Highlights
-
•
Enteroviruses cause many human diseases, yet no antiviral drugs are available.
-
•
Capsids and viral enzymes are promising targets for direct-acting antiviral therapy.
-
•
Fundamental research has unveiled host factors for broad-spectrum drug development.
-
•
Drug repurposing screens have yielded new promising enterovirus inhibitors.
Abstract
Enteroviruses (e.g., poliovirus, enterovirus-A71, coxsackievirus, enterovirus-D68, rhinovirus) include many human pathogens causative of various mild and more severe diseases, especially in young children. Unfortunately, antiviral drugs to treat enterovirus infections have not been approved yet. Over the past decades, several direct-acting inhibitors have been developed, including capsid binders, which block virus entry, and inhibitors of viral enzymes required for genome replication. Capsid binders and protease inhibitors have been clinically evaluated, but failed due to limited efficacy or toxicity issues. As an alternative approach, host-targeting inhibitors with potential broad-spectrum activity have been identified. Furthermore, drug repurposing screens have recently uncovered promising new inhibitors with disparate viral and host targets. Together, these findings raise hope for the development of (broad-range) anti-enteroviral drugs.
Current Opinion in Virology 2017, 24:1–8
This review comes from a themed issue on Antiviral strategies
Edited by Lieve Naesens and Fabien Zoulim
For a complete overview see the Issue and the Editorial
Available online 12th April 2017
http://dx.doi.org/10.1016/j.coviro.2017.03.009
1879-6257/© 2017 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The Picornaviridae constitutes a large family of non-enveloped, positive-stranded RNA (+RNA) viruses, currently consisting of 31 genera. The genus Enterovirus, which is by far the largest genus, comprises many human pathogens, including poliovirus, coxsackie A and B viruses, echoviruses, numbered enteroviruses (e.g., EV-A71 and EV-D68), and rhinoviruses. Infections with non-polio enteroviruses can result in a wide variety of symptoms, including hand-foot-and-mouth disease, conjunctivitis, aseptic meningitis, severe neonatal sepsis-like disease, and acute flaccid paralysis, whereas infections with rhinoviruses cause the common cold as well as exacerbations of asthma and chronic obstructive pulmonary disease (COPD) (reviewed in Ref. [1]). Vaccines are only available against poliovirus and EV-A71. Development of vaccines against all enteroviruses seems unfeasible, given the large number of (sero)types (i.e., >100 non-polio enteroviruses and >150 rhinoviruses). Hence, there is a great need for (broad-acting) antivirals against entero-viruses. Here, we will review recent efforts to develop direct-acting antivirals as well as host factor-targeting inhibitors to treat enterovirus infections (Table 1 ).
Table 1.
Overview of direct-acting or host-targeting inhibitors discussed in this review
| Type of inhibitor | Compounds | |
|---|---|---|
| Capsid binder | Pirodavir [5], Pleconarilb [5], Pocapavir (V-073)b [5], Vapendavir (BTA798)b [5] | |
| 3Cpro inhibitor | peptidic mimetic | Rupintrivir (AG7088)b [18] and its analogs (eg Compound 1a) [26, 27, 28] |
| non-peptidic mimetic | DC07090 [29] | |
| 3Dpol inhibitor | nucleoside/nucleotide analog | Gemcitabine [35], NITD008 [34], Ribavirin [31, 71] |
| non-nucleoside/nucleotide analog | Amiloride [5], Aurintricarboxilic acid [5], BPR-3P0128 [5], DTrip-22 [5], Gliotoxin [5], GPC-N114 [37••] | |
| 2CATPase inhibitor | Dibucaine [72], Fluoxetine [41], Guanidine hydrochloride [5], HBB [5], MRL-1237 [5], Pirlindole [72], TBZE-029 [5], Zuclopenthixol [72] | |
| Host factor inhibitor | HSP90 | Geldanamycin (analog 17-AAG) [51] |
| PI4KB | BF738735 [5], Enviroximeb [5], GW5074 [5], PIK93 [5], T-00127-HEV1 [5] | |
| OSBP | 25-hydroxycholesterol [5], AN-12-H5 [64], Itraconazole [61], OSW-1 [63], T-00127-HEV2 [64], TTP-8307 [65] | |
| Cyclophilins | Cyclosporin A [67•], HL05100P2 [67•], NIM-811 [69] | |
| Glutathione | Buthionine sulfoximine (BSO) [46•], TP219 [47•] | |
Phase 1 clinical trial.
Phase 2 clinical trial or completed.
Direct-acting antivirals
Entry inhibitors
Enterovirus capsids are icosahedral (pseudo T = 3) structures composed of 60 copies of each of the four capsid proteins (VP1 to VP4). The enterovirus replication cycle (Figure 1 b) is initiated by binding of a virion to its receptor. Most enterovirus receptors are protein receptors that belong to the Ig superfamily or the integrin receptor family (reviewed in Ref. [2]). The receptors usually bind in the ‘canyon’, a depression in the virion surface around the five-fold axes of symmetry [2]. Receptor-binding induces virion destabilization and release of the ‘pocket factor’, a fatty acid located in a hydrophobic pocket beneath the canyon, to initiate virion uncoating [2].
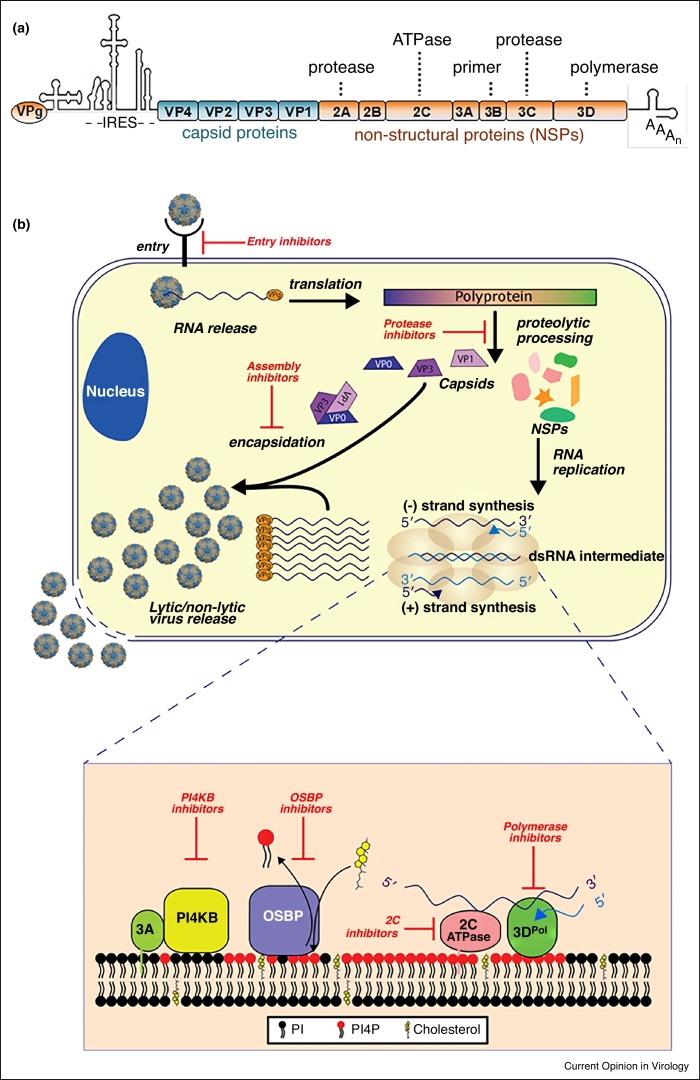
Figure 1.
Enterovirus genome, replication cycle, and antiviral targets. (a) The enterovirus genome encodes four structural proteins (VP1-VP4) and seven non-structural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D). IRES: internal ribosome entry site. (b) The enterovirus life cycle begins with the attachment of the virus particle to a cellular receptor followed by the internalization of the particle into the host cell. The genome is released and directly translated into a polyprotein, which is processed by virally encoded proteases into the individual viral proteins. Non-structural proteins rewire host cell membranes and generate replication organelles (ROs) for viral RNA replication. Several host proteins, such as PI4KB (phosphatidylinositol 4-kinase III beta) and OSBP (oxysterol-binding protein), are recruited to ROs by viral 3A protein, which results in ROs with a unique lipid composition. Genome replication starts with synthesis of complementary negative-stranded RNA, which is used as template for the synthesis of a large number of +RNA molecules. Newly synthesized +RNAs either enter a new round of genome replication or are packaged into capsid proteins to build infectious particles. Viruses are released by a non-lytic mechanism as well as upon cell lysis. Inhibitors of the different stages in the replication cycle are depicted in red.
So-called ‘capsid binders’ are the most extensively studied class of anti-enteroviral compounds [3, 4]. These compounds replace the pocket factor in the canyon and thereby block virion uncoating. Clinical trials for the capsid binders pleconaril, vapendavir (a.k.a. BTA798), and pocapavir (a.k.a. V-073) are currently in progress or have recently been completed, the status of which has been described last year [5]. Since then, another trial with pleconaril was conducted for the treatment of neonates with enterovirus sepsis, which showed greater survival among pleconaril recipients [6]. A major drawback of capsid binders is the rapid emergence of resistance. Indeed, in a clinical trial for the treatment of rhinovirus infections with pleconaril, compound-resistant viruses were isolated [7]. In addition, naturally occurring pleconaril-resistant viruses (e.g., an echovirus 11 strain) have been reported [8]. These resistance issues may complicate the application of capsid binders in the clinic.
Many capsid binders are active against rhinovirus A and B species members [3], but not against members of the rhinovirus C species [9, 10]. The recent elucidation of the atomic virion structure of rhinovirus-C15 by cryo-EM revealed a unique ‘spiky’ structure, vastly different from other enterovirus species. Furthermore, the hydrophobic pocket is filled with bulky hydrophobic residues, thereby not providing sufficient space for a pocket factor or a capsid binder. These features likely explain why rhinovirus C species are not responsive to pocket-binding compounds [11••].
The atomic structure of rhinovirus-C also revealed a potential binding site for sialic acids in a sequence-conserved surface depression [11••]. Sialic acids were recently shown to also facilitate entry of EV-D68 [12, 13]. Targeting sialic acid (reviewed in Ref. [14]), which has also been applied for influenza virus, could be an approach to inhibit rhinovirus-C and EV-D68 infections. One of the best-described compounds targeting sialic acids is DAS181, a bacterial sialidase that cleaves α2,3- and α2,6-sialic acid linkages, and that has been tested in a phase II clinical trial for (para)influenza infection [15, 16]. DAS181 also inhibits EV-D68 replication in vitro [17], but it remains to be tested in vivo.
Protease inhibitors
The 7.5 kb +RNA genome of enteroviruses encodes a single polyprotein harboring the structural P1 proteins and the non-structural P2 and P3 proteins (Figure 1a). This polyprotein is proteolytically processed into individual proteins by viral proteases 2Apro, 3Cpro, and 3CDpro.
The development of protease inhibitors has focused particularly on 3Cpro, since it is more conserved than 2Apro. One of the most potent 3Cpro inhibitors developed over the years is rupintrivir (a.k.a. AG7088). Rupintrivir is a peptidomimetic compound that irreversibly binds to the catalytic site of 3Cpro [18]. Because it is very active against a broad panel of enteroviruses [3, 19, 20, 21], this compound was selected for clinical trials, despite its poor oral bioavailability [22, 23]. Although the results of rhinovirus challenge trials were promising [24], rupintrivir did not reduce disease severity in naturally infected patients [25], hence the clinical development was halted. However, many rupintrivir derivatives are currently under development [26, 27, 28]. Furthermore, non-peptidomimetic small molecule inhibitors are developed to circumvent difficulties with bioavailability [29], but they have yet to be evaluated in clinical trials.
3Dpol inhibitors
The viral RNA-dependent RNA polymerase 3Dpol catalyzes viral RNA synthesis in replication complexes that are associated with so-called replication organelles (ROs, see below). Inhibitors of 3Dpol can be divided into two classes based on their structure, being nucleoside/nucleotide inhibitors (NIs) and non-nucleoside/nucleotide inhibitors (NNIs).
NIs
Antiviral NIs can act in two ways. They can be incorporated into the viral genome by mimicking nucleotides to induce lethal mutagenesis or terminate elongation of the nascent chain, or inhibit viral replication in an indirect manner by affecting the cellular nucleotide pools (reviewed in Ref. [30]). Until now, few NIs against enteroviruses have been developed, but compounds developed against other viruses offer promising results, such as ribavirin [31, 71], which is clinically used for the treatment of hepatitis C virus (HCV) infections [32, 33]. Another example is NITD008, which failed in preclinical studies for the treatment of dengue virus infection due to toxicity. However, a 10-fold lower concentration of NITD008 protected mice from a lethal challenge with EV-A71 without showing any cytotoxicity [34].
Drug repurposing – that is, the concept of using compounds developed for a certain disease to treat a different condition – offers an attractive alternative to de novo drug development, as profound pharmacological and toxicological profiles are already available allowing a bypass of expensive (pre-)clinical studies. For example, the NI gemcitabine, an anticancer drug, was recently found to exert broad-spectrum anti-enteroviral activity [35, 36]. Besides incorporation into nascent viral RNA, gemcitabine has been suggested to block access of nucleotides into the active side of the polymerase. Furthermore, gemcitabine blocks viral genome replication by inhibiting ribonucleotide reductase, the cellular enzyme that catalyzes the conversion of deoxyribonucleotides to ribonucleotides [35]. The dose of gemcitabine needed for antiviral activity is significantly lower than the dose for the anticancer activity, which raises hope for an application without toxic effects that are inherent to many anticancer drugs.
NNIs
Several NNIs of 3Dpol have been identified (e.g., gliotoxin, DTrip-22, aurincarboxilic acid, BPR-3P0128, and GPC-N114), but their mechanism of action is poorly understood, except for amiloride, which decreases the polymerase fidelity (reviewed in Ref. [5]). GPC-N114 was identified as a novel broad-range enterovirus inhibitor that targets the RNA template-primer site in the core of 3Dpol, making it the first anti-enteroviral compound with this mechanism of action [37••]. Unfortunately, the efficacy of GPC-N114 in animal models remains to be tested due to problems with formulating the compound for in vivo use. Alternative strategies for 3Dpol inhibition, although thus far unexplored, may be to interfere with posttranslational modifications of 3Dpol like sumoylation and ubiquitination, both of which are important for 3Dpol activity [38].
2CATPase inhibitors
The highly conserved viral protein 2C, an ATPase, is an attractive target for broad-spectrum enterovirus inhibitors. 2CATPase has several functions in genome replication (reviewed in Ref. [5]). Several structurally disparate 2CATPase inhibitors have been identified, such as guanidine hydrochloride, HBB, MRL-1237 and TBZE-029 [3]. In addition, drug repurposing screens have recently uncovered a number of FDA-approved drugs (fluoxetine, pirlindole, dibucaine, zuclopenthixol) that inhibit replication of enterovirus species B and D members [39, 40, 41]. Since mutations in 2CATPase provide resistance to these compounds, they are considered to target 2CATPase. Indeed, fluoxetine (i.e., Prozac) was shown to interfere with the ATPase activity of 2CATPase, but the mechanism of inhibition of the other drugs has yet to be unraveled [40]. Recent in vitro experiments have confirmed the long-presumed ATP-dependent RNA helicase activity and ATPase-independent RNA chaperone functions of 2CATPase [42••], paving the way for studies to elucidate the mechanism of action of 2CATPase inhibitors in more detail. So far, 2CATPase inhibitors have not been tested in clinical trials. However, fluoxetine was effective in an immunocompromised child with chronic enterovirus encephalitis [43•], implying that 2CATPase inhibitors have potential for clinical use.
Assembly inhibitors
Virion morphogenesis is a poorly understood, step-wise process [44]. The first step is the liberation of P1 from the polyprotein. Assisted by the chaperone heat shock protein 90 (Hsp90) [44, 45], P1 is processed into VP0 (i.e., the precursor of VP4 and VP2), VP1, and VP3, which spontaneously form a protomer. Five protomers subsequently assemble into a pentamer, twelve of which in turn form an empty capsid (a.k.a. procapsid). Assembly of pentamers and procapsids is supported by glutathione by an as yet unidentified mechanism [46•, 47•]. Governed by interactions between VP1/VP3 and 2CATPase [44, 48, 49, 50], actively replicating viral RNA is included in the procapsid to form a provirion. The final step in virion morphogenesis is the cleavage of VP0 into VP4 and VP2 to form a stable icosahedral particle.
Only a few assembly inhibitors have been identified so far. Geldanamycin and its analog 17-AAG target Hsp90 to inhibit the processing of P1 [51]. Buthionine sulfoximine, an inhibitor of glutathione synthesis, and TP219, a small molecule that covalently binds to glutathione, both impede the role of glutathione in morphogenesis [46•, 47•]. Yet, not all enteroviruses rely on glutathione [47•], thereby precluding glutathione as an important target for broad-spectrum inhibitors.
Inhibitors of host factors
Viruses critically depend on specific host factors. In recent years, several host factors required for enterovirus replication have been discovered, spurring host-directed drug development. Since some host factors appear to be used by many – or even all – enteroviruses, inhibitors of these cellular factors may have broad-spectrum activity.
PI4KB
Enteroviruses, like all +RNA viruses, induce specific alterations in intracellular membranes and lipid homeostasis to form replication organelles (ROs). The formation of enterovirus ROs is mediated by the concerted actions of viral proteins 2B, 2C, and 3A, as well as a selected set of hijacked host factors (recently reviewed in Ref. [52]). One of these pivotal host factors is phosphatidylinositol 4-kinase type IIIβ (PI4KB) [52, 54]. It is recruited to membranes by the viral protein 3A and enriches ROs in phosphatidylinositol 4-phosphate (PI4P) lipids, which is essential for genomic RNA replication [53••]. As PI4KB is essential for all enteroviruses, inhibitors of this enzyme (e.g., PIK93, GW5074, T-00127-HEV1 and BF738735) (reviewed in Ref. [5]) have broad-spectrum activity [53••, 54, 55, 56]. However, some PI4KB inhibitors showed lethal toxicity in mice and affected lymphocyte function in vitro, which has stalled the development of PI4KB inhibitors [57]. Besides the fact that PI4KB inhibitors may be toxic, their activity may also be overcome by some mutations in the viral 3A, as recently published [58, 59].
OSBP
Itraconazole, a clinically used antifungal drug that also has anti-cancer properties, was identified in drug repurposing screens as a broad-spectrum enterovirus inhibitor [36, 60••, 61]. We identified the oxysterol-binding protein (OSBP) as a novel target of itraconazole responsible for the antiviral effects [60••]. OSBP is a PI4P-binding protein that shuttles cholesterol and PI4P at ER-Golgi membrane contact sites [62]. OSBP is recruited to ROs through the PI4KB-mediated increase in PI4P and its lipid shuttling activity is essential for viral genome replication. Other OSBP inhibitors (e.g., 25-hydroxycholesterol, AN-12-H5, T-00127-HEV2, TTP-8307, and the natural compound OSW-1) also impaired enterovirus replication [56, 63, 64, 65]. In a rhinovirus mouse model, prophylactic intranasal treatment with itraconazole reduced viral titers and pathology, raising expectations for topically applied itraconazole to prevent or treat common colds [66].
Cyclophilins
Cyclophilin A plays a role during the uncoating process of EV-A71 [67•]. In line with this, cyclophilin A inhibitors HL05100P2 and cyclosporine A block EV-A71 replication [67•]. Cyclophilins facilitate protein folding by catalyzing peptide bond isomerization and also play a role in the replication of other +RNA viruses, including HCV and coronaviruses (reviewed in Ref. [68]). Because cyclophilin inhibitors like cyclosporine A have an immunosuppressive effect, non-immunosuppressive inhibitors (e.g., NIM-811 [69]) were developed and are currently in clinical trials for antiviral activity (e.g., alisporivir, a.k.a. Debio025, for HCV treatment). It remains to be established whether uncoating of other enteroviruses also relies on cyclophilins. Hence, the spectrum of anti-enteroviral activity of cyclophilin inhibitors remains to be explored.
Outlook
Currently, there are no antiviral drugs available for the treatment of enterovirus infections, while several potent antivirals are available against HCV, a +RNA virus with a similar replication strategy. Possibly, the small market for anti-enteroviral drugs impedes extensive (industrial) efforts to develop enterovirus inhibitors. Yet, antiviral drugs are urgently needed as enterovirus infections can be life-threatening especially in young children. Furthermore, antiviral drugs are expected to play a crucial role in poliovirus eradication and the post-eradication era.
Capsid binders are currently most advanced in clinical trials, but the inherent problem of rapid resistance development raises concerns. The development of protease inhibitors requires the synthesis of relatively complex peptidomimetic molecules. 2CATPase and 3Dpol may be more promising targets for direct-acting antiviral drugs as they can be inhibited by small molecules, and several inhibitors of these factors were found to have broad-range anti-enteroviral activity. Other targets for broad-spectrum antiviral drugs are host factors, as many host factors are shared by enteroviruses, but a possible downside is the chance of adverse effects and toxicity, as exemplified by PI4KB inhibitors. Lately, several enterovirus inhibitors have been discovered in drug repurposing screens. These compounds are already available or at least quite advanced in (pre-)clinical development for their respective conditions, thus shortening the development process. Importantly, several of those inhibitors have broad-range, sometimes even pan-enterovirus activity.
Besides targeting individual viral or cellular proteins, an emerging concept in drug design is to interfere with essential protein–protein interactions. In the case of enterovirus infections, one may pharmaceutically disrupt essential interactions between viral proteins and host factors, which likely hampers virus replication without causing the overt toxicity issues that may be associated with inhibition of host proteins. Unfortunately, most protein–protein interactions cannot be addressed by current drug formats, including small molecules. The recent development of small cell-permeating, synthetic protein scaffolds (e.g., Alphabodies) may potentially lead to a novel approach to target protein–protein interactions in (entero)virus-infected cells [70].
Since targeted drug discovery depends heavily on basic knowledge of virus replication, fundamental research on the role of viral enzymes as well as essential host factors for enterovirus replication remains needed for the development of broad-range antiviral drugs against these important pathogens.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We apologize to our colleagues whose work we could not cite due to space limitations. This work was supported by research grants from the Netherlands Organisation for Scientific Research (NWO-VENI-863.12.005 to HMvdS, NWO-VENI-722.012.066 to JRPMS, NWO-VICI-91812628 to FJMvK, ERASysApp project ‘SysVirDrug’ ALW project number 832.14.003 to FJMvK) and from the European Union (Horizon 2020 Marie Skłodowska-Curie ETN ‘ANTIVIRALS’, grant agreement number 642434 to FJMvK).
References
- 1.Tapparel C., Siegrist F., Petty T.J., Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013;14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J.M., Coyne C.B. Picornavirus entry. Adv. Exp. Med. Biol. 2013;790:24–41. doi: 10.1007/978-1-4614-7651-1_2. [DOI] [PubMed] [Google Scholar]
- 3.De Palma A.M., Vliegen I., De Clercq E., Neyts J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008;28:823–884. doi: 10.1002/med.20125. [DOI] [PubMed] [Google Scholar]
- 4.Thibaut H.J., De Palma A.M., Neyts J. Combating enterovirus replication: state-of-the-art on antiviral research. Biochem. Pharmacol. 2012;83:185–192. doi: 10.1016/j.bcp.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden L., Wolthers K.C., van Kuppeveld F.J. Replication and inhibitors of enteroviruses and parechoviruses. Viruses. 2015;7:4529–4562. doi: 10.3390/v7082832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abzug M.J., Michaels M.G., Wald E., Jacobs R.F., Romero J.R., Sanchez P.J., Wilson G., Krogstad P., Storch G.A., Lawrence R. A randomized, double-blind, placebo-controlled trial of pleconaril for the treatment of neonates with enterovirus sepsis. J. Pediatric Infect. Dis. Soc. 2016;5:53–62. doi: 10.1093/jpids/piv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pevear D.C., Hayden F.G., Demenczuk T.M., Barone L.R., McKinlay M.A., Collett M.S. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob. Agents Chemother. 2005;49:4492–4499. doi: 10.1128/AAC.49.11.4492-4499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benschop K.S., Wildenbeest J.G., Koen G., Minnaar R.P., van Hemert F.J., Westerhuis B.M., Pajkrt D., van den Broek P.J., Vossen A.C., Wolthers K.C. Genetic and antigenic structural characterization for resistance of echovirus 11 to pleconaril in an immunocompromised patient. J. Gen. Virol. 2015;96:571–579. doi: 10.1099/vir.0.069773-0. [DOI] [PubMed] [Google Scholar]
- 9.Basta H.A., Ashraf S., Sgro J.Y., Bochkov Y.A., Gern J.E., Palmenberg A.C. Modeling of the human rhinovirus C capsid suggests possible causes for antiviral drug resistance. Virology. 2014;448:82–90. doi: 10.1016/j.virol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao W., Bernard K., Patel N., Ulbrandt N., Feng H., Svabek C., Wilson S., Stracener C., Wang K., Suzich J. Infection and propagation of human rhinovirus C in human airway epithelial cells. J. Virol. 2012;86:13524–13532. doi: 10.1128/JVI.02094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Liu Y., Hill M.G., Klose T., Chen Z., Watters K., Bochkov Y.A., Jiang W., Palmenberg A.C., Rossmann M.G. Atomic structure of a rhinovirus C, a virus species linked to severe childhood asthma. Proc. Natl. Acad. Sci. U. S. A. 2016;113:8997–9002. doi: 10.1073/pnas.1606595113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes the crystal structure of rhinovirusC15 virion and reveals the mechanism of resistance to capsid binders.
- 12.Baggen J., Thibaut H.J., Staring J., Jae L.T., Liu Y., Guo H., Slager J.J., de Bruin J.W., van Vliet A.L., Blomen V.A. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1399–1404. doi: 10.1073/pnas.1524498113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Sheng J., Baggen J., Meng G., Xiao C., Thibaut H.J., van Kuppeveld F.J., Rossmann M.G. Sialic acid-dependent cell entry of human enterovirus D68. Nat. Commun. 2015;6:8865. doi: 10.1038/ncomms9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stencel-Baerenwald J.E., Reiss K., Reiter D.M., Stehle T., Dermody T.S. The sweet spot: defining virus-sialic acid interactions. Nat. Rev. Micro. 2014;12:739–749. doi: 10.1038/nrmicro3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss R.B., Hansen C., Sanders R.L., Hawley S., Li T., Steigbigel R.T. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J. Infect. Dis. 2012;206:1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholls J.M., Moss R.B., Haslam S.M. The use of sialidase therapy for respiratory viral infections. Antiviral Res. 2013;98:401–409. doi: 10.1016/j.antiviral.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhoden E., Zhang M., Nix W.A., Oberste M.S. In vitro efficacy of antiviral compounds against enterovirus D68. Antimicrob. Agents Chemother. 2015;59:7779–7781. doi: 10.1128/AAC.00766-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews D.A., Dragovich P.S., Webber S.E., Fuhrman S.A., Patick A.K., Zalman L.S., Hendrickson T.F., Love R.A., Prins T.J., Marakovits J.T. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11000–11007. doi: 10.1073/pnas.96.20.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binford S.L., Maldonado F., Brothers M.A., Weady P.T., Zalman L.S., Meador J.W., 3rd, Matthews D.A., Patick A.K. Conservation of amino acids in human rhinovirus 3C protease correlates with broad-spectrum antiviral activity of rupintrivir, a novel human rhinovirus 3C protease inhibitor. Antimicrob. Agents Chemother. 2005;49:619–626. doi: 10.1128/AAC.49.2.619-626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser L., Crump C.E., Hayden F.G. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antiviral Res. 2000;47:215–220. doi: 10.1016/s0166-3542(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 21.Patick A.K., Binford S.L., Brothers M.A., Jackson R.L., Ford C.E., Diem M.D., Maldonado F., Dragovich P.S., Zhou R., Prins T.J. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 1999;43:2444–2450. doi: 10.1128/aac.43.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsyu P.H., Pithavala Y.K., Gersten M., Penning C.A., Kerr B.M. Pharmacokinetics and safety of an antirhinoviral agent, ruprintrivir, in healthy volunteers. Antimicrob. Agents Chemother. 2002;46:392–397. doi: 10.1128/AAC.46.2.392-397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K.E., Hee B., Lee C.A., Liang B., Potts B.C. Liquid chromatography–mass spectrometry and liquid chromatography-NMR characterization of in vitro metabolites of a potent and irreversible peptidomimetic inhibitor of rhinovirus 3C protease. Drug Metab. Dispos. 2001;29:729–734. [PubMed] [Google Scholar]
- 24.Hayden F.G., Turner R.B., Gwaltney J.M., Chi-Burris K., Gersten M., Hsyu P., Patick A.K., Smith G.J., 3rd, Zalman L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003;47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patick A.K., Brothers M.A., Maldonado F., Binford S., Maldonado O., Fuhrman S., Petersen A., Smith G.J., 3rd, Zalman L.S., Burns-Naas L.A. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005;49:2267–2275. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan J., George S., Kusov Y., Perbandt M., Anemuller S., Mesters J.R., Norder H., Coutard B., Lacroix C., Leyssen P. 3C protease of enterovirus 68: structure-based design of Michael acceptor inhibitors and their broad-spectrum antiviral effects against picornaviruses. J. Virol. 2013;87:4339–4351. doi: 10.1128/JVI.01123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan Y.W., Ang M.J., Lau Q.Y., Poulsen A., Ng F.M., Then S.W., Peng J., Hill J., Hong W.J., Chia C.S. Antiviral activities of peptide-based covalent inhibitors of the Enterovirus 71 3C protease. Sci. Rep. 2016;6:33663. doi: 10.1038/srep33663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C., Zhang L., Li P., Cai Q., Peng X., Yin K., Chen X., Ren H., Zhong S., Weng Y. Fragment-wise design of inhibitors to 3C proteinase from enterovirus 71. Biochim. Biophys. Acta. 2016;1860:1299–1307. doi: 10.1016/j.bbagen.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Ma G.H., Ye Y., Zhang D., Xu X., Si P., Peng J.L., Xiao Y.L., Cao R.Y., Yin Y.L., Chen J. Identification and biochemical characterization of DC07090 as a novel potent small molecule inhibitor against human enterovirus 71 3C protease by structure-based virtual screening. Eur. J. Med. Chem. 2016;124:981–991. doi: 10.1016/j.ejmech.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Jordheim L.P., Durantel D., Zoulim F., Dumontet C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013;12:447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer J.K., Kirkegaard K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7289–7294. doi: 10.1073/pnas.1232294100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manns M.P., McHutchison J.G., Gordon S.C., Rustgi V.K., Shiffman M., Reindollar R., Goodman Z.D., Koury K., Ling M., Albrecht J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 33.Fried M.W., Shiffman M.L., Reddy K.R., Smith C., Marinos G., Goncales F.L., Jr., Haussinger D., Diago M., Carosi G., Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 34.Deng C.L., Yeo H., Ye H.Q., Liu S.Q., Shang B.D., Gong P., Alonso S., Shi P.Y., Zhang B. Inhibition of enterovirus 71 by adenosine analog NITD008. J. Virol. 2014;88:11915–11923. doi: 10.1128/JVI.01207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang H., Kim C., Kim D.E., Song J.H., Choi M., Choi K., Kang M., Lee K., Kim H.S., Shin J.S. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antiviral Res. 2015;124:1–10. doi: 10.1016/j.antiviral.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z., Yang E., Hu C., Cheng H., Chen C.Y., Huang D., Wang R., Zhao Y., Rong L., Vignuzzi M. Cell-based high-throughput screening assay identifies 2′,2′-difluoro-2′-deoxycytidine Gemcitabine as potential anti-poliovirus agent, ACS Infect. Dis. 2016;2017(3):45–53. doi: 10.1021/acsinfecdis.6b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.van der Linden L., Vives-Adrian L., Selisko B., Ferrer-Orta C., Liu X., Lanke K., Ulferts R., De Palma A.M., Tanchis F., Goris N. The RNA template channel of the RNA-dependent RNA polymerase as a target for development of antiviral therapy of multiple genera within a virus family. PLoS Pathog. 2015;11:e1004733. doi: 10.1371/journal.ppat.1004733. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies the novel non nucleoside analog GPC-N114 and identifies the RNA template channel of 3Dpol as novel target for broad-spectrum antiviral therapy.
- 38.Liu Y., Zheng Z., Shu B., Meng J., Zhang Y., Zheng C., Ke X., Gong P., Hu Q., Wang H. SUMO modification stabilizes enterovirus 71 polymerase 3D to facilitate viral replication. J. Virol. 2016;90:10472–10485. doi: 10.1128/JVI.01756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulferts R., de Boer S.M., van der Linden L., Bauer L., Lyoo H.R., Mate M.J., Lichiere J., Canard B., Lelieveld D., Omta W. Screening of a library of FDA-approved drugs identifies several enterovirus replication inhibitors that target viral protein 2C. Antimicrob. Agents Chemother. 2016;60:2627–2638. doi: 10.1128/AAC.02182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulferts R., van der Linden L., Thibaut H.J., Lanke K.H., Leyssen P., Coutard B., De Palma A.M., Canard B., Neyts J., van Kuppeveld F.J. Selective serotonin reuptake inhibitor fluoxetine inhibits replication of human enteroviruses B and D by targeting viral protein 2C. Antimicrob. Agents Chemother. 2013;57:1952–1956. doi: 10.1128/AAC.02084-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuo J., Quinn K.K., Kye S., Cooper P., Damoiseaux R., Krogstad P. Fluoxetine is a potent inhibitor of coxsackievirus replication. Antimicrob. Agents Chemother. 2012;56:4838–4844. doi: 10.1128/AAC.00983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Xia H., Wang P., Wang G.C., Yang J., Sun X., Wu W., Qiu Y., Shu T., Zhao X., Yin L. Human enterovirus nonstructural protein 2CATPase functions as both an RNA helicase and ATP-independent RNA chaperone. PLoS Pathog. 2015;11:e1005067. doi: 10.1371/journal.ppat.1005067. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper showing that 2CATPase possesses the long-sought ATP-dependent RNA helicase activity, allowing studies to elucidate the mode of action of 2C inhibitors with a yet unknown mechanism.
- 43•.Gofshteyn J., Cardenas A.M., Bearden D. Treatment of chronic enterovirus encephalitis with fluoxetine in a patient with X-linked Agammaglobulinemia. Pediatr. Neurol. 2016:64:94–6498. doi: 10.1016/j.pediatrneurol.2016.06.014. [DOI] [PubMed] [Google Scholar]; This is the first report showing that the repurposed drugs fluoxetine could be used as potential therapy in the clinic for chronic enterovirus infection.
- 44.Jiang P., Liu Y., Ma H.C., Paul A.V., Wimmer E. Picornavirus morphogenesis. Microbiol. Mol. Biol. Rev. 2014;78:418–437. doi: 10.1128/MMBR.00012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geller R., Vignuzzi M., Andino R., Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes. Dev. 2007;21:195–205. doi: 10.1101/gad.1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Ma H.C., Liu Y., Wang C., Strauss M., Rehage N., Chen Y.H., Altan-Bonnet N., Hogle J., Wimmer E., Mueller S. An interaction between glutathione and the capsid is required for the morphogenesis of C-cluster enteroviruses. PLoS Pathog. 2014;10:e1004052. doi: 10.1371/journal.ppat.1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]; First decription of the importance of glutathione in enterovirus morphogenesis.
- 47•.Thibaut H.J., van der Linden L., Jiang P., Thys B., Canela M.D., Aguado L., Rombaut B., Wimmer E., Paul A., Perez-Perez M.J. Binding of glutathione to enterovirus capsids is essential for virion morphogenesis. PLoS Pathog. 2014;10:e1004039. doi: 10.1371/journal.ppat.1004039. [DOI] [PMC free article] [PubMed] [Google Scholar]; First decription of the importance of glutathione in enterovirus morphogenesis.
- 48.Asare E., Mugavero J., Jiang P., Wimmer E., Paul A.V. A single amino acid substitution in poliovirus nonstructural protein 2CATPase causes conditional defects in encapsidation and uncoating. J. Virol. 2016;90:6174–6186. doi: 10.1128/JVI.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Wang C., Mueller S., Paul A.V., Wimmer E., Jiang P. Direct interaction between two viral proteins, the nonstructural protein 2C and the capsid protein VP3, is required for enterovirus morphogenesis. PLoS Pathog. 2010;6:e1001066. doi: 10.1371/journal.ppat.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C., Jiang P., Sand C., Paul A.V., Wimmer E. Alanine scanning of poliovirus 2CATPase reveals new genetic evidence that capsid protein/2CATPase interactions are essential for morphogenesis. J. Virol. 2012;86:9964–9975. doi: 10.1128/JVI.00914-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsou Y.L., Lin Y.W., Chang H.W., Lin H.Y., Shao H.Y., Yu S.L., Liu C.C., Chitra E., Sia C., Chow Y.H. Heat shock protein 90: role in enterovirus 71 entry and assembly and potential target for therapy. PLoS One. 2013;8:e77133. doi: 10.1371/journal.pone.0077133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Schaar H.M., Dorobantu C.M., Albulescu L., Strating J.R., van Kuppeveld F.J. Fat(al) attraction: picornaviruses usurp lipid transfer at membrane contact sites to create replication organelles. Trends Microbiol. 2016;24:535–546. doi: 10.1016/j.tim.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of PI4KB as an important host factor for enterovirus replication. This information spurred the development of PI4KB inhibitors as broad-range enterovirus replication inhibitors.
- 54.van der Schaar H.M., Leyssen P., Thibaut H.J., de Palma A., van der Linden L., Lanke K.H., Lacroix C., Verbeken E., Conrath K., Macleod A.M. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIbeta. Antimicrob. Agents Chemother. 2013;57:4971–4981. doi: 10.1128/AAC.01175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arita M., Kojima H., Nagano T., Okabe T., Wakita T., Shimizu H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 2011;85:2364–2372. doi: 10.1128/JVI.02249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roulin P.S., Lotzerich M., Torta F., Tanner L.B., van Kuppeveld F.J., Wenk M.R., Greber U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2014;16:677–690. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Lamarche M.J., Borawski J., Bose A., Capacci-Daniel C., Colvin R., Dennehy M., Ding J., Dobler M., Drumm J., Gaither L.A. Anti-hepatitis C virus activity and toxicity of type III phosphatidylinositol-4-kinase beta inhibitors. Antimicrob. Agents Chemother. 2012;56:5149–5156. doi: 10.1128/AAC.00946-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arita M. Mechanism of poliovirus resistance to host phosphatidylinositol-4 kinase III beta inhibitor. ACS Infect. Dis. 2016;2:140–148. doi: 10.1021/acsinfecdis.5b00122. [DOI] [PubMed] [Google Scholar]
- 59.van der Schaar H.M., van der Linden L., Lanke K.H., Strating J.R., Purstinger G., de Vries E., de Haan C.A., Neyts J., van Kuppeveld F.J. Coxsackievirus mutants that can bypass host factor PI4KIIIbeta and the need for high levels of PI4P lipids for replication. Cell Res. 2012;22:1576–1592. doi: 10.1038/cr.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Strating J.R., van der Linden L., Albulescu L., Bigay J., Arita M., Delang L., Leyssen P., van der Schaar H.M., Lanke K.H., Thibaut H.J. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015;10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]; Itraconazole, an FDA-aroved drug to target fungal infections, is identified as a broad-spectrum enterovirus inhibitor and shown to target the lipid shuttling activity of oxysterol-binding protein that is essential for viral replication organell formation and/or function.
- 61.Gao Q., Yuan S., Zhang C., Wang Y., Wang Y., He G., Zhang S., Altmeyer R., Zou G. Discovery of itraconazole with broad-spectrum in vitro antienterovirus activity that targets nonstructural protein 3A. Antimicrob. Agents Chemother. 2015;59:2654–2665. doi: 10.1128/AAC.05108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mesmin B., Bigay J., Moser von Filseck J., Lacas-Gervais S., Drin G., Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 63.Albulescu L., Strating J.R., Thibaut H.J., van der Linden L., Shair M.D., Neyts J., van Kuppeveld F.J. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antiviral Res. 2015;117:110–114. doi: 10.1016/j.antiviral.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Arita M., Kojima H., Nagano T., Okabe T., Wakita T., Shimizu H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. J. Virol. 2013;87:4252–4260. doi: 10.1128/JVI.03546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albulescu L., Bigay J., Biswas B., Weber-Boyvat M., Dorobantu C.M., Delang L., van der Schaar H.M., Jung Y.S., Neyts J., Olkkonen V.M. Uncovering oxysterol-binding protein (OSBP) as a target of the anti-enteroviral compound TTP-8307. Antiviral Res. 2017;140:37–44. doi: 10.1016/j.antiviral.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Shim A., Song J.H., Kwon B.E., Lee J.J., Ahn J.H., Kim Y.J., Rhee K.J., Chang S.Y., Cha Y., Lee Y.S. Therapeutic and prophylactic activity of itraconazole against human rhinovirus infection in a murine model. Sci. Rep. 2016;6:23110. doi: 10.1038/srep23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Qing J., Wang Y., Sun Y., Huang J., Yan W., Wang J., Su D., Ni C., Li J., Rao Z. Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential role in viral infection as an uncoating regulator. PLoS Pathog. 2014;10:e1004422. doi: 10.1371/journal.ppat.1004422. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of the role of cyclophilin in the uncoating process of an enterovirus and the potential of cyclophilin inhibitors to interfere with enterovirus infection.
- 68.Frausto S.D., Lee E., Tang H. Cyclophilins as modulators of viral replication. Viruses. 2013;5:1684–1701. doi: 10.3390/v5071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seizer P., Klingel K., Sauter M., Westermann D., Ochmann C., Schonberger T., Schleicher R., Stellos K., Schmidt E.M., Borst O. Cyclophilin A affects inflammation, virus elimination and myocardial fibrosis in coxsackievirus B3-induced myocarditis. J. Mol. Cell Cardiol. 2012;53:6–14. doi: 10.1016/j.yjmcc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Desmet J., Verstraete K., Bloch Y., Lorent E., Wen Y., Devreese B., Vandenbroucke K., Loverix S., Hettmann T., Deroo S. Structural basis of IL-23 antagonism by an Alphabody protein scaffold. Nat. Commun. 2014;5:5237. doi: 10.1038/ncomms6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crotty S., Maag D., Arnold J.J., Zhong W.D., Lau J.Y.N., Hong Z., Andino R., Cameron C. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 2001;7:255. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]