Summary
Background
Viral respiratory illnesses are common causes of outbreaks and can be fatal to some patients.
Aim
To investigate the association between laboratory-confirmed viral respiratory infections and potential sources of exposure during the previous 7 days.
Methods
In this nested case–control analysis, healthcare personnel from nine Canadian hospitals who developed acute respiratory illnesses during the winters of 2010/11–2013/14 submitted swabs that were tested for viral pathogens. Associated illness diaries and the weekly diaries of non-ill participants provided information on contact with people displaying symptoms of acute respiratory illness in the previous week. Conditional logistic regression assessed the association between cases, who were matched by study week and site with controls with no respiratory symptoms.
Findings
There were 814 laboratory-confirmed viral respiratory illnesses. The adjusted odds ratio (aOR) of a viral illness was higher for healthcare personnel reporting exposures to ill household members [7.0, 95% confidence interval (CI) 5.4–9.1], co-workers (3.4, 95% CI 2.4–4.7) or other social contacts (5.1, 95% CI 3.6–7.1). Exposures to patients with respiratory illness were not associated with infection (aOR 0.9, 95% CI 0.7–1.2); however, healthcare personnel with direct patient contact did have higher odds (aOR 1.3, 95% CI 1.1–1.6). The aORs for exposure and for direct patient contact were similar for illnesses caused by influenza.
Conclusion
Community and co-worker contacts are important sources of viral respiratory illness in healthcare personnel, while exposure to patients with recognized respiratory infections is not associated. The comparatively low risk associated with direct patient contact may reflect transmission related to asymptomatic patients or unrecognized infections.
Keywords: Respiratory, Healthcare worker, Hospital, Transmission, Viral, Exposure, Adult
Introduction
Acute respiratory tract infections are among the most commonly experienced illnesses worldwide and cause an estimated 4 million deaths annually [1]. Upper respiratory tract infections are almost exclusively caused by viruses [2,3] whose transmission persists in healthcare facilities despite current infection control practices. The contagiousness and short incubation time of these viruses promote outbreaks in healthcare facilities [[4], [5], [6]] where they can cause serious illness in vulnerable patients [[7], [8], [9], [10]].
Healthcare personnel may be exposed to respiratory viruses from a variety of sources including patients, co-workers, household members and other community contacts. Current literature regarding the relative contribution of community and occupational exposures to illness in healthcare personnel is conflicting [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. This study used the data from a large cohort study of Canadian healthcare personnel to determine the distribution of presumed (or likely) sources of exposure in the week before onset of laboratory-confirmed viral respiratory illness.
Methods
The Influenza Cohort Study was a multi-site prospective cohort study conducted during the 2010/11–2013/14 influenza seasons [22]. Participants were workers aged 18–69 years who worked >20 h/week at one of nine Canadian acute care hospitals: six in Toronto, two in Halifax and one in Hamilton. Participants could enrol for one or multiple seasons. The sample size was estimated to detect a two-fold difference in the likelihood of healthcare personnel developing influenza based on their work area. Seasons were defined as beginning the earlier of the first Monday following 1st November or the beginning of influenza activity (≥5% of specimens submitted to regional reference laboratories testing positive for influenza), and ending whichever was the later of the Monday following 30th April or 2 weeks after the end of the influenza season (<3% of specimens testing positive for 2 consecutive weeks). The study was approved by the research ethics boards of all participating hospitals and was conducted according to good clinical practice guidelines and the Declaration of Helsinki (2013).
Participants were recruited and completed a baseline questionnaire prior to onset of the influenza season. Throughout the season, they received weekly e-mails with a link to report the presence or absence of respiratory symptoms or illness. Each week, 20% of participants with no illness diary for the previous week answered additional questions about contact with people with symptoms of acute respiratory illness in the previous 7 days. Just prior to the first weekly e-mail of each season, participants were selected at random into five groups, with each group answering the additional questions every fifth week to reduce participant burden while providing a sufficiently large sample size to conduct analyses. Participants were asked to complete daily symptom diaries when ill and to submit a self-collected mid-turbinate swab if they developed one or more symptoms suggestive of acute respiratory illness (any one of cough, stuffy or runny nose, sore throat), a fever or felt generally unwell. The first day illness diary included the same questions about contact with symptomatic persons as the weekly diary assigned to 20% of participants. All questionnaires were pilot tested prior to their use in the 2009/10 influenza season [11], with slight adaptations made, as needed, prior to use in this study. Swabs were tested for respiratory viruses (influenza A or B, parainfluenza viruses 1–4, respiratory syncytial viruses A and B, human coronaviruses OC43/HKU1 and 229E/NL63, human metapneumovirus, human bocavirus, enterovirus, rhinovirus and adenovirus) using the Seeplex RV15 respiratory panel (Seegene Technologies, Seoul, South Korea).
For this nested case–control analysis, cases were defined as participants with a mid-turbinate swab testing positive for a respiratory virus and an associated first day illness diary (i.e. those with a laboratory-confirmed symptomatic viral respiratory illness). Controls were participants who completed a weekly diary with additional questions who reported being asymptomatic during the current, the previous and the following weeks (within an incubation period). The focal exposure was close contact (within arm's reach for ≥2 min) with person(s) with acute respiratory illness symptoms. Separate exposures were collapsed into one variable with six categories: 0) no known exposure, 1) patient(s), 2) household member(s), 3) co-worker(s), 4) social contacts (e.g. friends or non- household family members) or 5) multiple (more than one of these).
Self-reported compliance with the use of protective equipment when in close contact with patients with a febrile respiratory illness was defined as full (usually/always wash hands after patient contact and wear a surgical mask or N95 respirator, gloves, and a face shield or goggles), partial (usually/always wash hands after contact and wear a surgical mask or N95 respirator and gloves but sometimes/rarely/never wear face shield/goggles), limited (sometimes/rarely/never wash hands after contact, wear a surgical mask or N95 respirator, gloves and face shield/goggles) and not applicable (not in close contact with patients with a febrile respiratory illness). Participants were asked to rate their use of each type of protection on a four-point Likert-like scale of never/rarely, sometimes, usually/always or not applicable, and scored as full/partial/limited based on guidelines from the Public Health Agency of Canada [23]. Close contact was defined as within arm's reach. High-risk procedures were defined as nebulized therapies, manual ventilation, intubation, tube or needle thoracostomy, bronchoscopy/upper airway endoscopy, tracheostomy, continuous positive airway pressure, sputum induction or open suctioning.
Data analysis
The analysis was based on participant-weeks such that participants could be included more than once as a case and/or control. For the primary analysis, each case was matched with up to four controls from the same hospital site and study week (±1 week) to account for potential confounding associated with differences in circulating viruses. The post-hoc power analysis determined that there was 100% power to reject the null hypothesis of no association between exposure to symptomatic household contacts and a viral respiratory illness based on a type I error probability of 5%.
A multivariable conditional logistic regression model was used to assess the association between sources of acute respiratory illness exposure and viral respiratory infection or influenza occurrence, respectively. This was done to reduce confounding remaining beyond hospital site and study week. Variables associated with the outcome at P≤0.20 in bivariate analyses were entered into the full model and removed sequentially using the likelihood ratio test to assess changes in models. The variable whose elimination caused the least significant deterioration of model fit was removed and the process repeated until all variables were significant at P≤0.20 [24]. Age and sex were retained as they are associated with incidence of influenza and other viral respiratory infections [25]. Collinearity of eligible variables was investigated, and the final multivariable model was assessed for fit, influential observations and overfitting. Observations with missing data were dropped using listwise deletion.
Sensitivity analyses, using generalized estimating equations with exchangeable correlation, robust standard errors and logit link to account for clustering of data by individual (for those participating in more than one season), were conducted to assess the impact of ignoring correlated observations for multiple season participants in the conditional models. All parameter estimates were adjusted for week, season and hospital site to make them comparable with the conditional analyses' estimates. A second set of sensitivity analyses was conducted to determine the impact of assessing the exposures as one variable (against no exposure) as done in the primary analysis vs each exposure type individually, with multiple exposures as originally categorized (i.e. more than one per exposed participant). Stata Version 11 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
Of 3074 eligible participant-seasons, 2809 (91.4%) had complete baseline requirements and 2347 (76.4%) had complete illness diaries and submitted a swab for laboratory testing (cases) and/or complete weekly diaries with additional questions (controls). This analysis consists of data from 1735 healthcare personnel of whom 1303 participated for one season, 287 for two seasons, 110 for three seasons and 35 for all four seasons (2347 participant-seasons). In total, 814 swabs tested positive for a respiratory virus and were matched with an illness diary. There were 9005 weekly diaries with additional questions (median 4, range 1–13 per participant per season) from participants with no acute respiratory symptoms. The median age of participants was 44 years (interquartile range 34–52), 86% were female and 38% were nurses (see Table I ).
Table I.
Study participants, acute care hospital healthcare personnel, Canada, 2010/11–2013/14
| Cases (N=814) |
All controls (N=9005) |
Matched controlsa (N=3244) |
P-valueb | |
|---|---|---|---|---|
| Age in years, median (IQR) | 42 (34–50) | 45 (34–52) | 45 (35–52) | 0.79 |
| Sex | 0.57 | |||
| Female | 732 (89.9) | 7795 (86.6) | 2821 (87.0) | |
| Male | 82 (10.1) | 1210 (13.4) | 423 (13.0) | |
| Occupation | 0.19 | |||
| Nurse | 324 (39.8) | 3375 (37.5) | 1228 (37.8) | |
| Physician | 56 (6.9) | 501 (5.6) | 160 (4.9) | |
| Allied health staffc | 161 (19.8) | 1293 (14.4) | 505 (15.6) | |
| Support services staffc | 273 (33.5) | 3836 (42.6) | 1351 (41.6) | |
| Years working in profession, median (IQR) | 12 (6–24) | 13 (5–25) | 13 (5–24) | 0.97 |
| Hours worked per week, median (IQR) | 40 (37.5–40) | 40 (37.5–44) | 40 (37.5–43) | 0.42 |
| Asthma, self-report | 137 (16.8) | 1092 (12.1) | 381 (11.7) | 0.57 |
| Influenza vaccination, current season | 634 (77.9) | 6980 (77.5) | 2482 (76.5) | 0.23 |
| Housemates, median (IQR) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.26 |
| Close social contacts/week, median (IQR) | 10 (5–20) | 10 (5–20) | 10 (5–20) | 0.46 |
| Smoking habits | 0.19 | |||
| Never | 557 (68.4) | 6176 (68.6) | 2221 (68.5) | |
| Former | 197 (24.2) | 1984 (22.0) | 749 (23.1) | |
| Current (occasional or daily) | 59 (7.2) | 838 (9.3) | 273 (8.4) | |
| Hands-to-face habit | 552 (67.8) | 5746 (63.8) | 2088 (64.4) | 0.64 |
| Use refillable water bottle | 737 (90.5) | 7844 (87.1) | 2823 (87.0) | 0.90 |
| Hours of sleep per 24 h, median (IQR) | 7 (6–7) | 7 (6–7) | 7 (6, 7) | 0.73 |
| Reported stress level | 0.84 | |||
| Not at all/a bit | 556 (68.3) | 6139 (68.2) | 2218 (68.4) | |
| Quite a bit/extreme | 258 (31.7) | 2866 (31.8) | 1026 (31.6) | |
| Home handwashing, number per day, median (IQR) | 8 (5–10) | 8 (5–10) | 7 (5–10) | 0.72 |
| Physical contact with patientsd | 432 (53.1) | 4222 (46.9) | 1526 (47.0) | 0.88 |
| Protective equipment usee | 0.95 | |||
| Limited | 87 (10.7) | 1021 (11.4) | 367 (11.3) | |
| Partial | 201 (24.7) | 1807 (20.1) | 648 (20.0) | |
| Full | 278 (34.2) | 3077 (34.3) | 1128 (34.8) | |
| Not applicable (no exposure to patients with FRI) | 246 (30.3) | 3077 (34.3) | 1096 (33.8) | |
ARI, acute respiratory illness; FRI, febrile respiratory illness; IQR, interquartile range.
Cases were matched to four controls from the same hospital site and study week (±1 week).
P-value comparing all vs matched controls.
Allied health: technologists, pharmacists, therapists, social workers, others. Support services: administrative, patient attendants, housekeeping, others.
Direct physical contact with patients was more commonly reported by physician (90%), nursing (79%), and allied health (58%) staff than by support staff (11%) (P<0.001).
Limited: occasional use of surgical mask or N95 respirator, gloves and hand hygiene after contact. Partial: usually/always use surgical mask or N95 respirator, gloves and hand hygiene after contact. Full: usually/always use surgical mask or N95 respirator, gloves and hand hygiene after contact plus goggles/eye protection.
All 814 cases of viral respiratory infection were matched to 3244 controls from the same hospitals with diaries completed within 2 weeks of the case diary (807 cases to four controls, four cases to three controls each, one case to two controls, and one case to one control). Matching resulted in similar distributions of variables between matched and all controls.
As shown in Table II , 298 of the 814 (36.6%) participant-weeks with a viral respiratory illness had no known exposure during the previous week. Of the 836 (142 cases and 694 control) participant-weeks with more than one potential source, 721 reported two sources, 110 identified three sources, and five recounted four potential sources of exposure to respiratory illness (Table II). Exposure to ill patients was most frequently included in the multiple exposure category, while exposure to ill co-workers was included least often. Patients were the single identified sources of exposure for 73 of 814 (9%) cases, and may be responsible for an additional 34 (4%) viral respiratory illnesses (as a percentage of multiple exposures). In comparison, 20–24% of cases were exposed to household members with symptoms.
Table II.
Exposure to person(s) with symptoms of an acute respiratory illness in the previous 7 days by source(s), healthcare personnel, Canada, 2010/11–2013/14
| Source(s) of exposurea | Cases (N=814) |
All controls (N=9005) |
Matched controls (N=3244) |
|---|---|---|---|
| No exposures reported | 298 (36.6) | 5873 (65.2) | 2082 (64.2) |
| One source | N=374 | N=2438 | N=932 |
| Household | 166 (20.4) | 441 (4.9) | 173 (5.3) |
| Patient | 73 (9.0) | 1344 (14.9) | 523 (16.1) |
| Social contact | 73 (9.0) | 312 (3.5) | 107 (3.3) |
| Co-worker | 62 (7.6) | 341 (3.8) | 129 (4.0) |
| Multiple sources | N=142 | N=694 | N=230 |
| Household and co-worker | 12 (1.5) | 29 (0.3) | 9 (0.3) |
| Household and social | 24 (2.9) | 109 (1.2) | 42 (1.3) |
| Household and patient | 31 (3.8) | 122 (1.3) | 31 (1.0) |
| Co-worker and social | 15 (1.8) | 51 (0.6) | 16 (0.5) |
| Co-worker and patient | 18 (2.2) | 117 (1.3) | 42 (1.3) |
| Social and patient | 21 (2.6) | 172 (1.9) | 52 (1.6) |
| Household, co-worker and social | 5 (0.6) | 4 (0.04) | 3 (0.1) |
| Household, co-worker and patient | 5 (0.6) | 14 (0.2) | 6 (0.2) |
| Household, social and patient | 6 (0.7) | 27 (0.3) | 10 (0.3) |
| Co-worker, social and patient | 5 (0.6) | 44 (0.5) | 16 (0.5) |
| Household, co-worker, social and patient | 0 | 5 (0.01) | 3 (0.1) |
Source of exposure may include more than one person (e.g. exposure to a household may include exposure to more than one ill person in that household).
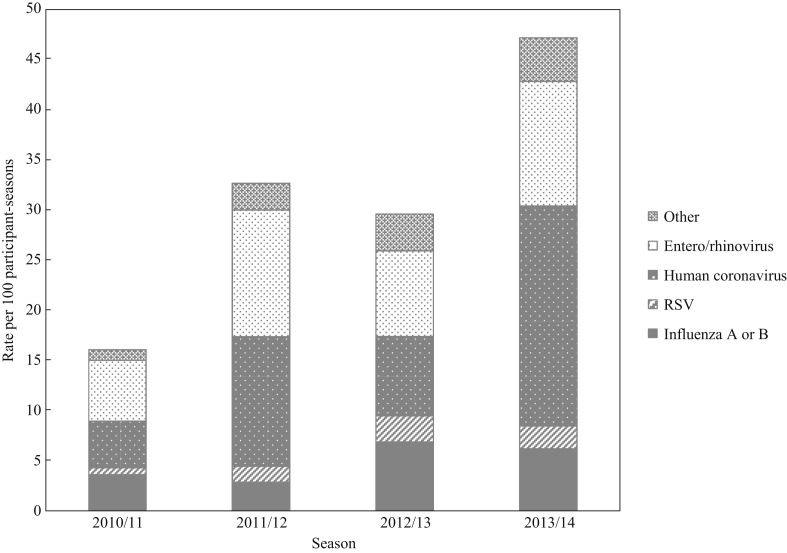
As shown in Figure 1 , human coronaviruses (N=318 or 13.5 per 100 participant-seasons), enterovirus/rhinovirus (N=250 or 10.7 per 100 participant-seasons) and influenza A or B (N=121 or 5.2 per 100 participant-seasons) were the top three viruses detected, with a higher rate of detection in 2013/14 than in earlier seasons.
Figure 1.
Seasonal rate of viral respiratory illness in hospital-based healthcare personnel, Canada, 2010/11–2013/14. RSV, respiratory syncytial virus; Other, human metapneumovirus, parainfluenza virus, adenovirus and bocavirus.
As shown in Table III , the OR that a healthcare worker with a viral respiratory infection was exposed to a patient with an acute respiratory illness in the previous week was not significantly higher than the OR that a healthcare worker without an illness was exposed. In contrast, the adjusted odds ratios (aORs) for exposure to household members, social contacts, co-workers and those reporting more than one exposure were significantly higher than for those with no known exposure. Participants who reported direct physical contact and/or care of patients had higher likelihood of infection, as did people with asthma and participants with hands-to-face habits (reported biting fingernails or frequently touching faces).
Table III.
Association of healthcare personnel participant characteristics with laboratory-confirmed viral respiratory illnesses, Canada, 2010/11–2013/14 winter seasons
| Factor | Crude OR |
aOR (95% CI)a |
|---|---|---|
| Exposure | ||
| No known | Referent | Referent |
| Patient | 1.02 (0.77–1.34) | 0.87 (0.65–1.17) |
| Household exposure | 7.16 (5.53–9.28)** | 7.00 (5.39–9.09)** |
| Co-worker exposure | 3.41 (2.43–4.79)** | 3.36 (2.39–4.74)** |
| Social exposure | 5.10 (3.65–7.12)** | 5.06 (3.62–7.09)** |
| Multiple | 4.28 (3.34–5.49)** | 3.84 (2.97–4.97)** |
| Age, 10-year increase | 0.91 (0.84–0.97)* | 0.96 (0.89–1.04) |
| Female (vs male) | 1.35 (1.05–1.74)* | 1.25 (0.95–1.65) |
| Asthma | 1.51 (1.22–1.87)** | 1.42 (1.13–1.79)* |
| Hands-to-face habits | 1.17 (0.99–1.38) | 1.21 (1.02–1.45)* |
| Use refillable water bottle | 1.42 (1.10–1.84)* | 1.28 (0.97–1.70) |
| Daily handwashing at home, N | 0.99 (0.98–1.01) | |
| Reported stress level | ||
| Not at all/a bit | Referent | |
| Quite a bit/extreme | 1.00 (0.85–1.18) | |
| Hours of sleep per 24 h | 1.05 (0.97–1.15) | |
| Smoking habits | ||
| Never | Referent | |
| Former | 1.05 (0.87–1.27) | |
| Current (occasional or daily) | 0.86 (0.64–1.16) | |
| Influenza vaccination, current season | 1.09 (0.90–1.32) | |
| Housemates, N | 1.09 (1.03–1.15)* | |
| Close non-household contacts/week, N | 1.00 (0.99–1.01) | |
| Occupation | ||
| Support services staffb | Referent | |
| Nurse | 1.31 (1.09–1.57)* | |
| Physician | 1.73 (1.24–2.41)** | |
| Allied health staffc | 1.58 (1.27–1.98)** | |
| Physical contact with patients | 1.29 (1.10–1.51)* | 1.31 (1.09–1.58)* |
| Years working in profession | 1.00 (0.99–1.01) | |
| Hours of work per week, 10-h increase | 0.99 (0.90–1.08) | |
| Protective equipment used | ||
| Limited | Referent | |
| Partial | 1.34 (0.99–1.78) | |
| Full | 1.05 (0.80–1.38) | |
| Not applicable | 0.95 (0.72–1.25) | |
aOR, adjusted odds ratio; AGMP, aerosol-generating medical procedure; ARI, acute respiratory illness; OR, odds ratio.
*P≤0.05.
**P≤0.001.
Adjusted for all column variables with estimates in column.
Administrative staff, patient attendants, housekeeping, laboratory technologists, infection control and others.
Medical imaging, pharmacists, physiotherapists, occupational and respiratory therapists, counsellors and others.
Limited: occasional use of surgical mask or N95 respirator, gloves and hand hygiene after contact. Partial: usually/always use surgical mask or N95 respirator, gloves and hand hygiene after contact. Full: usually/always use surgical mask or N95 respirator, gloves and hand hygiene after contact plus goggles/eye protection.
All 121 cases of influenza were successfully matched to four controls by hospital site, season and week. In the secondary analyses, exposure to patients with an acute respiratory illness did not increase the odds of influenza for healthcare personnel, but exposure to housemates, social contacts, co-workers and more than one source did increase the odds of influenza (Table IV ). Healthcare personnel with direct physical contact with patients had higher odds of influenza, while those who were vaccinated against influenza were protected. Between 9% and 15% of exposures resulting in influenza infection occurred following exposure to a patient with respiratory symptoms, while 22–24% followed exposure to a household member.
Table IV.
Association of healthcare personnel participant characteristics with laboratory-confirmed influenza, Canada 2010/11–2013/14 influenza seasons
| Factor | Crude OR (95% CI) |
aOR (95% CI)a |
|---|---|---|
| Exposure | ||
| No known | Referent | Referent |
| Patient | 1.11 (0.53–2.32) | 0.82 (0.37–1.80) |
| Household exposure | 6.73 (3.54–12.8)** | 6.44 (3.35–12.4)** |
| Co-worker exposure | 3.21 (1.40–7.36)* | 2.96 (1.22–7.15)* |
| Social exposure | 5.19 (2.23–12.1)** | 4.25 (1.74–10.4)* |
| Multiple | 3.73 (1.87–7.42)** | 3.05 (1.48–6.28)* |
| Age, 10-year increase | 0.85 (0.70–1.02) | 0.92 (0.75–1.14) |
| Female (vs male) | 1.19 (0.62–2.29) | 1.04 (0.50–2.15) |
| Asthma | 1.35 (0.77–2.37) | |
| Hands-to-face habits | 1.39 (0.90–2.14) | 1.49 (0.92–2.43) |
| Use refillable water bottle | 1.13 (0.63–2.03) | |
| Daily handwashing at home, N | 0.99 (0.96–1.02) | |
| Reported stress level | ||
| Not at all/a bit | Referent | |
| Quite a bit/extreme | 1.03 (0.67–1.58) | |
| Hours of sleep per 24 h | 0.90 (0.73–1.12) | |
| Smoking habits | Referent | |
| Never | 0.93 (0.58–1.51) | |
| Former | 1.28 (0.67–2.43) | |
| Current (occasional or daily) | ||
| Influenza vaccination, current season | 0.51 (0.32–0.80)* | 0.56 (0.33–0.93)* |
| Housemates, N | 1.06 (0.91–1.23) | |
| Close non-household contacts/week, N | 1.00 (0.99–1.02) | |
| Occupation | Referent | |
| Support services staffb | 1.77 (1.11–2.83)* | |
| Nurse | 1.10 (0.45–2.67) | |
| Physician | 2.25 (1.26–4.02)* | |
| Allied health staffc | ||
| Physical contact with patients | 1.68 (1.11–2.55)* | 1.96 (1.20–3.20)* |
| Years working in profession | 0.99 (0.98–1.01) | |
| Hours of work per week, 10-h increase | 0.85 (0.67–1.09) | |
| Protective equipment used | ||
| Limited | Referent | |
| Partial | 1.59 (0.71–3.59) | |
| Full | 1.52 (0.72–3.21) | |
| Not applicable | 1.10 (0.51–2.37) | |
aOR, adjusted odds ratio; AGMP, aerosol-generating medical procedure; ARI, acute respiratory illness; OR, odds ratio.
*P≤0.05.
**P≤0.001.
Adjusted for all column variables with estimates in column.
Administrative staff, patient attendants, housekeeping, laboratory technologists, infection control and others.
Medical imaging, pharmacists, physiotherapists, occupational and respiratory therapists, counsellors and others.
Limited: occasional use of surgical mask or N95 respirator, gloves and hand hygiene after contact. Partial: usually/always use surgical mask or N95 respirator, gloves and hand hygiene after contact. Full: usually/always use surgical mask or N95 respirator, gloves and hand hygiene after contact plus goggles/eye protection.
The results of generalized estimating equation models demonstrated no substantive differences in results (data not shown). Similarly, analyses comparing each exposure type as an individual variable, with multiple exposures counted under each applicable type, resulted in somewhat lower aORs per type but no difference in their relative sizes. There were also no differences in the aOR estimate or significance level of other (non-exposure) variables (data not shown).
Discussion
Acute care hospital based healthcare personnel who developed a laboratory-confirmed viral respiratory infection during the 2010/11–2013/14 winter seasons in Canada were significantly more likely to report exposure to a household member, co-worker or other community contact with a respiratory illness in the week prior to their own illness than participants who did not. In contrast, exposure to a patient with acute respiratory illness was not associated with increased odds of an infection. The same results occurred when the outcome was restricted to healthcare personnel who became ill with influenza.
The present data regarding exposure risk in households are compatible with the results of three studies of influenza during the 2009 influenza pandemic and one small study of respiratory illness in an intensive care unit setting during the 2008/09 winter season [11,12,14,15]. All of these studies identified the exposure of healthcare personnel to household contacts as having the highest odds of respiratory illness after exposure, with ORs ranging from 2.9 to 16.7. These data are also supported by several studies documenting that the number of children in a household is a risk for influenza in adults, which is to be expected since the seasonal attack rate for influenza in children is higher than that for adults [13,16,25].
Data regarding the risk of viral respiratory infection in healthcare personnel as a result of exposure to patients and colleagues are less consistent. Outbreaks of viral respiratory infection involving substantial transmission among staff have been identified [12,17]. Vanhems et al. [18] found the risk of influenza-like illness among patients and staff to be interlinked, but Choi et al. [19] identified no increased risk associated with exposure to ill colleagues. The proportion of influenza A (H1N1) 2009 pandemic illness in healthcare personnel thought to be due to transmission from colleagues ranged from 3.1% to 23% in different cohorts [11,14,15,17,[19], [20], [21],26]. Some of these differences may be due to study methods. For instance, Choi et al.‘s study was a retrospective questionnaire administered more than 1 year after the first wave of the pandemic, and illness in colleagues may have been under-reported. Other disparities may be due to true differences in hospital design or infection prevention practices. Another issue is the attribution of exposure sources. In some studies, multiple contacts were attributed to only one source – patients in some instances – while other studies failed to assess the impact of community contacts [17,20,27]. Future transmission of viral respiratory infection studies in healthcare personnel need to assess the impact of a variety of potential sources including patients, co-workers, household, non-household (social/community) and contemporaneous (i.e. non-attributable) exposures.
These data confirm the findings of other studies that most viral respiratory illnesses in healthcare personnel occur as a result of exposure at home rather than occupational exposure [[11], [12], [13], [14], [15]]. In this cohort, only 9–13% of viral illnesses were probably or possibly associated with exposure to a symptomatic patient. The facts that over 35% of viral infections had no known exposure, that HCPs with direct patient contact were more likely to develop infection, and that recognized exposure to patients with acute respiratory illness was not a risk, suggest that exposure to undetected and unsuspected viral respiratory illness may be a greater risk to healthcare providers than exposure to identified patients. This is not surprising given the evidence that influenza infection in the frail elderly often presents atypically [28,29], and that a non-trivial percentage of influenza infections are asymptomatic or pauci-symptomatic [30,31]. The present findings also suggest that isolating and measuring the risk to healthcare personnel from recognized patients with viral illness is difficult; the findings in different studies that healthcare personnel wearing different types of protective equipment have similar rates of viral infection may be because most infections are a result of non-occupational exposures or exposures to patients not recognized as ill, rather than because the different types of protective equipment provide similar levels of protection [32,33].
This study was unable to detect an association between infection and self-reported degree of adherence to personal protective equipment in caring for patients with acute respiratory illness. However, several other studies, including those summarized in at least three systematic reviews, provide evidence that personal protective equipment reduces the risk of transmission of infection [[34], [35], [36]]. The present results may be due to a lack of power or could be explained by differences in reported and actual behaviour, misuse of protective equipment and/or transmission from non-patient sources. Jessee and Mion report that nurses' self-reported adherence to precautions was significantly higher than observed behaviour, reflecting high levels of knowledge about precautions but also social desirability bias in answering survey questions [37]. Although some healthcare personnel may disregard or fail to recall recommended precautions (approximately 11% of healthcare personnel in this study reported limited use of protective equipment when caring for patients with a febrile respiratory illness), Krein et al. report that many healthcare personnel in their study made inadvertent mistakes when using protective equipment, putting themselves at risk of exposure [38].
Not unexpectedly, influenza vaccination was protective against influenza specifically, but not for viral respiratory infections in general. This finding is consistent with several recent publications in which influenza vaccination was confirmed to be protective against laboratory-confirmed influenza for healthcare personnel [[39], [40], [41]], and reinforces the benefits of ongoing vaccination programmes in healthcare facilities. In this cohort, household members, social contacts and co-workers represent approximately 80% of the known sources associated with influenza while exposure to patients represents the other 20%. These findings highlight the importance of vaccination programmes to protect vulnerable patients from influenza transmission via their healthcare provider.
This study has several strengths, including the enrolment of healthcare personnel from several different hospitals over four winter seasons. The prospective nature of the study and the completion of illness diaries at the time of swab collection (prior to the results being known) reduced the likelihood that cases biased their recall regarding who they were exposed to. Although the period of recall was only 7 days, participants may have been less likely to recall exposures on weeks when they were not ill and/or differentially recall exposures in different settings.
In conclusion, this study highlights the importance of community contacts as potential sources of viral respiratory infection in acute care healthcare personnel. Given that 95% of healthcare personnel in this cohort attended work while symptomatic with an acute respiratory illness [22], the potential for worker-to-worker and/or healthcare worker-to-patient transmission is high. Providing care to patients with undetected viral respiratory infection may explain why reported exposure to patients with an acute respiratory illness was not a risk factor for infection while having physical contact with patients was a risk factor.
Acknowledgements
The authors wish to thank the staff and study participants for their contributions to this research. The authors also wish to acknowledge the contribution of non-author members of the Canadian Healthcare Worker Study Group.
Conflict of interest statement
None declared.
Funding sources
This work was funded by the Canadian Institutes of Health Research (Grant No. 111187) and the Ontario Workplace Safety and Insurance Board (Grant No. 10031).
Contributor Information
B.L. Coleman, Email: b.coleman@utoronto.ca.
for the Canadian Healthcare Worker Study Group:
T. Hatchette, L. Holness, J. Raboud, J. Langley, T. Mazzulli, K. Nichol, L. Genesove, J. Oudyk, L. McCaskell, and N. Johnson
References
- 1.Ferkol T., Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404–406. doi: 10.1513/AnnalsATS.201311-405PS. [DOI] [PubMed] [Google Scholar]
- 2.Fahey T., Stocks N., Thomas T. Systematic review of the treatment of upper respiratory tract infection. Arch Dis Child. 1998;79:225–230. doi: 10.1136/adc.79.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau Y.F., Koh W.V., Kan C., Dua P.A., Lim A.E., Liaw C.J. Epidemiologic analysis of respiratory viral infections among Singapore military servicemen in 2016. BMC Infect Dis. 2018;18:123. doi: 10.1186/s12879-018-3040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monto A.S. Epidemiology of viral respiratory infections. Am J Med. 2002;112:4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 5.Aitken C., Jeffries D.J. Nosocomial spread of viral disease. Clin Microbiol Rev. 2001;14:528–546. doi: 10.1128/CMR.14.3.528-546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huttunen R., Syrjanen J. Healthcare workers as vectors of infectious diseases. Eur J Clin Microbiol Infect Dis. 2014;33:1477–1488. doi: 10.1007/s10096-014-2119-6. [DOI] [PubMed] [Google Scholar]
- 7.Horcajada J.P., Pumarola T., Martinez J.A., Tapias G., Bayas J.M., de la Prada M. A nosocomial outbreak of influenza during a period without influenza epidemic activity. Eur Respir J. 2003;21:303–307. doi: 10.1183/09031936.03.00040503. [DOI] [PubMed] [Google Scholar]
- 8.Harvala H., Gaunt E., McIntyre C., Roddie H., Labonte S., Curran E. Epidemiology and clinical characteristics of parainfluenza virus 3 outbreak in a haemato-oncology unit. J Infect. 2012;65:246–254. doi: 10.1016/j.jinf.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reese S.M., Thompson M., Price C.S., Young H.L. Evidence of nosocomial transmission of human rhinovirus in a neonatal intensive care unit. Am J Infect Control. 2016;44:355–357. doi: 10.1016/j.ajic.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuster S.P., Coleman B.L., Raboud J., McNeil S.A., De Serres G., Gubbay J.B. Risk factors for influenza among health care workers during 2009 pandemic, Toronto, Ontario, Canada. Emerg Infect Dis. 2013;19:606–615. doi: 10.3201/eid1904.111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stupica D., Lusa L., Petrovec M., Zigon N., Jevsnik M., Bogovic P. Respiratory viruses in patients and employees in an intensive care unit. Infection. 2012;40:381–388. doi: 10.1007/s15010-012-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams C.J., Schweiger B., Diner G., Gerlach F., Haaman F., Krause G. Seasonal influenza risk in hospital healthcare workers is more strongly associated with household than occupational exposures: results from a prospective cohort study in Berlin, Germany, 2006/07. BMC Infect Dis. 2010;10:11. doi: 10.1186/1471-2334-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seto W.-H., Cowling B.J., Lam H.-S., PTY Ching, To M.-L., Pittet D. Clinical and nonclinical health care workers faced a similar risk of acquiring 2009 pandemic H1N1 infection. Clin Infect Dis. 2011;53:280–283. doi: 10.1093/cid/cir375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du M., Suo J., Jia N., Xing Y., Xie L., Liu Y. The cross-transmission of 2009 pandemic influenza A(H1N1) infections among healthcare workers and inpatients in a Chinese tertiary hospital. Infect Control Hosp Epidemiol. 2012;33:295–298. doi: 10.1086/664050. [DOI] [PubMed] [Google Scholar]
- 16.Marshall C., Kelso A., McBryde E., Barr I.G., Eisen D.P., Sasadeusz J. Pandemic (H1N1) 2009 risk for frontline health care workers. Emerg Infect Dis. 2011;17:1000–1006. doi: 10.3201/eid1706.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magill S.S., Black S.R., Wise M.E., Kallen A.J., Lee S.J., Gardner T. Investigation of an outbreak of 2009 pandemic influenza A virus (H1N1) infections among healthcare personnel in a Chicago hospital. Infect Control Hosp Epidemiol. 2011;32:611–615. doi: 10.1086/660097. [DOI] [PubMed] [Google Scholar]
- 18.Vanhems P., Voirin N., Roche S., Escuret V., Regis C., Gorain C. Risk of influenza-like illness in an acute health care setting during community influenza epidemics in 2004–2005, 2005–2006, and 2006–2007: a prospective study. Arch Intern Med. 2011;171:151–157. doi: 10.1001/archinternmed.2010.500. [DOI] [PubMed] [Google Scholar]
- 19.Choi S.H., Chung J.W., Jeon M.H., Lee M.S. Risk factors for pandemic H1N1 2009 infection in healthcare personnel of four general hospitals. J Infect. 2011;63:267–273. doi: 10.1016/j.jinf.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise M.E., De Perio M., Halpin J., Jhung M., Magill S., Black S.R. Transmission of pandemic (H1N1) 2009 influenza to healthcare personnel in the United States. Clin Infect Dis. 2011;52(Suppl. 1):S198–S204. doi: 10.1093/cid/ciq038. [DOI] [PubMed] [Google Scholar]
- 21.Yeom J.S., Lee J.H., Bae I.G., Oh W.S., Moon C.S., Park K.H. 2009 H1N1 influenza infection in Korean healthcare personnel. Eur J Clin Microbiol Infect Dis. 2011;30:1201–1206. doi: 10.1007/s10096-011-1213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L., McGeer A., McNeil S., Katz K., Loeb M., Muller M.P. Which healthcare workers work with acute respiratory illness? Evidence from Canadian acute-care hospitals during 4 influenza seasons: 2010–2011 to 2013–2014. Infect Control Hosp Epidemiol. 2019;40:889–896. doi: 10.1017/ice.2019.141. [DOI] [PubMed] [Google Scholar]
- 23.Public Health Agency of Canada . Government of Canada; Ottawa, ON: 2016. Routine practices and additional precautions for preventing the transmission of infection in healthcare settings. [PubMed] [Google Scholar]
- 24.Vittinghoff E., Glidden D.V., Shiboski S.C., McCulloch C.E. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. In: Gail M., Tsiatis A., Krickeberg K., Wong W.J.S., editors. Statistics for biology and health. Springer; New York: 2005. [Google Scholar]
- 25.Huang Q.S., Bandaranayake D., Wood T., Newbern E.C., Seeds R., Ralston J. Risk factors and attack rates of seasonal influenza infection: results of the southern hemisphere influenza and vaccine effectiveness research and surveillance (SHIVERS) seroepidemiologic cohort study. J Infect Dis. 2019;219:347–357. doi: 10.1093/infdis/jiy443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Perio M.A., Brueck S.E., Mueller C.A., Milne C.K., Rubin M.A., Gundlapalli A.V. Evaluation of 2009 pandemic influenza A(H1N1) exposures and illness among physicians in training. Am J Infect Control. 2012;40:617–621. doi: 10.1016/j.ajic.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeger J.L., Patel M., Dharan N., Hancock K., Meites E., Mattson C. Transmission of 2009 pandemic influenza A(H1N1) virus among healthcare personnel-southern California, 2009. Infect Control Hosp Epidemiol. 2011;32:1149–1157. doi: 10.1086/662709. [DOI] [PubMed] [Google Scholar]
- 28.Monmany J., Rabella N., Margall N., Domingo P., Gich I., Vazquez G. Unmasking influenza virus infection in patients attended to in the emergency department. Infection. 2004;32:89–97. doi: 10.1007/s15010-004-3088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babcock H.M., Merz L.R., Fraser V.J. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol. 2006;27:266–270. doi: 10.1086/501539. [DOI] [PubMed] [Google Scholar]
- 30.Ip D.K.M., Lau L.L., Leung N.H., Fang V.J., Chan K.H., Chu D.K. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin Infect Dis. 2017;64:736–742. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung N.H.L., Xu C., Ip D.K.M., Cowling B.J. The fraction of influenza virus infections that are asymptomatic: a systematic review and meta-analysis. Epidemiology. 2015;26:862–872. doi: 10.1097/EDE.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeb M., Dafoe N., Mahony J., John M., Sarabia A., Glavin V. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 33.Radonovich L.J., Jr., Simberkoff M.S., Bessesen M.T., Brown A.C., Cummings D.A.T., Gaydos C.A. N95 respirators vs masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Offeddu V., Yung C.F., Low M.S.F., Tam C.C. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.French C.E., McKenzie B.C., Coope C., Rajanaidu S., Paranthaman K., Pebody R. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respir Viruses. 2016;10:268–290. doi: 10.1111/irv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jefferson T., Del Mar C.B., Dooley L., Ferroni E., Al-Ansary L.A., Bawazeer G.A. The Cochrane Library; Oxford: 2011. Physical interventions to interrupt or reduce the spread of respiratory viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jessee M.A., Mion L.C. Is evidence guiding practice? Reported versus observed adherence to contact precautions: a pilot study. Am J Infect Control. 2013;41:965–970. doi: 10.1016/j.ajic.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Krein S.L., Mayer J., Harrod M., Weston L.E., Gregory L., Petersen L. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: a qualitative study. JAMA Intern Med. 2018;178:1016–1057. doi: 10.1001/jamainternmed.2018.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuster S.P., Shah P.S., Coleman B.L., Lam P.P., Tong A., Wormsbecker A. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dini G., Toletone A., Sticchi L., Orsi A., Bragazzi N.L., Durando P. Influenza vaccination in healthcare workers: a comprehensive critical appraisal of the literature. Hum Vaccin Immunother. 2017;8:1–18. doi: 10.1080/21645515.2017.1348442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai C., Toizumi M., Hall L., Lambert S., Halton K., Merollini K. A systematic review and meta-analysis of the direct epidemiological and economic effects of seasonal influenza vaccination on healthcare workers. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198685. [DOI] [PMC free article] [PubMed] [Google Scholar]