Abstract
Epidemiological differences between tropical and temperate regions regarding viruses causing acute respiratory infection are poorly understood. This is in part because methodological differences limit the comparability of data from these two regions. Using identical molecular detection methods, we tested 1174 Ghanaian and 539 German children with acute respiratory infections sampled over 12 months for the 15 most common respiratory viruses by PCR. A total 43.2% of the Ghanaian and 56.6% of the German children tested positive for at least one respiratory virus. The pneumoviruses respiratory syncytial virus and human metapneumovirus were most frequently detected, in 13.1% and 25.1% within the Ghanaian and German children, respectively. At both study sites, pneumoviruses were more often observed at younger ages (p <0.001). In the Ghanaian rainy season, enveloped viruses were detected twice as often as non-enveloped viruses (prevalence rate ratio (PR) 2.0, 95% CI 1.7–2.4). In contrast, non-enveloped viruses were more frequent during the Ghanaian dry season (PR 0.6, 95% CI 0.4–0.8). In Germany, enveloped viruses were also more frequently detected during the relatively colder winter season (PR 1.6, 95% CI 1.2–2.1) and non-enveloped viruses during summer (PR 0.7, 95% CI 0.5–0.9). Despite a distance of about 5000 km and a difference of 44° latitude separating Germany and Ghana, virus spectra, age associations and seasonal fluctuation showed similarities between sites. Neither respiratory viruses overall, nor environmentally stable (non-enveloped) viruses in particular were more frequent in tropical Ghana. The standardization of our sampling and laboratory testing revealed similarities in acute respiratory infection virus patterns in tropical and temperate climates.
Keywords: Epidemiology, Germany, Ghana, infant, PCR, respiratory tract infections, viruses
Introduction
Acute respiratory infections (ARI) constitute a major public health burden and cause significant mortality in children under 5 years of age [1]. Using molecular diagnostic tools, viruses can be detected in up to 80% of ARI in developed countries [2]. In developing countries, there is little information on the epidemiology and seasonal variability of respiratory viruses. Some of the few available studies on children with ARI in Africa found similarly high viral detection rates [3], [4], [5], [6], [7]. Regarding epidemiological differences between viral ARI patterns, climate factors have been extensively studied for influenza viruses and respiratory syncytial virus (RSV). For influenza viruses, higher incidence has been associated with cold and dry climate in temperate regions, compared to higher incidence in warm and humid climate in some tropical regions [8]. These conditions may not uniformly favour a higher incidence of all respiratory viruses, as for RSV, higher incidence has been associated with cold and humid conditions in temperate settings [9]. For other respiratory viruses, very limited information exists on differences between tropical and temperate regions. This is partly because methodological differences limit the comparability of data regarding detection rates, composition of circulating viruses, and seasonality of detection.
Here we have studied two large cohorts of children with ARI in Ghana and Germany over an almost congruent 1-year period during 2008. Using standardized and identical molecular detection methods for 15 respiratory viruses, we observed similar infection patterns in both study sites.
Materials and Methods
Study sites
Children up to 13 years of age were selected at both study sites. At both sites, a paediatrician made the diagnosis of ARI at the time of the child's visit. An ARI was defined as an infection of the upper or lower airways with acute manifestations including dry or productive cough, sore throat, wheezing and coryza. In Ghana, nasopharyngeal flocked swabs were collected from outpatients with ARI, during February 2008 to February 2009 at the Child Welfare Clinic of the Agogo Presbyterian Hospital, Ashanti region. The Agogo hospital is the referral hospital of the Asante Akim North district of the Ashanti region of Ghana and sees about 6000 patients per year. Specimens were stored in previously prepared individual aliquots of RNAlater stabilizing solution (Qiagen, Hilden, Germany) until processing within the same day at the Kumasi Centre for Collaborative Research in Tropical Medicine. In Germany, nasopharyngeal flocked swabs were taken from inpatients with ARI at the University Paediatric Hospital, Bonn, during November 2007 to December 2008 and processed within 24 h at the Institute of Virology, Bonn University Medical Centre. The University Paediatric Hospital, Bonn sees about 3000 inpatients per year.
Sample processing and virus detection
RNA was purified using the Viral RNA Mini Kit (Qiagen). Testing was carried out using identical laboratory methods in both study sites by real-time RT-PCR using the Qiagen One-Step RT-PCR System and previously published assays for influenza A and B viruses (Flu A/B) [10], respiratory syncytial virus (RSV) [11], human metapneumovirus (hMPV) [12], human parainfluenza viruses 1–4 (hPiV1–4) [13], [14], human coronaviruses (hCoVs) -229E, -OC43, -NL63 and -HKU1 [15], enteroviruses (EV) [16], rhinoviruses (RhV) [17] and adenoviruses (AdV) [18]. Assay sensitivity and specificity were evaluated on photometrically quantified in vitro transcribed cRNA/DNA controls generated from cloned PCR amplicons containing the respective genomic target sites as described previously [19]. In Ghana, multiplex reactions were used to identify Flu A and Flu B, as well as RSV and hMPV, without the possibility of distinguishing individual viruses due to limited resources. RSV and hMPV are genetically related and constitute the Paramyxoviridae subfamily Pneumovirinae; from hereon they are referred to as pneumoviruses. Enveloped viruses under study included Flu A/B, pneumoviruses, hPiV1–4 and hCoVs. Non-enveloped viruses included EV, RhV and AdV.
Ethics, consent and approval
Ethical approval was obtained from the Committee on Human Research Publication and Ethics of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology in Ghana. Written informed consent was secured, expressed by the legal representatives of participants appending their signatures or thumbprint to the informed consent form. All study procedures were in accordance with the Helsinki Declaration of 1975 (as revised 1983 and 2000). No ethical approval was necessary in Bonn because these specimens were tested during routine diagnostics.
Statistics
Differences between virus detection rates were compared using the prevalence rate ratio (PR) with corresponding 95% CI [20]. Chi-square tests were applied to assess associations between categorical variables. Age-stratified analyses were conducted to assess differences among age groups. Mantel–Haenszel corrected PRs (PRMH) where calculated to adjust for age effects. Statistical analyses were performed using Stata 12 (StataCorp LP, College Station, TX, USA).
Results
Specimens
This study included individual upper respiratory tract specimens from 1174 Ghanaian children (45.4% female, 54.6% male) with a median age of 25 months (interquartile range (IQR), 12–41 months) and 539 German children (35.4% female, 64.6% male) with a median age of 16 months (IQR, 5–15 months). Because the median age of the two cohorts differed significantly (p <0.001, Mann–Whitney U-test), virus prevalence was analysed within age strata. For these analyses the age categories 0 to <1, 1 to <2, 2 to <5 and 5–13 years were established, within which 243 (22.6%), 269 (25.1%), 422 (39.3%) and 140 (13.0%) Ghanaian children and 226 (41.9%), 97 (18.0%), 131 (24.3%) and 85 (15.8%) German children were grouped, respectively.
Virus prevalence
In 507 Ghanaian (43.2%) and 305 German children (56.6%) at least one virus was identified, corresponding to a PRMH of 1.3 (95% CI 1.2–1.4) using Ghana as the reference group. Virus detection did not differ significantly between female and male patients in either of the two study sites (chi-square; Ghana, p 0.51 and Germany, p 0.45). The lowest difference in prevalence was observed in children below 1 year of age (PR 1.1; 95% CI 0.9–1.3), whereas the other age groups showed higher estimates with a combined PRMH of 1.4 (95% CI 1.2–1.6).
Among all viruses, the pneumoviruses (RSV or hMPV) were most frequently detected, contributing in total 13.1% (n = 154) and 25.1% (n = 135) of all virus detections within the Ghanaian and German cohorts, respectively. In Ghana, the next most frequent viruses were hPiVs1–4 (n = 114; 9.7%), RhVs (n = 93; 7.9%), hCoVs (n = 79; 6.7%), Flu A/B (n = 67; 5.7%), AdVs (n = 39; 3.3%) and EVs (n = 29; 2.5%). In Germany, the next most frequently detected viruses were RhVs (n = 63; 11.7%), AdVs (n = 48; 8.9%), hPiVs1–4 (n = 41; 7.6%), EVs (n = 36; 6.7%), hCoVs (n = 31; 5.8%) and Flu A/B (n = 22; 4.1%). RhVs, pneumoviruses, AdVs and EVs occurred more frequently in patients with ARI in Germany than in Ghana with PRMH of 1.6 (95% CI 1.2–2.2), 1.8 (95% CI 1.4–2.2), 2.6 (95% CI 1.7–3.9) and 3.0 (95% CI 1.8–5.0), respectively. PRs for individual viruses were comparable among age groups, except for EVs, where the strongest differences between study sites were observed in children aged 2 years (PR 9.7, 95% CI 2.7–35.2). In summary, no virus occurred more frequently in Ghana than in Germany at statistically significant PRs. Pneumoviruses afforded the majority of detections, whereas other viruses differed in prevalence between sites. Detection rates for all viruses and cohorts are listed in Table 1 .
Table 1.
Detection rates of respiratory viruses
| Kumasi (Ghana), n (%) |
Bonn (Germany), n (%) |
PRMH (95% CI)a | |
|---|---|---|---|
| Virusb | n = 1174 | n = 539 | |
| EV | 29 (2.5) | 36 (6.7) | 3.0 (1.8–5.0) |
| RhV | 93 (7.9) | 63 (11.7) | 1.6 (1.1–2.2) |
| AdV | 39 (3.3) | 48 (8.9) | 2.6 (1.7–3.9) |
| RSV/hMPV | 154 (13.1) | 135 (25.1) | 1.8 (1.4–2.2) |
| Flu A/B | 67 (5.7) | 22 (4.1) | 0.8 (0.5–1.2) |
| hPiV | 114 (9.7) | 41 (7.6) | 0.7 (0.5–1.1) |
| hCoV | 79 (6.7) | 31 (5.8) | 0.9 (0.6–1.3) |
| Any virus detectedc | 507 (43.2) | 305 (56.6) | 1.3 (1.2–1.4) |
Prevalence rate ratio age corrected with 95% CI, Ghana serves as reference group.
EV, enteroviruses; RhV, rhinoviruses; AdV, adenoviruses; RSV/hMPV, respiratory syncytial virus/human metapneumovirus; Flu A/B, influenza viruses A and B; hPiV, human parainfluenza viruses 1–4; hCoV, human coronaviruses.
Because of detections of multiple viruses in several patients, the sum of individual virus detections is higher than the number of patients testing positive for any of the viruses under study.
Patient age and virus detections
At both study sites, pneumoviruses were more often observed at younger ages (p <0.001). For some of the other viruses under study, different age trends between study sites were observed. In Ghana, hPiVs1–4 (p 0.03), EVs (p 0.001) and AdVs (p 0.03) were more often observed at younger ages, whereas Flu A/B was more often observed in older age groups (p 0.001). In Germany, only RhVs were more often observed at younger ages (p 0.03) beyond the pneumoviruses mentioned before. These data, taken together support a strong association of pneumoviruses with younger patient ages, whereas no general associations were observed for any other virus under study. Associations between patient age and virus detections are detailed in Table 2 .
Table 2.
Association between age and respiratory viruses
| Age (years) | No. of childrenb | EVa |
RhV |
AdV |
RSV/hMPV |
Flu A/B |
hPiV |
hCoV |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Ghana | ||||||||
| 0 to <1 | 243 | 8 (3.3) | 20 (8.2) | 13 (5.4) | 46 (18.9) | 9 (3.7) | 33 (13.6) | 19 (7.8) |
| 1 to <2 | 269 | 12 (4.5) | 18 (6.7) | 12 (4.5) | 37 (13.8) | 16 (6.0) | 31 (11.5) | 21 (7.8) |
| 2 to <5 | 422 | 3 (0.7) | 34 (8.1) | 9 (2.1) | 46 (10.9) | 19 (4.5) | 39 (9.2) | 23 (5.5) |
| 5 to <13 | 140 | 0 (–) | 6 (7.7) | 1 (0.7) | 10 (7.1) | 18 (12.9) | 6 (4.3) | 10 (7.1) |
| Chi-square test | p <0.01 | p 0.44 | p 0.03 | p <0.01 | p <0.01 | p 0.03 | p 0.56 | |
| Germany | ||||||||
| 0 to <1 | 226 | 11 (4.9) | 35 (15.5) | 19 (8.4) | 71 (31.4) | 4 (1.8) | 15 (6.6) | 10 (4.4) |
| 1 to <2 | 97 | 9 (9.3) | 13 (13.4) | 12 (12.4) | 25 (25.8) | 6 (6.2) | 7 (7.2) | 9 (9.3) |
| 2 to <5 | 131 | 9 (6.9) | 11 (8.4) | 14 (10.7) | 27 (20.6) | 7 (5.3) | 13 (9.9) | 9 (6.9) |
| 5 to <13 | 85 | 7 (8.2) | 4 (4.7) | 3 (3.5) | 12 (14.1) | 5 (5.9) | 6 (7.1) | 3 (3.5) |
| Chi-square test | p 0.46 | p 0.03 | p 0.17 | p <0.01 | p 0.14 | p 0.72 | p 0.26 | |
For 100 children from Ghana, no age information was available.
EV, enteroviruses; RhV, rhinoviruses; AdV, adenoviruses; RSV/hMPV, respiratory syncytial virus/human metapneumovirus; Flu A/B, influenza viruses A and B; hPiV, human parainfluenza viruses 1–4; hCoV, human coronaviruses.
Seasonality
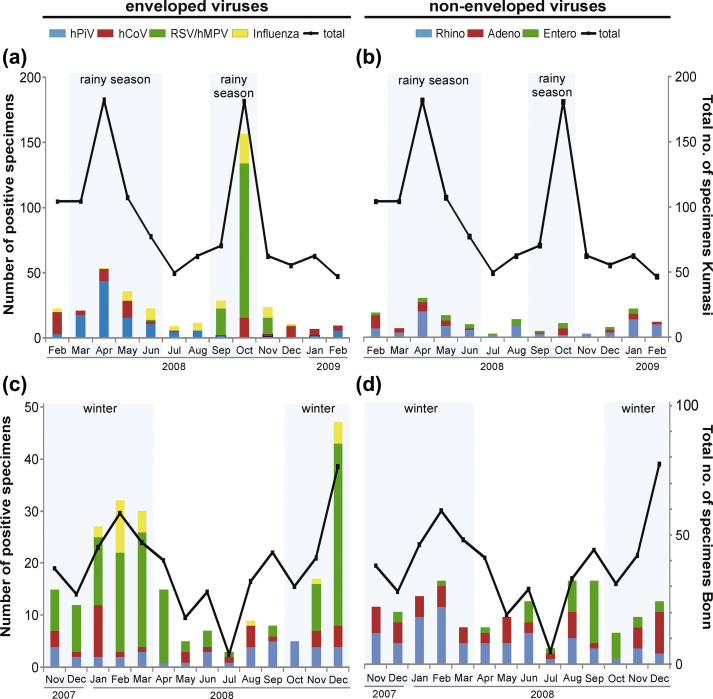
Seasonal differences showed similar patterns between sites. In the Ghanaian rainy season (relatively colder weather with clouded skies, March to June and September to October) enveloped viruses were detected twice as often as non-enveloped viruses (PR 2.0, 95% CI 1.7–2.4). As indicated in Fig. 1(a), this was mainly due to pneumoviruses (PR 5.8, 95% CI 3.4–9.7) and hPiVs (PR 2.6, 95% CI 1.7–4.1). Of note, peak pneumovirus detection rates coincided with the second rainy season only, matching a higher rainfall in these 2 months compared with the first rainy season (March to June 2008: 154 mm/month, peak in May: 224 mm; September to October 2008: mean 234 mm/month, peak in September: 283 mm). In contrast to enveloped viruses, non-enveloped viruses were more frequent during the Ghanaian dry season (PR 0.6, 95% CI 0.4–0.8). As indicated in Fig. 1(b), this difference was mainly driven by RhVs (PR 0.5, 95% CI 0.4–0.8).
Fig. 1.
Virus seasonality. Seasonality of enveloped viruses (a) and non-enveloped viruses (b) in Kumasi, Ghana and of enveloped viruses (c) and non-enveloped viruses (d) in Bonn, Germany. Blue-shaded months represent winter in Germany and rainy season in Ghana, the black line indicates the aggregate number of collected samples per month. In (c) and (d), primary and secondary y-scales differ to ensure visibility of individual viruses showing low detection rates.
In Germany, enveloped viruses were also more frequently detected during the relatively colder winter season (October to March; PR 1.6, 95% CI 1.2–2.1; Fig. 1c). As in Ghana, this was mainly due to increased detection of pneumoviruses (PR 2.5, 95% CI 1.6–3.8). Again, non-enveloped viruses were more frequently detected during the German summer (April to September; PR 0.7, 95% CI 0.5–0.9). As indicated in Fig. 1(d), this was mainly driven by EVs with a PR of 0.2 (95% CI 0.1–0.4). No seasonal patterns were observed for the enveloped Flu A/B and hCoVs, or the non-enveloped AdVs at either study site.
Co-infections
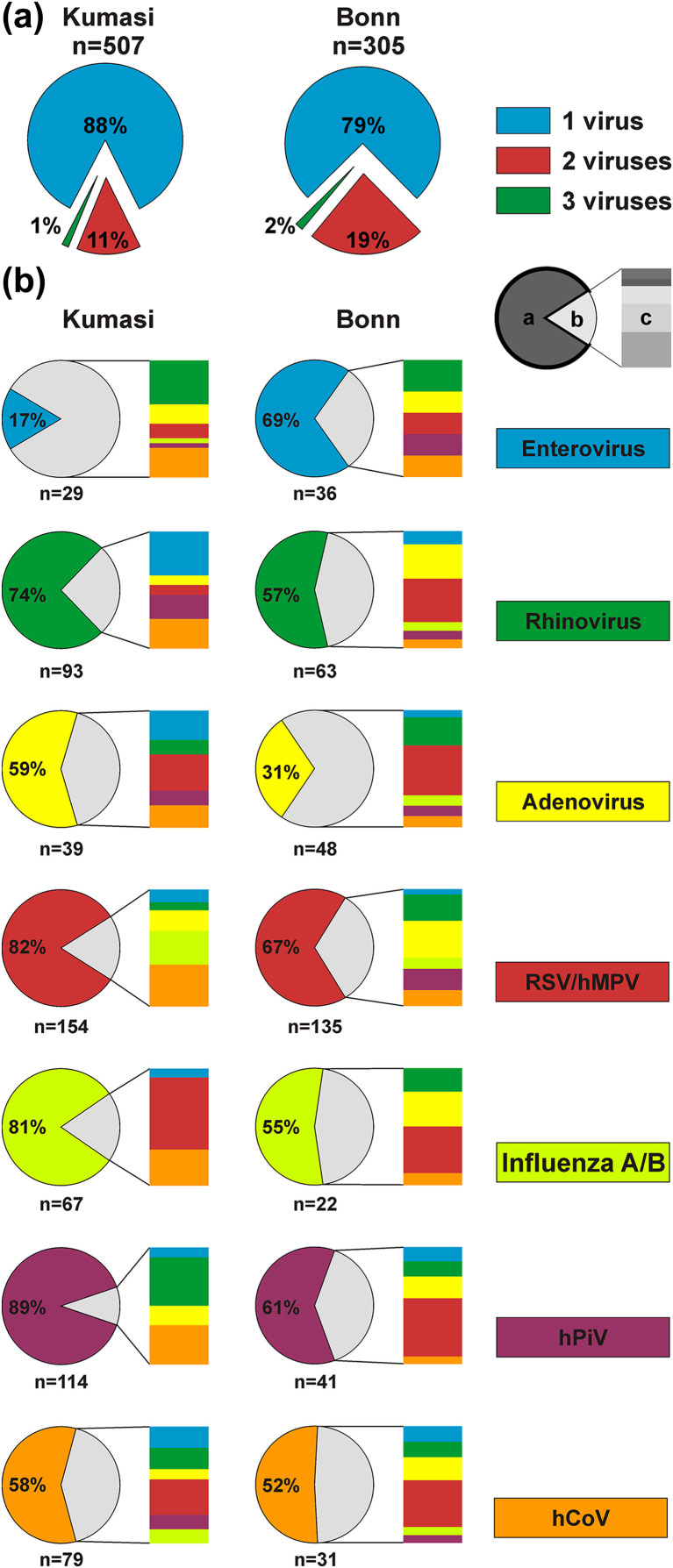
As shown in Fig. 2(a), significantly more viral co-infections were observed in Germany (21.0%) than in Ghana (12.0%; chi-square, p <0.001). As shown in Fig. 2(b), individual co-infection patterns differed between sites, suggesting that no generic property of any virus to cause mono-infections was evident from our data.
Fig. 2.
Viral co-infections. (a) Percentage of multiple infections at the two study sites. The total number of patients testing positive for any virus under study is indicated below sites. (b) Co-infections of individual viruses in Kumasi, Ghana and Bonn, Germany. The pie chart shows percentage of cases with a single infection of the respective pathogen (slice a), the share of multiple infections (slice b) and the co-infecting viruses identified by individual colours (slice c). The total number of individual virus detections is given below each pie chart per site.
Discussion
We employed sensitive molecular detection methodology to perform a comparative study on viral ARI between study sites located in a tropical and a temperate country.
Despite a distance of about 5 000 km and a latitude difference of about 44° separating our study sites, we observed an overall similar spectrum of viruses with a predominance of pneumoviruses, an association of pneumoviruses with younger patient age, and a seasonal predominance of pneumoviruses in cold and picornaviruses in warm weather conditions.
It is unclear to what extent climatic factors influence ARI viruses in temperate and tropical climates [21]. For the well-investigated virus Flu A, cold weather and low humidity probably favour transmission and incidence (summarized in ref. [22]). Additionally, a preferential transmission of Flu A by aerosol in temperate climates has been hypothesized, in contrast to predominant transmission by contact in the tropics [23]. Because Flu A/B detection rates were similar between Germany and Ghana, climatic conditions may have favoured incidence in Germany to a similar extent to the increased contact in Ghana. Contact-mediated transmission in crowded households during the tropical rainy season may also cause a higher incidence of RSV [24]. This is in line with our observation that the detection rate of pneumoviruses in Ghana peaked only in the second of two rainy seasons, coinciding with a higher rainfall at that time compared with the first rainy season. Increased transmission of pneumoviruses probably also contributed to the high number of pneumovirus detections during the German winter. Finally, the environmental stability of non-enveloped picornaviruses did not seem to correlate with an expected importance of these viruses as causes of ARI in the tropics [22].
Our study yielded lower overall virus detection rates than previous studies covering the majority of respiratory viruses by sensitive molecular techniques [1], [2]. In particular, the 43.2% rate in Ghana was lower than that in the few previous African studies using similar methodologies. However, strong region-specific and time-specific differences may exist in tropical ARI virus patterns. Examples for different viruses predominating in African studies include RhVs in a study from South Africa (37% of all virus detections; conducted during 2009–12) [6], hPiV1–3 and RhVs in Nigeria (35% each; 2009) [25], Flu A/B/C in Cameroon (28%; 2009) [3] and AdVs in Senegal (22%; 2012/13) [5]. Furthermore, African patient collectives may differ, for instance, based on the rate of underlying conditions such as human immunodeficiency virus type 1 (HIV-1) infection [6]. HIV prevalence in Ghana is 1.5%, whereas that of other African study sites yielding higher overall virus detection rates ranges from 0.5% (Senegal) to 18.9% (South Africa) [26]. The lower virus detection rate in Ghana compared with other African studies is therefore unlikely to be the result of HIV co-infection alone.
Regarding potential differences in patient collectives, it should be noted that our study contained two different clinical cohorts. In Ghana, specimens were collected from outpatients, whereas in Germany, specimens were collected from inpatients. We therefore cannot exclude that German inpatients may have presented with more severe ARI compared with Ghanaian outpatients, and that this may have influenced virus spectra and detection rates. This would be consistent with the higher pneumovirus detection rate in Germany. Unfortunately, we could not compare ARI severity between our sites. However, a previous study from our groups investigating Ghanaian inpatients with ARI yielded a pneumovirus detection rate of 14% [27]. This was near-identical to the 13% pneumovirus detection rate in Ghanaian outpatients in the present study. Altogether, these data speak against a major bias from cohort composition, suggesting that the German and Ghanaian cohorts can be compared regarding their ARI epidemiological patterns. Of note, we cannot exclude that only Ghanaian parents living in close proximity to the outpatient clinic or with children experiencing more severe disease sought medical attention, hence explaining the similar pneumovirus detection rates in Ghanaian inpatients and outpatients. This hypothesis is supported by previous work from our groups showing that travel distance to medical institutions significantly affects health care utilization in rural Ghana [28].
The strengths of our study include the largely overlapping period of patient specimen collection during 2008. This prevented a bias caused by pandemics of single viruses, such as the Flu A H1N1v pandemic in 2009/10 [29]. A major advantage over previous studies is the use of identical diagnostic tests under controlled conditions, including quantified controls to exclude variation in assay sensitivity during the study period.
In summary, our data suggest that poorer hygienic conditions in resource-limited tropical areas neither result in a generally higher detection rate of respiratory viruses, nor do they favour the detection of physically more stable non-enveloped viruses. To provide more robust data on the factors contributing to the burden of ARI in different economic and climatic conditions, population-based cross-sectional studies containing standardized clinical and sociological data will be required. Ideally, similar study sites from different countries representing tropical and temperate climates should be involved.
Contribution to authorship
The following contributions were made by authors: AA and FE acquisition, analysis and interpretation of data and drafting of manuscript; VMC, AMH, AS, and MP analysis of data; RK analysis and interpretation of data; YAS and TK conception of study; JM and JE acquisition of data; CD conception and design of study; and JFD design of study, analysis and interpretation of data and drafting of manuscript. All authors have read and approved the final manuscript.
Acknowledgements
We are grateful to Monika Eschbach-Bludau, Ulrike Reber, Sebastian Brünink, Ulrich Everling and Daniel Schacht for technical support and Alexander N. Lukashev and Ellis Owusu-Dabo for helpful suggestions. Work in Ghana was funded by the United Bank of Switzerland Optimus Foundation, and in Germany by the European Union FP7 project EMPERIE (Grant agreement number 223498).
Editor: L. Kaiser
Transparency Declaration
The authors have no conflict of interest to declare.
References
- 1.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahony J.B. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21:716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Njouom R., Yekwa E.L., Cappy P., Vabret A., Boisier P., Rousset D. Viral etiology of influenza-like illnesses in Cameroon, January–December 2009. J Infect Dis. 2012;206(Suppl. 1):S29–S35. doi: 10.1093/infdis/jis573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lekana-Douki S.E., Nkoghe D., Drosten C., Ngoungou E.B., Drexler J.F., Leroy E.M. Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC Infect Dis. 2014;14:373. doi: 10.1186/1471-2334-14-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dia N., Diene Sarr F., Thiam D., Faye Sarr T., Episé E., OmarBa I. Influenza-like illnesses in Senegal: not only focus on influenza viruses. PloS One. 2014;9:e93227. doi: 10.1371/journal.pone.0093227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen C., Walaza S., Moyes J., Groome M., Tempia S., Pretorius M. Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009–2012. Pediatr Infect Dis J. 2015;34:66–72. doi: 10.1097/INF.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Acremont V., Kilowoko M., Kyungu E., Philipina S., Sangu W., Kahama-Maro J. Beyond malaria—causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370:809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 8.Tamerius J.D., Shaman J., Alonso W.J., Bloom-Feshbach K., Uejio C.K., Comrie A. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9:e1003194. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J.W., Loh T.P. Correlations between climate factors and incidence—a contributor to RSV seasonality. Rev Med Virol. 2014;24:15–34. doi: 10.1002/rmv.1771. [DOI] [PubMed] [Google Scholar]
- 10.van Elden L.J., Nijhuis M., Schipper P., Schuurman R., van Loon A.M. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuypers J., Wright N., Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol. 2004;31:123–129. doi: 10.1016/j.jcv.2004.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dare R., Sanghavi S., Bullotta A., Keightley M.C., George K.S., Wadowsky R.M. Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol. 2007;45:548–552. doi: 10.1128/JCM.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuypers J., Wright N., Ferrenberg J., Huang M.L., Cent A., Corey L. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong S., Chern S.W., Li Y., Pallansch M.A., Anderson L.J. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J Clin Microbiol. 2008;46:2652–2658. doi: 10.1128/JCM.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dare R.K., Fry A.M., Chittaganpitch M., Sawanpanyalert P., Olsen S.J., Erdman D.D. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–1328. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dierssen U., Rehren F., Henke-Gendo C., Harste G., Heim A. Rapid routine detection of enterovirus RNA in cerebrospinal fluid by a one-step real-time RT-PCR assay. J Clin Virol. 2008;42:58–64. doi: 10.1016/j.jcv.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Lu X., Holloway B., Dare R.K., Kuypers J., Yagi S., Williams J.V. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–539. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heim A., Ebnet C., Harste G., Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70:228–239. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 19.Drexler J.F., Kupfer B., Petersen N., Grotto R.M., Rodrigues S.M., Grywna K. A novel diagnostic target in the hepatitis C virus genome. PLoS Med. 2009;6:e31. doi: 10.1371/journal.pmed.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zocchetti C., Consonni D., Bertazzi P.A. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997;26:220–223. doi: 10.1093/ije/26.1.220. [DOI] [PubMed] [Google Scholar]
- 21.Shek L.P.-C., Lee B.-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003;4:105–111. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 22.Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr Opin Virol. 2012;2:90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowen A., Palese P. Transmission of influenza virus in temperate zones is predominantly by aerosol, in the tropics by contact: a hypothesis. PLoS Curr. 2009;1:RRN1002. doi: 10.1371/currents.RRN1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simoes E.A.F. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:118–126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 25.Akinloye O.M., Rönkkö E., Savolainen-Kopra C., Ziegler T., Iwalokun B.A., Deji-Agboola M.A. Specific viruses detected in Nigerian children in association with acute respiratory disease. J Tropical Med. 2011;2011:690286. doi: 10.1155/2011/690286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UNAIDS . Joint United Nations Programme on HIV/AIDS; Geneva: 2014. HIV and AIDS estimates. [Google Scholar]
- 27.Kwofie T.B., Anane Y.A., Nkrumah B., Annan A., Nguah S.B., Owusu M. Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol J. 2012;9:78. doi: 10.1186/1743-422X-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krumkamp R., Sarpong N., Kreuels B., Ehlkes L., Loag W., Schwarz N.G. Health care utilization and symptom severity in Ghanaian children—a cross-sectional study. PloS One. 2013;8:e80598. doi: 10.1371/journal.pone.0080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak G.C., Wong A.H., Ho W.Y., Lim W. The impact of pandemic influenza a (H1N1) 2009 on the circulation of respiratory viruses 2009–2011. Influenza RespVirus. 2012;6:e6–10. doi: 10.1111/j.1750-2659.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]