current study had S1 hypervariable region sequences almost identical to either of the two vaccines. Uninvolved assessment might correctly raise the possibility that many 793B field viruses have equal identity in this region, and to confuse the picture further, it would be expected that mass use of vaccines A and B for some two decades, might have led to vaccine derived detections being added to the world sequence databases as field strains. Unfortunately this sequence issue cannot be unequivocally resolved without the availability of vaccine markers. Thirdly the two vaccines differed by eight nucleotides in the tested region and all detections matched the sequence of the vaccine which had been applied. Nonetheless the main evidence arises from 793B becoming undetectable after the withdrawal of the two 793B vaccines on several hundred Italian farms. It is reasonable to conclude that 793B vaccine use is no longer required for 793B virus control at the current time on these farms.
The paper confirms the importance to live vaccination strategies of distinguishing genuine field viruses from applied vaccines and their derivatives. For example for in human polio, vaccination strategies have needed to consider the occurrence of vaccine-associated paralytic poliomyelitis, which in turn has required the technical expertise for vaccine identification [19]. In another avian viral disease of poultry AMPV, the ability to clearly distinguish between vaccine and field virus has become practical and this would be expected to have benefits in future vaccination strategies [18]. For IBV it would be clearly undesirable to be adding millions or billions of doses of live vaccine virus to birds if, as results here suggest, they served little benefit. Furthermore the presence of unnecessary vaccine viruses, especially where multiple IBV genotypes might be involved, might be expected to drive the generation of recombinant viruses; indeed this notion has restricted certain concurrent IBV vaccination strategies in France. While to date there is no evidence of field recombinants with vaccine virus contribution causing major disease in the worldwide chicken industry, the possible mechanism is there, hence vaccine use should be ideally restricted to situations where there is a known protective benefit.
Acknowledgement
The work here described was supported by University of Padua grant 60A08-8005/14 (ex 60%, 2014).
References
- 1.Jia W., Karaca K., Parrish C.R., Naqi S.A. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch Virol. 1995;140(2):259–271. doi: 10.1007/BF01309861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanagh D., Mawditt K., Adzhar A., Gough R.E., Picault JP., Naylor C.J. Does IBV change slowly despite the capacity of the spike protein to vary greatly? Adv Exp Med Biol. 1998;440:729. doi: 10.1007/978-1-4615-5331-1_94. [DOI] [PubMed] [Google Scholar]
- 3.Dolz R., Pujols J., Ordonez G., Porta R., Majo N. Molecular epidemiology and evolution of avian infectious bronchitis virus in Spain over a fourteen-year period. Virology. 2008;374(1):50–59. doi: 10.1016/j.virol.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinley E.T., Jackwood M.W., Hilt D.A., Kissinger J.C., Robertson J.S., Lemke C. Attenuated live vaccine usage affects accurate measures of virus diversity and mutation rates in avian coronavirus infectious bronchitis virus. Virus Res. 2011;158(1–2):225–234. doi: 10.1016/j.virusres.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kant A., Koch G., van Roozelaar D.J., Kusters J.G., Poelwijk F.A., van der Zeijst B.A. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J Gen Virol. 1992;73(Pt 3):591–596. doi: 10.1099/0022-1317-73-3-591. [DOI] [PubMed] [Google Scholar]
- 6.Koch G., Hartog L., Kant A., van Roozelaar D.J. Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J Gen Virol. 1990;71(Pt 6):1929–1935. doi: 10.1099/0022-1317-71-9-1929. [DOI] [PubMed] [Google Scholar]
- 7.Ignjatovic J., Sapats S. Identification of previously unknown antigenic epitopes on the S and N proteins of avian infectious bronchitis virus. Arch Virol. 2005;150(9):1813–1831. doi: 10.1007/s00705-005-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 9.Sjaak de Wit J.J., Cook J.K., van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40(3):223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56(4):634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 11.Cook J. Coronaviridae. In: Pattison M., Bradbury A., editors. Poultry Diseases. Saunders Elsevier; Amsterdam: 2008. pp. 340–349. [Google Scholar]
- 12.Jones R.C., Worthington K.J., Capua I., Naylor C.J. Efficacy of live infectious bronchitis vaccines against a novel European genotype, Italy 02. Vet Rec. 2005;156(20):646–647. doi: 10.1136/vr.156.20.646. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massi P. Situazione epidemiologica della bronchite infettiva in Italia. Rivista di Medicina Veterinaria, Speciale. 2013:13–20. [Google Scholar]
- 15.Catelli E., Cecchinato M., Savage C.E., Jones R.C., Naylor C.J. Demonstration of loss of attenuation and extended field persistence of a live avian metapneumovirus vaccine. Vaccine. 2006;24(42–43):6476. doi: 10.1016/j.vaccine.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 16.Listorti V., Lupini C., Cecchinato M., Pesente P., Rossi G., Giovanardi D. Rapid detection of subtype B avian metapneumoviruses using RT-PCR restriction endonuclease digestion indicates field circulation of vaccine-derived viruses in older turkeys. Avian Pathol. 2014;43(1):51–56. doi: 10.1080/03079457.2013.866212. [DOI] [PubMed] [Google Scholar]
- 17.Lupini C., Cecchinato M., Ricchizzi E., Naylor C.J., Catelli E. A turkey rhinotracheitis outbreak caused by the environmental spread of a vaccine-derived avian metapneumovirus. Avian Pathol. 2011;40(5):525–530. doi: 10.1080/03079457.2011.607428. [DOI] [PubMed] [Google Scholar]
- 18.Cecchinato M., Catelli E., Lupini C., Ricchizzi E., Prosperi S., Naylor C.J. Reversion to virulence of a subtype B avian metapneumovirus vaccine: is it time for regulators to require availability of vaccine progenitors? Vaccine. 2014;32(36):4660–4664. doi: 10.1016/j.vaccine.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Kew O.M., Sutter R.W., de Gourville E.M., Dowdle W.R., Pallansch M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]


