Abstract
Acute flaccid myelitis (AFM) is a rare condition associated with spinal cord gray matter abnormalities and frequent persistent motor deficits in the limbs. We present our experience with the diagnosis, management, and outcomes of affected children in 2014 and 2016, emphasizing features that should trigger early consideration of AFM. Early viral testing may increase the rate of detecting associated viruses.
Keywords: weakness, pediatric, enterovirus, acute flaccid myelitis, myelitis
Abbreviations: AFM, Acute flaccid myelitis; CDC, Centers for Disease Control and Prevention; EV, Enterovirus; IVIG, Intravenous immunoglobulin; MRI, Magnetic resonance imaging; mRS, modified Rankin Scale; PCR, Polymerase chain reaction
AFM is defined as the acute onset of focal limb weakness with corresponding spinal cord gray matter-specific abnormalities spanning one or more spinal segments on magnetic resonance imaging (MRI), sometimes with associated brainstem and posterior fossa abnormalities.1 AFM can be associated with a variety of viruses, including enteroviruses (eg, poliovirus, enterovirus [EV] D68, and EV A71).2, 3 Weakness typically begins in the setting of a recent or current respiratory and/or febrile illness and may be rapidly progressive.2, 4 Despite increased cases in the US in 2014 and 2016, the diagnosis is often delayed, optimal treatment regimens are not defined, and outcomes are poorly understood.
Using a case series of children diagnosed with AFM at a single tertiary care children's hospital in Philadelphia in 2014 and 2016, we summarized clinical, infectious, and imaging findings, with attention to details that should trigger a clinician to consider the diagnosis of AFM at initial clinical presentation. We also summarized our experience with treatment regimens, and report the 1- to 15-month outcome data for these children.
Methods
We performed a retrospective chart review of all children diagnosed with AFM at the Children's Hospital of Philadelphia between September 2014 and October 2016. This study was approved by the Children's Hospital of Philadelphia Institutional Review Board.
Data Collection and Study Definitions
All cases met the current Council of State and Territorial Epidemiologist's case definition of AFM at the time of diagnosis.1 Specifically, all cases had acute onset of focal limb weakness and an MRI demonstrating a spinal cord lesion largely restricted to gray matter and spanning ≥1 spinal segment. All cases were confirmed by the US Centers for Disease Control and Prevention (CDC). Probable cases were excluded.
We abstracted demographic, epidemiologic, clinical, treatment, and outcome data from medical records on all confirmed cases. We used the modified Rankin Scale (mRS), an ordinal scale of 0 (no symptoms) to 3 (moderate disability and requiring some help, but able to walk without assistance) to 4 (moderately severe disability and unable to walk without assistance or attend to bodily needs) to 6 (death) as a measure of disability.5
Procedures
Respiratory virus multiplex polymerase chain reaction (PCR) panels testing for respiratory syncytial virus; parainfluenza virus types 1, 2, and 3; adenoviruses; metapneumovirus; rhinoviruses (RV); and coronaviruses from respiratory specimens were performed using laboratory developed, in-house, real-time, qualitative assays. Because respiratory panels are often unable to differentiate RVs from some enteroviruses owing to genetic similarities, positive results for RVs were reported as RV/EV positive. EV-specific monoplex PCR testing was performed using the Roche MagnaPure 2.0 system (Roche, Basel, Switzerland) and amplified by the Quanstudio DX system by Thermo Fisher Scientific (Waltham, Massachusetts). EV typing was performed on any available acute sample from children with suspected AFM, regardless of in-house EV testing results, at the CDC (Atlanta, Georgia).
Data Analyses and Statistical Method
Data were analyzed using non-parametric methods in STATA version 14.2 (Stata Corp, College Station, Texas). Interval and ordinal variables were described using medians and ranges, and intergroup differences compared using Wilcoxon rank-sum tests. Categorical variables were described using counts and frequencies, and inter-group differences compared using the χ2 test. Statistical significance was determined a priori as a 2-tailed P value of < .05.
Results
Fourteen children met the case definition for a confirmed case of AFM during the study time period (n = 5 in 2014 and n = 9 in 2016). No children were diagnosed at our institution in 2015, consistent with the biennial variation in incidence reported nationally. Demographics, clinical characteristics, diagnostic testing, therapies, and outcomes are summarized in Table I . All children had weakness on presentation, which was more pronounced proximally. Five children had prominent single limb involvement (4 upper extremity, 1 lower extremity), and 9 had multiple limb involvement.
Table I.
Demographics, clinical features, treatment, and outcomes of children with confirmed AFM by CDC criteria in 2014 and 2016
| Characteristics∗ | All |
By year |
P value† | |
|---|---|---|---|---|
| (n = 14) | 2014 (n = 5) | 2016 (n = 9) | ||
| Age (years) | 2.6 (0.5-8.8) | 5.4 (0.5-7.1) | 2.6 (1-8.8) | .640 |
| Male sex | 10 (71) | 3 (60) | 7 (78) | .480 |
| Clinical presentation | ||||
| Fever at the time of presentation | 8 (57) | 1 (20) | 7 (78) | .036 |
| Multiple limb involvement | 9 (64) | 1 (20) | 8 (89) | .010 |
| Cranial nerve involvement | 1 (7) | 0 (0) | 1 (13) | .439 |
| Pain on presentation | 5 (36) | 2 (40) | 3 (33) | .803 |
| CSF pleocytosis‡ | 11 (85) | 5 (100) | 6 (75) | .224 |
| mRS at symptom nadir | 4 (2-5) | 2 (2-5) | 4 (2-5) | .183 |
| Enteroviral testing | ||||
| Samples procured ≤1 day from admission | 9 (64) | 1 (20) | 6 (67) | .010 |
| EV-specific PCR+ in ≥1 sample§ | 10 (77) | 2 (40) | 8 (100) | .012 |
| Nasopharyngeal swab or tracheal aspirate | 9 (69) | 2 (40) | 7 (88) | .071 |
| Serum | 2 (17) | 0 (0) | 2 (25) | .224 |
| Urine | 1 (8) | 0 (0) | 1 (13) | .411 |
| Stool | 6 (46) | 1 (20) | 5 (63) | .135 |
| CSF | 0 (0) | 0 (0) | 0 (0) | – |
| Enteroviral typing (CDC)¶ | (n = 12) | (n = 5) | (n = 7) | |
| EV D68+ | 6 (50) | 0 (0) | 6 (86) | .003 |
| EV A71+ | 1 (8) | 1 (20) | 0 (0) | .217 |
| Treatment | ||||
| IV methylprednisolone | 7 (50) | 3 (60) | 4 (44) | .577 |
| IVIG | 14 (100) | 5 (100) | 9 (100) | – |
| Plasma exchange | 2 (14) | 0 (0) | 2 (22) | .255 |
| Fluoxetine | 7 (50) | 0 (0) | 7 (78) | .005 |
| Hospital course | ||||
| ICU care for AFM | 5 (36) | 1 (20) | 4 (44) | .360 |
| Intubated for weakness | 3 (14) | 1 (20) | 2 (22) | .923 |
| Feeding assistance | ||||
| Acute nasogastric tube | 4 (29) | 1 (20) | 3 (38) | .597 |
| Gastrostomy tube >3 months | 2 (14) | 0 (0) | 2 (22) | .255 |
| Inpatient rehabilitation recommended | 7 (50) | 1 (20) | 6 (67) | .094 |
| Follow-up | ||||
| Years at follow-up evaluation | 1.0 (0.1-1.3) | 1.0 (0.1-1.3) | 1.0 (0.7-1.2) | .739 |
| mRS at follow-up evaluation | 3 (0-5) | 1 (0-4) | 4 (0-5) | .084 |
| Gastrostomy tube | 2 (14) | 0 (0) | 2 (22) | .255 |
| Mechanical ventilation | 2 (14) | 0 (0) | 2 (22) | .255 |
Bold values indicate P ≤ .05.
CSF, cerebrospinal fluid; ICU, intensive care unit.
Categorical variables are described using number (%). Continuous variables are described using median (range).
P values to compare characteristics between children in 2014 and 2016 were calculated using χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Eight of 9 children underwent lumbar puncture in 2016. Pleocytosis defined as CSF white blood cells ≥5 cells/high-powered field.
Thirteen of 14 (5 in 2014, 8 in 2016) children had EV-specific PCR testing in ≥1 site.
Twelve of 14 (5 in 2014, 7 in 2016) children had samples sent to CDC for EV typing.
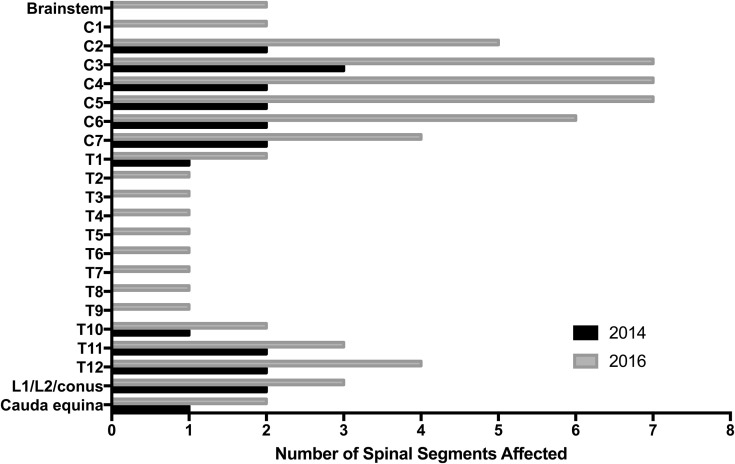
Thirteen patients underwent lumbar puncture, which demonstrated a lymphocytic predominant pleocytosis and variably elevated protein, consistent with other AFM literature.2, 4, 6, 7 Spinal MRI in all children demonstrated intraspinal gray matter T2 hyperintensities consistent with the case definition, most commonly in mid-cervical and lower thoracic regions (Figures 1 and 2; available at www.jpeds.com). Twelve of the 14 children underwent brain MRI; abnormalities were found in 3 (25%). Brain abnormalities included meningeal enhancement and T2 hyperintense lesions extending from the spinal cord into the brainstem and basal ganglia (Figure 2).
Figure 1.
Spinal cord abnormalities by levels involved. The cervical region was the most common site of involvement, followed by the thoracic region.
Figure 2.
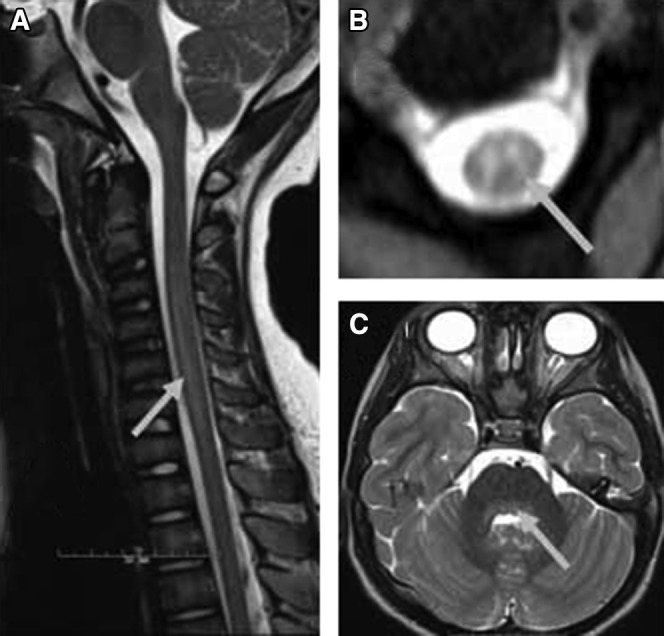
Spinal cord and brain imaging findings typical of AFM. A, Sagittal turbo spin echo T2 and B, T2 axial imaging of the spinal cord demonstrated longitudinal spinal cord lesion impacting primarily gray matter (arrows). C, Axial T2/fluid-attenuated inversion recovery brain images demonstrating posterior brainstem involvement, (grey arrow).
All children had ≥1 EV test performed, including screening respiratory virus PCR panel and/or EV-specific PCR testing (from nasopharyngeal swab, tracheal aspirate, serum, cerebrospinal fluid, urine, and/or stool). EV was detected in 11/14 (79%; Tables I and II ). Ten children were screened with a respiratory virus PCR panel, 4 (40%) of whom were RV/EV positive. Among these children, follow-up EV-specific PCR testing confirmed infection in 3. The 1 child with negative follow-up EV-specific PCR testing was initially cared for at an outside institution, so follow-up testing was delayed by 27 days. Among the 6 children with negative respiratory virus PCR panels, 5 had follow-up EV-specific PCR testing of nasopharyngeal swabs performed, and 3 were positive.
Table II.
EV testing by patient
| Year | Patient | EV+ at any site | Screening respiratory virus PCR panel | Multisite EV-specific PCR testing | EV type (CDC) | Days from admission to EV test |
|---|---|---|---|---|---|---|
| 2014 | 1 | Yes | + RV/EV | Negative | Negative | 27 |
| 2 | No | Negative | Negative | Negative | 3 | |
| 3 | Yes | Negative | + NP aspirate | Negative | 0 | |
| 4 | No | Negative | Negative | Negative | 6 | |
| 5 | Yes | Negative | + NP aspirate + stool |
EV-71 (NP) | 2 | |
| 2016 | 6 | Yes | Negative | + NP aspirate | EV-D68 (NP) | 1 |
| 7 | Yes | + RV/EV +parainfluenza +RSV |
+ NP aspirate + stool +serum |
EV-D68 (NP) | 0 | |
| 8 | Yes | Not done | + NP aspirate | EV-D68 (NP) | 0 | |
| 9 | Yes | + RV/EV | + NP aspirate | EV-D68 (NP) | −1 | |
| 10 | Yes | + RV/EV | + NP aspirate + stool |
EV-D68 (NP) | 1 | |
| 11 | No | Negative | Not done | Not done | 0 | |
| 12 | Yes | Not done | + NP aspirate + stool |
EV-D68 (NP) | 1 | |
| 13 | Yes | Not done | + stool + serum |
EV/RV, not EV-D68 (stool, NP swab not sent) | 0 | |
| 14 | Yes | Not done | + NP aspirate + stool + urine |
Not done | 5 |
NP, nasopharyngeal; RSV, respiratory syncytial virus.
EV-specific PCR testing from one or more sites was conducted in 13 patients (n = 5/5 in 2014, n = 8/9 in 2016), including 8 of the 10 children who were screened by respiratory virus PCR panel. Ten of the 13 patients (77%) had positive testing in ≥1 site; positive testing was more common in 2016 compared with 2014 (n = 8/8 vs n = 2/5). Most positive samples (n = 9/10) were from nasopharyngeal swabs or tracheal aspirates; cerebrospinal fluid samples were all negative (Table I). Of the 9 patients with positive nasopharyngeal or tracheal aspirates, 6 were also positive in other sites (3 in both serum and stool, 1 in urine and stool, and 3 in stool). One patient in 2016 was positive for EV in both serum and stool, but did not have nasopharyngeal aspirate sent. The median time from admission to EV-specific PCR testing was 1 day (range, 1-27 days), and was longer in 2014 compared with 2016 (3 days vs 0 days).
EV typing of samples from all 5 children in 2014 was performed at the CDC; one of these children was positive for EV A71. Typing was performed on samples from 7 of 9 children in 2016, and 6 of these children were positive for EV D68, all from nasopharyngeal samples. The remaining 2 children had delayed presentations to our institution, so acute samples were not available to type. Children who had samples procured within 1 day of admission more frequently tested positive for EV D68 than those whose samples were drawn later (n = 6/9 vs n = 0/5); this earlier testing was more common in 2016 compared with 2014 (1/5 in 2014 vs 8/9 in 2016).
Treatment strategies differed at our institution between 2014 and 2016. Cases in 2014 were treated as idiopathic myelitis with intravenous methylprednisolone (n = 3/5) and/or intravenous immunoglobulin (IVIG; n = 5/5). In 2016, cases were treated with intravenous methylprednisolone (n = 4/9), IVIG (n = 9/9), fluoxetine (n = 7/9), and/or plasma exchange (n = 2/9).
Most children demonstrated incomplete recovery at a median follow-up of 1 year (range, 0.1-1.3 years), with a median follow-up mRS of 3 (range, 0-5). This score represented only a slight improvement compared with the median mRS at symptom nadir of 4. Only 2 children recovered fully. One of these children presented in 2014 with isolated leg weakness and was positive for EV A71. The other child presented in late 2016 with 4-extremity weakness in the setting of a febrile illness, and was positive for an un-typed EV in respiratory samples, stool, and urine. Two children, both positive for EV D68, had persistent flaccid quadriplegia requiring tracheostomy and gastrostomy tube at follow-up. Follow-up mRS was significantly higher among children who tested positive for EV D68 compared with those who were negative or not tested (median 4 [n = 6] vs 1 [n = 8]; P = .011). There was no significant difference in follow-up mRS scores between those who did and did not receive steroids (median 1 [n = 7] vs 4 [n = 7]; P = .233). All children received IVIG. Fluoxetine was empirically added to the treatment protocol at our institution in 2016 and administered to 7 of the 9 children that year. Children who received fluoxetine had higher mRS scores at follow-up than those who did not (median of 4 vs 1), as did children who received plasma exchange (median of 5.0 vs 1.5), but the numbers were small and children who received these therapies had higher nadir mRS scores, reflecting worsened pretreatment function compared with other children.
Among the 14 total cases, 6 (43%) initially received an alternate diagnosis despite weakness at presentation, including a viral syndrome with neck pain (n = 3), Guillain-Barre syndrome (n = 1), viral syndrome with brachial nerve palsy (n = 1), and toxic synovitis (n = 1). Four of these 5 children were initially discharged from an emergency room, and upon re-presentation with worsening symptoms, 2 required emergent intubation and ultimately required tracheostomy.
Discussion
AFM is an increasingly recognized cause of focal, flaccid limb weakness in children that is associated with significant morbidity. Protocols for early diagnosis and prompt sample acquisition may increase the yield of diagnostic testing. Despite meeting the clinical criteria for AFM at presentation, 43% of the children in our case series had a delay in the initial diagnosis.
Although AFM first came to national attention in 2014 following a case series of 9 affected children in Colorado, reports of similar paralytic illnesses had occurred in the past.4, 8, 9, 10 Case series of AFM have typically demonstrated a pattern of asymmetric proximal greater than distal weakness in young children with antecedent or concurrent febrile illness.2, 6 Our data are consistent with those prior reports. Pain was also a common complaint at diagnosis in our case series, which may be underestimated given the young age of our patients.11, 12
There is increasing evidence of an association between AFM and EV infection, but whether this association is related to direct viral invasion of the spinal cord, para-infectious autoimmune response to infection, or a combination of the two, remains unclear.3, 13, 14 We did not find EV by PCR testing in cerebrospinal fluid from the 13 children that underwent lumbar puncture. We did, however, find evidence of EV infection in multiple other sites. More children in 2016 had positive EV-specific PCR testing compared with 2014, but a new institutional testing protocol in 2016 ensured rapid procurement of standardized multisite samples in suspected AFM cases, so it is unclear whether this is a true difference or a sampling bias. This observation underscores the importance of rapid sample acquisition in the search for associated pathogens. EV typing in previously reported case series has revealed both D68 and A71 strains in cases of AFM, and anecdotally, children with EV A71 neuroinvasive disease have been reported to have better outcomes than those associated with EV D68.2, 15 Although only one of the children in our case series was positive for EV A71, that child had a good outcome compared with the other children. The 6 children who tested positive for EV D68 had overall poorer outcomes than those who tested negative or were not tested, but it is unclear if other factors may have confounded this association. It is also possible that another unidentified virus may have influenced outcomes, because multiple other viruses, including flaviviruses, adenoviruses, and other types of EV, have also been associated with an AFM phenotype.
Anti-inflammatory treatment and immune-modulating therapies did not result in improved outcomes in our case series, nor have they nationally.2, 7 This lack of treatment effect could argue against an autoimmune neuropathogenesis. However, these data may be confounded by indication, with more severe cases receiving more aggressive therapy. Whether outcomes were improved from what they otherwise would have been without therapy is unknown.
There has been some concern that steroids may actually be detrimental if AFM is indeed caused by a direct viral infection of the spinal cord. These concerns are based on historical data from human EV A71 outbreaks, in which steroids were felt to worsen clinical course, a murine model of EV D68-associated AFM that found mice treated with high-dose steroids had increased mortality compared with untreated mice, and older poliovirus data demonstrating accelerated poliovirus replication in chick embryos when treated with cortisone.13, 16, 17 However, definitive evidence of an adverse effect has never been clearly shown in humans. These concerns, as well as a perceived lack of efficacy of methylprednisolone in 2016, led to changes in our internal treatment protocol in 2016 with more judicious use of steroids, primarily restricted to concern for spinal cord edema with risk of secondary injury. We treated all children with IVIG given the relatively low risk of complications, the potential for modification of infection as in the mouse model, and theoretical benefit if there were an autoimmune component to the pathophysiology of AFM.13 Fluoxetine treatment was implemented in 2016 based on in vitro data that it inhibited the replication of EV D68, but there was no clear treatment effect in retrospective analysis so this agent has since been removed from our institutional treatment protocol.11, 18, 19 This highlights the observation that adding new therapies at the leading edge of knowledge is not always successful, and rational use must be constantly reassessed clinically to streamline treatment protocols.
An AFM diagnosis was delayed in almost one-half of the children in our case series. Pain may have masked the presence of early subtle weakness. Weakness in AFM is rapidly progressive, with a median of 4 days between symptom onset and nadir of symptoms.11 Proximal weakness, in particular, can be easy to miss on a screening general medical examination. The presence of a recent or current febrile illness with asymmetry in motor function, even if initially attributed to pain, should prompt a thorough neurologic examination and consideration of AFM.
Our study had several limitations. First, our sample size was small and limited to a single center's experience, so analyses are underpowered, and significant associations may be underestimated. Future studies of AFM will require multisite, standardized protocols to increase sample sizes and to detect subtle associations while accounting for possible confounders using multivariable regression models. Second, our study was retrospective. We examined motor outcomes using mRS because it could be applied retrospectively. However, the mRS is an insensitive measure for subtle motor changes and has not been validated in pediatric populations. Developing sensitive and specific outcome measures for AFM will be critical to understand fully the natural history of illness and to assess the efficacy of potential therapeutic options. Third, patients presented to our institution at variable times in their acute illness; we were not able to determine reliably the date of their initial symptom onset in this retrospective analysis. Therefore, the timing of etiologic testing was inconsistent, which may have introduced false-negative results. Timing to initiation of treatment was also variable, so if there is an optimal window for intervention, this may have been missed. Finally, steroids and plasma exchange were largely given to sicker patients. Therefore, a confounding by indication may obscure any potential treatment effects. Randomized controlled clinical trials would address these limitations, but are logistically difficult in a rare disease.
AFM is a severe, life-threatening, but under-recognized cause of acute onset, flaccid limb weakness. The pattern of rapidly progressive asymmetric and proximal more than distal weakness in the context of current or recent fever but the absence of sensory finding should trigger consideration of AFM. Prompt recognition, which has improved at our institution with the development of internal protocols for suspected cases of AFM, is critical to appropriate clinical monitoring, ongoing epidemiologic surveillance, and acquisition of samples for laboratory evaluation in a timely fashion to facilitate potential pathogen identification. Larger future epidemiologic studies and multisite clinical trials may lend insight to neuropathogenic mechanisms and possible therapeutic strategies.
Footnotes
Funded by National Institutes of Health (National Institute of Neurological Disorders and Stroke) (K23 NS094069 [to J.M.]). S.H. receives support from the CDC for activities related to AFM surveillance. The other authors declare no conflicts of interest.
Portions of this study were presented at the American Academy of Neurology annual meeting in April 22-28, 2017, Boston, Massachusetts.
Appendix
References
- 1.Acute Flaccid Myelitis Case Definitions. www.cdc.gov/acute-flaccid-myelitis/hcp/case-definition.html
- 2.Messacar K., Schreiner T.L., Van Haren K., Yang M., Glaser C.A., Tyler K.L. Acute flaccid myelitis: a clinical review of US cases 2012–2015. Ann Neurol. 2016;80:326–338. doi: 10.1002/ana.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyda A., Stelzer-Braid S., Adam D., Chughtai A.A., Macintyre R. The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68)-what is the evidence for causation? Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.3.17-00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sejvar J.J., Lopez A.S., Cortese M.M., Leshem E., Pastula D.M., Miller L. Acute flaccid myelitis in the United States, August-December 2014: results of nationwide surveillance. Clin Infect Dis. 2016;63:737–745. doi: 10.1093/cid/ciw372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonita R., Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 6.Gordon-Lipkin E., Muñoz L.S., Klein J.L., Dean J., Izbudak I., Pardo C.A. Comparative quantitative clinical, neuroimaging, and functional profiles in children with acute flaccid myelitis at acute and convalescent stages of disease. Dev Med Child Neurol September. 2019;61:366–375. doi: 10.1111/dmcn.14030. [DOI] [PubMed] [Google Scholar]
- 7.Nelson G.R., Bonkowsky J.L., Doll E., Green M., Hedlund G.L., Moore K.R. Recognition and management of acute flaccid myelitis in children. Pediatr Neurol. 2016;55:17–21. doi: 10.1016/j.pediatrneurol.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Van Haren K., Ayscue P., Waubant E., Clayton A., Sheriff H., Yagi S. Acute flaccid myelitis of unknown etiology in California, 2012-2015. JAMA. 2015;314:2663–2671. doi: 10.1001/jama.2015.17275. [DOI] [PubMed] [Google Scholar]
- 9.Solomon T., Kneen R., Dung N.M., Khanh V.C., Thuy T.T., Ha D.Q. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094–1097. doi: 10.1016/S0140-6736(97)07509-0. [DOI] [PubMed] [Google Scholar]
- 10.Sejvar J.J., Davis L.E., Szabados E., Jackson A.C. Delayed-onset and recurrent limb weakness associated with West Nile virus infection. J Neurovirol. 2010;16:93–100. doi: 10.3109/13550280903586378. [DOI] [PubMed] [Google Scholar]
- 11.Messacar K., Sillau S., Hopkins S.E., Otten C., Wilson-Murphy M., Wong B. Safety, tolerability, and efficacy of fluoxetine as an antiviral for acute flaccid myelitis. Neurology. 2019;92:e2118–e2126. doi: 10.1212/WNL.0000000000006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen E.W., Kornberg A.J., Freeman J.L., Leventer R.J., Ryan M.M. Acute flaccid myelitis in childhood: a retrospective cohort study. Eur J Neurol. 2017;24:1077–1083. doi: 10.1111/ene.13345. [DOI] [PubMed] [Google Scholar]
- 13.Hixon A.M., Yu G., Leser J.S., Yagi S., Clarke P., Chiu C.Y. A mouse model of paralytic myelitis caused by enterovirus D68. Coyne CB. PLOS Pathog. 2017;13:e1006199. doi: 10.1371/journal.ppat.1006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messacar K., Asturias E.J., Hixon A.M., Van Leer-Buter C., Niesters H.G.M., Tyler K.L. Enterovirus D68 and acute flaccid myelitis—evaluating the evidence for causality. Lancet Infect Dis. 2018;18:e239–e247. doi: 10.1016/S1473-3099(18)30094-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casas-Alba D., de Sevilla M.F., Valero-Rello A., Fortuny C., García-García J.J., Ortez C. Outbreak of brainstem encephalitis associated with enterovirus-A71 in Catalonia, Spain (2016): a clinical observational study in a children's reference centre in Catalonia. Clin Microbiol Infect. 2017;23:874–881. doi: 10.1016/j.cmi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Seiff A. Cambodia unravels cause of mystery illness. Lancet. 2012;380:205. doi: 10.1016/s0140-6736(12)61200-8. [DOI] [PubMed] [Google Scholar]
- 17.Dunham W.B., Ewing F.M. Apparent multiplication of the Lansing poliomyelitis virus in cortisone treated chick embryos. J Bacteriol. 1953;65:224. doi: 10.1128/jb.65.2.224-224.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyler K.L. Rationale for the evaluation of fluoxetine in the treatment of enterovirus d68-associated acute flaccid myelitis. JAMA Neurol. 2015;72:493–494. doi: 10.1001/jamaneurol.2014.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo J., Quinn K.K., Kye S., Cooper P., Damoiseaux R., Krogstad P. Fluoxetine is a potent inhibitor of coxsackievirus replication. Antimicrob Agents Chemother. 2012;56:4838–4844. doi: 10.1128/AAC.00983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]