Abstract
A case of canine parvovirus type 2c (CPV-2c) infection in a 3-month-old feral kitten with a cerebral abscess and neurological disease is reported. The cat displayed ataxia and convulsions together with signs of gastroenteritis and profound alteration of the total and differential white blood cell counts. A parvovirus strain was detected by a TaqMan assay in the blood and faeces of the affected kitten, which was characterised as CPV by means of molecular assays but did not react with any of the CPV type-specific probes. By sequence and phylogenetic analyses of the VP2-protein gene, the CPV-2c strain displayed a non-coding mutation in the probe-binding region. Although the role of CPV-2c in this particular case is unclear, it is possible that it predisposed the kitten to the clinical signs seen. Continuous surveillance is needed to monitor future spreading of this CPV-2c mutant, and any associated clinical signs, in the dog and cat population.
Feline panleukopenia (FPL) is a contagious disease of cats which is traditionally caused by feline panleukopenia virus (FPLV), a member of the genus Parvovirus closely related to canine parvovirus type 2 (CPV-2) and other parvoviruses of carnivores. 1,2 Carnivore parvoviruses are small, non-enveloped, single-stranded DNA viruses that are very prone to genetic evolution. However, while FPLV since its first identification in 1920 has not undergone significant changes in the antigenic and biological properties, 3 CPV-2 has progressively evolved under partly positive selection, giving rise to antigenic variants that replaced the original type and possess different biological and antigenic properties. 4–6 Three antigenic variants of CPV-2 are currently known, namely CPV-2a, CPV-2b and CPV-2c, that have completely replaced the original type 2, still used in most commercial vaccines. 7,8 In comparison to the original type 2, the antigenic variants are more aggressive and have re-gained the ability to replicate in vivo in the feline host. 9,10 FPL outbreaks caused by CPV-2a and CPV-2b have been described worldwide. 11,12 In contrast, cases of CPV-2c infection in cats have been reported only in Italy 12,13 and there is a single report of overt disease associated to this antigenic variant. 12
FPL is usually characterised by profound leukopenia involving both lymphocytes and polymorphocytes and acute gastroenteritis with fever (40–41.5°C), abdominal pain, vomiting and diarrhoea. In-utero and immediate post-mortem infections may lead to the development of neurological disease as a consequence of virus colonisation of Purkinje cells of cerebellum, with newborn kittens displaying signs referable to cerebellar damage such as ataxia, circling and muscular weakness. 1
In this paper, a CPV-2c infection is reported in a kitten with intracranial abscess and convulsions.
Materials and methods
Clinical case, physical, neurological and laboratory investigations
On June 19, 2010, a European shorthair feral kitten, 3 months of age, was presented at a veterinary clinic of Bari, Apulia region, Italy, with a soft subcutaneous swelling at the head from which a purulent material was oozing out. Anamnesis of the cat, including the vaccination history, was not known. At clinical examination, an alopecic area, 4 cm of diameter, was evident in correspondence to the subcutaneous swelling. The general health conditions were good and the appetite was preserved despite the presence of fever (39.8°C). The cat was discharged with a therapy consisting of oral administration of antibiotics (amoxicillin–clavulanic acid 25 mg/kgq12 h for 6 days). The day after the cat was again submitted to clinical examination due to the onset of neurological signs that consisted of ataxia and difficulty in standing and walking. Neurological examination revealed depressed postural reactions in all limbs, whereas oculo-vestibular responses, spinal reflexes, pain sensation and urinary and faecal continence were normal. Fever (40.0°C) and anorexia were also observed. New antibiotics were administered (enrofloxacin 5 mg/kgq24 h, clindamycin 25 mg/kgq12 h) in association with carprofen (2 mg/kg at q48 h). Ethylenediamine tetraacetic acid (EDTA)-blood, serum and faecal samples were collected during the clinical examination for haematology, serum electrophoresis and immunochromatographic testing for detection of feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) (Snap Feline Combo Plus; Idexx Laboratories, Westbrook, Maine, USA) and CPV/FPLV (Snap; Canine Parvovirus Antigen Test, Idexx Laboratories) antigens.
Blood cell counts obtained from the EDTA-blood sample showed a marked modification of white blood cell (WBC) counts (28.5×109 cells/l, reference interval (RI) 5.5–19.5×109 cells/l), with lymphopenia (0.5×109 cells/l, RI 1.5–7.0×109 cells/l) and increased neutrophil counts (23.9×109 cells/l, RI 2.5–12.5×109 cells/l). Reductions of haematocrit (23.9%, RI 30–45%) and platelets (79×109 cells/l, RI 175–600×109 cells/l) were also observed. Serum electrophoresis did not reveal any important modification of the serum proteins and FIV/FeLV testing was negative, whereas the Snap Canine Parvovirus Antigen Test gave a positive result. In the subsequent days, the cat underwent a progressive clinical improvement, regaining appetite and a normal rectal temperature and losing the neurological signs. On June 23, EDTA-blood, serum, nasal and faecal samples were collected and sent to our laboratory for further investigations. Five days later the kitten was presented again at the veterinary clinic with loss of appetite and diarrhoea and an antibiotic therapy was started with sulfamethopyrazine (30 mg/kgq24 h). On July 2, the appearance of convulsions was observed with more than 10 episodes per day. By post-contrast computed tomography (CT), carried out using a Philips MX8000 MultiSlice CT scanner, a focal area of markedly enhanced signal intensity compatible with an intracranial abscess was evident in the left parietal region in close proximity to the subcutaneous abscess. An adjacent area of decreased signal intensity of possible oedematous origin was observed together with reduction of the third ventricle and deviation of the falx cerebri. In contrast, there were no cerebellar abnormalities, and no signs of recent, or old, fractures of the cranial bones. Abscess and cerebrospinal fluid samples for cytology, bacteriology and/or virology were not collected at the clinical examination nor during CT. Administration of phenobarbital (2.5 mg/kgq12 h) resulted in the rapid recovery of the kitten and loss of the neurological signs. The abscesses underwent progressive adsorption leading to complete recovery.
Molecular investigations
Nucleic acids were extracted from the nasal, faecal and EDTA-blood samples using the DNeasy Tissue Kit and QIAamp Viral RNA Mini Kit (Qiagen, Milan, Italy) and stored at −70°C until their use. DNA extracts were subjected to TaqMan assay for detection of CPV/FPLV 14 and to a minor groove binder probe (MGB) assay for rapid discrimination between true FPLV strains and the antigenic variants of CPV-2. 15 Parvovirus strains were characterised as FPLV and CPV on the basis of the detected fluorescent reporter probe (VIC and FAM) signals, respectively. CPV-2 molecular characterisation was achieved by means of a panel of real-time polymerase chain reaction (PCR) assays using MGB probes able to identify the antigenic variants 2a, 2b and 2c 16 and to discriminate between CPV vaccine and field strains. 17,18
Faecal RNA extracts were screened by either real-time or conventional reverse transcriptase PCR assays specific for carnivore coronaviruses 19 and caliciviruses. 20 The blood extracts were also used to detect the proviral DNA of FIV 21 and FeLV 22 and the DNA of feline haemoplasmas. 23 Feline herpesvirus and feline calicivirus were searched for in the nasal swab extract. 24
Virus isolation attempts
Parvovirus isolation was attempted as described previously. 25,26 Briefly, the parvovirus positive samples were treated with antibiotics (penicillin 5000 IU/ml, streptomycin 2500 μg/ml, amphotericin B 10 μg/ml) at 37°C for 30 min and inoculated onto freshly trypsinised Crandell feline kidney (CrFK) and canine A-72 cells grown in Dulbecco's minimal essential medium containing 5% foetal calf serum. After an incubation period of 5 days at 37°C, the cells were tested by an immunofluorescence (IF) assay using a dog serum positive for CPV-2 and a rabbit anti-dog IgG conjugated with fluorescein isothiocyanate (Sigma–Aldrich, Milan, Italy). Three serial passages were carried out before virus isolation was considered unsuccessful.
Detection of CPV antibodies
Antibodies against CPV were searched for in the cat serum by means of a haemagglutination-inhibition (HI) test, as previously described. 27,28 The test was performed at +4°C in 96-well V-plates, using 10 haemagglutinating units of CPV-2b antigen and 1% pig erythrocytes. Two-fold dilutions of each serum sample in phosphate buffered saline, starting from 1:10, were tested. The HI titre was indicated as the highest serum dilution completely inhibiting viral haemagglutination.
Sequence and phylogenetic analyses
The sequence of the full-length VP2 gene of the identified parvovirus strain (300/10) was determined through PCR amplification of three partially overlapping fragments, as previously described. 3,6 The obtained sequences were assembled, edited and compared with FPLV and CPV reference strains and with a sequence database of parvovirus strains detected in Italy, 3,6,12 using the BioEdit software package, version 7.0.1 (www.mbio.ncsu.edu/BioEdit/bioedit.html). The VP2 gene sequence was deposited in the GenBank database under accession number HQ025913.
The phylogenetic relationships were evaluated by using Mega3; 29 pair-wise genetic distances were calculated by using the Kimura's two-parameter model and phylogenetic trees were constructed by the neighbor-joining and maximum parsimony methods. A bootstrap analysis with 1000 replicates was undertaken to assess the confidence level of the branch pattern. Canine and feline strains used for tree constructions were selected from previous studies. 3,6,12
Results
All molecular assays for detection of the most common pathogens of the cat gave negative results with the exception of the parvovirus TaqMan assay, which detected low viral DNA titres of 7.43×103/mg and 2.50×104/ml for the faecal and blood samples, respectively. Surprisingly, the parvovirus strain was characterised as CPV by means of the FPLV/CPV MGB probe assay and resulted as a CPV field strain by additional MGB probe assays. However, due to the lack of reaction with any of the type-specific probes of the 2a/2b and 2b/2c MGB probe assays, the strain was not fully characterised.
Virus isolation attempts on feline and canine cells were unsuccessful which is in agreement with the low viral titres detected in clinical samples. The HI test revealed the presence of CPV antibodies in the cat serum (titre of 1:640), which confirmed the exposure of the cat to a parvovirus strain.
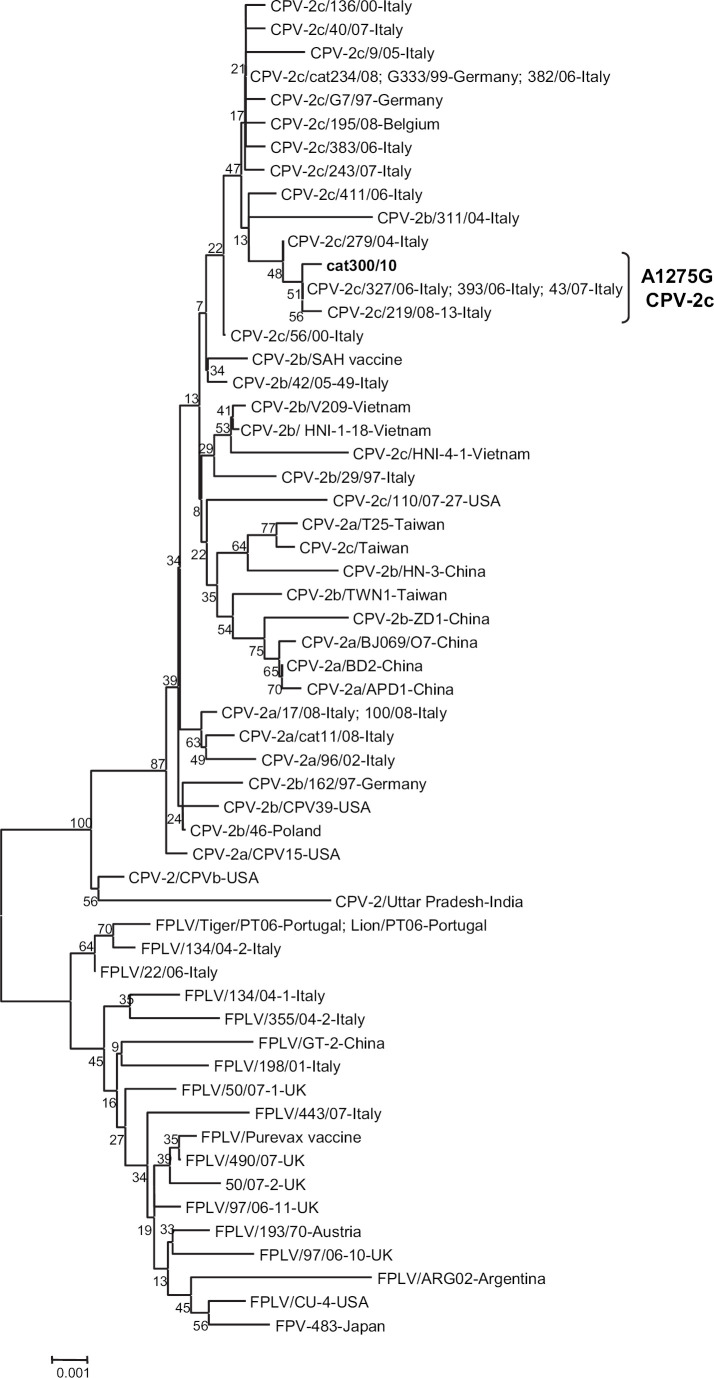
By sequence analysis of the full-length VP2-protein gene, strain 300/10 displayed residues typical of CPV-2 strains, Leu-87, Thr-101, Gly-300, Tyr-305, and Asp-375. The presence of codon GAA at position 1276–1278 corresponding to residue E426 of the encoded protein led us to type definitively the strain as CPV-2c. The change A1275G was detected in the binding region of the type 2c-specific probe, which accounted for the absence of VIC fluorescence in the 2b/2c assay. 16 This nucleotide change did not determine an amino acid substitution at residue 425 of the VP2 protein. The CPV-2c strain 300/10 of feline origin displayed the highest nucleotide identity (99%) to Italian type 2c strains displaying the same non-coding mutation at codon 425. 6 By phylogeny obtained using the neighbor-joining method, strain 300/10 was found to fall in the same cluster as Italian type 2c CPVs (Fig 1), which was separate from that including a previous CPV-2c strain isolated from a cat with FPL. 12 The maximum parsimony method confirmed the topology of the neighbor-joining tree.
Fig 1.
Neighbor-joining tree based on the full-length VP2 gene sequences (1755 nucleotides) of FPLVs and CPVs. The GenBank accession numbers for the reference strains used for phylogenetic tree construction are reported in previous papers. Canine and feline strains used for tree constructions were selected from previous studies. 6,12,15 A statistical support was provided by bootstrapping over 1000 replicates. The scale bar indicates the estimated numbers of nucleotide substitutions per site.
Discussion
In this paper, a CPV-2c infection in a 3-month-old feral kitten has been reported. Apart from the occurrence of marked lymphopenia, the haematological findings were not typical of FPL as total WBC and neutrophil counts were increased as a consequence of the concurrent abscess, whereas FPL is usually characterised by a dramatic reduction of all leukocyte subsets. Clinically, the occurrence of diarrhoea was compatible with the parvoviral infection, whereas fever could be attributed to either FPL or suppurative process. The prevalent sign in the cat was the appearance of neurological disease which was characterised by ataxia and subsequently by convulsions. FPL may cause neurological disorders only in newborn kittens due to the virus replication in Purkinje cells of the cerebellum subsequent to perinatal infection. Thus, the major finding is represented by ataxia due to cerebellar hypoplasia. 1 In the present case, ataxia was observed only in the early stage of the disease and was subsequently followed by severe seizures. In addition, the CT scanning showed focal lesions compatible with a parietal abscess without any involvement of the cerebellum, so that the neurological signs could be mainly attributed to the suppuration rather than to a viral infection. However, the recovery of the cat together with the lack of analyses on the cerebrospinal fluid prevented a definitive diagnosis for the neurological disorders. Although the clinical history and vaccination status of the cat were unknown, based on the low CPV-2c titres detected in the faeces and blood, it could be speculated that the cat was recovering from the infection when the neurological disease appeared. In this perspective, a preceding CPV-2c infection may have determined a general immunosuppression which could have been responsible for the parietal-brain colonisation by pyogenes.
In recent years, FPL cases caused by the antigenic variants of CPV-2 have been repeatedly reported. 11,12,30,31 However, to date there is a single report on clinical signs induced in a cat by the new variant CPV-2c. 12 Despite the full vaccination against FPL through administration of two doses of a classical FPLV vaccine, that cat was affected by overt clinical signs which included non-haemorrhagic diarrhoea, depression and loss of appetite but not alterations of the leukocyte counts. In the case reported in the present paper, there are no anamnestic data about previous vaccinations as the feral kitten was found already ill. This further detection of CPV-2c in a cat confirms the circulation of the new canine variant in the feline population, stressing again our recommendation to carefully evaluate the efficacy of classical FPLV vaccine against the CPV-2 antigenic variant.
Importantly, the feline CPV-2c was not characterised by the type-specific MGB probe assays as it displayed a mutation in the probe-binding region that prevented the correct hybridisation of the CPV-2c-specific probe. Untypeable CPV-2c strains with the same nucleotide change as the feline strain 300/10 have been previously detected in dogs. 6 Residue 425 is part of a major antigenic site located over the three-fold spike of the CPV-2 capsid and considered to affect viral immunogenicity. 32 Considering that this mutation is non-coding, the antigenicity of the virus is not altered. Further studies are needed to determine the distribution of this atypical CPV-2c in the canine and feline populations and any disease that might be associated with it.
Acknowledgements
This work was supported by grants from University of Bari, Italy, Ricerca di Ateneo 2009, project ‘Epidemiologia molecolare del parvovirus del cane nei carnivori domestici e selvatici’ and contribution to PRIN07, project ‘Infezione da parvovirus nei carnivori: aspetti molecolari, patogenetici ed immunologici’. We are grateful to Dr Gianfranco Pastorelli (Veterinary Clinic Santa Fara, Bari, Italy) for providing anamnestic data and clinical samples and to Dr Alice Casali (Veterinary Hospital Pingry, Bari, Italy) for the computed tomography scanning.
References
- 1.Greene C.E., Addie D.D. Feline parvovirus infections. Greene C.E. Infectious diseases of the dog and cat, 3rd edn, 2006, Saunders Elsevier: St Louis, USA, 78–88. [Google Scholar]
- 2.Truyen U., Addie D., Belák S., et al. Feline panleukopenia. ABCD guidelines on prevention and management, J Feline Med Surg 11, 2009, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decaro N., Desario C., Miccolupo A., et al. Genetic analysis of feline panleukopenia viruses from cats with gastroenteritis, J Gen Virol 89, 2008, 2280–2289. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi M., Yamaguchi Y., Gojobori T., et al. Differences in the evolutionary pattern of feline panleukopenia virus and canine parvovirus, Virology 249, 1998, 440–452. [DOI] [PubMed] [Google Scholar]
- 5.Shackelton L.A., Parrish C.R., Truyen U., Holmes E.C. High rate of viral evolution associated with the emergence of carnivore parvovirus, Proc Natl Acad Sci USA 102, 2005, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decaro N., Desario C., Parisi A., et al. Genetic analysis of canine parvovirus type 2c, Virology 385, 2009, 5–10. [DOI] [PubMed] [Google Scholar]
- 7.Parrish C.R., O’Connel P.H., Evermann J.F., Carmichael L.E. Natural variation of canine parvovirus, Science 230, 1985, 1046–1048. [DOI] [PubMed] [Google Scholar]
- 8.Buonavoglia C., Martella V., Pratelli A., et al. Evidence for evolution of canine parvovirus type-2 in Italy, J Gen Virol 82, 2001, 1555–1560. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael L.E. An annotated historical account of canine parvovirus, J Vet Med B Infect Dis Vet Public Health 52, 2005, 303–311. [DOI] [PubMed] [Google Scholar]
- 10.Truyen U. Evolution of canine parvovirus-A need for new vaccines?, Vet Microbiol 117, 2006, 9–13. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki M., Harasawa R., Nakatani H. Antigenic and genomic variabilities among recently prevalent parvoviruses of canine and feline origin in Japan, Vet Microbiol 38, 1993, 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Decaro N., Buonavoglia D., Desario C., et al. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia, Res Vet Sci 89, 2010, 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battilani M., Bassani M., Forti D., Morganti L. Analysis of the evolution of feline parvovirus (FPV), Vet Res Commun 30, 2006, 223–226. [Google Scholar]
- 14.Decaro N., Elia G., Martella V., et al. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs, Vet Microbiol 105, 2005, 19–28. [DOI] [PubMed] [Google Scholar]
- 15.Decaro N., Desario C., Lucente M.S., et al. Specific identification of feline panleukopenia virus and its rapid differentiation from canine parvoviruses using minor groove binder probes, J Virol Methods 147, 2008, 67–71. [DOI] [PubMed] [Google Scholar]
- 16.Decaro N., Elia G., Martella V., et al. Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology, J Virol Methods 133, 2006, 92–99. [DOI] [PubMed] [Google Scholar]
- 17.Decaro N., Elia G., Desario C., et al. A minor groove binder probe real-time PCR assay for discrimination between type 2-based vaccines and field strains of canine parvovirus, J Virol Methods 136, 2006, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decaro N., Martella V., Elia G., et al. Diagnostic tools based on minor groove binder probe technology for rapid identification of vaccinal and field strains of canine parvovirus type 2b, J Virol Methods 138, 2006, 10–16. [DOI] [PubMed] [Google Scholar]
- 19.Gut M., Leutenegger C.M., Huder J.B., Pedersen N.C., Lutz H. One-tube fluorogenic reverse transcription-polymerase chain reaction for the quantitation of feline coronaviruses, J Virol Methods 77, 1999, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X., Huang P.W., Zhong W.M., Farkas T., Cubitt D.W., Matson D.O. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR, J Virol Methods 83, 1999, 145–154. [DOI] [PubMed] [Google Scholar]
- 21.Stiles J., Bienzle D., Render J.A., Buyukmihci N.C., Johnson E.C. Use of nested polymerase chain reaction (PCR) for detection of retroviruses from formalin-fixed, paraffin-embedded uveal melanomas in cats, Vet Ophthalmol 2, 1999, 113–116. [DOI] [PubMed] [Google Scholar]
- 22.Quackenbush S.L., Dean G.A., Mullins J.I., Hoover E.A. Analysis of FeLV-FAIDS provirus burden and productive infection in lymphocyte subsets in vivo, Virology 223, 1996, 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Tasker S., Binns S.H., Day M.J., et al. Use of a PCR assay to assess the prevalence and risk factors for Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in cats in the United Kingdom, Vet Rec 152, 2003, 193–198. [DOI] [PubMed] [Google Scholar]
- 24.Di Martino B., Di Francesco C.E., Meridiani I., Marsilio F. Etiological investigation of multiple respiratory infections in cats, New Microbiol 30, 2007, 455–461. [PubMed] [Google Scholar]
- 25.Desario C., Decaro N., Campolo M., et al. Canine parvovirus infection: which diagnostic test for virus?, J Virol Methods 121, 2005, 179–185. [DOI] [PubMed] [Google Scholar]
- 26.Decaro N., Martella V., Desario C., et al. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain, J Vet Med B Infect Dis Vet Public Health 53, 2006, 468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decaro N., Desario C., Campolo M., et al. Evaluation of lactogenic immunity to canine parvovirus in pups, New Microbiol 27, 2004, 375–379. [PubMed] [Google Scholar]
- 28.Decaro N., Campolo M., Desario C., et al. Maternally-derived antibodies in pups and protection from canine parvovirus infection, Biologicals 33, 2005, 261–267. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment, Brief Bioinform 5, 2004, 150–163. [DOI] [PubMed] [Google Scholar]
- 30.Truyen U., Platzer G., Parrish C.R. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany, Vet Rec 138, 1996, 365–366. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda Y., Mochizuki M., Naito R., et al. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats, Virology 278, 2000, 13–19. [DOI] [PubMed] [Google Scholar]
- 32.Parrish C.R., Aquadro C.F., Strassheim M.L., Evermann J.F., Sgro J.-Y., Mohammed H.O. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus, J Virol 65, 1991, 6544–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]