Abstract
Characteristics such as versatility, stability and high-level expression make adenovirus vectors invaluable tools for the expression of transgenes in mammalian cells for the development of recombinant viral vaccines and for delivery of therapeutic genes.
Keywords: Adenoviruses, Vectors, Gene transfers, Gene therapy
Adenoviruses are used extensively to deliver genes into mammalian cells, particularly where there is a requirement for high-level expression of transgene products in cultured cells, or for use as recombinant viral vaccines or in gene therapy (reviewed in Ref. 1). The boundaries between the latter two applications are somewhat blurred, as the use of viral vectors as vaccines (e.g. for immunotherapy of cancer) is not fundamentally different from their use in gene therapy. These viruses are particularly well suited for many applications for several reasons: their stability and ability to grow to high titres; their ease of manipu-lation and purification; and their ability to transduce many mammalian cell types from numerous species, including both dividing and nondividing cells in vitro and in vivo.
Vectorology
The adenovirion2 is a nonenveloped icosahedral capsid of approximately 700 nm comprising only protein and DNA, the latter consisting of a linear double-stranded DNA of approximately 30–40 kb (Fig. 1a ). DNA replication and virion assembly take place in the nucleus of infected cells, and the production of huge amounts of virions and virion products results in cell death and the release of several thousand infectious viruses per cell at the end of the replication cycle. There are many kinds of adenovirus vectors and many ways of constructing them3. At one extreme are the nondefective vectors that retain all essential viral genes and have inserts of foreign DNA in nonessential regions of the genome, and at the other extreme are the vectors from which all viral genes have been deleted and substituted with foreign DNA (up to 36 kb)4.
Fig. 1.
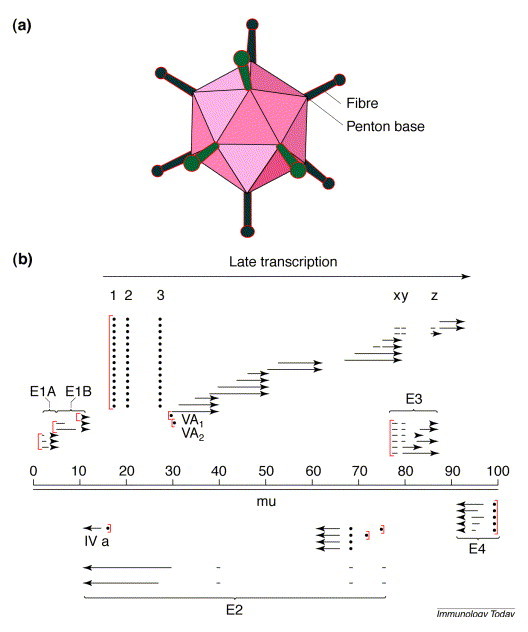
(a) The adenovirus virion is an icosahedron with protrusions, called fibres, attached to a penton base at each of the 12 vertices. The capsid protein that forms the major component of the 20 facets is called hexon. A dozen or so additional proteins make up the capsid and core of the virion. Approximately 20% of the molecular mass of the particle comprises DNA packaged as a linear double-stranded molecule. (b) Organization of the viral genome [100 map units (mu)=36 kb]. Promoters are shown as [ (in red). Transcription from the major late promoter at 16 mu generates a single long transcript that is spliced into late mRNAs as indicated. 1, 2, 3 and x, y, z represent leader RNAs attached to various late messages. The viral genome has four regions that are transcribed early in the replication cycle, of which only E3 is not essential for virus replication; E3 is primarily involved in regulating the host immune response to viral infection. E1 encodes essential functions but can also be deleted to produce viruses that are severely attenuated and must be propagated in cells, such as 293, that express E1. The DNA-packaging capacity of the virion is limited to approximately 105% of the wild-type genome but deletion of E1 and E3 sequences increases the packaging capacity of adenovirus vectors to as much as 8 kb. The only sequences needed in cis for viral DNA replication and packaging of DNA into virions are the inverted terminal repeats (ITRs) of approximately 100 bp and a packaging signal (Ψ) located adjacent to the left ITR and spanning approximately 200 bp. Thus, if all necessary gene products are provided in trans, virtually the entire genome can be deleted and substituted with as much as 36 kb of foreign DNA. This is the basis for development of fully deleted (FD) vectors, also called ‘helper-dependent’ vectors because the only currently available technology for propagating these vectors is in cells coinfected with a helper virus.
The transcriptional organization of a typical adenovirus genome is illustrated in Fig. 1b. From the perspective of adenovectorology, the most important regions are the early regions 1 and 3 (E1 and E3). E3 is nonessential and can be deleted without interfering with the ability of the virus to replicate, and E1, although essential, can also be deleted, resulting in a defective virus that is propagated in E1-expressing cells such as 293 cells (Ad5-transformed human embryonic kidney cells).
The most commonly used vectors are those containing deletions of E1 and E3, with inserts of foreign DNA in E1. Such vectors, which are generally referred to as first-generation (FG) vectors, are defective for replication in normal cells but can efficiently transduce most cells. FG vectors are particularly useful for gene transfer into cultured cells and for gene therapy applications that require transient gene expression. FG vectors are not suitable for long-term expression because they retain most viral genes and express them at low levels, resulting in an immune response against transduced cells in vivo. Currently, the best available adenovirus vectors for long-term expression in vivo are ones from which all viral genes have been deleted5. These fully deleted (FD) vectors must be propagated in the presence of a helper virus that provides all the viral functions and virion capsid proteins needed in trans for virus replication, and are often referred to as ‘helper-dependent’ vectors4.
Applications
FG vectors are easy to engineer, propagate and purify, and have numerous uses where efficient gene delivery and high-level expression are desired. Thus, they are excellent research tools, and will be used increasingly as novel genes are discovered and their products become a subject for investigation. Because the vectors can deliver genes encoding antigens and express them at high levels in vivo in any mammalian species, they are excellent candidates as recombinant viral vaccines. Indeed, vectors capable of immunizing animals against rabies6, herpes viruses7, rotaviruses8 and coronaviruses9 have all been developed. FG vectors are particularly suited for use in cancer immunotherapy strategies because of the ability of the vector to tranduce most cell types, including nondividing cells, and its ability to express transgene products to high levels. In these regimens, transient expression is preferred over long-term expression, and the inflammatory response and cytotoxic T lymphocyte (CTL) activity associated with administration of FG vectors may be advantageous. Several FG vectors have been produced that express a variety of cytokines and other immunomodulatory proteins10, 11. These have yielded encouraging results when tested in tumour models in animals and some have been used in clinical trials12.
FD vectors are technically more difficult to engineer, propagate and purify than FG vectors but have a much higher therapeutic index and give much longer expression in vivo 5. Thus, FD vectors may find use in ‘classical’ gene therapy such as enzyme replacement, where the desired outcome is permanent expression of the transgene product.
Concluding remarks
In summary, adenovirus vectors come in many forms and have great versatility and high efficacy when designed and used appropriately. They will play an increasingly important role as agents for gene transfer into mammalian cells.
Acknowledgements
This work was supported by grants from the National Institutes of Health, Medical Research Council of Canada (MRC) and the National Cancer Institute of Canada (NCIC), and by Merck Research Laboratories.
References
- 1.Hitt M. In: Advances in Pharmacology – Gene Therapy. August J.T., editor. Academic Press; 1997. pp. 137–206. [Google Scholar]
- 2.Shenk T. In: Fields Virology (3rd edn) Fields B.N., editor. Lippincott-Raven; 1996. pp. 2111–2148. [Google Scholar]
- 3.Hitt M. In: Cell Biology: A Laboratory Handbook (Vol. 1, 2nd edn) Celis J.E., editor. Academic Press; 1998. pp. 500–512. [Google Scholar]
- 4.Parks R.J. A new helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morral N. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarosh O.K. Human adenovirus type 5 vectors expressing rabies glycoprotein. Vaccine. 1996;14:1257–1264. doi: 10.1016/s0264-410x(96)00012-6. [DOI] [PubMed] [Google Scholar]
- 7.McDermott M.R. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989;169:244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 8.Both G.W. Protective immunity to rotavirus-induced diarrhoea is passively transferred to new-born mice from naive dams vaccinated with a single dose of a recombinant adenovirus expressing rotavirus VP7sc. Virology. 1993;193:940–950. doi: 10.1006/viro.1993.1203. [DOI] [PubMed] [Google Scholar]
- 9.Wesseling J.G. Mouse hepatitis virus spike and nucleocapsid proteins expressed by adenovirus vectors protect mice against a lethal infection. J. Gen. Virol. 1993;74:2061–2069. doi: 10.1099/0022-1317-74-10-2061. [DOI] [PubMed] [Google Scholar]
- 10.Addison C.A. Intratumoural injection of an adenovirus expressing interleukin-2 induces tumour regression and long term immunity in a murine breast cancer model. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8522–8526. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putzer B.M. Interleukin-12 and B7-1 costimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10889–10894. doi: 10.1073/pnas.94.20.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart A.K. Adenovector-mediated gene delivery of interleukin 2 in metastatic breast cancer and melanoma: results of a phase I clinical trial. Gene Ther. 1999;6:350–363. doi: 10.1038/sj.gt.3300833. [DOI] [PubMed] [Google Scholar]


