Abstract
Background
Several animal studies, and one inoculation study in adult asthmatics have shown that interleukin-33 (IL-33) is a major contributor to type-2 inflammation in acute asthma. However, the link between IL-33 and type-2 inflammation has not been shown in naturally occurring asthma exacerbations.
Objectives
To determine if airway IL-33 is associated with type-2 inflammation measured by type-2 cytokines, FeNO and sputum eosinophils in patients presenting to the Emergency Department with an asthma exacerbations.
Methods
Adult patients hospitalized due to acute asthma were enrolled. Upper airways were sampled with nasal swabs and lower airways with induced sputum. Cytokines were measured at protein level using a Luminex® assay and mRNA expression level using droplet-digital-PCR. Airway sampling was repeated four weeks after exacerbation.
Results
At the time of exacerbation, upper airway IL-33 correlated with upper airway IL-5 and IL-13 (R = 0.84, p < 0.01 and R = 0.76, p < 0.01, respectively) and with lower airway IL-13 (R = 0.49, p = 0.03). Similar associations were observed for mRNA expression. Lower airway IL-33 positively correlated with lower airway IL-13 (R = 0.84, p < 0.01). IL-13 and IL-33 were positively correlated with FeNO, and IL-5 with eosinophils. The association between IL-33 and type-2 cytokines were still present four weeks after exacerbation.
Conclusion
This is the first study to demonstrate that airway IL-33 is associated with type-2 cytokines in naturally occurring asthma exacerbations in adults, providing in vivo evidence supporting that IL-33 may be driving type-2 inflammation in acute asthma. Thus supporting IL-33 as a potential future drug target due to its role, upstream in the immunological cascade.
Keywords: Interleukin-33, Acute asthma, Exacerbation, Type 2 cytokines, Adults
Abbreviations: ACQ, asthma control questionnaire; COPD, chronic obstructive pulmonary disease; DD PCR, droplet digital polychain reaction; ED, emergency department; FeNO, fractional exhaled nitric oxide; ICS, inhaled corticosteroids; UTM, universal transport medium
Highlights
-
•
IL-33 correlates to type 2 cytokines in hospitalized patients with acute asthma.
-
•
Airway IL-13 and IL-33 positively correlate with fractional exhaled nitric oxide.
-
•
Airway IL-5 correlates to blood eosinophils in naturally occurring acute asthma.
Key message
Airway IL-33 correlates with type 2 cytokines and FeNO in naturally occurring asthma exacerbations.
Summary at a glance
Upper and lower airways of adult asthmatics admitted to hospital with an acute exacerbation, were sampled. Levels of IL-33 were found to correlate with IL-5 and IL-13, providing in vivo evidence of the role of IL-33 in acute asthma.
1. Introduction
Every year asthma exacerbations are a major cause of morbidity and increased health care expenses and can be fatal, although this is rare. The airway epithelium has been suggested to play an active role in promoting type 2 inflammation in the airways during asthma exacerbations, by releasing cytokines such as IL-33 in response to epithelial damage [1,2]. IL-33 is increased in asthmatics and is associated with asthma severity [3,4].
Previous studies have shown that respiratory viral infections trigger exacerbation in 40–80% of patients with adult asthma [[5], [6], [7], [8], [9], [10]]. In an experimental rhinovirus study, Jackson et al. demonstrated that mild to moderate asthma patients had increased IL-33 and that it correlated with type 2 cytokines after virus inoculation [11]. This possibly explains why asthma patients experience worsening of symptoms, loss of control and ultimately exacerbation, when infected with common viruses, but the immunological pathways involved in such acute episodes of asthma are yet to be fully understood.
The potential relationship between IL-33, type 2 cytokines, airway eosinophilia and fractional exhaled nitric oxide (FeNO) have not been examined in naturally occurring asthma exacerbations.
Thus, the aim of this study was to determine whether IL-33 is up-regulated in naturally occurring asthma exacerbations, and furthermore, if IL-33 is associated with increased type 2 airway inflammation as measured by type 2 cytokines, FeNO, sputum- and blood eosinophils.
2. Methods
2.1. Study design and subjects
This study was conducted at Bispebjerg University Hospital. Patients between 18 and 60 years of age admitted with an acute exacerbation of asthma were enrolled during a 4 month period beginning in June 2015. Patients with pulmonary disease other than asthma (e.g. COPD) were excluded. To reflect a representative real-life group of patients admitted to hospital due to acute asthma, all current and former smokers were also included in the study. Written informed consent was obtained, and the study was approved by the local ethics committee (H-15003691).
Patients were examined within 24 h of admission and again after 4 weeks. All patients were initially treated according to local guidelines with inhalations of salbutamol/ipratropium and 80 mg methylprednisolone i. v., followed by nine days of oral prednisone 37.5 mg once daily.
2.2. Asthma assessment
Fractional exhaled nitric oxide (FeNO) was measured using NIOX® (Aerocrine AB, Sweden) following the recommendations of ERS/ATS [12], using the mean of two measurements.
Lung function was measured using EasyOne™ diagnostic Spirometer (Medical Technologies, Switzerland) and performed according to ERS guidelines.
Sputum was induced according to the ERS guidelines and processed as described by Pavord et al. [13]: After pre-treatment with terbutaline sputum was induced using the EasyNeb 3 (Flaem Nuova, Italy). The patients inhaled hypertonic saline solutions with increasing concentrations (3, 4 and 5% sodium chloride solutions) for 5 min each. Sputum plugs was processed in 0.2% dithiothreitol (Sputolysin®, Calbiochem, USA) and PBS. Cytospins were prepared, stained and differential cell counts were obtained from 400 non-squamous cells expressed as the percentage of non-squamous cells. Supernatant and cell pellets were added to RNAlater ® (Ambion inc., USA) and stored at −80 °C until protein and mRNA analysis were performed. These samples were considered representative of lower airways.
Nasal swabs were collected using flocked swabs (FLOQSwabs™, Copan, Italia).
The swab was inserted into each nostril until resistance was met and rotated six times clockwise. The swab was then placed in the universal transport medium (UTM) supplied. The collected material from upper airways was released into the media from the swab. The swab was then removed and the vial containing UTM was spun at 600 g and the supernatant and cell pellet were kept at −80 °C until protein and mRNA analysis were performed.
After completing the nasal swab sampling, a nasal lavage were performed as described by Gern and colleges [14] using 5 mL of preheated Hanks Balanced Salt Solutions with 0.5% gelatine. The expelled fluid was spun at 600 g and the supernatant and cell pellet were kept at −80 °C until virus detection were performed.
Blood samples were collected from each subject in the ED shortly after admission and a full white blood cell differential count was completed by the hospital laboratory.
Asthma control was assessed with the 5-item Asthma Control Questionnaire (ACQ-5) [15].
2.3. Protein analysis
IL-5, IL-13 and IL-33 protein were measured using multiplex analyte detection Luminex® assay (Merck Millipore) according to the manufacturer's instructions.
The analyses were performed at the laboratories of Medimmune, Cambridge.
2.4. mRNA analysis
RNA-extraction was done using TRIzol® (Ambion, USA) and RNeasy MinElute (Qiagen, Germany) as per manufacturers instructions.
Droplet digital PCR (DD-PCR) is a novel 3rd generation, highly sensitive PCR technique (Bio-rad, USA) that is able to detect low levels of mRNA expression [16]. The PCR was done using TaqMan master mix, qPCR primer and probes (Applied Biosystems, USA). The mRNA-concentrations were calculated using the Bio-rad DD-PCR-software ‘QuantaSoft™’.
2.5. Detection of respiratory pathogens
Virus typing was performed using a tandem multiplex real-time PCR assay to detect a comprehensive range of respiratory viruses (human adenovirus species B-D; human bocavirus; coronaviruses OC43, 229E, HKU1 and NL63; influenza viruses A, B and C; parainfluenzaviruses 1–4; KI and WU polyomaviruses; respiratory syncytial virus types A and B and human metapneumovirus) [17]. Rhinoviruses were detected with a separate RT-PCR [18,19]. Bacterial cultures were completed by the hospital laboratory on spontaneous expectorate gathered at exacerbation.
2.6. Statistical analysis
The statistical analysis was undertaken using SPSS version 22 (IBM, USA). Normally distributed data were reported as mean and SD. Non-normally distributed data were reported as median and range.
The correlation analyses used Pearsons Correlations for normally distributed data, and for data presenting as normally distributed after log-transformation. Spearman Correlation was used for variables without normal distribution even after log-transformation.
Un-paired analysis used the Mann–Whitney-test and the paired-analysis was done using the Wilcoxon matched-pairs test.
3. Results
A total of 19 patients (female: 57.9%) agreed to participate in the study aged from 22 to 52 years (median: 34). The characteristics of the subjects are shown in Table 1 . At exacerbation, peakflow in the ED was reduced to 64.5% (SD: 16.6) of predicted after salbutamol/ipratropium-nebulizing treatment. Around two thirds of patients had sputum eosinophils ≥3% (63.6%) and a similar proportion had FeNO > 25 ppb (73.7%), although we did not observe a statistically significant overlap between the two (Fisher's Exact test, p= 0.109).
Table 1.
Characteristics of study participants.
| Characteristics at exacerbation | |
|---|---|
| Age (years) | 34.0 (22–52) |
| Gender (%female) | 57.9% |
| BMI (kg/m2) | 25.5 (19.1–39.5) |
| Blood eosinophils (x10ˆ/L) | 0.3 (0–1.2) |
| Peakflow % of pred. post beta2 – In the ED | 64.5 (±16.6) |
| Current smoker (%) | 26.3% |
| Former smoker (%) | 26.3% |
| Pack-years (20 cigarettes/day for a year) | 2.5 (0–40) |
| Daily ICS dose* (n = 7) | 800 (350–1440) |
| FEV1 (% of predicted) | 67.9 (±24.6) |
| FeNO > 25 ppb (%) | 73.7% |
| FeNO (ppb) | 34.0 (7–102) |
| 5-item ACQ score | 3.8 (0.2–5.4) |
| Sputum Eosinophils > 3% | 63.6% |
| Sputum Eosinophils (%) | 6.5 (0–36.5) |
ED: Emergency Department, ICS: Inhaled Corticosteroids, ppb: parts per billion, ACQ: asthma control questionnaire. * Budesonide equivalent dose.
Seven subjects had a positive PCR rhinovirus (36.8%) at the time of exacerbation, five of whom had RV-A and two had RV-C. No pathogen (viral or bacterial) other than rhinovirus was detected.
To reflect a representative real-life group of patients admitted to hospital due to acute asthma, all current and former smokers were additionally included in the study. Five were current smokers (26.3%) and five were former smokers (26.3%), with a median of 2.5 pack years (0–40 pack years).
3.1. IL-33 and the type 2 cytokines at exacerbation
Using the Luminex® protein assay, it was possible to detect IL-33 from the upper airways nasal swab samples and from the lower airway induced sputum samples as well as in serum.
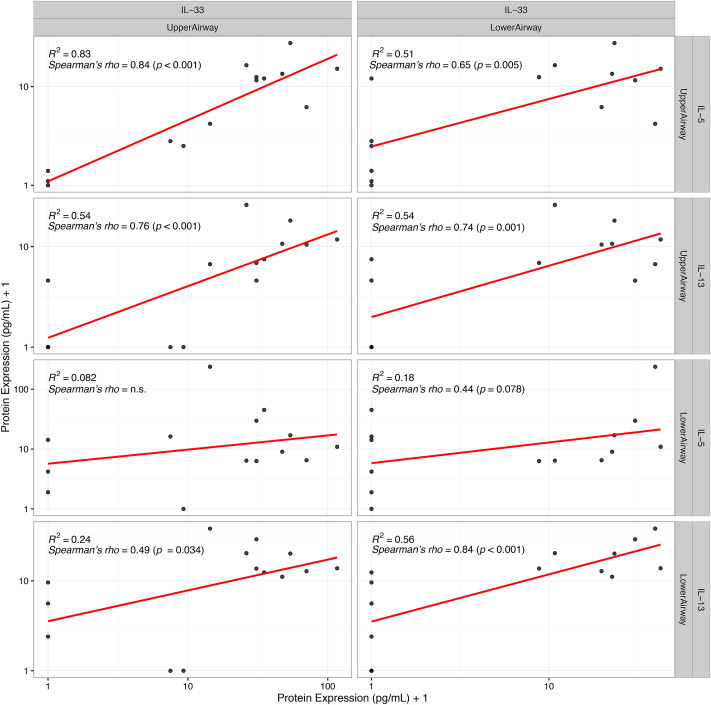
Upper Airway IL-33 protein correlated with IL-5 (R = 0.84, p < 0.01) and IL-13 (R = 0.76, p < 0.01) in the upper airways (Fig. 1 ). Furthermore, IL-33 protein in the upper airways correlated with lower airway IL-13 (R = 0.49, p = 0.034), whereas no relationship with lower airway IL-5 was detected (R = 0.12, p = 0.65) (Fig. 1).
Fig. 1.
Protein IL-33 in upper and lower airways and the association with type 2 cytokines in both upper and lower airways at exacerbation. In all compartments IL-33 is positively correlated with the type 2 cytokines IL-5 and IL-13. Correlations shown on plot are done with exact protein concentration. For illustrative purposes all cytokine values have been added 1 in this plot due to the use of a log scale.
Associations between lower airway IL-33 and type 2 cytokines were similar to those found in the upper airways: IL-33 protein in lower airways correlated with lower airway IL-13 (R = 0.84, p < 0.01), and a trend towards a positive correlation was found with lower airway IL-5 (R = 0.44, p = 0.08) (Fig. 1).
3.2. IL-33 and markers of eosinophilic airway inflammation at exacerbation
FeNO was positively correlated with IL-33 protein in both upper (R = 0.53, p = 0.02) and lower (R = 0.58, p = 0.01) airways at exacerbation. IL-33 did not correlate with sputum eosinophils. However, there was a trend towards a positive correlation between blood-eosinophils and IL-33 protein levels in lower airways (R = 0.40, p = 0.09).
3.3. Type 2 cytokines and markers of eosinophilic airway inflammation at exacerbation
The level of IL-13 protein in both upper (R = 0.53, p = 0.031) and lower airways (R = 0.47, p = 0.044) correlated with FeNO at the time of exacerbation. Furthermore, the level of IL-5 protein in the lower airways (R = 0.49, p = 0.047) correlated with blood eosinophils.
3.4. Serum levels
IL-33 protein levels could be detected in serum. However, these levels did not correlate to lower airway (R = 0.18, p = 0.45) or upper airway (R = 0.01, p = 0.97) IL-33 levels. No relationship between serum IL-33 levels and markers of type 2 inflammation, in either serum or airways, were found.
3.5. mRNA expression
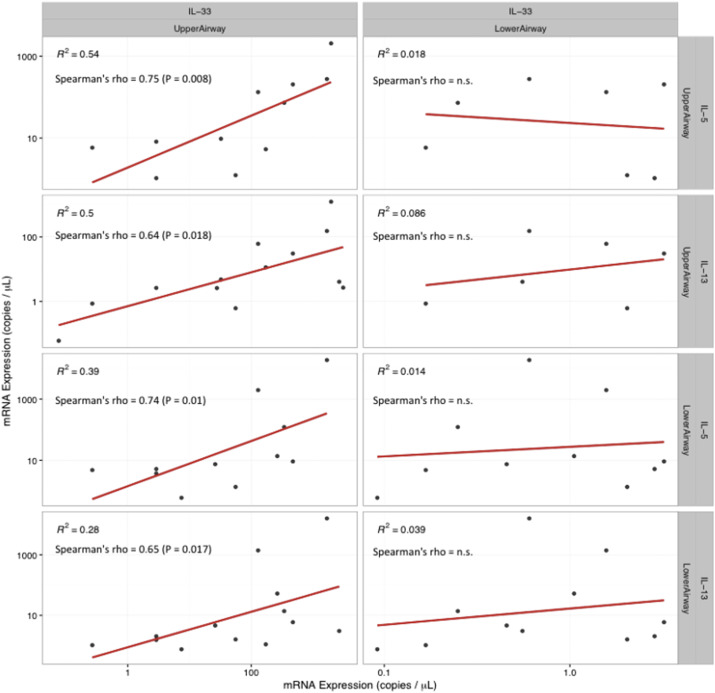
Droplet digital PCR was used to quantify IL-33, IL-5, and IL-13 mRNA expression. The relationship between IL-33 mRNA expression in the upper airways and the type 2 cytokines in both upper and lower airways followed the same pattern found at the protein level (Fig. 2 ): IL-33 mRNA expression in the upper airways correlated with upper airway IL-5 (R = 0.75, p = 0.008) and upper airway IL-13 (R = 0.64, p = 0.018). Similar associations between IL-33 mRNA expression in the upper airways and lower airway IL-5 (R = 0.74, p = 0.010) and IL-13 (R = 0.65, p = 0.017) were found. No association between lower airway IL-33 mRNA expression and the type 2 cytokines IL-5 and IL-13 were found. Additionally, no associations between IL-33 and markers of eosinophilic airway inflammation was found on mRNA expression level at exacerbation.
Fig. 2.
mRNA expression of IL-33 in upper and lower airways and the association with type 2 cytokines in both upper and lower airways at exacerbation. mRNA expression levels at least at exacerbation supports the positive correlations between IL-33 and the type 2 cytokines IL-5 and IL-13 found on protein level.
3.6. Symptoms and clinical features at 4 weeks follow-up
The four-week follow-up was completed by 15 patients (79%). The 5-item ACQ score improved with 1.8 (p = 0.0001) from exacerbation to follow-up. FEV1 showed similar pattern of remission with increasing lung function (p = 0.015) (Table 2 ). However, in contrast, FeNO, sputum and blood eosinophil levels did not change from exacerbation to follow-up, indicating that the type 2 inflammation was not in significant remission four weeks after an acute exacerbation.
Table 2.
ACQ, FEV1, FeNO, blood and sputum eosinophils 4 weeks after exacerbation.
| Exacerbation | 4 weeks follow-up | Delta-values | p-valuea | |
|---|---|---|---|---|
| ACQ | 3.3 (±1.47) | 1.5 (±1.26) | −1.80 (±1.32) | 0.0001 |
| FEV1 -Percentage of predicted | 68.3 (±23.5) | 78.6 (±21.3) | 10.3 (±14.4) | 0.015 |
| FeNO (ppb) | 42.2 (±23.6) | 31.5 (±29.3) | −10.7 (±35.83) | 0.27 |
| Blood eosinophils (x10ˆ/L) | 0.3 (±0.4) | 0.4 (±0.2) | 0.01 (±0.35) | 0.91 |
| Sputum Eosinophils (%) | 10.9 (±13.3) | 14.4 (±26.9) | 3.52 (±16.6) | 0.52 |
ACQ: asthma control questionnaire, FeNO: fractional expiratory nitric oxide, ppb: parts per billion.
Pairwise t-test of exacerbation versus 4 weeks follow-up.
3.7. IL-33 protein and mRNA expression levels at the time of exacerbations versus at the 4-week follow-up
There were no difference in IL-33 levels from exacerbation to follow-up in the upper or lower airways. With the type 2 cytokines, there was a trend towards lower IL-5 protein in the lower airways at follow-up compared to levels at exacerbation (p = 0.077), whereas IL-13 did not change.
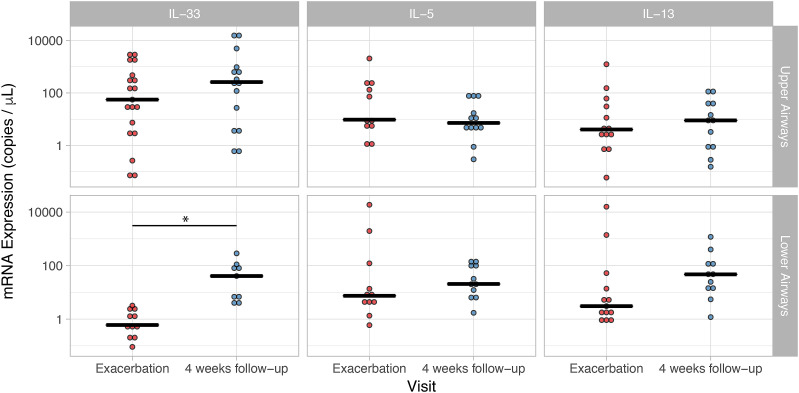
In contrast, mRNA levels of IL-33 in lower airways were significantly increased at follow-up (40.88 copies/μl) compared to exacerbation (0.60 copies/μl, p = 0.028). This was not seen with mRNA expression in upper airway (Fig. 3 ). IL-5 and IL-13 mRNA expression level did not change from exacerbation to follow-up (Fig. 3).
Fig. 3.
mRNA expression levels of IL-33, IL-5 and IL-13 in upper and lower airways during exacerbation and follow-up. Only IL-33 mRNA expression levels in lower airways were significantly increased at 4 weeks follow-up compared to levels at exacerbation. * indicate a p-value of 0.028.
IL-33 levels in either upper or lower airways did not differ between patients with rhinovirus compared to patients that tested negative for rhinovirus (p = 0.7 and 0.9, respectively).
3.8. IL-33 protein and mRNA expression levels and the type 2 cytokines at 4 weeks follow-up
The association between IL-33 protein and the type 2 cytokines at follow-up showed similar patterns as seen at exacerbation (Table 3 ) indicating that the relationship between IL-33 and type 2 cytokines remains 4 weeks after exacerbation.
Table 3.
Association between IL-33 and type 2 cytokine proteins at 4 weeks follow-up.
| 4 weeks follow-up | Upper airway IL-5 |
Upper airway IL-13 |
Lower airway IL-5 |
Lower airway IL-13 |
||||
|---|---|---|---|---|---|---|---|---|
| Spear-mans rho | p-value | Spear-mans rho | p-value | Spear-mans rho | p-value | Spear-mans rho | p-value | |
| Upper airway IL-33 | 0.73 | 0.001 | 0.72 | 0.001 | 0.46 | 0.062 | 0.68 | 0.001 |
| Lower airway IL-33 | 0.44 | 0.08 | 0.56 | 0.02 | 0.73 | 0.001 | 0.90 | <0.01 |
Only upper airway IL-33 mRNA and upper airway IL-5 mRNA (R = 0.59, p = 0.045) correlated 4 weeks after exacerbation.
3.9. IL-33 and markers of eosinophilic airway inflammation at 4 weeks follow-up
IL-33 protein, as well as IL-5 or IL-13 protein, did not correlate with either FeNO, sputum or blood eosinophils at follow-up. However, IL-33 mRNA expression levels in lower airways unexpectedly correlated negatively with blood eosinophils (−0.79, p = 0.04) at follow-up. Similarly, there was a trend towards decreased IL-33 mRNA expression levels in lower airways in the group of subjects with >3% sputum eosinophils (p = 0.07).
4. Discussion
This study is the first to demonstrate a correlation between airway IL-33 and the type 2 cytokines IL-5 and IL-13, in naturally occurring asthma exacerbations. Airway IL-5 was also associated with increased airway eosinophilia, while IL-13 was associated with increased FeNO. These findings are similar to those found in an experimental exacerbation model of asthma, using RV-16 [11], and provides real world evidence in patients of IL-33 being a potential driver of type 2 inflammation during exacerbations.
Our group recently reported that IL-33 in bronchial biopsies from stable asthma patients correlated with FeNO, but to our knowledge, this association has not previously been demonstrated in acute asthma [20]. This is now further supported by the data from this acute study which shows a strong correlation between airway IL-33 and FeNO in acute asthma. This finding, along with the positive correlations found between IL-33 and IL-5/IL-13, may further indicate that IL-33 is indeed driving type 2 inflammation during exacerbations via IL-5 & IL-13. Although we did not observe an association with sputum eosinophils, there was a trend towards IL-33 being positively correlated with blood eosinophils. Overall the mRNA expression results supported the protein findings. Interestingly, despite improvement in lung function and symptom control at the four-week follow-up, the level of eosinophilic inflammation and type 2 cytokine did not decrease, nor did IL-33. This suggests that airway inflammation persists for a significant period of time after an exacerbation, and that IL-33 is part of this perpetuated inflammatory response.
One slightly surprising finding was that the mRNA level of IL-33 increased at follow-up despite stable protein levels. This could indicate independent transcriptional and translational regulation of IL-33. A possible explanation could be the timing of sampling during the exacerbation: Patients underwent sampling up to 24 h after being admitted to hospital, and after having received systemic steroid treatment. Hence, most likely, mRNA expression could have been suppressed at time of exacerbation sampling due to corticosteroids anti-inflammatory actions, as mRNA was being degraded faster than protein [21].
One other unexpected finding was the negative correlation between mRNA expression levels of IL-33 in lower airways and blood eosinophils at follow-up. Supportive of this result was the trend towards mRNA expression of lower airway IL-33 being decreased in patients with >3% sputum eosinophils, again at follow-up. This was not seen at the protein level. This may potentially represent a negative feedback loop, in which mRNA expression is suppressed in patients with type 2 inflammation. In keeping with this observation, we have previously reported a study that showed IL-33 immunoreactivity was negatively correlated with an extended panel of markers of airway and systemic eosinophilia in stable asthma [20]. Such a negative feedback loop could represent a physiological regulation of the inflammatory response after asthma exacerbations, but requires further study.
IL-33 belongs to the IL-1 family and is a nuclear as well as a secreted cytokine. It is released during epithelial necrosis and cell damage including infection [4]. Asthma exacerbations are commonly caused by external triggers such as respiratory infection and allergens, all of which cause damage to the epithelial cells of the lung, suggesting that epithelial activation plays a role in driving inflammation during asthma exacerbations [4]. In the present study, we did not observe an association between viral infection and IL-33, which may reflect that IL-33 is a more universal inflammatory mediator that is released in relation to a number of triggers [4].
Several genome-wide association studies have found that both IL-33 and its receptor ST2 associates with asthma [22,23]. Asthmatics have higher levels of IL-33 and ST2 in serum compared to non-asthmatics and high levels are associated with severe asthma [3,4]. In animal studies, IL-33 has been shown to induce eosinophilic airway inflammation and hyperresponsiveness [[24], [25], [26]], and administration of anti-IL-33 reduces the production of the type 2 cytokines [4,27,28].
Eosinophilic asthma with activation of the type 2 pathway occurs in a large subgroup of asthmatics [29,30]. This makes IL-33 an interesting pharmacological target, because of its position upstream to the complex cascade leading to increased airway inflammation and exacerbation. Our study provides further evidence that IL-33 might be driving type 2 inflammation in acute asthma.
Being an observational study, limited conclusions can be drawn on the causality between IL-33 and type 2 inflammation. Another limitation is the relatively small sample size, which limited the statistical power. In spite of this, we were able to demonstrate strong correlations between IL-33 and IL-5 and IL-13, providing supportive evidence that IL-33 plays a significant role in acute asthma. The lack of statistical power however made it impossible to conclude on any difference in certain subgroups of patients e.g. rhinovirus positive/negative patients. Another potential limitation of this study is the timing issue of when to sample patients: Patients were examined as soon as their clinical state allowed. However, this represents different stages of the natural course of an exacerbation introducing additionally heterogeneity between the samples.
Acute studies are important, but difficult to perform, on a large scale. Furthermore, new standardised and easy to use collecting techniques need to be developed. An important result of this study is the identification of associations between upper airway cytokines and lower airway cytokines. This suggests that for future studies, nasal swab samples might be used to indirectly assess the immune responses of the lower airways. Nasal swabs would represent a relatively easy airway sampling methods but need to be further validated in a larger sample of asthma patients. Furthermore it doesn't require complex laboratory resources and is therefore suited for the clinical setting. It can be done bedside in the emergency room immediately after admission, and while the stabilising treatment is given, therefore providing airway material before corticosteroid administration, since it is established that corticosteroids can alter gene expression. Nasal swabs were obtained within 24 h of admission to hospital, and not immediately after admission. However, we suggest, that this would be possible in future studies.
In comparison, even though we were able to measure IL-33 in serum, it correlated poorly with airway levels, and no relationship to markers of type 2 inflammation could be detected. This suggests that the systemic IL-33 response might be less relevant than the local IL-33 response.
At present, there is a need for larger prospective studies examining patients before, during and after an exacerbation using appropriate sampling methods with enough epithelial cells to be able to measure epithelial cytokines.
In conclusion, this study is the first to show a relationship between airway IL-33 and the markers of type 2 inflammation IL-5, IL-13 in naturally occurring asthma exacerbations, indicating that IL-33 is central to the increased airway inflammation seen in naturally occurring exacerbations. In the future, blocking IL-33 could be a novel therapeutic option for preventing asthma exacerbations.
Declaration of funding
Grants were received from ‘University of Copenhagen’, Torben og Alice Frimodts Fond’, ‘Fonden af 17-12-1981’, ‘Kong Christian den Tiendes Fond’, ‘Frimodt-Heineke Fonden’.
Copan, Italia S.P.A. provided the flocked swabs for this study. MedImmune Ltd, Cambridge performed the protein analysis of IL-33, IL-5 and IL-13.
Acknowledgements
Lab technician Öznur Turan.
No conflicts of interests.
References
- 1.C. Hammond, M. Kurten, J.L. Kennedy, Rhinovirus and Asthma: a Storied History of Incompatibility, (n.d.). doi:10.1007/s11882-014-0502-0. [DOI] [PMC free article] [PubMed]
- 2.Jarjour N.N., Esnault S., Thymic Stromal D.G. Interleukin-33: a potential link between rhinovirus infections and asthma exacerbation. J. Allergy Clin. Immunol. 2005;115116 doi: 10.1164/rccm.201411-1949ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R., Yang G., Yang R., Peng X., Li J. Interleukin-33 and receptor ST2 as indicators in patients with asthma: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8:14935–14943. http://www.ncbi.nlm.nih.gov/pubmed/26628975 [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd C.M. IL-33 family members and asthma – bridging innate and adaptive immune responses. Curr. Opin. Immunol. 2010;22:800–806. doi: 10.1016/j.coi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grissell T.V., Powell H., Shafren D.R., Boyle M.J., Hensley M.J., Jones P.D., Whitehead B.F., Gibson P.G. Interleukin-10 gene expression in acute virus-induced asthma. Am. J. Respir. Crit. Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 6.Wark P.A.B., Johnston S.L., Moric I., Simpson J.L., Hensley M.J., Gibson P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 7.Jent K., Nicholson K.G., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atmar R.L., Guy E., Guntupalli K.K., Zimmerman J.L., Bandi V.D., Baxter B.D., Greenberg S.B. Respiratory tract viral infections in inner-city asthmatic adults. Arch. Intern. Med. 2009;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 9.Tan W.C., Xiang X., Qiu D., Ng T.P., Lam S.F., Hegele R.G. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am. J. Med. 2003;115:272–277. doi: 10.1016/S0002-9343(03)00353-X. [DOI] [PubMed] [Google Scholar]
- 10.Kistler A., Avila P.C., Rouskin S., Wang D., Ward T., Yagi S., Schnurr D., Ganem D., DeRisi J.L., Boushey H.A. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D.J. Jackson, H. Makrinioti, B.M.J. Rana, B.W.H. Shamji, M.-B. Trujillo-Torralbo, J. Footitt, J. Del-Rosario, A.G. Telcian, A. Nikonova, J. Zhu, J. Aniscenko, L. Gogsadze, E. Bakhsoliani, S. Traub, J. Dhariwal, J. Porter, D. Hunt, T. Hunt, T. Hunt, L.A. Stanciu, M. Khaitov, N.W. Bartlett, M.R. Edwards, O.M. Kon, P. Mallia, N.G. Papadopoulos, C.A. Akdis, J. Westwick, M.J. Edwards, D.J. Cousins, R.P. Walton, S.L. Johnston, IL-33–Dependent Type 2 Inflammation during Rhinovirus-induced Asthma Exacerbations In Vivo, (n.d.). doi:10.1164/rccm.201406–1039OC. [DOI] [PMC free article] [PubMed]
- 12.Silkoff P.E. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. Am. J. Respir. Crit. Care Med. 1999;160:2104–2117. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 13.Pavord I.D., Pizzichini M.M.M., Pizzichini E., Hargreave F.E. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome experimental rhinovirus infection. Am. J. Respir. Crit. Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 15.Juniper E.F., Svensson K., Mörk A.-C., Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir. Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hiddessen A.L., Legler T.C., Kitano T.K., Hodel M.R., Petersen J.F., Wyatt P.W., Steenblock E.R., Shah P.H., Bousse L.J., Troup C.B., Mellen J.C., Wittmann D.K., Erndt N.G., Cauley T.H., Koehler R.T., So A.P., Dube S., Rose K.A., Montesclaros L., Wang S., Stumbo D.P., Hodges S.P., Romine S., Milanovich F.P., White H.E., Regan J.F., Karlin-Neumann G.A., Hindson C.M., Saxonov S., Colston B.W. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chidlow G.R., Harnett G.B., Shellam G.R., Smith D.W. An economical tandem multiplex real-time PCR technique for the detection of a comprehensive range of respiratory pathogens. Viruses. 2009;1:42–56. doi: 10.3390/v1010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W.M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T., Jakiela B., Lemanske R.F., Shult P.A., Gern J.E. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bochkov Y.A., Grindle K., Vang F., Evans M.D., Gern J.E. Improved molecular typing assay for rhinovirus species A, B, and C. J. Clin. Microbiol. 2014;52:2461–2471. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porsbjerg C., Baines K., Gibson P., Bergqvist A., Erjefält J.S., Sverrild A., Backer V. IL-33 is related to innate immune activation and sensitization to HDM in mild steroid-free asthma. Clin. Exp. Allergy. 2016;46:564–574. doi: 10.1111/cea.12702. [DOI] [PubMed] [Google Scholar]
- 21.C. Vogel, E.M. Marcotte, Insights into the regulation of protein abundance from proteomic and transcriptomic analyses, (n.d.). [DOI] [PMC free article] [PubMed]
- 22.Saluja R., Zoltowska A., Ketelaar M.E., Nilsson G. IL-33 and Thymic Stromal Lymphopoietin in mast cell functions. Eur. J. Pharmacol. 2016;778:68–76. doi: 10.1016/j.ejphar.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 23.N. Eomi, S. Grotenboer, M.E. Ketelaar, G.H. Koppelman, M.C. Nawijn, Decoding asthma: Translating genetic variation in IL33 and IL1RL1 into disease pathophysiology, (n.d.). doi:10.1016/j.jaci.2012.11.028. [DOI] [PubMed]
- 24.Morita H., Arae K., Unno H., Toyama S., Motomura K., Matsuda A., Suto H., Okumura K., Sudo K., Takahashi T., Saito H., Matsumoto K., Nakae S. IL-25 and IL-33 contribute to development of eosinophilic airway inflammation in epicutaneously antigen-sensitized mice. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell P.D., O'Byrne P.M. Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol. Ther. 2016 doi: 10.1016/j.pharmthera.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Zoltowska A.M., Lei Y., Fuchs B., Rask C., Adner M., Nilsson G.P. The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma. Clin. Exp. Allergy. 2016;46:479–490. doi: 10.1111/cea.12683. [DOI] [PubMed] [Google Scholar]
- 27.Borish L., Steinke J.W. 2010. Interleukin-33 in asthma: how big of a role does it play? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa H., Hayakawa M., Kume A., Tominaga S.-I. 2007. Soluble ST2 blocks Interleukin-33 signaling in allergic airway inflammation. [DOI] [PubMed] [Google Scholar]
- 29.F. Aleman, F. Hui, M. Lim, P. Nair, Eosinophilic Endotype of Asthma KEYWORDS Asthma Eosinophil Sputum Airway inflammation Endotype, (n.d.). doi:10.1016/j.iac.2016.03.006.
- 30.Simpson J.L., Scott R., Boyle M.J., Gibson P.G. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]