Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) crosses the placenta during late gestation and productively infects the fetus. Virus replication and cytokine responses were measured in tissues of fetuses recovered at 109–112 days of gestation, just prior to parturition. At the time of recovery, gross anatomical abnormalities were evident in both infected and non-infected fetuses from the infected dams. Virus isolation and immunohistochemistry identified the thymus as the primary site of virus replication. Steady state RT-PCR amplification of inflammatory, Th1 and Th2 cytokines, showed elevated IFN-γ and TNF-α mRNAs in tissues from infected fetuses, which corresponded to elevated cytokine proteins in serum but not amniotic fluid. Further evidence for induction of immunity was found in the hyperplastic response of lymph nodes, which included the development of germinal centers occupied CDw75+ B cells. Collectively, these data support the notion that the immunocompetent fetus is capable of initiating an antiviral response, which is compartmentalized within the infected fetus. Furthermore, fetal pathology may not be a direct result of virus replication in the fetus.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), Congenital infection, Tumor necrosis factor-α (TNF-α), Interferon-γ (IFN-γ)
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is caused by an enveloped positive-stranded RNA virus, PRRSV, belonging to the family Arteriviridae (Benfield et al., 1992, Cavanagh, 1997, Nelsen et al., 1999, Wensvoort et al., 1991). Other members of the arterivirus group include lactate dehydrogenase-elevating virus (LDV) of mice, equine arteritis virus (EAV), and simian hemorrhagic fever virus (SHFV; for review see Plagemann, 1996). The arteriviruses, toroviruses, roniviruses and coronaviruses form a single order, Nidovirales. Arteriviruses structurally resemble togaviruses, but similar to coronaviruses, replicate via a nested 3′-co-terminal set of subgenomic mRNAs that possesses a common leader and a poly-A tail (reviewed in Snijder and Mulenberg, 1998). The arteriviruses exhibit several important properties relevant to the study of viral pathogenesis, including cytopathic replication in macrophages, the capacity to establish and maintain an asymptomatic infection, as well as cause severe and fatal disease (Plagemann, 1996).
Infection of adult pigs with PRRSV usually produces a non-fatal disease, characterized by flu-like symptoms, a transient elevation in temperature and inappetance (reviewed in Benfield et al., 1999, Christianson et al., 1992). The reproductive form of PRRS occurs following the infection of late gestation pregnant gilts or sows. Natural infection of the fetus with PRRSV is initiated with the infection of gilts and sows at 90 days gestation. After productive replication on the maternal side, the virus crosses the placenta and productively infects the fetus. The mechanism of transplacental infection is unknown, but could be similar to the infected “Trojan Horse” macrophage, described for LDV (Cafruny and Bradley, 1996). Since the pig fetus becomes immunocompetent at about 70 days of gestation, PRRSV infection occurs in an immune environment containing functional B and T cells.
Accordingly, virus-induced reproductive failure can present clinically as delayed returns to estrus, as well as abortions, mummified fetuses, stillborn and weak-born pigs (Benfield et al., 1999, Christianson et al., 1993, Collins et al., 1992, Mengeling et al., 1994, Rossow et al., 1999, Rowland et al., 2003). Surviving neonates can exhibit the severest form of respiratory disease with mortality sometimes reaching 100% within three weeks after birth (Feng et al., 2001, Rossow et al., 1994, Rossow, 1998). The complex pathology following exposure to PRRSV in utero represents a unique form of the disease referred to as congenital PRRS (Rowland et al., 2003). The purpose of this study was to characterize the interaction between PRRSV and the pig fetus by (1) identifying sites of virus replication, (2) measuring immune and inflammatory cytokines in different compartments, and (3) evaluating the response of lymph nodes.
2. Materials and methods
2.1. Virus and infection
Experiments involving animals were approved by the Kansas State University IACU Committee. Pregnant sows, obtained from a closely monitored PRRSV-negative herd, were challenged at 90 days gestation with a sixth passage isolate of SD-23983, a typical North American field isolate (Rowland et al., 2001). The methods for the preparation of the PRRSV inoculum on MARC-145 cells and infection of pigs are described in Rowland et al. (2003). Virus was cultivated on MARC-145 cells in MEM supplemented with antibiotics (pen/step) and 7% FBS. Dams, at 90 days gestation were challenged with approximately 105 TCID50 of virus diluted in 5 ml of culture medium. One half of the inoculum was administered by intramuscular injection in the neck. The remaining dose was administered intranasally. Mock-infected sows were challenged with medium recovered from MARC-145 cells. Dams were monitored for clinical signs and blood collected weekly. At between 109 and 112 days of an approximate 114 days gestation period, the dams were euthanized. The uterine horns were immediately removed and the individual fetuses with intact placenta were carefully removed and immediately necropsied. A sample of amniotic fluid was collected prior to removal. The brachial artery of each fetus was severed and blood collected using a disposable syringe and serum stored at −80 °C. Maternal, accessory and fetal tissues were collected and stored in formalin for histological staining and immunohistochemistry (IHC), or storage in RNAlater (Ambion) for RT-PCR of cytokine mRNAs. PRRSV-specific antibody was measured in sera using the HerdCheck® PRRS ELISA (IDEXX) and performed by personnel at Kansas State University Veterinary Diagnostic Laboratory. Serology results were reported as a sample/positive (S/P) ratio. An S/P ratio greater than 0.39 was considered positive for PRRSV antibody.
2.2. Virus isolation and titration
Virus isolation (VI) in serum and tissues was performed as described in Rowland et al. (2003). Briefly, serum was serially diluted in MEM supplemented with pen/step antibiotics and 7% FBS and placed on 96 well plates of confluent MARC-145 cells. After three days, plates were fixed in 80% acetone and stained with FITC-SDOW-17 anti-nucleocapsid antibody, diluted in PBS with 5% FBS (Nelson et al., 1993). The results were reported as the log10 of the inverse dilution of the last positive well. Virus isolation from tissues was the same except that tissues were weighed and homogenized in Hanks balanced salt solution and then centrifuged at 500 × g for 20 min to remove debris.
2.3. PRRSV ORF5 sequencing
The sequencing of the hypervariable region of ORF5 is described in Rowland et al. (1999). Total RNA was prepared from serum or infected MARC-145 cells using an RNeasy kit (Qiagen) according manufacturer's instructions. For PCR, cDNA was prepared using MLV reverse transcriptase (Promega) and 4MSB as the primer. The sense and antisense primers for the outer amplification were 4MSA, 5′-CTTCGTCCCTTCTTTTCCTCGTGG, and 4MSB, 5′-CCGCTCTAGAGCCAACGATAGAGTCTGC, respectively. The product was re-amplified with a nested set of sense and antisense primers, 04A, 5′-ACCGTGTATGTTACCATCACAGCC and 04B, ACGGGAAAGATGACAAAACTCTCC. Thirty-two cycles of amplification were performed for each primer pair. The conditions for both amplifications included a 95 °C denaturing step (25 s), a 58 °C annealing step (10 s), and a 74 °C (25 s) polymerization step. The final PCR product, which contained the last 312 nucleotides of ORF4, the 10 nucleotide untranslated region (UTR), and the first 215 nucleotides of ORF5 was sequenced directly by automated DNA sequencing. PCR products were cloned into a pCR2.1 TA cloning vector (Invitrogen), propagated in Escherichia coli and individual plasmids sequenced using M13 forward and reverse primers. Sequences were analyzed using Gene Jockey II software.
2.4. RT-PCR and ELISA for detection of porcine cytokines
Tissue samples for RT-PCR were immediately placed in RNA-Later (Ambion) and stored at −80 °C. Total RNA was extracted from approximately 50 μg of tissue using RNeasy kit (Qiagen) according to manufacturer's instructions. The design of cytokine-specific primers and RT-PCR procedures were performed according to Reddy and Wilkie (2000). Primer sequences are listed in Table 1 . RNA was diluted to a final volume of 50 μl in nuclease free water. cDNA was prepared from10 μl of total RNA by reverse transcription using Molony murine leukemia virus reverse transcriptase (Promega) and random hexamers as primers. The amplification of β2m mRNA was used as an internal control. PCR amplification of cytokine and control cDNAs consisted of 35 cycles (45 s at 94 °C, 45 s at 55 °C, and 45 s at 72 °C) and DNA products electrophoresed on a 2.0% agarose gel and visualized using ethidium bromide. The identity of the DNA products was confirmed by DNA sequencing.
Table 1.
PCR primers used for amplification of porcine cytokines.
| Primer name | Primer sequence (5′–3′) | GenBank accession no. | |
|---|---|---|---|
| β2m | Forward | CTGCTCCACTGTCTGG | L13854 |
| Reverse | ATCGAGAGTCACGTGCT | ||
| IFN-γ | Forward | CCAGAATGCAAGTACCTCAG | X53085 |
| Reverse | TCTCTGGCCTTGGAACATAG | ||
| TNF-α | Forward | CACTGAGAGCATGATCCGAG | X57321 |
| Reverse | GGCTGATAGTGGTAGTGAGG | ||
| IL-1α | Forward | CAGCTATGAGCCACTTCCTG | X52731 |
| Reverse | GTCACAGGAAGTTGCGAATC | ||
| IL-2 | Forward | GATTTACAGTTGCTTTTGAA | X58428 |
| Reverse | CATCCTGGAGAGATCAGCATTC | ||
| IL-4 | Forward | GTCTCACATCGTCAGTGC | L12991 |
| Reverse | TCATGCACAGAACAGGTC | ||
| IL-6 | Forward | GGAACGCCTGGAAGAAGATG | M80258 |
| Reverse | ATCCACTCGTTCTGTGACTG | ||
| IL-8 | Forward | TGCAGCTTCATGGACCAG | X61151 |
| Reverse | GTACAACCTTCTTCTGCACC | ||
| IL-10 | Forward | GCTCTATTGCCTGATCTTCC | L20001 |
| Reverse | GCACTCTTCACCTCCTCCAC | ||
| IL-12α | Forward | CCGTCAGCAACACACTTC | NM 213993 |
| Reverse | TTCAGAGCCTGCATCAGC | ||
| IL-12β | Forward | GTGCTGGAAGCTGTTCAC | NM 214013 |
| Reverse | GGTCTGCTCCATCATGTC | ||
2.5. Histology and immunohistochemistry
Tissue samples were collected and immediately placed in 10% buffered formalin. Paraffin-embedded thin sections were mounted on slides, deparaffinized and stained with hematoxylin and eosin (H and E). Immunohistochemistry (IHC) and H and E staining procedures were performed by personnel in the Kansas State Veterinary Diagnostic Laboratory. For IHC staining, thin sections were mounted on Vectabond-treated slides (Vector Laboratories) and deparaffinized and processed using an automated NexES IHC Staining Module (Ventana Medical). For the detection of PRRSV antigen, slides were incubated for 30 min with a 1:100 dilution of mAb SR-30 anti-nucleocapsid antibody (Rural Technologies). Other antibodies included a polyclonal anti-human CD3 and B cell antibodies, anti-CDw75 and anti-CD79α. Bound antibody was detected with biotinylated goat anti-mouse or anti-rabbit Ig followed by avidin-HRPO and DAB chromagen (Ventana Medical). Slides were counterstained with hematoxylin.
3. Results
3.1. Clinical signs, gross pathology and infection status
The experiment incorporated four PRRSV-infected and two-mock-infected dams. All maternal serum samples were VI-negative and seronegative for PRRSV prior to infection. Between one and two weeks after virus challenge, all infected sows were VI-positive in serum, confirming the presence of an active infection. By the time of necropsy, the concentration of circulating virus in the dams had dipped to below detectable levels by VI. A total of 44 viable fetuses were recovered from the four infected dams (see Table 2 for summary). Two fetuses were dead and partially autolysed and not subjected to further study. 10 (22%) of the 44 viable fetuses were positive for PRRSV by VI. Since dams were VI-negative in blood at the time of necropsy, it was concluded that the presence of virus in the fetal circulation was the result of virus replication in the fetus and not from contamination with maternal blood. (The virus isolation technique is not subject to false positives from minute amounts of cross-contamination with viral protein or RNA.) The number of infected fetuses in each litter varied from no infected fetuses (dam no. 2) to five of 14 (36%) infected fetuses for dam no. 4. Fetuses that were VI-negative in serum were confirmed as PRRSV-negative by VI-negative results in placenta, lung, lymph nodes and thymus (data not shown). One fetus, 1-1, was seropositive for PRRSV (S/P ratio = 0.94). Since there is no maternal transfer of antibody from mother to fetus (Tizard, 1996), it was concluded that this fetus generated an antibody response to PRRSV in utero. 7 of the 10 infected fetuses showed some form of gross pathology, including growth retardation (two fetuses) or reduced amounts and/or merconium-stained amniotic fluid (five fetuses). Ongoing virus replication in the fetus as the source of these gross pathological changes is questionable, since non-infected fetuses from infected dams exhibited similar changes. For example, fetuses from dam no. 2 (see Fig. 1 ) were either merconium stained (two fetuses), non-viable, possessed reduced amniotic fluid levels (four fetuses) or small (one fetus). Except for one autolysed fetus, the 23 fetuses from the two control dams showed no evidence of gross pathology (data not shown).
Table 2.
Fetal infection and mortality.
| Dam no. | Day of gestation | Number of fetuses | Number of infected fetuses | Number of dead fetusesa |
|---|---|---|---|---|
| 1 | 109 | 8 | 2 (25%) | 1 |
| 2 | 111 | 12 | 0 (0%) | 0 |
| 3 | 111 | 12 | 3 (25%) | 1 |
| 4 | 109 | 14 | 5 (36%) | 0 |
| Total | 46 | 10 (22%) | 2 |
Dead fetuses were not tested for the presence of virus or antibody.
Fig. 1.
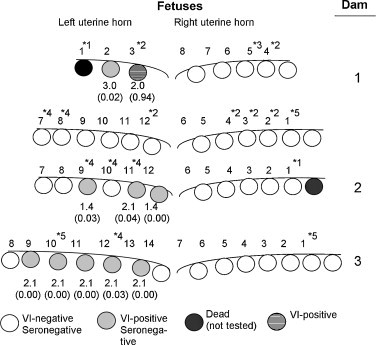
Status of fetuses from infected dams. Circles represent the relative location of each fetus in the left and right uterine horns. The number above each fetus identifies the order of removal from the uterus. The serum VI titer is below each infected fetus. Serology results, shown in parentheses, are presented as the S/P ratio. S/P ratios greater than 0.4 were considered positive for PRRSV antibody. Dead fetuses were partially autolysed and not tested. Gross pathology key: *1, partially mummified; *2, non-viable fetus or relatively low quantity of amniotic fluid; *3, necrotic placenta; *4, merconium- and/or blood-stained amniotic fluid; *5, small, underdeveloped fetus.
3.2. Appearance of a novel viral sequence in dams and fetuses
RNA viruses frequently exist as a heterogeneous population, frequently referred to as a quasispecies. The appearance or disappearance of individual viral sequences within a quasispecies population is often used as evidence to support the existence of positive or negative selection during infection (Elena et al., 2000, Forns et al., 1999, Tsibris et al., 2009). Mutations that appear in the PRRSV genome are useful as markers to identify and follow the appearance and disappearance of viruses within the population (Allende et al., 2000, Rowland et al., 1999). DNA sequencing of ORF5 PCR products, amplified directly from the sera of infected dams, identified one dam, no. 4, which possessed a virus with a mutation within the hypervariable region of ORF5. The change detected by sequencing the PCR product was a C to T (U for RNA) nucleotide transition at position 77 that resulted in a non-conserved amino acid change from threonine to isoleucine in GP5. The T-77 mutation was not detected after sequencing the ORF5 PCR products from the other dams (see Table 3 ). PCR products were cloned into a TA plasmid and the individual plasmids sequenced. Even though T-77 was detected in the PCR product from dam no. 4, the sequence of individual clones showed that two of the five sequences possessed a C at position 77 mutation, which indicated that viruses with the wild-type ORF5 sequence were still present in the population. Whole PCR and plasmid-cloned PCR products were sequenced for the five infected fetuses from dam no. 4. The frequency of the T-77 mutation ranged from 25% (fetus 4–11) to 100% (fetuses 4–12 and 4–13). These results indicate that the fetus is capable of selecting for a particular virus population, which either arises in the dam or fetus. Therefore, fetal infection is a potential source of PRRSV diversity.
Table 3.
Frequency of T at nucleotide position 77 of ORF5.
| Source of ORF5 sequence | Consensus nucleotide at position 77 of ORF5 | T:C ratio in cloned PCR products (percent) |
|---|---|---|
| 23983-P6 | C | 0/5 (0) |
| Dam-1 | C | 0/2 (0) |
| Dam-3 | C | 0/2 (0) |
| Dam-4 | T | 3/5 (60) |
| Fetus 4-09 | T | 6/7 (86) |
| Fetus 4-10 | T | 3/6 (50) |
| Fetus 4-11 | C | 1/4 (25) |
| Fetus 4-12 | T | 6/6 (100) |
| Fetus 4-13 | T | 5/5 (100) |
3.3. The fetal thymus as the primary site of PRRSV replication
During acute infection of the postnatal pig, the largest quantity of virus and greatest number of cells supporting virus replication are found in the lung, a consequence of targeting alveolar macrophages. During the later stages of PRRSV infection, secondary lymphoid organs, including tonsil and lymph nodes, become sources of virus replication (Allende et al., 2000, Rossow, 1998, Rowland et al., 1999). Virus replication in fetal tissues was assessed using a combination of virus isolation and IHC detection of nucleocapsid antigen in formalin-fixed tissues. As summarized in Table 4 , virus was isolated from all tissues from infected fetuses, including placenta, umbilical cord, heart, lung, spleen, lymph nodes and thymus. Overall, the thymus contained the largest quantity of virus. Nine of ten fetuses yielded measurable amounts of virus with five of the ten fetal thymuses producing titers greater than 3.0. For lung, 6 of 10 fetuses were VI-positive and only one fetal lung yielded a virus titer greater than 2.0. The recovery of virus from a tissue can represent virus in circulating blood. Therefore, to determine if cells in the thymus and other tissues were a source of PRRSV, tissue thin sections were stained with PRRSV anti-nucleocapsid antibody. The IHC staining procedure included two sets of negative controls: tissue thin sections from non-infected fetuses and from infected fetuses stained with only secondary antibody. Both controls were negative for staining (data not shown). The results in Table 4 showed the largest number of positive tissues for the thymus (8 of 10 positive), followed by spleen (2 of 8 positive) and lymph node (1 of 9 positive). Within the thymus, antigen-positive cells were located in both medullar and cortical regions (data not shown). PRRSV antigen-positive cells were not detected in lung or tonsil. Taken together, the virus titration and IHC results identify the thymus as a principal source of virus replication in the PRRSV-infected fetus.
Table 4.
PRRS virus isolation and immunohistochemistry (IHC) in fetal tissues.a
| Dam no. | 1 |
3 |
4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fetus no. | 2 | 3 | 9 | 11 | 12 | 9 | 10 | 11 | 12 | 13 |
| Placenta | 3.6 | n.m.b | 1.0 | 1.6 | 1.9 | 2.4 | n.m. | n.m. | 2.7 | 1.3 |
| Umbilical Cord | n.d.c | 1.4 | 1.3 | 2.2 | 1.6 | 2.2 | 2.0 | 1.2 | 1.1 | 1.9 |
| Heart | 1.7 | 1.8 | 2.5 | 2.6 | 1.4 | 2.8 | n.m. | 1.3 | 1.0 | 2.0 |
| Lung | 2.1 | n.m. | 1.0 | n.m. | n.m. | 0.8 | n.m. | 0.7 | 0.5 | 1.9 |
| (IHC) | (−)d | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Lymph nodee | 3.6 | n.m. | 2.2 | 2.2 | n.m. | 3.0 | n.m. | n.m. | 1.3 | n.m. |
| (IHC) | (−) | (−) | n.d. | (−) | n.d. | (−) | (−) | (+) | (−) | (+) |
| Spleen | 2.3 | n.m. | 2.1 | 3.1 | 2.1 | 2.7 | 1.9 | 1.0 | n.m. | 1.6 |
| (IHC) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (+) |
| Tonsil | 1.3 | 1.4 | 1.2 | 1.2 | 2.2 | 3.2 | n.m | n.m. | n.m. | n.m. |
| (IHC) | (−) | (−) | (n.d.) | (−) | (−) | (−) | (−). | (−) | (−) | (−) |
| Thymus | 4.1 | n.m. | 1.6 | 2.3 | 3.6 | 1.6 | 3.5 | 3.5 | 2.2 | 4.2 |
| (IHC) | (+) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
VI reported as titer per gram of tissue.
n.d., not determined.
n.m., no virus detected at a dilution of 1:5.
The presence (+) or absence (−) of PRRSV antigen-positive cells for a single thin section of tissue.
VI was performed on mandibular lymph node. IHC, in parentheses, included results for mandibular and medial inguinal lymph node tissues.
3.4. Response of fetal lymph node to infection
In the post-natal pig, PRRSV infection results in distinct pathology in the lung, including the appearance of interstitial pneumonia. Representative lymph node and lung tissues from non-infected and infected fetuses are shown in Fig. 2 . There was no discernable difference between lungs from infected and non-infected fetuses (compare Fig. 2 panels C and D). Lymph nodes from PRRSV-negative fetuses appeared largely undeveloped and devoid of well-defined germinal centers. In contrast, the lymph nodes from PRRSV-infected fetuses appeared much more pronounced and enlarged. At the microscopic level, the increased lymph node volume was associated with increased numbers of cells and the formation of distinct germinal centers (see Fig. 2 panels A and B). The overall appearance is consistent with an antigen-activated lymph node. To determine the source of the increased cell volume, formalin-fixed thin sections of lymph nodes were stained with T cell-specific (CD3) and B cell-specific (CDw75 and CD79α) antibodies. Representative results for mandibular lymph nodes from infected and non-infected fetuses are shown in Fig. 3 . The lymph nodes from both infected and non-infected fetuses showed extensive areas of staining with anti-CD3, indicating the presence of T cells. Lymph nodes from non-infected fetuses were negative for CwD75 staining, but possessed some regions that were positive for CD79α. The B cell receptor for antigen (BCR) signal transduction complex is composed of a heterodimer of Igα and Igβ chains, which are also known as CD79α and CD79β, respectively. CD79α staining in the lymph node from non-infected fetuses is consistent with the presence of pro- and pre-B cells (Lee et al., 2008). The principle difference between infected and non-infected fetuses was found in an overall increase in CD79α+ cells as well as the appearance of CDw75+ cells, which were associated with germinal centers. CDw79 is beta-galactoside alpha-2,6-sialyltransferase, which is up-regulated in activated B cells (Erikstein et al., 1992). The absence of CDw75 staining in non-infected fetuses is consistent with the overall quiescent nature of the non-stimulated fetal immune system. The up-regulation of CDw75 after PRRSV infection is consistent with B cell activation and the formation of mature germinal centers in response to infection. It should be noted that infected fetuses showed different degrees of staining, a likely consequence of the different stages of fetal infection; i.e. less staining was the result of early infection. Together, the overall morphology and lymphocyte marker expression results indicate that the increased volume in the lymph nodes of infected fetuses is largely the result of increased numbers of mature activated B cells, which occupy germinal centers.
Fig. 2.
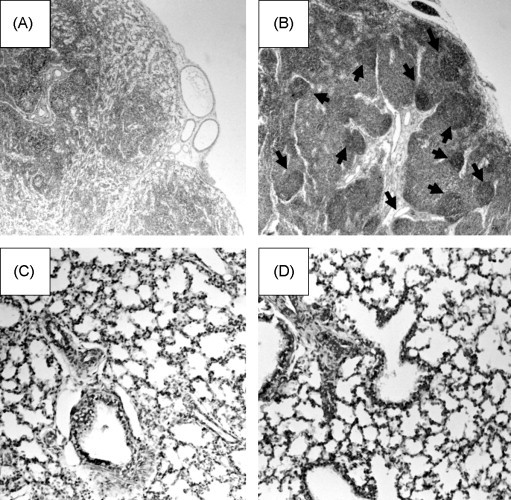
Microhistology of lung and lymph nodes. Panels (A) and (C) show a hematoxylin and eosin stained (HE) lymph node (A) and lung (C) of a fetus from a mock-infected dam. Panels (B) and (D) show representative lymph node and lung tissues from a PRRSV-infected fetus. Arrows identify germinal centers.
Fig. 3.
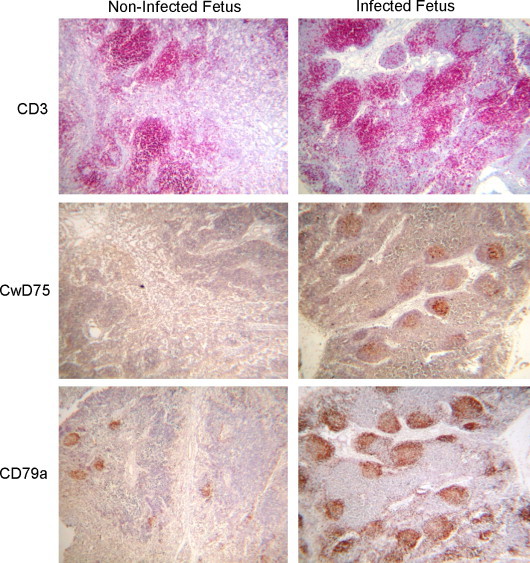
Antibody staining for B and T cell markers. Tissues were prepared and stained as described in Materials and Methods. T cells were identified by staining with anti-CD3. B cells that express Ig are identified by CD79α staining. Activated B cells are identified by CDw75 staining.
3.5. Cytokine gene expression
Immune cytokines are important factors in antiviral immunity and can influence the outcome of pregnancy (Arck et al., 1999, Basurko et al., 2009). The detection of cytokine gene expression in tissues was performed using a steady state RT-PCR procedure. RT-PCR was performed on RNA isolated from four fetuses randomly chosen from the two mock-infected dams and four randomly selected PRRSV-infected fetuses. The tissues selected for RT-PCR amplification were lung, lymph node and placenta. Lung and lymph node represent sites cytokine alterations in the post-natal pig. Placenta was selected as an accessory tissue located at the fetal maternal interface. Amplification of mRNAs included cytokines associated with inflammatory (IL-6, IL-8), Th1 (IL-12, IFN-γ, IL-2) and Th2/regulatory (IL-4, IL-10) responses. The amplification of β2m mRNA was included as an internal control. The determination of a cytokine response was based on the presence or absence of a PCR product. IL-6, IL-8, IL-12 and IL-10 products were detected in tissues from both control and infected fetuses. Because of the qualitative nature of the PCR method, it was not possible to accurately determine quantitative differences between control and infected fetuses; and therefore, these cytokines were not subjected to further study (data not shown). IL-4 mRNA was not detected in any of the selected tissues for control and infected fetuses. Marked differences in expression were observed for TNF-α and IFN-γ mRNAs. The results for IFN-γ and TNF-α from lung, mandibular lymph node and placenta are presented in Fig. 4 . The results for IL-10 are also shown. The internal control mRNA, β2m, was amplified from all tissues, indicating that the RNA was intact. IL-10 was amplified from lung and mandibular lymph nodes from two of the four control fetuses and from all infected fetuses. The presence of IL-10 was not unexpected, since increased IL-10 production is associated with fetal T cell responses (Lin et al., 1993, Rainsford and Reen, 2002). As shown in Fig. 4A, IFN-γ and TNF-α PCR products were not detected in lung, lymph node or placenta from the non-infected fetuses. However, IFN-γ PCR products were obtained for lung and lymph nodes from infected fetuses. One infected fetus, 4–3, yielded a faint IFN-γ product for placenta. For infected fetuses, TNF-α mRNA was amplified from lung, but not lymph node or placenta (Fig. 4B). To determine if cytokine gene expression was the direct result of PRRSV infection, RT-PCR for IFN-γ and TNF-α was performed on the same tissues from fetuses of infected dam no. 2, which produced only PRRSV VI-negative fetuses (see Fig. 1). The analysis of mRNA expression in lungs and lymph nodes from six fetuses from dam no. 2 showed only the amplification of the β2m, but not TNF-α or IFN-γ mRNAs (data not shown). Similar results were obtained from virus-negative fetuses located immediately adjacent to infected fetuses (data not shown).
Fig. 4.
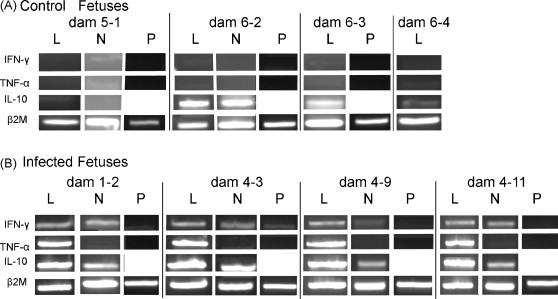
Cytokine gene expression in fetal tissues. RT-PCR for cytokine mRNAs was performed on lung (L), mandibular lymph node (N) and placenta (P) for control (panel A) and infected (panel B) fetuses as described in the text using the primers in Table 2. Amplification of β2-microglobulin mRNA was used as an internal control. PCR products were electrophoresed on a 1.2% agarose gel and stained with ethidium bromide.
In order to confirm that TNF-α and IFN-γ were produced during infection, cytokine protein levels were measured in fetal sera and amniotic fluid from infected fetuses and fetuses from mock-infected dams. As shown in Fig. 5 , the concentration of TNF-α in serum for PRRSV-infected fetuses ranged between 20 and 100 pg/ml compared to less than 30 pg/ml for control fetuses. Even though the maximum quantities obtained for infected fetuses were near the lower limit of detection for the ELISA test, it was clear that TNF-α was elevated during infection. Detectable concentrations of TNF-α (>23 pg/ml) were found in amniotic fluid from only two control and two infected fetuses. Compared to fetuses from mock-infected dams, IFN-γ concentrations were detected in sera, with values ranging from a low of 60 to more than 500 pg/ml. A maximum level of 60 pg/ml was obtained for a single non-infected fetus. IFN-γ was not detected in amniotic fluid from either the infected or control fetuses. The presence of IFN-γ and TNF-α in serum, but not amniotic fluid, further supports the notion that the IFN-γ and TNF-α responses are primarily restricted to the PRRSV-infected fetus and do not extend to the accessory tissue compartments.
Fig. 5.
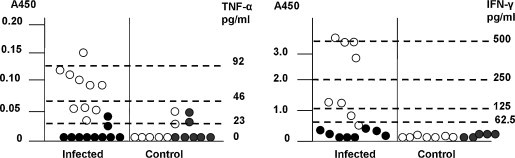
Measurement of TNF-α and IFN-γ proteins in serum and amniotic fluid. ELISA was used to measure the amount of immunoreactive cytokine proteins in serum (open circles) and amniotic fluid (closed circles) in infected fetuses and fetuses from mock-infected dams. Each cytokine was measured in 100 μl of sample and samples from control and infected fetuses assayed on a single ELISA plate. Each data point is the mean A450 values of triplicate and duplicate samples for serum and amniotic fluids, respectively. The horizontal lines show the A450 values for the cytokine standards.
4. Discussion
This study characterizes the unique biology associated with the interaction between PRRSV and the late gestation fetus. Consistent with the body of published literature, the fetuses from the infected dams obtained in this study exhibited several anatomic pathological features typical of PRRSV infection of the fetus, including lesions associated with the accessory organs, such as umbilical cord and amniotic sac (Lager and Halbur, 1996, Mengeling et al., 1996). One interesting observation from this study was the apparent absence of a correlation between the presence of gross abnormalities and productive fetal infection. For example, several fetuses from dam no. 2 exhibited several types of gross pathology, including death, growth retardation, or merconium/blood stained amniotic fluid. There were also examples of productively infected fetuses that showed no evidence of gross pathology (see fetuses 1–2, 3–12, 4–9, 4–11, 4–13 in Fig. 1). The mechanism for fetal pathology remains unclear, but the results suggest that the source pathology is likely result of the infection of tissues on the maternal side and damage to maternal tissues or production of maternal factors that affect the fetus. In this study we did not observe lesions in the myometrium or placenta; however, Stockhofe-Zurwieden et al. (1995) reported PRRS virions budding from maternal vascular endothelial cells at the maternal-fetal interface. Lager and Halbur (1996) reported damage to the myometrium during PRRSV infection. Virus-associated lesions in the myometrium are observed for horses infected with EAV (Coignoul and Cheville, 1984). The apparent discrepancy between pathology and infection helps to explain the stealthy nature of the PRRSV. A significant number of apparently healthy, but infected fetuses, are likely go on to become healthy growing pigs with the capacity to shed virus.
RNA viruses often exist as a population of closely related sequences, frequently referred to as a quasispecies. The appearance or disappearance of individual gene sequences in the population over the course of infection is used as evidence for changes in fitness that result from selection. PRRSV variants with mutations in ORF5 typically appear during the serial passage of virus in culture and during infection of pigs (Rowland et al. 1999; Allende et al., 2000). The significance of mutations in ORF5 as a source for increased fitness during infection is not completely understood; but is useful for following the fate of individual PRRSV subpopulations over the course of a long-term infection (Rowland et al., 1999). In this study, one dam, no. 4, showed evidence of a mixed PRRSV infection as indicated by the presence of two different ORF5 sequences, which were distinguished from each other by a nucleotide substitution at position 77 in ORF5. The C to T change was observed in approximately 60% of the cloned plasmid products obtained from dam no. 4 (see Table 2). However, the same frequency was not transferred to the individual fetuses, which included two fetuses that possessed only viruses with the T-77 mutation and a single fetus with a virus population dominated by C at position 77. These results suggest that fetal infection can alter the selection of PRRSV variants and may represent a source of PRRSV genetic diversity.
To identify the targets of virus replication in the fetus, a variety of tissues were assessed for the presence of virus and virus-infected cells. VI, as opposed to more sensitive approaches, such as PCR, was selected as the means for measuring virus, because the relative insensitivity of VI avoids the possibility of false positive PCR results that might result from small amounts of contaminating maternal material. Within the group of selected tissues, virus could be isolated from all tissues. However, the most consistent source and largest quantity of virus were obtained from the thymus. Collectively, these data identify the thymus as a primary site of virus replication in the PRRSV-infected fetus and confirm an earlier observation for PRRSV by Benson et al. (2002). The specific cell population in the thymus targeted PRRSV replication remains unknown.
The natural predisposition of maternal immunity towards Th2-like responses aids in protecting the fetus by blocking cell-mediated Th1-related allorejection responses (Arck et al., 1999, Entrican, 2002, Raghupathy, 2001; recently reviewed in Challis et al., 2009). The negative influence of Th1 cytokines on fetal development can be demonstrated experimentally in mice, which show that a single injection of IL-2 or IFN-γ into certain strains induces fetal resorption (Lala, 1990, Chaouat et al., 1990). TNF-α, when infected into mice is abortifacient (Clark et al., 2004). Th1 cytokines can also have important long-term impacts. For example, newborn mice that survive maternal infection with influenza virus exhibit behavior similar to hyperanxiety and autism. The behavioral changes are a consequence of the altered distribution of dopamine and glutamine receptors during fetal brain development. The effect of influenza virus infection on the fetal brain can be mimicked by the administration of poly I:C, an inducer of IFN (Shi et al., 2003). Furthermore, the addition of IFN-γ to hippocampal neuron cultures alters the distribution of glutamate receptors, sufficient to affect synaptic activity between neurons (Vikman et al., 2001). Virus infections during pregnancy present an interesting paradox: those cytokines that protect the fetus from viral infection, tend to inhibit fetal development or potentiate rejection of the fetus; while those cytokines that maintain and promote fetal development are associated with the inhibition of antiviral immune responses. Because of the potential negative impact of Th1 cytokines on fetal outcome, there is a natural predisposition for the fetus to block the induction of Th1-associated cytokines. For example, the IFN-γ response in the developing fetus can be blocked by a combination of factors, including (1) defects in the capacity of fetal dendritic cells to express MHC class II and synthesize IL-12, (2) hypermethylation of the IFN-γ gene in fetal T cells, (3) the absence of target macrophages, and (4) the presence of an immune environment dominated by Th2/regulatory cytokines (Goriely et al., 2001, Langrish et al., 2002, Melvin et al., 1995, Marodi et al., 2001, Murphy et al., 2009, Prescott et al., 1998, White et al., 2002,). With this in mind, there are examples of viruses, such as Epstein–Barr virus (EBV), which are capable of stimulating antigen-specific IFN-γ responses in human umbilical cord blood lymphocytes (Ito et al., 1998, Wilson and Morgan, 2002). These and other data provide a description of the fetus as capable of initiating a robust antiviral Th1 immune response (Chaouat et al., 2002, Chipeta et al., 2000, Murphy et al., 2009). In this study IFN-γ and TNF-α mRNAs were identified in tissues from infected fetuses. RNA message was not identified in tissues of fetuses from mock-infected dams or non-infected fetuses from infected dams. This indicates that altered gene expression is the direct result of fetal infection. Up-regulated expression was confirmed by the presence of detectable levels of IFN-γ and TNF-α proteins in serum. Furthermore, the results indicate that INF-α and IFN-γ cytokines response are compartmentalized within the fetus and do not extend to other compartments, such as amniotic fluid or placenta, thus lessening the probability of allorejection by the dam. From these data, it is apparent that the fetus is capable of initiating a Th1-like response; however, the capacity of IFN-γ and TNF-α to control PRRSV infection in the fetus is not known. Additional evidence for induction of virus-specific immunity was found in the development of germinal centers containing activated (CDw75+) B cells and seroconversion in at least one fetus.
Pro-inflammatory cytokines, such as TNF-α and IFN-γ can contribute to pulmonary distress through the activation of alveolar macrophages and other cell populations. The increased quantities of IFN-γ and TNF-α found in the lungs of PRRSV-infected fetuses may not be sufficient to cause pulmonary damage to the fetal lung, primarily because fetal macrophages have a reduced capacity to respond to inflammatory stimuli. In addition, IL-10, up-regulated in response to PRRSV infection, is a potent antagonist of IFN-γ and TNF-α activation of macrophages (Burchett et al., 1992, Marodi et al., 2001, Johnsen et al., 2002, Thanawongnuwech et al., 2003). However, within days after birth, adult macrophages, including mature alveolar and intravascular macrophages emerge into an environment already enriched in IFN-γ and TNF-α. The outcome is the rapid recruitment, activation, and cytopathic killing of large numbers of virus-permissive macrophages (Choi and Chae, 2002). This scenario as a cause of severe interstitial pnuemonia in the PRRSV-infected newborn requires further investigation, but has obvious implications in the etiology of postnatal pulmonary complications following virus infections of the fetus.
Acknowledgements
This work was partially supported by the USDA National Research Initiative for Competitive Grants Program Grants # 97-35204-5071.
References
- Allende R., Laegreid W.W., Kutish G.F., Galeota J.A., Wills R.W., Osario F.A. Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J. Virol. 2000;74:10834–10837. doi: 10.1128/jvi.74.22.10834-10837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck P.C., Ferrick D.A., Steele-Norwood D., Egan P.J., Croitoru K., Carding S.R., Dietl J., Clark D.A. Murine T cell determination of pregnancy outcome. Cell Immunol. 1999;196:71–79. doi: 10.1006/cimm.1999.1535. [DOI] [PubMed] [Google Scholar]
- Basurko C., Carles G., Youssef M., Guindi W.E. Maternal and foetal consequences of dengue fever during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;147:29–32. doi: 10.1016/j.ejogrb.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (Isolate ATCC VR-2332) J. Vet. Diag. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Collins J.E., Dee S.A., Halbur P., Joo H.S., Lager K.M., Mengeling W.L., Murtaugh M.P., Rossow K.D., Stevenson G.W., Zimmerman J.J. Porcine reproductive and respiratory syndrome virus. In: Straw B.E., D’Allare S., Sl Mengeling D.J., Taylor, editors. Diseases of Swine. 8th ed. Iowa State University Press; Ames, IA: 1999. pp. 201–232. [Google Scholar]
- Benson J.E., Yeager M.J., Christopher-Hennings J., Lager K., Yoon K. A comparison of virus isolation, immunohistochemistry, fetal serology, and reverse-transcription polymerase chain reaction assay for identification of porcine reproductive and respiratory syndrome virus transplacental infection in the fetus. J. Vet. Diagn. Invest. 2002;14:8–14. doi: 10.1177/104063870201400103. [DOI] [PubMed] [Google Scholar]
- Burchett S.K., Corey L., Mohan K.M., Westall J., Ashley R., Wilson C.B. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J. Infect. Dis. 1992;165:813–818. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- Cafruny W.A., Bradley S.E. Trojan Horse macrophages: studies with the murine lactate dehydrogenase-elevating virus and implications for sexually transmitted virus infection. J. Gen. Virol. 1996;77:3005–3012. doi: 10.1099/0022-1317-77-12-3005. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales. A new order comprising Coronaviridae and Arterivirdae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Challis J.R., Lockwood C.J., Myatt L., Norman J.E., Strauss J.F., Petraglia F. Inflammation and pregnancy. Reprod. Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Chaouat G.S., Zourbas S., Ostojic G., Lappree-Delage S., Dubanchet N., Ledee J., Martal J. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J. Reprod. Immunol. 2002;53:241–256. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- Chaouat G., Menu E., Clark D.A., Dy M., Minkowski M., Wegmann T.G. Control of fetal survival in CBA × DBA/2 mice by lymphokine therapy. J. Reprod. Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- Chipeta J., Komada Y., Zhang X.L., Azuma E., Yamamoto H., Sakurai M. Neonatal (cord blood) T cells can competently raise type 1 and 2 immune responses upon polyclonal activation. Cell Immunol. 2000;205:109–110. doi: 10.1006/cimm.2000.1718. [DOI] [PubMed] [Google Scholar]
- Choi C., Chae C. Expression of tumour necrosis factor-alpha is associated with apoptosis in lungs of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Res. Vet. Sci. 2002;72:45–49. doi: 10.1053/rvsc.2001.0519. [DOI] [PubMed] [Google Scholar]
- Christianson W.T., Choi C.S., Collins J.E., Molitor T.W., Morrison R.B., Joo H.S. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid gestation sows and fetuses. Can. J. Vet. Res. 1993;57:262–268. [PMC free article] [PubMed] [Google Scholar]
- Christianson W.T., Collins J.E., Benfield D.A., Harris L., Gorcyca D.E., Chladek D.W., Morrison R.B., Joo H.S. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am. J. Vet. Res. 1992;53:485–488. [PubMed] [Google Scholar]
- Clark D.A., Manuel J., Lee L., Chaouat G., Gorczynski R.M., Levy G.A. Ecology of danger-dependent cytokine-boosted spontaneous abortion in the CBA x DBA/2 mouse model. I. Synergistic effect of LPS and (TNF-alpha + IFN-gamma) on pregnancy loss. Am. J. Reprod. Immunol. 2004;52:370–378. doi: 10.1111/j.1600-0897.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Coignoul F.L., Cheville N.F. Pathology of maternal genital tract, placenta, and fetus in equine viral arteritis. Vet. Pathol. 1984;21:333–340. doi: 10.1177/030098588402100311. [DOI] [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J., Shaw D.P., Goyal S.M., Gorcyca D., Chladek D., McCullough S., Morrison R.B., Joo H.S. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Elena S.F., Miralles R., Cuevas J.M., Turner P.E., Moya A. The two faces of mutation: extinction and adaptation in RNA viruses. IUBMB Life. 2000;49:5–9. doi: 10.1080/713803585. [DOI] [PubMed] [Google Scholar]
- Entrican G. Immune regulation during pregnancy and host–pathogen interactions in infectious abortion. J. Comp. Pathol. 2002;126:79–94. doi: 10.1053/jcpa.2001.0539. [DOI] [PubMed] [Google Scholar]
- Erikstein B.K., Funderud S., Beiske K., Aas-Eng A., De Lange Davies C., Blomhoff H.K., Smeland E.B. Cell cycle-dependent regulation of CDw75 (beta-galactoside alpha-2,6-sialyltransferase) on human B lymphocytes. Eur. J. Immunol. 1992;22 doi: 10.1002/eji.1830220507. [DOI] [PubMed] [Google Scholar]
- Feng W., Laster S.M., Tompkins M., Brown T., Xu J.S., Altier C., Gomez W., Benfield D., McCaw M.B. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis type II. J. Virol. 2001;75:4889–4895. doi: 10.1128/JVI.75.10.4889-4895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns X., Purcell R.H., Bukh J. Quasispecies in viral persistence and pathogenesis of hepatitis C virus. Trends Microbiol. 1999;7:402–410. doi: 10.1016/s0966-842x(99)01590-5. [DOI] [PubMed] [Google Scholar]
- Goriely S., Vincart B., Stordeur P., Vekemans J., Willems F., Goldman M., De Wit D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- Ito M., Koide W., Sakurai M. Changes in intracellular cytokine levels in newborn and adult lymphocytes induced by HSV-1. J. Med. Virol. 1998;56:145–150. [PubMed] [Google Scholar]
- Johnsen C.K., Botner A., Kamstrup S., Lind P., Nielsen J. Cytokine mRNA profiles in bronchoalveolar cells of piglets experimentally infected in utero with porcine reproductive and respiratory syndrome virus: association of sustained expression of IFN-gamma and IL-10 after viral clearance. Viral Immunol. 2002;15:549–556. doi: 10.1089/088282402320914494. [DOI] [PubMed] [Google Scholar]
- Lager K.M., Halbur P.G. Gross and microscopic lesions in porcine fetuses infected with porcine reproductive and respiratory syndrome virus. J. Vet. Diag. Invest. 1996;8:275–282. doi: 10.1177/104063879600800301. [DOI] [PubMed] [Google Scholar]
- Lala P.K. Interruption of murine pregnancy by activation of antigen-non-specific killer cells in the endometrium with endomethacin, high dose IL-2, or a combination. Res. Immunol. 1990;141:159. doi: 10.1016/0923-2494(90)90136-m. [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Buddle J.C., Thrasher A.J., Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Kim S.J., Park C.G., Park J., Kim J.H., Chun T. Molecular cloning and expression analysis of pig CD79alpha. Vet. Immunol. Immunopathol. 2008;125:368–374. doi: 10.1016/j.vetimm.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Lin H., Mosmann T.R., Guilbert L., Tuntipopipat S., Wegmann T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- Marodi L., Goda K., Palicz A., Szabo G. Cytokine receptor signalling in neonatal macrophages: defective STAT-1 phosphorylation in response to stimulation with IFN-gamma. Clin. Exp. Immunol. 2001;126:456–460. doi: 10.1046/j.1365-2249.2001.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin A.J., McGurn M.E., Bort S.J., Gibson C., Lewis D.B. Hypomethylation of the interferon-gamma gene correlates with its expression by primary T-lineage cells. Eur. J. Immunol. 1995;25:426–430. doi: 10.1002/eji.1830250218. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L., Lager K.M., Vorwald A.C. Temporal characterization of transplacental infection of porcine fetuses with porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 1994;55:1391–1398. [PubMed] [Google Scholar]
- Mengeling W.L., Vorwald A.C., Lager K.M., Brockmeier S.L. Comparison among strains of porcine reproductive and respiratory syndrome virus for their ability to cause reproductive failure. Am. J. Vet. Res. 1996;57:834–839. [PubMed] [Google Scholar]
- Murphy S.P., Tayade C., Ashkar A.A., Hatta K., Zhang J., Croy B.A. Interferon gamma in successful pregnancies. Biol. Reprod. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C.J., Murtaugh M.P., Faaberg K.S. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.A., Christopher-Hennings J., Drew T., Wensvoort G., Collins J.E., Benfield D.A. Differentiation of United States and European isolates of porcine reproductive and respiratory syndrome (PRRS) virus using monoclonal antibodies. J. Clin. Microbiol. 1993;31:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P.G.W. Lactate dehydrogenase-elevating virus and related viruses. In: Fields B., editor. Fields Virology. 3rd ed. Lippincott-Raven; Philadelphia: 1996. pp. 1105–1120. [Google Scholar]
- Prescott S.L., Macaubas C., Holt B.J., Smallacombe T.B., Loh R., Sly P.D., Holt P.G. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J. Immunol. 1998;160:4730–4737. [PubMed] [Google Scholar]
- Raghupathy R. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Sem. Immunol. 2001;13:219–227. doi: 10.1006/smim.2001.0316. [DOI] [PubMed] [Google Scholar]
- Rainsford E., Reen D.J. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br. J. Haematol. 2002;116:702–709. doi: 10.1046/j.0007-1048.2001.03321.x. [DOI] [PubMed] [Google Scholar]
- Reddy N.R., Wilkie B.N. Quantitation of porcine cytokine and beta 2-microglobulin mRNA expression by reverse transcription polymerase chain reaction. J. Immunol. Methods. 2000;233:83–93. doi: 10.1016/s0022-1759(99)00188-x. [DOI] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Morrison R.B., Goyal S.M., Singh G.S., Collins J.E. Lymph node lesions in neonatal pigs congenitally exposed to porcine reproductive and respiratory syndrome virus. J. Vet. Diagn. Invest. 1994;6:368–371. doi: 10.1177/104063879400600316. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Shivers J.L., Yeske P.E., Polson D.D., Rowland R.R.R., Lawson S.R., Murtaugh M.P., Nelson E.A., Collins J.E. Porcine reproductive and respiratory syndrome virus infection in neonatal pigs characterised by marked neurovirulence. Vet. Rec. 1999;144:444–448. doi: 10.1136/vr.144.16.444. [DOI] [PubMed] [Google Scholar]
- Rowland R.R.R., Steffen M., Ackerman T., Benfield D.A. The evolution of porcine reproductive and respiratory syndrome virus: quasispecies and emergence of a virus subpopulation during infection of pigs with VR-2332. Virology. 1999;259:262–266. doi: 10.1006/viro.1999.9789. [DOI] [PubMed] [Google Scholar]
- Rowland R.R., Lawson S., Rossow K., Benfield D.A. Lymphoid tissue tropism of porcine reproductive and respiratory syndrome virus replication during persistent infection of pigs originally exposed to virus in utero. Vet. Microbiol. 2003;96:219–235. doi: 10.1016/j.vetmic.2003.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland R.R.R., Kim T.S., Robinson B., Stefanick J., Guanghua L., Lawson S.R., Benfield D.A. Inhibition of porcine reproductive and respiratory syndrome virus by interferon-gamma and recovery of virus replication with 2 aminopurine. Archiv. Virol. 2001;146:539–555. doi: 10.1007/s007050170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Fatemi S.H., Sidwell R., Patterson P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Mulenberg J.M. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Stockhofe-Zurwieden N., Navarro-Camarro J.A., Grosse-Beilage E., Chavez J., Pohlenz J. Uterine and placental alterations in pregnant sows associated with the porcine epidemic abortion and respiratory syndrome (PEARS) Zentralbl. Veterinamed. B. 1995;40:261–271. doi: 10.1111/j.1439-0450.1993.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Rungsipipat A., Disatian S., Saiyasombat R., Napakanaporn S., Halbur P.G. Immunohistochemical staining of IFN-gamma positive cells in porcine reproductive and respiratory syndrome virus-infected lung. Vet. Immunol. Immunopathol. 2003;91:73–77. doi: 10.1016/s0165-2427(02)00268-4. [DOI] [PubMed] [Google Scholar]
- Tizard I.R. Veterinary Immunology: An Introduction, Chapter 19. WB Saunders Company; Philadelphia, PA: 1996. Immunity in the fetus and newborn; pp. 237–254. [Google Scholar]
- Tsibris A.M., Korber B., Arnaout R., Russ C., Lo C.C., Leitner T., Gaschen B., Theiler J., Paredes R., Su Z., Hughes M.D., Gulick R.M., Greaves W., Coakley E., Flexner C., Nusbaum C., Kuritzkes D.R. Quantitative deep sequencing reveals dynamic HIV-1 escape and large population shifts during CCR5 antagonist therapy in vivo. PLoS One. 2009;25:e5683. doi: 10.1371/journal.pone.0005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman K.S., Owe-Larsson B., Brask J., Kristensson K.S., Hill R.H. Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Res. 2001;896:18–29. doi: 10.1016/s0006-8993(00)03238-8. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M.A., ter Laak E.A., Bloemrad M., deKluyer E.P., Kragten C., van Buiten L., den Besten A., Wagenaar F., Broekhuijsen J.M., Moonen P.L.J.M., Zetstra T., de Boer E.A., Tibben H.J., de Jong M.F., van’t Veld P., Groenland G.J.R., van Gennep J.A., Voets M.T.H., Verheijden J.H.M., Braamskamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- White G.P., Watt P.M., Holt B.J., Holt P.G. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO-T cells. J. Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- Wilson A.D., Morgan A.J. Primary immune responses by cord blood CD4(+) T cells and NK cells inhibit Epstein–Barr virus B-cell transformation in vitro. J. Virol. 2002;76(10):5071–5081. doi: 10.1128/JVI.76.10.5071-5081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


