Abstract
Objectives
We aimed to assess the accuracy of PCR detection of viruses and bacteria on nasopharyngeal and oropharyngeal swabs (NPS) for the diagnosis of pneumonia in elderly individuals.
Methods
We included consecutive hospitalized elderly individuals suspected of having pneumonia. At inclusion, NPS were collected from all participants and tested by PCR for the presence of viral and bacterial respiratory pathogens (index test, defined as comprehensive molecular testing). Routine diagnostic tests (blood and sputum culture, urine antigen detection) were also performed. The reference standard was the presence of pneumonia on a low-dose CT scan as assessed by two independent expert radiologists.
Results
The diagnosis of pneumonia was confirmed in 127 of 199 (64%) included patients (mean age 83 years, community-acquired pneumonia in 105 (83%)). A pathogen was identified by comprehensive molecular testing in 114 patients (57%) and by routine methods in 22 (11%). Comprehensive molecular testing was positive for viruses in 62 patients (31%) and for bacteria in 73 (37%). The sensitivity and specificity were 61% (95% CI 53%–69%) and 50% (95% CI 39%–61%) for comprehensive molecular testing, and 14% (95% CI 82%–21%) and 94% (95% CI 86%–98%) for routine testing, respectively. Positive likelihood ratio was 2.55 for routine methods and 1.23 for comprehensive molecular testing.
Conclusion
Comprehensive molecular testing of NPS increases the number of pathogens detected compared with routine methods, but results are poorly predictive of the presence of pneumonia. Hence, comprehensive molecular testing is unlikely to impact clinical decision-making (NCT02467192).
Clinical Trials Registration
Keywords: Diagnosis, Elderly patients, Low-dose CT scan, Multiplex PCR, Nasopharyngeal swabs, Pneumonia
Introduction
Identification of the pathogen causing pneumonia is useful to guide antibiotic therapy, to help with differential diagnosis and for epidemiological reasons. Hence, most current guidelines recommend microbiological investigations in patients hospitalized for suspected community-acquired pneumonia (CAP) [1], [2], [3]. Nevertheless the microorganism causing CAP is identified in only a minority of patients [4]. The difficulty in obtaining high-quality respiratory samples for microbiological analysis (e.g. sputum cultures) is an important limitation in the elderly [5]. Use of molecular biology technology improves the diagnostic yield in suspected pneumonia and is often prescribed by physicians, but it is unclear how it impacts clinical management [6]. Oosterheert et al. showed in a randomized controlled trial (107 individuals with lower respiratory tract infections, mean age 65 years) that PCR for viruses and atypical bacteria in nasopharyngeal and oropharyngeal swabs (NPS) allowed the identification of additional pathogens but did not reduce antibiotic use or costs [7]. A pragmatic randomized controlled trial (720 individuals with acute respiratory illness, mean age 63 years) showed that molecular point-of-care testing for respiratory viruses did not reduce the proportion of patients treated with antibiotics [8]. Finally, in a prospective observational study (147 individuals with lower respiratory tract infection, mean age 78 years), PCR detection of respiratory viruses had no impact on antibiotic use and length of stay [9].
We aimed to assess the diagnostic accuracy of PCR detection of viral and bacterial pathogens on NPS for the diagnosis of pneumonia in elderly individuals.
Methods
Individuals admitted to hospital for suspected pneumonia had NPS collected at inclusion for the detection of multiple bacterial and viral pathogens using multiplex PCR (comprehensive molecular testing), in addition to routine testing. A chest low-dose computed tomography scan (LDCT) was performed as soon as possible. Results regarding the diagnostic performance of LDCT have been published elsewhere [10]. The present study is a pre-planned secondary analysis evaluating the accuracy of comprehensive molecular testing in patients with suspected pneumonia. Sample size is based on the power calculation of the original study.
Context and design
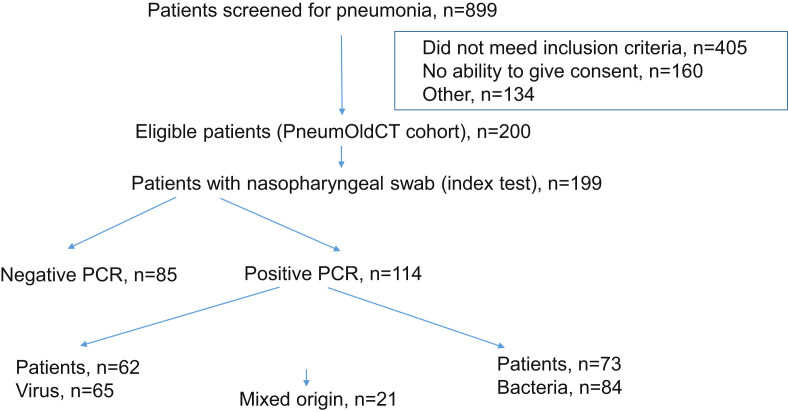
This study took place in the geriatric and internal medicine wards of Geneva University Hospitals, an 1800-bed tertiary-care institution serving approximately 500 000 inhabitants. The study was approved by Geneva's Institutional Review Board (CER 14-250) and registered at clinicaltrials.gov (NCT02467192). Informed consent was obtained from all patients or next of kin. Consecutive hospitalized individuals ≥65 years old, suspected of having community-, nursing-home- or hospital-acquired pneumonia were enrolled between 1 May 2015 and 30 April 2016. Individuals included had at least one respiratory symptom and at least one symptom or laboratory finding compatible with infection [10]. Individuals treated for pneumonia during the previous 6 months or treated with antimicrobial therapy for more than 48 h before inclusion were excluded (Fig. 1 ). Demographic data, co-morbidities, vital signs, clinical findings, severity scores, results of standard laboratory tests, blood, sputum and urine cultures, urinary antigen detection, PCR for respiratory viruses on NPS, and antimicrobial therapy administered were recorded prospectively.
Fig. 1.
Flowchart.
Low-dose CT scan and reference standard
Images were interpreted as consistent or inconsistent with pneumonia by two independent radiologists experienced in thoracic radiology. Discordant cases were reviewed together to reach a consensus. The radiological diagnoses were taken as the reference standard and patients with a seemingly infectious infiltrate were considered to have pneumonia. The radiologists were blinded to patients' results.
Nucleic acid analysis using multiplex real-time PCR (index test)
The NPS were performed on all individuals at inclusion. These were placed in COPAN® 305 Universal Transport Medium (COPAN Italia spa, Brescia, Italy), sent to the central virology laboratory as soon as possible and processed directly (neither frozen nor thawed). Nucleic acids were extracted with Qiasymphony (Qiagen, Hombrechtikon, Switzerland) using a Virus/Pathogen kit (937055, Qiagen). Real-time PCR was performed on a Viia7 thermocycler (Life Technologies, Carlsbad, California, USA). The FTD Respiratory pathogens 21 panel (FTD-2, FastTrack Diagnostics, Esch-sur-Alzette, Switzerland) was used to target 17 viruses (coronaviruses 229E, NL63, OC43, HKU1; human metapneumovirus; influenza A, A(H1N1) pdm2009 and B viruses; parainfluenza viruses 1, 2, 3 and 4; respiratory syncytial viruses A and B; rhinovirus; adenovirus; bocavirus; enterovirus; parechovirus). The Bacterial pneumonia CAP (for community-acquired pneumonia, FTD-29.19, FastTrack Diagnostics) and the Bacterial pneumonia HAP (for hospital-acquired pneumonia, FTD-30, FastTrack Diagnostics) tests were used to detect nine bacterial species/genera: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella spp., Klebsiella pneumoniae and Pseudomonas aeruginosa. The results of the PCR for viruses were available to the treating physicians whereas the results of PCR for bacteria were performed subsequently as a batch on nucleic acid extraction, which were kept at –80°C and were not reported to the treating physicians. Comprehensive molecular testing was defined as the results of both viral and bacterial PCR.
Routine testing
Routine microbiological tests were performed according to recommendations, including blood cultures, sputum cultures in patients able to expectorate and pneumococcal and Legionella pneumophila urinary antigen detection [3]. Sputum samples with 25 or more neutrophils and fewer than ten epithelial cells were evaluated after Gram-staining, and were subsequently cultured.
Statistical analysis
We used frequencies, percentage, mean with range and median with interquartile range for descriptive purposes. Variables including results of comprehensive molecular testing, viral PCR alone, bacterial PCR alone and routine methods, were compared between patients with and without pneumonia in univariate analysis using the Mann–Whitney–Wilcoxon test or the Kruskal–Wallis method for continuous variables, and Fisher's exact test or chi-square test for categorical variables, as appropriate. Comprehensive molecular testing was the index test. The test characteristics (sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios (LR), and diagnostic odds ratios) of comprehensive molecular testing, viral PCR, bacterial PCR, and routine methods, were computed using two-by-two tables. A p value of <0.05 was considered significant. Analyses were performed using the R statistical software package, version 3.1.1 (www.cran.r-project.org).
Results
Participants
Baseline characteristics of included patients are shown in Table 1 . NPS were performed in 199/200 patients (99.5%) and presence of pneumonia was confirmed in 127/199 (64%). Pneumonia was community-acquired in 105/127 (83%). The median delay between inclusion and LDCT was 2.2 h (interquartile range 0.9–15.4). Among the 72 patients without pneumonia, the most frequent diagnoses were non-respiratory sepsis, viral upper respiratory tract infection, bronchitis or exacerbation of chronic obstructive pulmonary disease, and heart failure. Mean duration of antimicrobial therapy was 6.8 days. Eighty-six of 199 patients (43.2%) received a combination antimicrobial therapy for a mean of 3.1 days (most frequently a β-lactam and a macrolide, according to institutional guidelines).
Table 1.
Demographic and clinical characteristics comparing participants with or without pneumonia
| All patients (n = 199) n (%) or mean (min–max) | Pneumonia (n = 127) n (%) or mean (min–max) | No pneumonia (n = 72) (n (%) or mean (min–max) | p value (pneumonia versus no pneumonia) | |
|---|---|---|---|---|
| Age (years) | 84 (65–103) | 83 (65–101) | 85 (70–103) | 0.13 |
| CURB65 score | 2.4 (1–4) | 2.4 (1–4) | 2.4 (1–4) | 0.96 |
| Place of acquisition of symptoms | 0.67 | |||
| Home | 161 (81) | 105 (83) | 56 (78) | |
| Nursing home | 22 (11) | 13 (10) | 9 (12) | |
| Hospital | 16 (8) | 9 (7) | 7 (10) | |
| Temperature ≥38.0°C on admission | 116 (58) | 77 (60.6) | 39 (54.2) | 0.45 |
| Cough | 169 (85) | 115 (90.6) | 54 (75.0) | 0.006 |
| Dyspnoea | 144 (72.4) | 92 (72.4) | 52 (72.2) | 1 |
| Sputum production | 73 (36.7) | 46 (36.2) | 27 (37.5) | 0.88 |
| Chest pain | 34 (17) | 23 (18.1) | 11 (15.3) | 0.70 |
| Crackles | 170 (85.4) | 105 (82.7) | 65 (90.3) | 0.21 |
| Decreased breath sounds | 51 (25.6) | 37 (29.1) | 14 (19.4) | 0.18 |
| Respiratory rate >30/min | 35 (17.6) | 25 (19.7) | 10 (13.9) | 0.34 |
| White blood cell count at inclusion, 103/mm3 | 11.6 (0.8–40.9) | 12.0 (2.0–40.9) | 10.9 (0.8–22.3) | 0.36 |
| C-reactive protein, mg/L | 119 (1–512) | 135 (1–512) | 91 (11–305) | 0.006 |
| Antimicrobial therapy before inclusion | 10 (5) | 7 (5.5) | 3 (4.2) | 0.75 |
| 30-day mortality | 14 (7) | 11 (14.2) | 3 (4.2) | 0.39 |
Laboratory values and vital signs were obtained at admission.
Definitions: CURB65 is a pneumonia severity score taking into account confusion, respiratory rate, blood pressure and age ≥65 years.
PCR findings on NPS
Results are depicted in Table 2 and the Supplementary material (Table S1). Comprehensive molecular testing was positive in 114/199 patients (57%). Sixty-two patients (31%) had a positive PCR for at least one virus, 73 patients (37%) had a positive PCR for at least one bacterium, and 21 (11%) had a positive PCR for both virus and bacteria. Antimicrobial therapy was stopped in 6/62 patients (9.7%) with a positive viral PCR.
Table 2.
Microbiological results according to the presence of pneumonia
| All patients (n = 199) | Pneumonia (n = 127) | No pneumonia (n = 72) | p value (pneumonia versus no pneumonia) | |
|---|---|---|---|---|
| Comprehensive molecular testing | 114 (57%) | 78 (61%) | 36 (50%) | 0.14 |
| Viral PCRa | 62 (31%) | 45 (35.4%) | 17 (23.6%) | 0.111 |
| Bacterial PCRa,b | 73 (36.7%) | 50 (39%) | 23 (32%) | 0.484 |
| Routine methodsc | 22 (11%) | 18 (14%) | 4 (6%) | 0.097 |
One patient could have more than one positive result.
Twenty-one patients had PCR positive for both virus and bacteria (18 with pneumonia, three without pneumonia, p 0.097).
Eleven patients had two bacteria identified using PCR.
Five patients had two bacteria identified using routine methods.
Routine findings
Results are displayed in Table 2 and the Supplementary material (Table S1). Blood cultures were performed in 176/199 patients (88%), urinary antigens for Legionella spp. and Streptococcus pneumoniae in 178/199 patients (89%) and 183/199 patients (92%), respectively. Sputum was obtained in 81/199 patients (41%), with only 12/81 (15%) of sufficient quality to warrant further evaluation. Routine methods led to the identification of a pathogen in 22/199 patients (11%). Twelve patients (6%) had bacteraemia, six (3%) of which had a non-respiratory origin (four urinary and two abdominal).
Microbiological results according to the presence of pneumonia on LDCT
Details for each pathogen are available in the Supplementary material (Table S1). Comprehensive molecular testing was positive in 78/127 patients (61%) with pneumonia and 36/72 patients (50%) without. At least one viral PCR was positive in 45/127 patients (35%) with pneumonia and 17/72 (24%) without. Bacterial PCR was positive in 50/127 patients (39%) with pneumonia and 23/72 (32%) without. Routine methods were positive in 18/127 patients (14%) with pneumonia and 4/72 (6%) without.
Test characteristics of comprehensive molecular testing and routine methods
Results are depicted in Table 3 . Sensitivity and specificity were 61% and 50% for comprehensive molecular testing, 14% and 94% for routine methods, 35% and 76% for viral PCR, and 39% and 68% for bacterial PCR, respectively. The positive LR was 2.55 for routine methods and 1.23 for comprehensive molecular testing. Negative LRs of routine and molecular methods were 0.91 and 0.77, respectively.
Table 3.
Accuracy of microbiological tests for the diagnosis of pneumonia
| Comprehensive molecular testing | Viral PCR | Bacterial PCR | Routine methods | |
|---|---|---|---|---|
| Sensitivity | 61% (53–69) | 35% (28–44) | 39% (31–48) | 14% (82–21) |
| Specificity | 50% (39–61) | 76% (65–85) | 68% (57–78) | 94% (86–98) |
| Positive predictive value | 68% (62–74) | 73% (62–81) | 68% (59–76) | 81% (61–93) |
| Negative predictive value | 42% (35–50) | 40% (36–45) | 39% (34–44) | 38% (36–41) |
| Positive likelihood ratio | 1.23 (0.94–1.61) | 1.50 (0.93–2.42) | 1.23 (0.83–1.84) | 2.55 (0.90–7.25) |
| Negative likelihood ratio | 0.77 (0.56–1.06) | 0.85 (0.70–1.01) | 0.90 (0.72–1.10) | 0.91 (0.83–0.99) |
| Diagnostic odds ratio | 1.59 (0.89–2.85) | 1.77 (0.92–3.41) | 1.38 (0.75–2.55) | 2.81 (0.91–8.65) |
Discussion
Our main findings are that results of comprehensive molecular testing of NPS are poorly predictive of the presence of pneumonia. Positive (1.23) and negative (0.77) LR of comprehensive molecular testing are too low to affect the probability of having pneumonia.
Comprehensive molecular testing increased the sensitivity to 61% in comparison with 14% with routine methods. Gadsby et al. achieved pathogen detection in 87% of patients hospitalized for CAP using comprehensive molecular testing [6]. This higher sensitivity is probably explained by collection of sputum in 96% of their patients, who were much younger (median age 67 years). In comparison, sputum of adequate quality was obtained in only 12/199 (6%) of our patients. Though sputum culture is recommended in international guidelines [3], [11], it is hard to obtain in elderly patients [12]. Our results compare with those of Putot et al., who were able to collect sputum from only 15% of elderly patients hospitalized for pneumonia [5]. Obtaining more good-quality lower respiratory tract samples would require an invasive procedure [13], [14].
In our cohort, 31% of the patients had a positive result for a virus, mainly rhinovirus and influenza virus. Forty-five (22.6%) had both a positive viral PCR and pneumonia. Positive and negative LRs were 1.50 and 0.85, respectively. This is in accordance with previous results in the literature. In another study assessing the performance of PCR on NPS for the prediction of CT-scan-confirmed pneumonia, prevalence of positive viral PCR was 28%, and positive and negative LRs were 1.39 and 0.87, respectively [15]. Certainly, many viruses, including influenza virus, respiratory syncytial virus, human metapneumovirus, parainfluenza virus and rhinovirus, are known causes of pneumonia [16], [17], [18], [19], [20], [21]. Jain et al. showed that respiratory viruses were more frequently detected than bacteria in patients hospitalized for non-severe CAP (median age 57 years) [22]. But viruses are also detected in NPS in individuals with bronchitis, exacerbation of chronic obstructive pulmonary disease, and even in asymptomatic patients [23], [24], [25]. In the present study, many patients with non-respiratory sepsis or cardiac failure had positive NPS. Our results confirm that viruses are frequently identified in patients with symptoms of a respiratory illness, including those without pneumonia. Finally, antimicrobial therapy was stopped in only 10% of patients with a positive viral PCR, suggesting that comprehensive molecular testing might not be an adequate means of reducing antimicrobial therapy prescription in patients with symptoms of lower respiratory tract infection.
We had hypothesized that a positive bacterial PCR in NPS might be a surrogate for bacterial pneumonia, allowing us to surpass the aforementioned difficulties to obtain good-quality sputum samples. However, bacterial PCR had poor diagnostic accuracy (positive and negative LRs of 1.23 and 0.90, respectively), probably because they are not able to differentiate between pharyngeal carriage and lower respiratory tract infection. Compared with the results of the CAPiTA cohort, pharyngeal carriage rate of Staphylococcus aureus and Streptococcus pneumoniae were lower in our cohort (14% versus 21% and 3% versus 17%, respectively), whereas the carriage rate was similar for Haemophilus influenzae (8% versus 7%) and higher for Moraxella catarrhalis (14% versus 8%) [26]. This differences may stem from the different population enrolled, the CAPiTA cohort including community-dwelling elderly people (mean age 72 years), a far younger and healthier population than ours.
Finally, the identification of a pathogen with routine methods did not result in a high diagnostic accuracy, although better than with comprehensive molecular testing.
Our study has several strengths. It was conducted in a consecutive cohort of unselected elderly patients who were submitted to extensive testing. We used a robust reference standard based on LDCT scan. The radiologists were blinded to participants' clinical, biological and microbiological results, so incorporation bias could be attenuated. According to recent findings, a reference standard for pneumonia based on chest X-ray may lead to frequent misclassifications, which can flaw the evaluation of microbiological test accuracy [10], [27]. Our comprehensive molecular testing included more respiratory bacterial pathogens than previous works [6], [7].
Our study also had some limitations. The lack of a control arm prevented the assessment of the impact of comprehensive molecular testing on patient management. We did not perform PCR analyses on a quantitative basis, and multiplex PCR have different analytical sensitivities according to the viruses sought. Sputum was not tested with PCR because good-quality sputum could only be obtained in a small minority of patients. Finally, the presence of an infiltrate on an LDCT scan may be an imperfect reference standard for the diagnosis of infectious pneumonia. However, using microbiological results of the patients in the reference definition would have led to a risk of incorporation bias.
Conclusion
The present study highlights the difficulties in identifying a causative agent in elderly patients with suspected pneumonia. Viruses and bacteria are frequently isolated by PCR in the upper airway of elderly patients but their presence is not useful for predicting the presence or absence of pneumonia. Hence they are unlikely to be helpful in making patient management decisions. Further investigation is needed to assess the usefulness of PCR sampling in patients with proven pneumonia to direct treatment.
Transparency declaration
The authors declare no conflict of interest.
Funding
The study was supported by grants from the Geneva University Hospitals (HUG) (Research & Development Grant, Medical Directorate, HUG) and the Ligue Pulmonaire Genevoise, a non-profit association involved in the care of patients with respiratory diseases.
Acknowledgements
This work was presented at the 28th ECCMID (Madrid). We thank the patients and their families for their participation in this study. We also thank the PneumOldCT study group, clinicians, radiology technicians, research nurses and case managers who helped us to enrol our participants, and our translator, Mr Darren Hart. We acknowledge the contribution of the ESCMID Study Group for Infections in the Elderly (ESGIE, www.escmid.org/esgie).
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2018.12.037.
Contributions of authors
VP and JS conceived the study, wrote the grant to obtain funding, obtained ethical approval, analysed the results and wrote the manuscript. VP, MPM and AM participated in recruitment and followed the participants and the samples. MS and XM were the radiologist experts. BH, NG, CM, AM, PEF, JPJ, JLR and LK helped design the study and contributed to the manuscript. JS performed the statistical plan and analyses.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim W.S., Baudouin S.V., George R.C., Hill A.T., Jamieson C., Le Jeune I. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64:iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 3.Woodhead M., Blasi F., Ewig S., Garau J., Huchon G., Leven M. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect. 2011;17:E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musher D.M., Abers M.S., Bartlett J.G. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of Pneumococcus. Clin Infect Dis. 2017;65:1736–1744. doi: 10.1093/cid/cix549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putot A., Tetu J., Perrin S., Bailly H., Piroth L., Besancenot J.F. Impact of microbiological samples in the hospital management of community-acquired, nursing home-acquired and hospital-acquired pneumonia in older patients. Eur J Clin Microbiol Infect Dis. 2016;35:489–495. doi: 10.1007/s10096-015-2565-9. [DOI] [PubMed] [Google Scholar]
- 6.Gadsby N.J., Russell C.D., McHugh M.P., Mark H., Conway Morris A., Laurenson I.F. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 2016;62:817–823. doi: 10.1093/cid/civ1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oosterheert J.J., van Loon A.M., Schuurman R., Hoepelman A.L., Hak E., Thijsen S. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41:1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brendish N.J., Malachira A.K., Armstrong L., Houghton R., Aitken S., Nyimbili E. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernes S.S., Quarterstaves H., Hamre R., Hagen E., Bjorvatn B., Bakke P.S. A comparison of nasopharyngeal and oropharyngeal swabbing for the detection of influenza virus by real-time PCR. Eur J Clin Microbiol Infect Dis. 2013;32:381–385. doi: 10.1007/s10096-012-1753-0. [DOI] [PubMed] [Google Scholar]
- 10.Prendki V., Scheffler M., Huttner B., Garin N., Herrmann F. Low-dose computed tomography for the diagnosis of pneumonia in elderly patients: a prospective, interventional cohort study. Eur Resp J. 2018;51 doi: 10.1183/13993003.02375-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eccles S., Pincus C., Higgins B., Woodhead M. Diagnosis and management of community and hospital acquired pneumonia in adults: summary of NICE guidance. BMJ. 2014;349:g6722. doi: 10.1136/bmj.g6722. [DOI] [PubMed] [Google Scholar]
- 12.Janssens J.P., Krause K.H. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 13.Karhu J., Ala-Kokko T.I., Vuorinen T., Ohtonen P., Syrjala H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S.-H., Hong S.-B., Ko G.-B., Lee Y., Park H.J., Park S.-Y. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Resp Crit Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 15.Das D., Le Floch H., Houhou N., Epelboin L., Hausfater P., Khalil A. Viruses detected by systematic multiplex polymerase chain reaction in adults with suspected community-acquired pneumonia attending emergency departments in France. Clin Microbiol Infect. 2015;21:608. doi: 10.1016/j.cmi.2015.02.014. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsey A.R., Walsh E.E. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518–524. doi: 10.1086/499955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falsey A.R., McElhaney J.E., Beran J., van Essen G.A., Duval X., Esen M. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209:1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panda S., Mohakud N.K., Pena L., Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014;25:45–52. doi: 10.1016/j.ijid.2014.03.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo H.J., Lim S., Choe J., Choi S.H., Sung H., Do K.H. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 20.Kim M.C., Kim M.Y., Lee H.J., Lee S.O., Choi S.H., Kim Y.S. CT findings in viral lower respiratory tract infections caused by parainfluenza virus, influenza virus and respiratory syncytial virus. Med (Balt) 2016;95:e4003. doi: 10.1097/MD.0000000000004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drysdale S.B., Mejias A., Ramilo O. Rhinovirus—not just the common cold. J Infect. 2017;74:S41–S46. doi: 10.1016/S0163-4453(17)30190-1. [DOI] [PubMed] [Google Scholar]
- 22.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuya-Kanamori L., Cox M., Milinovich G.J., Magalhaes R.J., Mackay I.M., Yakob L. Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerg Infect Dis. 2016;22:1052–1056. doi: 10.3201/eid2206.151080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira L.P., Watanabe A.S.A., Camargo C.N., Melchior T.B., Granato C., Bellei N. Respiratory syncytial virus evaluation among asymptomatic and symptomatic subjects in a university hospital in Sao Paulo, Brazil in the period of 2009 to 2013. Influenza Other Respir Virus. 2018;12:326–330. doi: 10.1111/irv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ip D.K., Lau L.L., Leung N.H., Fang V.J., Chan K.H., Chu D.K. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin Infect Dis. 2017;64:736–742. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Deursen A.M.M., van Houten M.A., Webber C., Patton M., Scott D., Patterson S. The impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal carriage in the Community Acquired Pneumonia Immunization Trial in Adults (CAPiTA) Study. Clin Infect Dis. 2018;67:42–49. doi: 10.1093/cid/ciy009. [DOI] [PubMed] [Google Scholar]
- 27.Claessens Y.E., Debray M.P., Tubach F., Brun A.L., Rammaert B., Hausfater P. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974–982. doi: 10.1164/rccm.201501-0017OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.