Abstract
Background
The causes of acute febrile illness (AFI) in Latin America are diverse and their complexity increases as the proportion of fever due to malaria decreases, as malaria control measures and new pathogens emerge in the region. In this context, it is important to shed light on the gaps in the epidemiological characteristics and the geographic range for many AFI aetiologies.
Objectives
To review studies on community-acquired fever aetiology other than malaria in Latin America, and to highlight knowledge gaps and challenges needing further investigation.
Sources
PubMed from 2012 to April 2018.
Content
We found 17 eligible studies describing 13 539 patients. The median number of pathogens tested per individual was 3.5 (range 2–17). A causative pathogen could be determined for 6661 (49.2%) individuals. The most frequently reported pathogen during the study periods was dengue virus (DENV) (14 studies), followed by chikungunya virus (nine studies) and Zika virus (seven studies). Among the studies reporting concurrent infections, 296 individuals (2.2%) were found to have co-infections. In-hospital mortality was reported in eight (47%) studies, ranging between 0% and 18%.
Implications
DENV fever is the febrile illness most frequently reported, reflecting its importance, while chikungunya and zika viruses present increasing trends since their emergence in the region. Studies with systematic and harmonized approaches for detection of multiple pathogens are needed and would probably reveal a higher burden of neglected pathogens such as Rickettsia spp. and arenaviruses. The lack of point-of-care tests and harmonized approach limits the care provided by health professionals and the efficacy of surveillance for AFI in the region.
Keywords: Acute febrile illness, Co-infection, Dengue, Fever, Latin America, Malaria
Introduction
Fever is a common reason for seeking healthcare in Latin America associated with substantial morbidity and mortality [1]. A typical feature of many infectious and non-infectious diseases, the aetiological diagnosis of fever poses a considerable challenge for health professionals and surveillance systems, especially when confirmatory tests are not available at point-of-care [2]. Environmental conditions, socio-economic factors and availability of vaccines are some of the factors influencing the incidence and aetiology of infectious febrile illness in the region.
In tropical areas in the American continent (i.e. Amazon basin), the predominant cause of fever has historically been malaria; however, significant progress towards malaria control during the last decades has resulted in decreased incidence [3], along with a higher proportion of patients with acute febrile illness (AFI) that do not present malaria [4]. The progression from malaria to non-malaria febrile illness (NMFI) has shaped the fever epidemiology landscape and exposed new challenges for management and control in the region [5]. Hence, identifying the causes of NMFI is of paramount importance for improving patient care, for more efficient surveillance systems that include detection of emerging pathogens, and to guide implementation of diagnostic and preventive tools.
Arthropod-borne viruses, also known as arboviruses, represent one of the predominant aetiological agents responsible for human febrile illness in Latin America [6], where high temperatures, humidity and poor sanitation contribute to the proliferation of transmitting mosquitoes. Among these, dengue virus (DENV) has recently shown an expanding geographic range, moving from urban to rural areas, with efforts to control Aedes aegypti being largely unsuccessful [7]. The recent introduction into Latin America of zika virus (ZIKV) and chikungunya virus (CHIKV), which share many epidemiological and clinical features with DENV, highlights the lack of preparedness of local health systems to promptly and accurately ascertain the aetiology of AFI, resulting in inadequate management of and delayed responses to epidemics.
In this review, we summarize evidence about the aetiologies of community-acquired AFI in Latin America focusing on causes other than malaria, and highlight the important gaps in current knowledge that should be addressed to improve our understanding of AFI in the region.
Sources and selection criteria
We searched PubMed using medical subject headings and keywords such as ‘non-malaria febrile illness’, ‘acute febrile illness’, ‘acute undifferentiated febrile illness’ and other terms relevant to our review. For this report, we identified studies published in any language from 2012 to 1 April 2018. Reference lists of included studies were cross-checked to identify additional relevant studies. An additional search was performed in LILACS but did not reveal additional publications.
Eligibility criteria
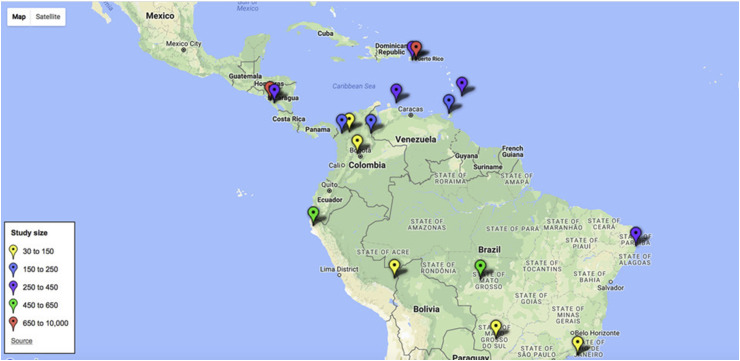
We included observational studies enrolling individuals suffering from AFI in Latin America that were evaluated for at least two febrile diseases using laboratory-confirmed case definitions. For this review, Latin America was defined as the group of countries or territories that are situated south of the USA, thus including the Caribbean (see Fig. 1 ). We excluded studies conducted in other geographical areas; studies focused on a single aetiology of fever (e.g. outbreaks, seroepidemiology surveys; case reports/case series); studies of healthcare-associated infections, commentaries/editorials and those published before 2012. The complete search strategy is described in the Supplementary material (Appendix S1).
Fig. 1.
Location of studies included in the review.
Study selection and data extraction
Two authors screened the title and abstracts. One author extracted the data, which were verified by a second author. The following variables were then extracted from each included paper and entered into a piloted extraction form: first author, year of publication, geographical location of the healthcare facility, study time dates and duration, study inclusion criteria, study size, age range, diagnostic techniques evaluated for each infection, timing of the sample collection, number of patients tested for each infection and those who were positive, co-infections, and in-hospital mortality rate.
The frequency of pathogens was recorded as zero if the study individually tested for them, but no pathogens were detected or when specific testing was not recorded. Next, we compared the frequency of pathogen isolation considering when the study was published. We categorized 2015 articles as the threshold, as after that CHIKV and ZIKV were introduced in the region.
Results
We found 17 eligible publications describing 13 539 patients [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. Table 1 summarizes characteristics of the included studies. All except one study (in Spanish) were published in English. Ten (58.8%) were published before 2015 and seven (41%) from 2015 onwards. All studies were conducted between 2007 and 2016 and published between 2012 and 2018. Three (17.64%) were multicentre, and none included a healthy control group. The median number of pathogens sought in each study was 3.5 (range 2–17). The countries where the studies took place were mostly Colombia (n = 4), Brazil (n = 4) and Peru (n = 2), whereas studies conducted in the Caribbean were scarce. The study size ranged from 30 to 8996 participants, and the average duration of study enrolment was 11.35 (range:2–36) months. Seven (41.17%) were conducted for 1 year or longer. Each study site was mapped, and data related to study size were included (Fig. 1). Most studies enrolled febrile patients without age limits (91%) and were performed in urban settings (58.8%). Six (35.2%) studies were conducted in outpatient units, three (17.6%) in inpatient units, and seven (41.1%) evaluated both settings.
Table 1.
Acute Febrile Illness studies evaluating more than one pathogen in Latin America, 2007-2016
| [Reference] design | Study location/type of healthcare type/[setting] | Study time | Inpatient/outpatient | Inclusion criteria | N | Age group | Diagnostic tests conducted | Pathogens detected (%) |
|---|---|---|---|---|---|---|---|---|
| Caribe | ||||||||
| [24] [prospective] | Bridgetown, Barbados [tertiary hospital/urban] | 2009–2011 | Inpatient | Febrile children admitted to hospital with negative testing for DENV | 272 | Children [<15 years] | DENV/Hantavirus serology | Hantavirus (14) DENV (0) Undetermined (86) |
| [16] [prospective] | Trinidad and Tobago [primary health clinic] | 2013–2014 | Outpatient | Febrile (axillary temperature of ≥38°C) or history of fever <7 days without an identifiable focus of infection | 158 | Adult [18–88 years] | DENV/CHIKV serology DENV/CHIKV RT-PCR |
CHIKV(19) DENV (66) Undetermined (29) |
| [8] [prospective] | Willemstad, Curacao [tertiary hospital/urban] | 2008–2009 | Both [55.6% were admitted] | Febrile patients (rectal temperature ≥ 38.5° C) presenting at ED | 403 | Adult [18–80 years] | Bacterial cultures DENV serology |
Klebsiela pneumoniae (92) DENV (3.72) Parasite/fungi (1.98) |
| [11] [prospective] | Ponce, Puerto Rico [tertiary hospital/urban] Guayama, Puerto Rico [hospital/urban] |
2012–2015 | Both [24.9% hospitalized] | AFI (≥38 C [oral] or ≥ 38.5 C [axillary] or reported a history of fever ≤7 days duration) | 8996 | Children & Adult | DENV/CHIKV/FLUA/B/AdV/HRSV/HMPV/PIV/HRV/HCoV/Coxiella burnetii/Rickettsia rickettsia/R. typhi/Ehrlichia chaffensis/EV RT-PCR DENV/CHIKV/Lepto/Burkholderia pseudomallei/Rickettsia spp./Ehrlichia spp./Coxiella spp. serology |
CHIKV(18.2) FLUA/B (11.9) DENV (10.8) Respiratory viruses (10.3) EV (0.9) Lepto (0.1) Melioidosis (0.02) |
| [18] [prospective] | Ponce, Puerto Rico [tertiary hospital/urban] | 2009 | Both [19% admitted] | Surveillance of AFI: All ages; fever (≥38° C) or history of fever <7 days, without an identified source | 284 | Children & Adult [6 months–82 years] | Influenza RT-PCR Influenza RDT DENV RT-PCR DENV serology Lepto serology EV PCR Blood Culture Urine Culture |
FLUA (80) DENV(18.6) Lepto (0.58) EV (1.74) E coli (0.58) S saprophyticus (0.58) |
| Central & South America | ||||||||
| [10] [prospective] | Cordoba, Colombia [tertiary hospital/urban] | 2012–2013 | Inpatient | UTFI (fever without a focus of infection) admitted to ED | 100 | Children & Adult [1–79 years] | DENV serology Lepto MAT Hantavirus serology Rickettsia serology Brucellosis antigen Malaria smear HAV/HBV serology |
Lepto (27) DENV(26) Hanta (4) Malaria (4) Rickett (2) HAV (1) Brucellosis (1) HBV (0) |
| [19] [prospective] | Multicenter, Nicaragua [health facilities/hospitals/urban/rural] | 2015–2016 | Both [68.9% hospitalized] | AFI (within 7 days of illness onset) suspected of arboviruses | 346 | Adult [>15 years] | ZIKV/CHIKV/DENV RT-PCR | ZIKV (13.6) CHIKV (26.3) DENV (15.6) Undetermined (24) |
| [20] [retrospective] | Mato Grosso, Brazil [urban] | 2011–2012 | NR | AFI (<5 days) and suspected of DENV | 604 | Children & Adult [10–59 years] | MAYV/Aura virus/East/West/Venezuelan equine encephalitis virus/DENV RT-PCR | MAYV (2.5) DENV (1.98) |
| [17] [cross-sectional] | Campo Grande, MS, Brazil [primary health clinic/urban] | 2016 | Both [9 admitted] | Suspicion of arboviral infections and <7 days of illness | 134 | Adult [>15 years] | DENV/CHIKV/ZIKV serology DENV/CHIKV/ZIKV/MAYV RT-PCR |
Undetermined (20.8) ZIKV(28.3) DENV(26.8) CHIKV (38) MAYV (0) |
| [31] [prospective] | Léon, Nicaragua [tertiary Hospital/rural/urban] | 2008–2009 | Both | Febrile (≥38.5° C) patients ≥ 1 month old | 825 | Children & Adult [≥1 month old] | Rickettsia serology Rickettsia RT-PCR Q Fever serology Q fever RT-PCR |
Rickett (4.54) Q fever (1.87) Undetermined (95.4%) |
| [23] [prospective] | Recife, Brazil [health clinic/urban] | 2015–2016 | Both | Age ≥ 5 years; fever or history of fever ≤72 h; undifferentiated fever; non-severe illness | 263 | Children & Adult [6–67 years] | ZIKV/CHIKV/DENV serology ZIKV/CHIKV/DENV RT-PCR DENV/ZIKV PRNT |
DENV(0.38) ZIKV (15.9) CHIKV (52) Undetermined (68.4) |
| [21] [cross-sectional] | Puerto Maldonado, Peru [primary health care units/urban/rural] | 2016 | Outpatients | AFI (≥38 C < 7 days) but without an identified focus of fever | 139 | Children & Adult [0–45 years] | DENV/OROV/CHIKV/MAYV/ZIKV RT-PCR | CHIKV (9.35) OROV (8.63) DENV (6.47) ZIKV (5) Undetermined (70.5) |
| [12] [prospective] | Rio de Janeiro, Brazil [tertiary hospital/urban] | 2015–2016 | Inpatients | Suspected DENV | 30 | NR | DENV/CHIKV/ZIKV serology DENV/CHIKV/ZIKV RT-PCR |
ZIKV (56.6) CHIKV(3.33) Undetermined (40%) |
| [13] [cross-sectional] | Villa del Rosario, Colombia [hospital/urban/rural] | 2015–2016 | Outpatient | Any age, suspected of arboviruses and <7 of fever | 157 | Children & Adult [0–50 years] | DENV/CHIKV/ZIKV RT-PCR | DENV(40) CHIKV (57) ZIKV (35) Undetermined (47.7) |
| [14] [cross-sectional] | Piura, Peru [primary health clinics/rural] | 2016 | Outpatient | AFI (axillary temperature ≥ 38° C) and <7 days | 496 | Children & Adult [0–60 years] | DENV/CHIKV/ZIKV RT-PCR | DENV (34.2) ZIKV (7.86) CHIKV (4.6) Undetermined (53.2) |
| [15] [prospective] | Villeta, Cundinamarca, Colombia [hospital/urban/rural] | 2011–2013 | Outpatient | AUFS and suspected of DENV | 104 | Children & Adult [10–60 years] | DENV/Lepto/Rickett/Anaplasma /Q fever serology |
DENV (16.3) Lepto (24) Rickett (5.76) Q fever (0) Undetermined (53.8) |
| [9] [retrospective] | Uraba antioqueno, Colombia [health clinics/urban/rural] | 2007–2008 | Outpatients | NMFI [malaria smear negative]; axillary temperature ≥ 38° C; <7 days; without an source of infection; 5–65 years | 220 | Children & Adult [10–40 years] | DENV/Lepto/Rickett/Hanta/Arena serology | DENV (37.2) Lepto (14) Rickett (2.72) Arenavirus (0.45) Hantavirus (0) Undetermined (52.2%) |
AdV, adenovirus; AFI, acute febrile illness; AUFS, acute undifferentiated febrile syndrome; CHIKV, chikungunya virus; DENV, dengue virus; EV, enterovirus; FLU A/B, influenza A/B; HAV, hepatitis A virus; HBV, hepatitis B virus; HCoV, human coronaviruses; HMPV, human metapneumovirus; HRSV, human respiratory syncytial virus; HRV, human rhinovirus; Lepto, Leptospirosis; MAT, microscopic agglutination test; MAYV, mayaro virus; NMFI, non-malaria febrile illness; NR, not reported; OROV, oropouche virus; PIV, parainfluenza virus; PRNT, plaque reduction neutralization assay; RDT, rapid diagnostic test; Rickett, rickettsioses; RT-PCR, reverse transcriptase polymerase chain reaction; ZIKV, zika virus.
Of the 17 studies included, ten (58.8%) reported collection of paired acute-phase and convalescent-phase samples, whereas in seven (41.2%) only acute-phase was included. The inclusion criteria differed substantially between the studies, and a precise and harmonized definition of AFI was not provided.
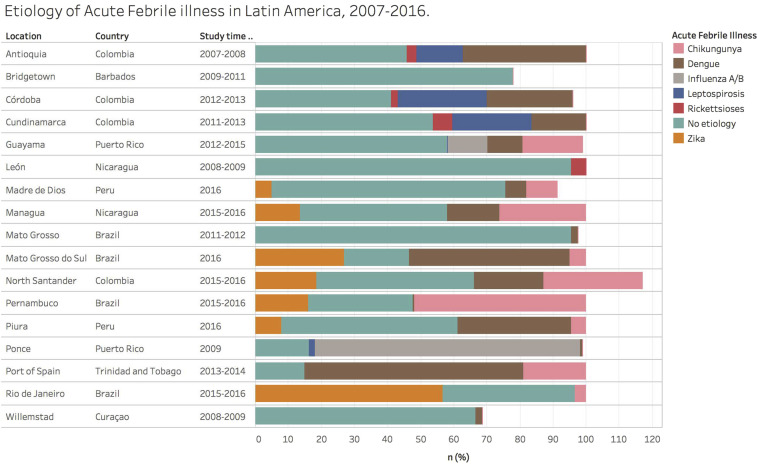
Approximately half of the 13 539 people with febrile illness had at least one pathogen detected (49.2%). The proportion of detection in each study ranged from 4.5% to 79%. The most frequently reported pathogen was DENV (n = 14), followed by CHIKV (n = 9), ZIKV (n = 7), Leptospira spp. (n = 5), Rickettsia spp. (n = 4) and Hantavirus spp. (n = 3). Fig. 2 shows the frequency of isolation of each pathogen sought in the studies according to study periods.
Fig. 2.
Aetiology of acute febrile illness in Latin America, 2007–2016. The frequency of main pathogens isolated in each febrile illness study conducted in Latin America during 2007–2016 is shown for studies conducted after (top panel) or before (bottom panel) 2015. In some studies, the frequency exceeds 100% because patients were co-infected with more than one pathogen. DENV, dengue virus; CHIKV, chikungunya virus; FLU A/B, influenza A/B; Lepto, leptospirosis; rickettsioses; and ZIKV, zika virus.
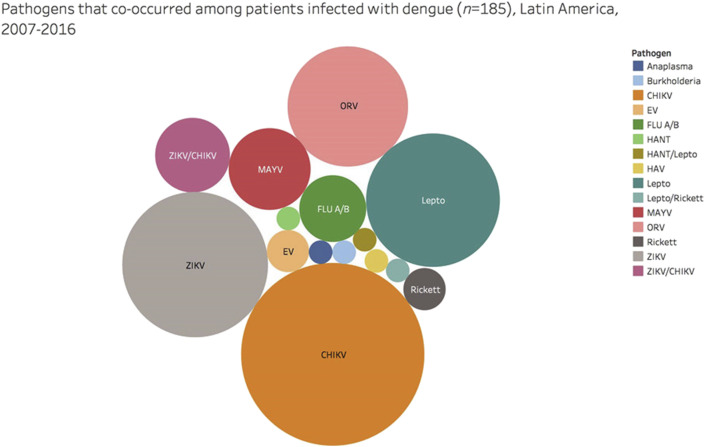
Dengue virus was tested in 16 studies, comprising 12 714 patients of whom 9873 (77.6%) were tested with both PCR and serology, 1742 (13.7%) by PCR alone, and 1099 (8.6%) by serology only. Co-infection was described in 16 studies (13 136 patients), with 296 individuals (2.2%) found to have a co-infection. Identification of the co-infection by molecular detection only occurred in 141 (1%) patients. The most frequent febrile illness among co-infected participants was DENV (62.5%). Common concurrent pathogens were CHIKV (30%), followed by ZIKV (19%), Leptospira spp. (16%), and Oropouche virus (13%) (Fig. 3 ).
Fig. 3.
Aetiologies of dengue co-infection cases from the included studies. The proportions of other pathogens co-occurring with dengue virus in studies evaluating acute febrile illness in Latin America, 2007–2016.
In-hospital mortality was reported in eight (47%) studies, and the mortality upon hospital admission ranged between 0% and 18% varying according to setting and prevalence of each aetiology.
Discussion
This review of studies published in the last 5 years—conducted between 2007 and 2016–that focused on the epidemiology of AFI in Latin America using laboratory-based case definitions showed a broad range of infectious causes of AFI in the region, with high variability according to geographical location and time. It is important to underscore the low number of studies aimed at screening and describing multiple causes of fever—only 17 in a 15-year period. Furthermore, these studies differed largely in the clinical definitions used and lacked standardization and uniformity in terms of study design and laboratory approach. This number reflects an essential gap in the understanding of fever epidemiology in Latin America and a need for standardized and multicentre initiatives.
The variability of design and approaches make it difficult to compare studies. First, the definition of AFI (i.e. the primary inclusion criteria) varied substantially, as some of them defined AFI as fever within the last 7 days and without an identifiable focus of infection [10], [16], [18], whereas others included febrile patients regardless of identifiable focus [8], [11]. Similarly, others were more restricted and enrolled individuals with <72 h history of fever [11]. Second, most studies were conducted in reference hospitals, where diagnostics tests are more commonly available, contrasting with the reality of primary healthcare clinics. For these reference centres, most studies did not describe explicit referral and catchment area information, making it difficult to infer what occurs at the community level. Most studies also focused on non-severe febrile illnesses in the outpatient setting [16], [21]. The inclusion of severe febrile illness such as febrile patients admitted to hospitals could shed light on the burden caused by morbidity and mortality at these settings. Third, laboratory test definitions applied for confirmation also varied, and assays with different diagnostic accuracy performance were used. Fourth, scarce fever aetiology studies aimed to provide a more comprehensive investigation of causes, focusing instead on groups of pathogens such as arboviruses.
Investigations including a broader number of pathogens performed at diverse settings across Latin America representing its distinct demography, geography and epidemiology are critically needed to inform evidence-based public health policies on local fever epidemiology.
Our regional AFI estimation has limitations. The data provide a guide to frequency of isolation, and therefore a rough guide of likelihood of encountering the pathogens, but should not be confused with prevalence or frequency of infection in view of the wide variation in standards and sampling used and the broad time-frame analysed (i.e. some pathogens were introduced after mass event gatherings in the region such as CHIKV followed by ZIKV in late 2013 and 2014, respectively). Further, pathogens that are known to occur but not routinely tested were not evaluated in a significant proportion of the studies included, so the burden of such pathogens is probably underestimated.
We underscore hereafter selected pathogens associated with AFI that fit into two categories: (1) those which are known to be transmitted in Latin America but that are usually neglected and underdiagnosed and underreported; and (2) those agents for which transmission characteristics in the region are largely unknown and that are candidates for emergence in Latin America.
Leptospirosis
Leptospirosis is a worldwide zoonotic disease caused by bacteria belonging to the genus Leptospira. Apart from the endemic transmission, several epidemic outbreaks in human population have occurred in Latin America, following massive flooding [25], [26], [27]. The most frequent species isolated in Latin America from infected animals or humans are Leptospira interrogans, Leptospira licerasiae, Leptospira borgpeteresenii, Leptospira kirschneri and Leptospira noguchi [26]. Prospective studies enrolling patients with acute undifferentiated febrile illness found a prevalence ranging from 6% to 14% [1], [9], [28].
Q fever
Testing for Coxiella burnetii is infrequently reported among febrile patients, although it was diagnosed in 24.4% of individuals with community-acquired pneumonia in French Guyana [29]. A study in Brazil found nine molecularly confirmed Q fever infections among 272 patients suspected of having DENV infection (3.3.%), one of them with co-infection with both agents confirmed [30]. In other Latin American countries, the proportion of fever attributable to Q fever varied from 2% to 5% [1], [31]. Patients with C. burnetti pneumonia had more severe symptoms at presentation [29].
Bartonellosis
Bartonellosis or Carrion's disease is caused by Bartonella bacilliformis, a bacterium presumed to be transmitted between humans through the bites of infected female phlebotomine sand flies. The disease is endemic to arid, high-altitude valleys in the Andes Mountains of Peru, Ecuador and Colombia [32]. Oroya fever—the bacteraemic phase—is associated with high case-fatality rate (40%–88%) if left untreated [33], [34]. There is a lack of point-of-care diagnostics, and treatment is usually presumptive with marked gaps in epidemiology. Bartonella bacilliformis bartonellosis represents the highest reported lethality rate by far for any Bartonella species.
Rickettsiosis
Rocky Mountain spotted fever, spotted fever group and murine typhus are associated with significant morbidity and mortality in the region, in part due to unawareness of the diagnosis among clinicians and the lack of specificity of the clinical symptoms. It occurs mainly in rural or sylvatic environments, although domestic dogs can be implicated in more populated settings [35]. Among the main species associated with human illness in Latin America are Rickettsia rickettsia, Rickettsia parkeri, Rickettsia typhi and Rickettsia massiliae. In surveys of febrile illness, the proportion of rickettsia-attributed fever varied from 0.9% to 18% [9], [15], [31], [36], [37]. The case-fatality of Rocky Mountain spotted fever ranges between 40% and 95% [38]. More recently, Orientia tsutsugamushi, the agent of scrub typhus, has been found in Chile and Peru [39], [40], [41], and it is likely that the disease is largely underdiagnosed in other areas of Latin America as well.
Hantavirus
Hantavirus are emerging viruses transmitted by inhalation of rodent secretions, and responsible for Hantavirus Pulmonary Syndrome in Latin America [42]. Patients with confirmed acute hantavirus infection are mistakenly assumed to have DENV infection or leptospirosis, due to their similar clinical features and the endemicity of the latter two diseases in the region [24]. Epidemiological studies have shown noticeable differences in seroprevalence of antibodies against hantaviruses in humans ranging from 1% to 13.5%, according to geographical and ethnic differences [43], [44]. Exposure to rural areas and history of peridomestic activities are some of the risk factors most reported [45]. Studies investigating recent hantavirus infection in febrile patients found a prevalence that ranges from 0.3% to 14% [10], [24], [44], [46].
Chagas disease
Although there has been remarkable progress in controlling transmission of Trypanosoma cruzi in Latin America, it remains an important public health problem [47], [48]. Acute disease is characterized by undifferentiated fever, which can progress to severe complications, especially myocarditis and encephalitis [49]. A recent feature of these diseases has been the occurrence of outbreaks of orally transmitted infections—usually through consumption of acai berry and sugar cane juice—in the Amazon region [49], [50], [51]. Surveillance provided by malaria microscopists has been important in the early recognition of its occurrence and other actions are needed [52].
Endemic invasive mycosis
Endemic mycoses that are prevalent in Latin America and frequently present as AFI include Histoplasma capsulatum, Paracoccidioides brasiliensis and Coccidioides immitis [53]. The areas of main occurrence of these fungi do not coincide; however, as there are no point-of-care diagnostics for remote areas, these pathogens remain largely underdiagnosed and underdetected [54].
Under the radar: pathogens waiting to emerge?
Among the pathogens whose occurrence is believed to be underestimated are the arboviruses that are usually identified in the Amazon or the Caribbean, such as Mayaro and Oropouche viruses [20], [21], [55], [56], for which the recent outbreaks in areas without previous identification of these agents demonstrate their potential for expansion. Likewise, West Nile virus is believed to be a likely cause of epidemics in the near future [57], [58].
Implications for clinicians, researchers and policy makers
Due to its clinical and epidemiological importance [7], DENV has been the most studied AFI aetiology and was the most prevalent cause of diseases in our review. However, recent studies have demonstrated displacement of DENV by ZIKV and CHIKV, or a concurrent circulation of all three arboviruses in the same place [19], [23], [59]. From a clinical perspective, there is no clinical algorithm robust enough to distinguish between these three arboviruses. Clinical guidelines in Latin America recommend patients to be managed in the initial phase as DENV, as lack of early intervention can result in high rates of complications. Point-of-care diagnostics are of limited value, due to low sensitivity for some of the serotypes, while serology interpretation is challenged by previous exposure to DENV and other flaviviruses.
Primary-level clinicians need to (i) be prepared to conduct differential diagnosis of AFI on a syndrome-based approach (see Supplementary material, Table S1); (ii) recognize alarming signs of severe febrile illness (i.e. neck stiffness, severe abdominal pain, unconsciousness, or respiratory distress); and (iii) stratify the mortality risk with prediction scores, such as the quick sequential organ failure assessment (qSOFA). A positive qSOFA score (≥2) suggests a high risk of poor outcome in patients with suspected infection [60]. However, studies validating the performance of qSOFA in resource-poor countries are lacking, and the role of such scores in Latin American settings should be investigated.
Knowing the local causes of fever and the predictable patterns of antimicrobial resistance can provide useful local epidemiological data to inform and validate empirical management recommendations. For instance, in a region with a considerable burden of Q fever or rickettsioses, empirical initiation of tetracycline is recommended because other antimicrobials might not act on the latter pathogens. Similarly, the empirical antimicrobial therapy for Enterobacteriaceae related-bacteraemia will require a combination of different agents (i.e. piperacillin/tazobactam, ciprofloxacin, meropenem) as the proportion of Gram-negative bacilli that are extended-spectrum β-lactam-positive is high in countries such as Brazil, Argentina, Mexico and Chile [61]. Investigation of the impact of different empirical treatment strategies for a febrile illness that best matches the local epidemiology and prevents development of antimicrobial resistance merits further research.
Finally, with the development of a robust fever surveillance programme, policy makers and other key stakeholders could point toward the need for development of pharmaceutical therapies for the treatment of the most common AFI in the region. Those surveillance programmes would also allow for more efficient and robust disease forecasting systems and detection of emerging pathogens, assisting health systems to reduce the burden of such diseases. The development of rapid and inexpensive diagnostic tools should be given high research priority, particularly those focused on distinguishing bacterial from non-bacterial causes of infection (i.e. host biomarkers) [62].
Next steps: putting research into context
Our findings can be used to help guide future research in the field. We argue that the scientific community should work together to redefine and harmonize the fever aetiology research agenda. It is vital that standardized case definitions and laboratory methods be in place to ensure the generalizability of the study results. Moreover, the inclusion of a healthy control group (i.e. afebrile patients) would allow the pathogen-specific attributable fraction of illness to be calculated. Finally, although expensive and time-consuming, there is an unmet need to develop multicentre international studies evaluating fever aetiology in different patient settings (i.e. hospitalized and ambulatory patients). To address some of the gaps highlighted here, studies are underway to better understand the major causes of febrile illness in adults and children in Sub-Saharan Africa and Latin America (i.e. NCT03047642).
Conclusions
We identified 17 studies conducted in Latin America during 2007–2016 that focused on fever epidemiology using a laboratory-based case definition. DENV was the febrile illness most frequently reported and occurred concurrently with other diseases. The fatality rate for admitted febrile patients was not negligible. Our current understanding of the fever landscape in Latin America is incomplete and requires a coordinated response from different stakeholders.
Transparency declaration
None of the authors declare conflict of interest related to this manuscript.
Editor: E. Bottieau
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cmi.2018.05.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Manock S.R., Jacobsen K.H., de Bravo N.B., Russell K.L., Negrete M., Olson J.G. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg. 2009;81:146–151. [PubMed] [Google Scholar]
- 2.Chappuis F., Alirol E., d’Acremont V., Bottieau E., Yansouni C.P. Rapid diagnostic tests for non-malarial febrile illness in the tropics. Clin Microbiol Infect. 2013;19:422–431. doi: 10.1111/1469-0691.12154. [DOI] [PubMed] [Google Scholar]
- 3.Recht J., Siqueira A.M., Monteiro W.M., Herrera S.M., Herrera S., Lacerda M.V.G. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J. 2017:1–18. doi: 10.1186/s12936-017-1925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitor-Silva S., Siqueira A.M., de Souza Sampaio V., Guinovart C., Reyes-Lecca R.C., de Melo G.C. Declining malaria transmission in rural Amazon: changing epidemiology and challenges to achieve elimination. Malar J. 2016;15:266. doi: 10.1186/s12936-016-1326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cifuentes S.G., Trostle J., Trueba G., Milbrath M., Baldeon M.E., Coloma J. Transition in the cause of fever from malaria to dengue, Northwestern Ecuador, 1990–2011. Emerg Infect Dis. 2013;19:1642–1645. doi: 10.3201/eid1910.130137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forshey B.M., Guevara C., Laguna-Torres V.A., Cespedes M., Vargas J., Gianella A. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San Martin J.L., Brathwaite O., Zambrano B., Solorzano J.O., Bouckenooghe A., Dayan G.H. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limper M., Gerstenbluth I., Duits A.J., van Gorp E.C.M. Epidemiology of febrile diseases in the emergency department of a Caribbean island: the Curaçao experience. West Indian Med J. 2012;61:76–80. [PubMed] [Google Scholar]
- 9.Arroyave E., Londono A.F., Quintero J.C., Agudelo-Florez P., Arboleda M., Diaz F.J. Etiology and epidemiological characterization of non-malarial febrile syndrome in three municipalities of Uraba (Antioquia), Colombia. Biomedica. 2013;33:99–107. [PubMed] [Google Scholar]
- 10.Mattar S., Tique V., Miranda J., Montes E., Garzon D. Undifferentiated tropical febrile illness in Cordoba, Colombia: not everything is dengue. J Infect Public Health. 2017;10:507–512. doi: 10.1016/j.jiph.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Tomashek K.M., Lorenzi O.D., Andujar-Perez D.A., Torres-Velasquez B.C., Hunsperger E.A., Munoz-Jordan J.L. Clinical and epidemiologic characteristics of dengue and other etiologic agents among patients with acute febrile illness, Puerto Rico, 2012-2015. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabral-Castro M.J., Cavalcanti M.G., Peralta R.H.S., Peralta J.M. Molecular and serological techniques to detect co-circulation of DENV, ZIKV and CHIKV in suspected dengue-like syndrome patients. J Clin Virol. 2016;82:108–111. doi: 10.1016/j.jcv.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Carrillo-Hernandez M.Y., Ruiz-Saenz J., Villamizar L.J., Gomez-Rangel S.Y., Martinez-Gutierrez M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect Dis. 2018;18:61. doi: 10.1186/s12879-018-2976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Carbonel J., Tantalean-Yepez D., Aguilar-Luis M.A., Silva-Caso W., Weilg P., Vasquez-Achaya F. Identification of infection by chikungunya, zika, and dengue in an area of the Peruvian coast. Molecular diagnosis and clinical characteristics. BMC Res Notes. 2018;11:175. doi: 10.1186/s13104-018-3290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faccini-Martinez A.A., Ramirez-Hernandez A., Barreto C., Forero-Becerra E., Millan D., Valbuena E. Epidemiology of spotted fever group rickettsioses and acute undifferentiated febrile illness in Villeta, Colombia. Am J Trop Med Hyg. 2017;97:782–788. doi: 10.4269/ajtmh.16-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahadeo N., Mohammed H., Allicock O.M., Auguste A.J., Widen S.G., Badal K. Molecular characterisation of chikungunya virus infections in Trinidad and comparison of clinical and laboratory features with dengue and other acute febrile cases. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azeredo E.L., Dos Santos F.B., Barbosa L.S., Souza T.M.A., Badolato-Correa J., Sanchez-Arcila J.C. Clinical and laboratory profile of zika and dengue infected patients: lessons learned from the co-circulation of dengue, zika and chikungunya in Brazil. PLoS Curr. 2018;10 doi: 10.1371/currents.outbreaks.0bf6aeb4d30824de63c4d5d745b217f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzi O.D., Gregory C.J., Santiago L.M., Acosta H., Galarza I.E., Hunsperger E. Acute febrile illness surveillance in a tertiary hospital emergency department: comparison of influenza and dengue virus infections. Am J Trop Med Hyg. 2013;88:472–480. doi: 10.4269/ajtmh.12-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waggoner J.J., Gresh L., Vargas M.J., Ballesteros G., Tellez Y., Soda K.J. Viremia and clinical presentation in Nicaraguan patients infected with zika virus, chikungunya virus, and dengue virus. Clin Infect Dis. 2016;63:1584–1590. doi: 10.1093/cid/ciw589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuchi N., Heinen LB. da S., Santos MAM dos, Pereira F.C., Slhessarenko R.D. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem Inst Oswaldo Cruz. 2014;109:820–823. doi: 10.1590/0074-0276140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alva-Urcia C., Aguilar-Luis M.A., Palomares-Reyes C., Silva-Caso W., Suarez-Ognio L., Weilg P. Emerging and reemerging arboviruses: a new threat in Eastern Peru. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reller M.E., de Silva A.M., Miles J.J., Jadi R.S., Broadwater A., Walker K. Unsuspected dengue as a cause of acute febrile illness in children and adults in western Nicaragua. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magalhaes T., Braga C., Cordeiro M.T., Oliveira A.L.S., Castanha P.M.S., Maciel A.P.R. Zika virus displacement by a chikungunya outbreak in Recife, Brazil. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A., Krishnamurthy K., Nielsen A.L. Hantavirus infection among children hospitalized for febrile illness suspected to be dengue in Barbados. J Infect Public Health. 2016;9:81–87. doi: 10.1016/j.jiph.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Ko A.I., Galvão Reis M., Ribeiro Dourado C.M., Johnson W.D., Riley L.W. Urban epidemic of severe leptospirosis in Brazil. Salvador leptospirosis study group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 26.Petrakovsky J., Bianchi A., Fisun H., Najera-Aguilar P., Pereira M.M. Animal leptospirosis in Latin America and the Caribbean countries: reported outbreaks and literature review (2002–2014) Int J Environ Res Public Health. 2014;11:10770–10789. doi: 10.3390/ijerph111010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trevejo R.T., Rigau-Perez J.G., Ashford D.A., McClure E.M., Jarquin-Gonzalez C., Amador J.J. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J Infect Dis. 1998;178:1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 28.Reller M.E., Wunder E.A., Miles J.J., Flom J.E., Mayorga O., Woods C.W. Unsuspected leptospirosis is a cause of acute febrile illness in Nicaragua. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epelboin L., Chesnais C., Boulle C., Drogoul A.-S., Raoult D., Djossou F. Q fever pneumonia in French Guiana: prevalence, risk factors, and prognostic score. Clin Infect Dis. 2012;55:67–74. doi: 10.1093/cid/cis288. [DOI] [PubMed] [Google Scholar]
- 30.Lemos E.R.S., Marraschi S., dos Ferreira M.S., Mares-Guia M.A.M.M., Bochner R., Botticini R.D.G. Molecular identification of Q fever in patients with a suspected diagnosis of dengue in Brazil in 2013–2014. Am J Trop Med Hyg. 2016;94:1090–1094. doi: 10.4269/ajtmh.15-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reller M.E., Chikeka I., Miles J.J., Dumler J.S., Woods C.W., Mayorga O. First identification and description of rickettsioses and Q fever as causes of acute febrile illness in Nicaragua. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minnick M.F., Anderson B.E., Lima A., Battisti J.M., Lawyer P.G., Birtles R.J. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis Electron. Resour. 2014;8 doi: 10.1371/journal.pntd.0002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray G.C., Johnson A.A., Thornton S.A., Smith W.A., Knobloch J., Kelley P.W. An epidemic of oroya fever in the Peruvian Andes. Am J Trop Med Hyg. 1990;42:215–221. doi: 10.4269/ajtmh.1990.42.215. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlin J., Laughlin L.W., Romero S., Solorzano N., Gordon S., Andre R.G. Epidemiology of endemic Bartonella bacilliformis: a prospective cohort study in a Peruvian mountain valley community. J Infect Dis. 2002;186:983–990. doi: 10.1086/344054. [DOI] [PubMed] [Google Scholar]
- 35.Moreira-Soto A., Carranza M.V., Taylor L., Calderon-Arguedas O., Hun L., Troyo A. Exposure of dogs to spotted fever group rickettsiae in urban sites associated with human rickettsioses in Costa Rica. Ticks Tick Borne Dis. 2016;7:748–753. doi: 10.1016/j.ttbdis.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Schoeler G.B., Moron C., Richards A., Blair P.J., Olson J.G. Human spotted fever rickettsial infections. Emerg Infect Dis. 2005;11:622–624. doi: 10.3201/eid1104.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocher C., Morrison A.C., Leguia M., Loyola S., Castillo R.M., Galvez H.A. Rickettsial disease in the Peruvian Amazon basin. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abarca K., Oteo J.A. Clinical approach and main tick-borne rickettsiosis present in Latin America. Rev Chilena Infectol. 2014;31:569–576. doi: 10.4067/S0716-10182014000500009. [DOI] [PubMed] [Google Scholar]
- 39.Weitzel T., Dittrich S., Lopez J., Phuklia W., Martinez-Valdebenito C., Velasquez K. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954–961. doi: 10.1056/NEJMoa1603657. [DOI] [PubMed] [Google Scholar]
- 40.Kocher C., Jiang J., Morrison A.C., Castillo R., Leguia M., Loyola S. Serologic evidence of scrub typhus in the Peruvian Amazon. Emerg Infect Dis. 2017;23:8–10. doi: 10.3201/eid2308.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balcells M.E., Rabagliati R., Garcia P., Poggi H., Oddo D., Concha M. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659–1663. doi: 10.3201/eid1709.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueiredo L.T., Souza W.M., Ferres M., Enria D.A. Hantaviruses and cardiopulmonary syndrome in South America. Virus Res. 2014;187:43–54. doi: 10.1016/j.virusres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Mattar S., Parra M. Serologic evidence of hantavirus infection in humans, Colombia. Emerg Infect Dis. 2004;10:2263–2264. doi: 10.3201/eid1012.040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castillo Ore R.M., Forshey B.M., Huaman A., Villaran M.V., Long K.C., Kochel T.J. Serologic evidence for human hantavirus infection in Peru. Vector Borne Zoonotic Dis. 2012;12:683–689. doi: 10.1089/vbz.2011.0820. [DOI] [PubMed] [Google Scholar]
- 45.Pini N. Hantavirus pulmonary syndrome in Latin America. Curr Opin Infect Dis. 2004;17:427–431. doi: 10.1097/00001432-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Matheus S., Meynard J.B., Rollin P., Maubert B., Morvan J. New world hantavirus in humans, French Guiana. Emerg Infect Dis. 2006;12:1294–1295. doi: 10.3201/eid1208.051619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotez P.J., Bottazzi M.E., Franco-Paredes C., Ault S.K., Periago M.R. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2 doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015:33–44. [PubMed] [Google Scholar]
- 49.Coura J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions – a comprehensive review. Mem Inst Oswaldo Cruz. 2015;110:277–282. doi: 10.1590/0074-0276140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anez N., Crisante G., Rojas A., Rojas R.O., Bastidas J. A new acute oral Chagas disease outbreak in Merida, Venezuela: a comprehensive study. Int J Clin Med Res. 2016;3:29–37. [Google Scholar]
- 51.de Góes Costa E., dos Santos S.O., Sojo-Milano M., Amador E.C.C., Tatto E., Souza D.S.M. Acute Chagas disease in the Brazilian Amazon: epidemiological and clinical features. Int J Cardiol. 2017;235:176–178. doi: 10.1016/j.ijcard.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 52.Coura J.R., Junqueira A.C.V. Surveillance, health promotion and control of chagas disease in the Amazon Region—Medical attention in the Brazilian Amazon Region: a proposal. Mem Inst Oswaldo Cruz. 2015;110:825–830. doi: 10.1590/0074-02760150153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sifuentes-Osornio J., Corzo-León D.E., Ponce-De-León L.A. Epidemiology of invasive fungal infections in Latin America. Curr Fungal Infect Rep. 2012;6:23–34. doi: 10.1007/s12281-011-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Cayenne C.H., Franc G. Disseminated histoplasmosis in Central and South America, the invisible elephant. AIDS. 2016;30:167–170. doi: 10.1097/QAD.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 55.Mourão M.P.G., de Bastos M.S., de Figueiredo R.P., Gimaque J.B.L., dos Santos Galusso E., Kramer V.M. Mayaro fever in the city of Manaus, Brazil, 2007–2008. Vector Borne Zoonotic Dis. 2012;12:42–46. doi: 10.1089/vbz.2011.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mavian C., Rife B.D., Dollar J.J., Cella E., Prosperi M.C.F., Lednicky J. Emergence of recombinant Mayaro virus strains from the Amazon basin. Sci Rep. 2017:1–11. doi: 10.1038/s41598-017-07152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano A.P.M., Almeida-Neto W.S., Vieira M.A.C.S., Rodrigues S.G., Azevedo R.S.S., Eulálio K.D. West Nile virus encephalitis: the first human case recorded in Brazil. Am J Trop Med Hyg. 2015;93:377–379. doi: 10.4269/ajtmh.15-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inst R., Trop M., Paulo S. Letter to the Editor West Nile Fever in Brazil: sporadic case, silent endemic disease or epidemic in its initial stages? Rev Inst Med Trop Sao Paulo. 2015;57:2015. doi: 10.1590/S0036-46652015000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Netto E.M., Moreira-Soto A., Pedroso C., Hoser C., Funk S., Kucharski A.J. High zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. MBio. 2017;8 doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 61.Gales A.C., Castanheira M., Jones R.N., Sader H.S. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010) Diagn Microbiol Infect Dis. 2012;73:354–360. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Kapasi A.J., Dittrich S., Gonzalez I.J., Rodwell T.C. Host biomarkers for distinguishing bacterial from non-bacterial causes of acute febrile illness: a comprehensive review. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.