Abstract
Maintaining leanness and a physically active lifestyle during adulthood reduces systemic inflammation, an underlying factor in multiple chronic diseases. The anti-inflammatory influence of near-daily physical activity in lowering C-reactive protein, total blood leukocytes, interleukin-6, and other inflammatory cytokines may play a key role in lowering risk of cardiovascular disease, certain types of cancer, type 2 diabetes, sarcopenia, and dementia. Moderate exercise training causes favorable perturbations in immunity and a reduction in incidence of upper respiratory tract infection (URTI). During each bout of moderate exercise, an enhanced recirculation of immunoglobulins, neutrophils, and natural killer cells occurs that persists for up to 3-h post-exercise. This exercise-induced surge in immune cells from the innate immune system is transient but improves overall surveillance against pathogens. As moderate exercise continues on a near-daily basis for 12–15 weeks, the number of symptoms days with URTI is decreased 25%–50% compared to randomized sedentary controls. Epidemiologic and animal studies support this inverse relationship between URTI risk and increased physical activity.
Keywords: Inflammation, Natural killer cells, Neutrophils, Physical activity, Upper respiratory tract infection
1. Introduction
Exercise immunology, a relatively new area of scientific endeavor, is the study of acute and chronic effects of various exercise workloads on the immune system and immunosurveillance against pathogens.1 Two areas of investigation from exercise immunology have clinical and public health implications: (1) the chronic anti-inflammatory influence of exercise training; (2) the reduction in risk of upper respiratory tract infections (URTI) from regular moderate exercise training.
2. Anti-inflammatory influence of exercise training
Acute inflammation is a normal response of the immune system to infection and trauma. Intense and prolonged exercise similar to marathon race competition causes large but transient increases in total white blood cells (WBC) and a variety of cytokines including interleukin-6 (IL-6), IL-8, IL-10, IL-1 receptor antagonist (IL-1ra), granulocyte colony stimulating factor (GCSF), monocyte chemoattractant protein 1 (MCP-1). macrophage inflammatory protein 1β (MIP-1β), tumor necrosis factor-α (TNF-α), and macrophage migration inhibitory factor (MIF)2, 3 C-reactive protein (CRP) is also elevated following heavy exertion, but the increase is delayed in comparison to most cytokines.
Despite regular increases in these inflammation biomarkers during each intense exercise bout, endurance athletes have lower resting levels in contrast to overweight and unfit adults. For example, mean CRP levels in long distance runners (rested state) typically fall below 0.5mg/L in comparison to 4.0mg/L and higher in obese, postmenopausal women.3, 4
The persistent increase in inflammation biomarkers is defined as chronic or systemic inflammation, and is linked with multiple disorders and diseases including atherosclerosis and cardiovascular disease (CVD), the metabolic syndrome, diabetes mellitus, sarcopenia, arthritis, osteoporosis, chronic obstructive pulmonary disease, dementia, depression, and various types of cancers.5, 6, 7 CRP is the most frequently measured inflammatory biomarker, and individuals with CRP values in the upper tertile of the adult population (<3.0mg/L) have a 2-fold increase in CVD risk compared to those with a CRP concentration below 1.0mg/L.7 An elevated fasting IL-6 concentration is a significant component of the chronic low-grade inflammation that underlies the metabolic syndrome, CVD, diabetes, and various cancers.8 Athletes typically have plasma IL-6 concentrations that fall below 1.0 pg/mL in contrast to values above 2.0pg/mL in older and obese individuals.3, 8
2.1. Physical activity, fitness, and chronic inflammation
Large population observational studies consistently show reduced WBC, CRP, IL-6, TNF-α, and other inflammatory biomarkers in adults with higher levels of physical activity and fitness, even after adjustment for potential confounders.9, 10, 11, 12, 13, 14 The inverse association between physical activity/fitness and inflammation is related in part to the effect of activity on fat mass.11 In most studies, however, adjustment for body mass index (BMI) and adiposity attenuates but does not negate the strength of the relationship between inflammatory biomarkers and physical activity/fitness.11, 15 For example, in a study of 1002 community-dwelling adults (18–85 years), a general linear model (GLM) analysis adjusted CRP means for frequency of physical activity, BMI, and several other lifestyle and demographic factors.15 BMI had the strongest effect on CRP followed by gender (higher in females), exercise frequency, age, and smoking status (see Fig. 1 ).
Fig. 1.

C-reactive protein (CRP) adjusted means for frequency of physical activity, body mass index (BMI), and several other lifestyle and demographic factors.15
Randomized, controlled exercise-intervention studies provide equivocal support for the inverse relationship between increased physical activity and reduced systemic inflammation.11, 16, 17, 18, 19, 20, 21, 22 One explanation is that in comparison to the large variance evaluated in observational studies, the change in aerobic fitness and activity levels is typically of low magnitude in randomized exercise trials, the duration of training seldom extends beyond 6 months, and the number of subjects is relatively low.17, 18, 20, 21 Nonetheless, data from both study formats support that in order for reductions in chronic inflammation to be experienced, a large change in a combination of lifestyle factors is needed including weight loss, near-daily moderate-to-vigorous physical activity of 30–60-min duration, avoidance of cigarette smoking, and increased intake of fruits and vegetables.22, 23 For example, if an obese, older individual adds three weekly 30-min walking sessions to the lifestyle, reductions in chronic inflammation are unlikely to be experienced unless the exercise workload is increased in combination with significant weight loss and improved diet quality.
2.2. Potential mechanisms
When successful, exercise training may exert anti-inflammatory influences through a reduction in visceral fat mass and the induction of an acute anti-inflammatory environment with each bout of exercise that over time becomes chronic.24, 25 These effects may be mediated in part through muscle-derived peptides or myokines, but this proposed mechanism needs further testing.25 Contracting skeletal muscles release myokines (e.g., IL-6, IL-8, IL-15) that may exert both direct and chronic anti-inflammatory effects.
The first identified and most studied myokine is IL-6. During prolonged and intense exercise, IL-6 is produced by muscle fibers and stimulates the appearance in the circulation of other anti-inflammatory cytokines such as IL-1ra and IL-10.26 IL-6 also inhibits the production of the proinflammatory cytokine TNF-α and stimulates lipolysis and fat oxidation.26 With weight loss from energy restriction and exercise, plasma levels of IL-6 fall, skeletal muscle TNF-α decreases, and insulin sensitivity improves.27, 28 Thus IL-6 release from the exercising muscle may help mediate some of the health benefits of exercise including metabolic control of type 2 diabetes.27, 28
Muscle IL-6 release, however, is very low during moderate physical activity. For example, during a 30-min brisk walk on a treadmill, plasma IL-6 concentrations increased from1.3pg/mL to 2.0pg/mL in female subjects.29 The increase in IL-6 during brisk walking is probably insufficient to mediate anti-inflammatory and other beneficial health effects, and additional research is needed to determine the relative contribution of myokines compared to other exercise-induced factors. The acute exercise-induced increase in IL-6 after heavy exertion (e.g., typically above 5pg/mL, 10pg/mL, and 50pg/mL following 1-h, 2-h, and marathon race running bouts, respectively) may indeed orchestrate anti-inflammatory influences, lipolysis, and improved insulin sensitivity, but this amount of physical activity is beyond levels achievable by most overweight/obese individuals.
A moderate exercise program of near daily 30-min walking bouts, without diet control, has small influences on visceral fat, even in long-term studies.30 This is further evidence that the myokine hypothesis does not apply at the activity level attainable by most middle-aged and elderly individuals. Thus moderate physical activity training must be increased to the highest levels acceptable to an individual (e.g., 60 min a day) and combined with weight loss through tight control of energy intake and improved diet quality to achieve reductions in systemic inflammation.
3. URTI risk reduction from regular moderate exercise training
URTI is the most frequently occurring infectious disease in humans worldwide.31, 32, 33 More than 200 different viruses cause the common cold, and rhinoviruses and coronaviruses are the culprits 25%–60% of the time. The National Institute of Allergy and Infectious Diseases reports that people in the USA suffer one billion colds each year with an incidence of 2–4 for the average adult and 6–10 for children.31 URTI imposes an estimated USD40 billion burden in direct and indirect costs on the U.S. economy.32
Low to high exercise workloads have a unique effect on URTI risk.34 Regular physical activity improves immune function and lowers URTI risk while sustained and intense exertion has the opposite effect. Marathon race competitions and heavy exercise training regimens increase URTI risk, but relatively few individuals exercise at this level, limiting public health concerns. The second half of this chapter will review the benefits of regular, moderate activity in improving immunosurveillance against pathogens and lowering URTI risk. This information has broad public health significance and appeal, and provides the clinician with an additional inducement to encourage increased physical activity among patients.
3.1. Moderate physical activity and URTI risk
Several lines of evidence support the linkage between moderate physical activity and improved immunity and lowered infection rates: survey, animal, epidemiologic, and randomized training data. Survey data consistently support the common belief among fitness enthusiasts that regular exercise confers resistance against infection. In surveys, 80%–90% of regular exercisers perceive themselves as less vulnerable to viral illnesses compared to sedentary peers.35, 36
Animal studies are difficult to apply to the human condition, but in general, support the finding that moderate exercise lowers morbidity and mortality following pathogen inoculation, especially when compared to prolonged and intense exertion or physical inactivity. Mice infected with the herpes simplex virus, for example, and then exposed to 30-min of moderate exercise experience a lower mortality during a 21-day period compared to higher mortality rates after 2.5h of exhaustive exercise or rest.37 Another study with mice showed that 3.5 months of moderate exercise training compared to no exercise prior to induced influenza infection decreased symptom severity and lung viral loads and inflammation.38
Retrospective and prospective epidemiologic studies have measured URTI incidence in large groups of moderately active and sedentary individuals. Collectively, the epidemiologic studies consistently show reduced URTI rates in physically active or fit individuals. A one-year epidemiological study of 547 adults showed a 23% reduction in URTI risk in those engaging in regular versus irregular moderate-to-vigorous physical activity.39 In a group of 145 elderly subjects, URTI symptomatology during a one-year period was reduced among those engaging in higher compared to lower amounts of moderate physical activity.40 During a one-year study of 142 males aged 33–90 years, the odds of having at least 15 days with URTI was 64% lower among those with higher physical activity patterns.41 A cohort of 1509 Swedish men and women aged 20–60 years were followed for 15 weeks during the winter/spring.42 Subjects in the upper tertile for physical activity experienced an 18% reduction in URTI risk, but this proportion improved to 42% among those with high perceived mental stress.
A group of 1002 adults (18–85 years, 60% female, 40% male) were followed for 12 weeks (half during the winter, half during the fall) while monitoring URTI symptoms and severity using the Wisconsin Upper Respiratory Symptom Survey.43, 44 Subjects reported frequency of moderate-to-vigorous aerobic activity, and rated their physical fitness level using a 10-point Likert scale. The number of days with URTI was 43% lower in subjects reporting an average of five or more days of aerobic exercise (20-min bouts or longer) compared to those who were largely sedentary (≤1 day per week) (see Fig. 2 ). This relationship occurred after adjustment for important confounders including age, education level, marital status, gender, BMI, and perceived mental stress. The number of days with URTI was 46% lower when comparing subjects in the highest versus lowest tertile for perceived physical fitness, even after adjustment for confounders.
Fig. 2.
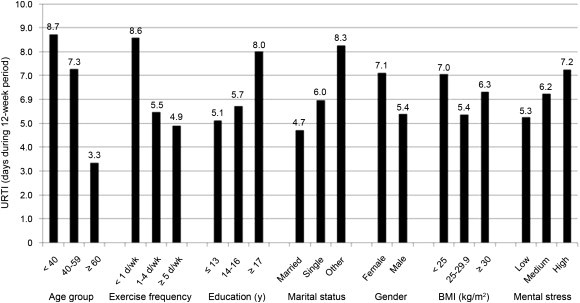
The number of days with upper respiratory tract infection (URTI) by exercise frequency tertiles after adjustment for age, education level, marital status, gender, body mass index (BMI), and perceived mental stress.43
Regular physical activity may lower rates of infection for other types of diseases, but data are limited due to low disease prevalence. For example, women with a high frequency of walking experienced an 18% lower risk of pneumonia compared with women who walked the least.45 In the same cohort, women who reported running or jogging more than 2h per week had a reduced pneumonia risk compared with women who spent no time running or jogging.45
Randomized experimental trials provide important data in support of the viewpoint that moderate physical activity reduces URTI symptomatology. In a randomized, controlled study of 36 women (mean age, 35 years), subjects walked briskly for 45-min, five days a week, and experienced one-half the days with URTI symptoms (5.1 vs. 10.8) during the 15-week period compared to that of the sedentary control group.46
The effect of exercise training (five 45-min walking sessions/week at 60%–75% maximum heart rate) and/or moderate energy restriction (1200–1300 kcal per day) on URTI was studied in obese women (n = 91, BMI 33.1±0.6kg/m2) randomized to one of four groups: control, exercise, diet, exercise and diet.47 Energy restriction had no significant effect on URTI incidence, and subjects from the two exercise groups were contrasted with subjects from the two nonexercise groups. The number of days with URTI for subjects in the exercise groups was reduced 40% relative to the nonexercise groups (5.6 vs. 9.4), similar to the level of nonobese, physically active controls (n = 30, 4.8 days with URTI).
In another study, 30 sedentary elderly women (mean age, 73 years) were assigned to walking or sedentary groups.48, 49 The exercise group walked 30–40 min, 5 days per week, for 12 weeks at 60% heart rate reserve. Incidence of URTI in the walking groups was 21% compared to 50% in the calisthenic control group during the study (September–November).
A one-year randomized study of 115 overweight, postmenopausal women showed that regular moderate exercise (166 min per week, ∼4days per week) lowered URTI risk compared to controls (who engaged in a stretching program).50 In the final three months of the study, the risk of colds in the control group was more than threefold that of the exercisers.
3.2. Moderate physical activity and enhanced immunosurveillance
During moderate exercise several transient changes occur in the immune system.29, 51, 52, 53 Moderate exercise increases the recirculation of immunoglobulins, and neutrophils and natural killer cells, two cells that play a critical role in innate immune defenses. Animal data indicate that lung macrophages play an important role in mediating the beneficial effects of moderate exercise on lowered susceptibility to infection.54 Stress hormones, which can suppress immunity, and pro- and anti-inflammatory cytokines, indicative of intense metabolic activity, are not elevated during moderate exercise.29
Although the immune system returns to pre-exercise levels within a few hours after the exercise session is over, each session may represent an improvement in immune surveillance that reduces the risk of infection over the long term. Other exercise-immune related benefits include enhanced antibody-specific responses to vaccinations. For example, several studies indicate that both acute and chronic moderate exercise training improves the body’s antibody response to the influenza vaccine.55, 56, 57, 58 In one study, a 45-min moderate exercise bout just before influenza vaccination improved the antibody response.55
These data provide additional evidence that moderate exercise favorably influences overall immune surveillance against pathogens. Taken together, the data on the relationship between moderate exercise, enhanced immunity, and lowered URTI risk are consistent with guidelines urging the general public to engage in near-daily brisk walking.
4. Conclusion
Although methodology varies widely and evidence is still emerging59 epidemiologic and randomized exercise training studies consistently report a reduction in URTI incidence or risk of 18%–67%. This is the most important finding that has emerged from exercise immunology studies during the past two decades. Animal and human data indicate that during each exercise bout, transient immune changes take place that over time may improve immunosurveillance against pathogens, thereby reducing URTI risk. The magnitude of reduction in URTI risk with near-daily moderate physical activity exceeds levels reported for most medications and supplements, and bolsters public health guidelines urging individuals to be physically active on a regular basis.
Regular physical activity should be combined with other lifestyle strategies to more effectively reduce URTI risk. These strategies include stress management, regular sleep, avoidance of malnutrition, and proper hygiene.33, 60, 61, 62, 63 URTI is caused by multiple and diverse pathogens, making it unlikely that a unifying vaccine will be developed.33 Thus lifestyle strategies are receiving increased attention by investigators and public health officials, and a comprehensive lifestyle approach is more likely to lower the burden of URTI than a focus on physical activity alone.
The anti-inflammatory effect of near-daily physical activity may play a key role in many health benefits, including reduced cardiovascular disease, type 2 diabetes, various types of cancer, sarcopenia, and dementia.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 This is an exciting area of scientific endeavor, and additional research is needed to determine how immune perturbations during each exercise bout accumulate over time to produce an anti-inflammatory influence. As with URTI, multiple lifestyle approaches to reducing chronic inflammation should be employed with a focus on weight loss, high volume of physical activity, avoidance of smoking, and improved diet quality.
Footnotes
Available online at www.sciencedirect.com
Peer review under responsibility of Shanghai University of Sport
References
- 1.Shephard R.J. Development of the discipline of exercise immunology. Exerc Immunol Rev. 2010;16:194–222. [PubMed] [Google Scholar]
- 2.Nieman D.C., Henson D.A., Smith L.L., Utter A.C., Vinci D.M., Davis J.M. Cytokine changes after a marathon race. J Appl Physiol. 2001;91:109–114. doi: 10.1152/jappl.2001.91.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Nieman D.C., Dumke C.L., Henson D.A., McAnulty S.R., Gross S.J., Lind R.H. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav Immun. 2005;19:398–403. doi: 10.1016/j.bbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Arsenault B.J., Earnest C.P., Després J.P., Blair S.N., Church T.S. Obesity, coffee consumption and CRP levels in postmenopausal overweight/obese women: importance of hormone replacement therapy use. Eur J Clin Nutr. 2009;63:1419–1424. doi: 10.1038/ejcn.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 6.Devaraj S., Valleggi S., Siegel D., Jialal I. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010;12:110–118. doi: 10.1007/s11883-010-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., III, Criqui M. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 8.Dekker M.J., Lee S., Hudson R., Kilpatrick K., Graham T.E., Ross R. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56:332–338. doi: 10.1016/j.metabol.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Hsu F.C., Kritchevsky S.B., Liu Y., Kanaya A., Newman A.B., Perry S.E. Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009;64:581–589. doi: 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavoie M.E., Rabasa-Lhoret R., Doucet E., Mignault D., Messier L., Bastard J.P. Association between physical activity energy expenditure and inflammatory markers in sedentary overweight and obese women. Int J Obes (Lond) 2010;34:1387–1395. doi: 10.1038/ijo.2010.55. [DOI] [PubMed] [Google Scholar]
- 11.Beavers K.M., Brinkley T.E., Nicklas B.J. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford E.S. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Borodulin K., Laatikainen T., Salomaa V., Jousilahti P. Associations of leisure time physical activity, self-rated physical fitness, and estimated aerobic fitness with serum C-reactive protein among 3,803 adults. Atherosclerosis. 2006;185:381–387. doi: 10.1016/j.atherosclerosis.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Brooks G.C., Blaha M.J., Blumenthal R.S. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Shanely R.A., Nieman D.C., Henson D.A., Jin F., Knab A., Sha W. Inflammation and oxidative stress are lower in physically fit and active adults. Scand J Med Sci Sports. 2011 Aug 18 doi: 10.1111/j.1600-0838.2011.01373.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Church T.S., Earnest C.P., Thompson A.M., Priest E.L., Rodarte R.Q., Saunders T. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42:708–716. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arsenault B.J., Cote M., Cartier A., Lemieux I., Després J.P., Ross R. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley G.A., Kelley K.S. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006;55:1500–1507. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Stewart L.K., Earnest C.P., Blair S.N., Church T.S. Effects of different doses of physical activity on C-reactive protein among women. Med Sci Sports Exerc. 2010;42:701–707. doi: 10.1249/MSS.0b013e3181c03a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson D., Markovitch D., Betts J.A., Mazzatti D., Turner J., Tyrrell R.M. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol. 2010;108:769–779. doi: 10.1152/japplphysiol.00822.2009. [DOI] [PubMed] [Google Scholar]
- 21.Stewart L.K., Flynn M.G., Campbell W.W., Craig B.A., Robinson J.P., Timmerman K.L. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen T., Paulsen S.K., Bruun J.M., Pedersen S.B., Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298:E824–E831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 23.Herder C., Peltonen M., Koenig W., Sutfels K., Lindstrom J., Martin S. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia. 2009;52:433–442. doi: 10.1007/s00125-008-1243-1. [DOI] [PubMed] [Google Scholar]
- 24.Brandt C., Pedersen B.K. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [Epub 2010 Mar 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen B.K. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol. 2009;587(Pt 23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen A.M., Pedersen B.K. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 27.Ryan A.S., Nicklas B.J. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27:1699–1705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]
- 28.Ferrier K.E., Nestel P., Taylor A., Drew B.C., Kingwell B.A. Diet but not aerobic exercise training reduces skeletal muscle TNF-alpha in overweight humans. Diabetologia. 2004;47:630–637. doi: 10.1007/s00125-004-1373-z. [DOI] [PubMed] [Google Scholar]
- 29.Nieman D.C., Henson D.A., Austin M.D., Brown V.A. The immune response to a 30-minute walk. Med Sci Sports Exerc. 2005;37:57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- 30.Nicklas B.J., Wang X., You T., Lyles M.F., Demons J., Easter L. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute of Allergy and Infectious Diseases. The common cold. Available at: http://www.niaid.nih.gov/topics/commoncold [accessed 03.07.2010].
- 32.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 33.Monto A.S. Epidemiology of viral respiratory infections. Am J Med. 2002;112(Suppl 6A):S4–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 34.Nieman D.C. Is infection risk linked to exercise workload? Med Sci Sports Exerc. 2000;32(Suppl 7):S406–S411. doi: 10.1097/00005768-200007001-00005. [DOI] [PubMed] [Google Scholar]
- 35.Nieman D.C. Immune function responses to ultramarathon race competition. Med Sportiva. 2009;13:189–196. [Google Scholar]
- 36.Shephard R.J., Kavanagh T., Mertens D.J., Qureshi S., Clark M. Personal health benefits of Masters athletics competition. Br J Sports Med. 1995;29:35–40. doi: 10.1136/bjsm.29.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis J.M., Kohut M.L., Colbert L.H., Jackson D.A., Ghaffar A., Mayer E.P. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol. 1997;83:1461–1466. doi: 10.1152/jappl.1997.83.5.1461. [DOI] [PubMed] [Google Scholar]
- 38.Sim Y.J., Yu S., Yoon K.J., Loiacono C.M., Kohut M.L. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200:1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews C.E., Ockene I.S., Freedson P.S., Rosal M.C., Merriam P.A., Hebert J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Kostka T., Praczko K. Interrelationship between physical activity, symptomatology of upper respiratory tract infections, and depression in elderly people. Gerontology. 2007;53:187–193. doi: 10.1159/000100017. [DOI] [PubMed] [Google Scholar]
- 41.Kostka T., Drygas W., Jegier A., Praczko K. Physical activity and upper respiratory tract infections. Int J Sports Med. 2008;29:158–162. doi: 10.1055/s-2007-965806. [DOI] [PubMed] [Google Scholar]
- 42.Fondell E., Lagerros Y.T., Sundberg C.J., Lekander M., Bälter O., Rothman K.J. Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc. 2011;43:272–279. doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- 43.Nieman D.C., Henson D.A., Austin M.D., Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med. 2011;45:987–992. doi: 10.1136/bjsm.2010.077875. [DOI] [PubMed] [Google Scholar]
- 44.Barrett B., Brown R., Mundt M., Safdar N., Dye L., Maberry R. The Wisconsin upper respiratory symptom survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuman M.I., Willett W.C., Curhan G.C. Physical activity and the risk of community-acquired pneumonia in US women. Am J Med. 2010;123:281.e7–281.e11. doi: 10.1016/j.amjmed.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieman D.C., Nehlsen-Cannarella S.L., Markoff P.A., Balk-Lamberton A.J., Yang H., Chritton D.B. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11:467–473. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 47.Nieman D.C., Nehlsen-Cannarella S.L., Henson D.A., Koch A.J., Butterworth D.E., Fagoaga O.R. Immune response to exercise training and/or energy restriction in obese women. Med Sci Sports Exerc. 1998;30:679–686. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Nieman D.C., Henson D.A., Gusewitch G., Warren B.J., Dotson R.C., Butterworth D.E. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Nieman D.C. Immune function. In: Gisolfi C.V., Lamb D.R., Nadel E., editors. Perspectives in exercise science and sports medicine. vol. 8. Cooper Publishing Group; Carmel, IN: 1995. pp. 435–461. (Exercise in older adults). [Google Scholar]
- 50.Chubak J., McTiernan A., Sorensen B., Wener M.H., Yasui Y., Velasquez M. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119:937–942. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 51.Nehlsen-Cannarella S.L., Nieman D.C., Jessen J., Chang L., Gusewitch G., Blix G.G. The effects of acute moderate exercise on lymphocyte function and serum immunoglobulins. Int J Sports Med. 1991;12:391–398. doi: 10.1055/s-2007-1024700. [DOI] [PubMed] [Google Scholar]
- 52.Nieman D.C. Exercise effects on systemic immunity. Immunol Cell Biol. 2000;78:496–501. doi: 10.1111/j.1440-1711.2000.t01-5-.x. [DOI] [PubMed] [Google Scholar]
- 53.Nieman D.C., Nehlsen-Cannarella S.L. The immune response to exercise. Sem Hematol. 1994;31:166–179. [PubMed] [Google Scholar]
- 54.Murphy E.A., Davis J.M., Brown A.S., Carmichael M.D., Van Rooijen N., Ghaffar A. Role of lung macrophages on susceptibility to respiratory infection following short-term moderate exercise training. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1354–R1358. doi: 10.1152/ajpregu.00274.2004. [DOI] [PubMed] [Google Scholar]
- 55.Edwards K.M., Burns V.E., Reynolds T., Carroll D., Drayson M., Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006;20:159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Kohut M.L., Arntson B.A., Lee W., Rozeboom K., Yoon K.J., Cunnick J.E. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22:2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 57.Kohut M.L., Lee W., Martin A., Arnston B., Russell D.W., Ekkekakis P. The exercise-induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain Behav Immun. 2005;19:357–366. doi: 10.1016/j.bbi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Lowder T., Padgett D.A., Woods J.A. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12:97–111. [PubMed] [Google Scholar]
- 59.Fondell E., Christensen S.E., Bälter O., Bälter K. Adherence to the Nordic nutrition recommendations as a measure of a healthy diet and upper respiratory tract infection. Public Health Nutr. 2011;14:860–869. doi: 10.1017/S136898001000265X. [DOI] [PubMed] [Google Scholar]
- 60.Cohen S. Keynote presentation at the eight international congress of behavioral medicine: the Pittsburgh common cold studies: psychosocial predictors of susceptibility to respiratory infectious illness. Int J Behav Med. 2005;12:123–131. doi: 10.1207/s15327558ijbm1203_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spiegel K., Sheridan J.F., Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 62.Cohen S., Doyle W.J., Alper C.M., Janicki-Deverts D., Turner R.B. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keusch G.T. The history of nutrition: malnutrition, infection and immunity. J Nutr. 2003;133:S336–S340. doi: 10.1093/jn/133.1.336S. [DOI] [PubMed] [Google Scholar]



