Graphical abstract
Food- and water-borne diseases (FWBD) are among climate and environmental sensitive infectious diseases. Climate change can highly exacerbate FWBD through vulnerable water systems.
Keywords: Climate change, Environmental health, Food-borne diseases, Infectious diseases, Water-borne diseases
Abstract
This paper provides a view of the major facts and figures related to infectious diseases with a focus on food-borne and water-borne diseases and their link with environmental factors and climate change. The global burden of food-borne diseases for 31 selected hazards was estimated by the World Health Organization at 33 million disability-adjusted life years (DALYs) in 2010 with 40% of this burden concentrated among children under 5 years of age. The highest burden per population of food-borne diseases is found in Africa, followed by Southeast Asia and the Eastern Mediterranean sub-regions. Unsafe water used for the cleaning and processing of food is a key risk factors contributing to food-borne diseases. The role of quality and quantity of water to the general burden of infectious diseases deserves attention, particularly in low- and middle-income countries, as its effects go beyond the food chain. Water-related infectious diseases are a major cause of mortality and morbidity worldwide, and climate change effects will exacerbate the challenges for the public health sector for both food-borne and water-borne diseases. Selected case studies from Africa and Asia show that (i) climate change extreme events, such as floods, may exacerbate the risks for infectious diseases spreading through water systems, and (ii) improvements related to drinking water, sanitation and hygiene could result in a significant reduction of intestinal parasitic infections among school-aged children. There is a need to better anticipate the impacts of climate change on infectious diseases and fostering multi-stakeholder engagement and multi-sectoral collaborations for integrated interventions at schools, community and household levels. The paper calls for giving priority to improving the environmental conditions affecting food-borne and water-borne infectious diseases under climate change.
1. Introduction
The World Health Organization (WHO) estimates that, in 2012, 12.6 million deaths globally, representing 23% of all deaths, were attributable to the environment (WHO, 2016). When accounting for premature mortality and disability, the fraction of the global burden of disease due to the environment was 22% (95% confidence interval (CI) 13–32%) (WHO, 2016). In children aged below 5 years, up to 26% (95% CI: 16–38%) of all deaths could be prevented if environmental risks were removed. Environmental factors might play a role in more than 80% of major diseases and injuries around the world and are among the biggest killers. Diseases with the largest environmental contribution in children under the age of 5 years include lower respiratory infections (32%), diarrhoeal diseases (22%), neonatal conditions (15%) and parasitic and vector-borne diseases (12%) (WHO, 2017).
Among the environmental factors, food and water contaminations are of particular relevance in the transmission of diseases. Several millions of children die each year from acute diarrheal diseases, and the majority of these deaths are likely due to contaminated food or water. Hence, it is important for public health interventions to pay particular attention to these two diseases. However, the true burden of food-borne and water-borne diseases is difficult to quantify. The challenges in many parts of the world concern how to generate evidence on risks related to environmental contaminations under climate change in different contexts, particularly in low-and middle-income countries (LMICs).
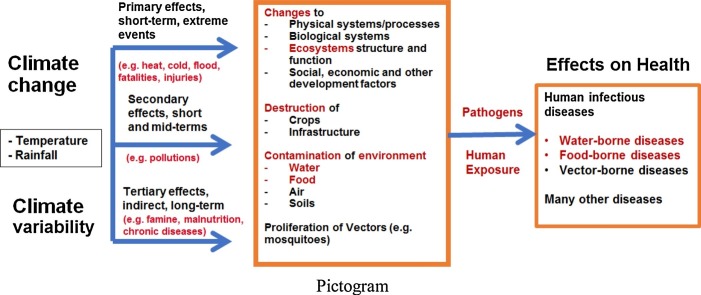
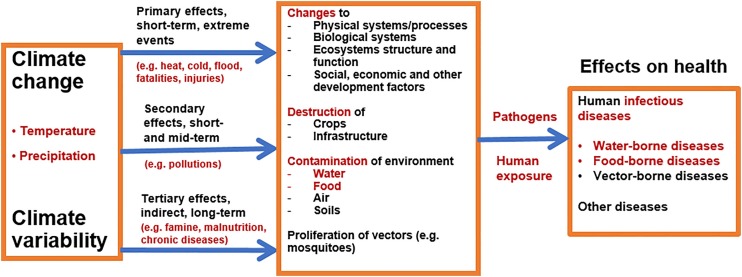
The recent Intergovernmental Panel on Climate Change (IPCC) “Special Report on the Impacts of Global Warming of 1.5 °C” (IPCC, 2018) states that “climate-related risks to health, livelihoods, food security, water supply, human security, and economic growth are projected to increase with global warming of 1.5 °C and to increase further at 2 °C. Any increase in global warming is projected to affect human health, with primarily negative consequences (high confidence). Risks from some vector-borne diseases, such as malaria and dengue fever, are projected to increase with warming from 1.5 °C to 2 °C, including potential shifts in their geographical range (high confidence)”. The pathways through which climate change and climate variability will affect human health are through different processes linked to various social, environmental, ecological and economic factors, and the spread, survival and growth of pathogens play a central role in diseases transmission (see Fig. 1 ).
Fig. 1.
Pathways of climate change effects on health with particular highlights of human infectious diseases (water-, food- and vector-borne diseases).
Humans must be exposed to pathogens to contract a number of diseases. Pathogens, vectors and hosts survive and reproduce within a range of optimal climatic conditions (WHO, 2003). Temperature and precipitation, in particular, play a major role in the transmission of diseases (Confalonieri et al., 2015; Dennis and Fisher, 2018). Precipitation can influence the transport and dissemination of infectious agents, particularly through water and sanitation systems (Cissé et al., 2016), while temperature can affect the growth and survival of pathogens and vectors (WHO, 2003).
There are three important categories of infectious diseases sensitive to climate change: (i) water-borne diseases; (ii) food-borne diseases; and (iii) vector-borne diseases (Cissé et al., 2018). Human exposure to water-borne infections occurs by contact with contaminated drinking water, recreational water or food. Water-and food-borne diseases are linked to the ingestion of pathogens via contaminated water or food, while vector-borne diseases are linked to the infections transmitted by arthropods, such as mosquitoes.
Climate change and climate variability will, therefore, affect the burden of climate-sensitive infectious diseases, particularly water-borne and food-borne diseases. There are warnings that in many regions of the world, it is likely that climate change trends will exacerbate the health risks associated with deficiencies in water, sanitation and hygiene (Cissé et al., 2011). Climate change can affect food- and water-borne diseases through the following pathways (Walker, 2018):
-
-
direct impacts, in the case of extreme events like floods and sea-level rise, where water can be contaminated due to presence in the environment of fecal-oral pathogens; and
-
-
indirect impacts, through climatic factors (like temperature and humidity) that influence processes of pathogens multiplication and survival, and other issues (e.g. agriculture, water resource management, conflicts, displacements, etc.).
Climate change related health effects also present a huge inequity dimension, as the risks are linked to the environmental systems and the social conditions (McMichael, 2013). It is widely acknowledged that LMICs are particularly affected by climate change impacts, while there is less research undertaken in many of these most affected regions (Liang and Gong, 2017). In LMICS, a number of important issues require particular attention, such as: (i) What are the likely climate change effects on infectious diseases, particularly for the most vulnerable areas and groups? (ii) How can we implement integrated approaches (e.g. Ecohealth and One Health) which are able to tackle the particular challenges of water and food contaminations, from the source to the consumption by the end-users? (iii) How and where do we need to relevantly implement successful multi-sectorial collaboration for more integrated risks assessment and interventions?
In this paper, I highlight updated knowledge related to the burden of food-borne and water-borne diseases and the importance of environmental factors in the prevention of infectious diseases. I will illustrate two major dimensions of the challenges with selected case studies: (i) the importance of health risks associated to vulnerabilities of water systems under climate change; and (ii) the importance of preventing parasitic infections by integrated water, sanitation and hygiene (WASH) interventions in school environments.
2. Methodology
I undertook a convenience literature review on food-borne and water-borne diseases (mainly on PubMed) and visited the web pages of major public and global health institutions (e.g. World Health Organization, WHO and the United States Center for Diseases Control and Prevention, CDC). The key terms used in different single searches or in combinations were as follows: water-borne diseases, food-borne diseases, climate change, selected countries (e.g. Burkina Faso, Mauritania and Nepal), Africa, Asia, Oceania, Europe, and America. There was no limitation of the time period, but I gave priority to scientific papers, reports and literature issued in the past 10 years, with a few exceptions related generally to immutable definitions and concepts. The objectives of the review were: (i) to obtain relevant information on the major concepts, facts and figures related to the two disease clusters (i.e. food- and water-borne diseases) and the links between the diseases and environmental factors and (ii) to identify and describe examples of projects related to the particular role of WASH among environmental factors in the prevention of infectious diseases under climate change. For these examples, following the clarification provided by Fig. 1 and the text above, showing the potential links between climate change and food- and water-borne diseases, I looked after two illustrative project-based case studies, one from each: (i) the potential impact of climate extreme events on infectious diseases through water systems, and (ii) the potential effects of integrated WASH interventions on infectious diseases among school-aged children. For these two case studies, I undertook a review of the respective projects’ literature (published protocols and papers, policy briefs and other documentation).
3. Results
3.1. Food-borne diseases
In 2007, the World Health Organization (WHO) established the Food-borne Disease Burden Epidemiology Reference Group (FERG), in order to estimate the global burden of food-borne diseases (WHO, 2015c). The report estimated that 31 selected hazards with food-borne diseases resulted in over 600 million illnesses and 420,000 deaths worldwide. Moreover, it was estimated that the 31 selected hazards induced 33 million DALYs in 2010; and 40% of the food-borne disease burden was among children under 5 years of age. The highest burden per population of food-borne diseases was observed in Africa, and unsafe water used for the cleaning and processing of food was one of the main drivers.
Other estimates have been made for different regions of the world (Devleesschauwer et al., 2015; Ford et al., 2015; Gibb et al., 2015; Havelaar et al., 2015; Hoffmann et al., 2017; Park et al., 2015; Torgerson et al., 2015). For example, Kirk et al. (2015) conducted an estimate for 22 diseases that contributed in 2010 for 2 billion (95% uncertainty interval [UI] 1.5–2.9 billion) cases, over one million (95% UI 0.89–1.4 million) deaths and 78.7 million (95% UI 65.0–97.7 million) DALYs (Kirk et al., 2015). The authors estimate that 29% (95% UI 23–36%) of cases caused by the selected diseases, or 582 million (95% UI 401–922 million), were transmitted by contaminated food, resulting in 25.2 million (95% UI 17.5–37.0 million) DALYs. Norovirus was the leading cause of food-borne illness, causing 125 million (95% UI 70–251 million) cases, while Campylobacter spp. caused 96 million (95% UI 52–177 million) food-borne illnesses. Of all food-borne diseases, diarrhoeal and invasive infections due to non-typhoidal Salmonella enterica infections resulted in the highest burden, causing 4.07 million (95% UI 2.49–6.27 million) DALYs.
Food-borne diseases occur through the ingestion of foodstuffs (including water) contaminated with microorganisms or chemicals. The risks of contamination exist in the food chain, from food production to consumption (“farm to fork”), and involve the pollution of water, soil or air. The estimation of food-borne disease burden is complicated because most of the hazards causing food-borne diseases are not transmitted solely by food (Hald et al., 2016); most have several potential exposure routes consisting of transmission from animals, by humans and via environmental routes, including water. As water plays a major role in the burden of food-borne diseases, the separation of food and water as exposure vehicles is difficult, particularly at the community level. Table 1 presents the different bacterial, viral, protozoan and toxic agents that are associated with food-borne and water-borne illnesses in humans (Acheson, 2009).
Table 1.
Bacterial, viral, protozoan, helminth and toxic agents associated with food-borne and water-borne diseases in humans.
| Categories of pathogens | Sub-groups and agents |
|---|---|
| Bacterial pathogens | Bacteria causing disease primarily mediated by a performed toxin |
| |
| Bacteria causing disease by production of toxins within the intestine | |
| |
| Bacteria causing diseases primarily by invading the intestinal epithelial cells | |
| |
| Other bacterial causes | |
| |
| Viral pathogens | Astroviruses Coronaviruses Enteric adenovirus Hepatitis A virus Hepatitis E virus Noroviruses Parvoviruses Picobirnaviruses Reoviruses Rotavirus Saporo-like viruses Toroviruses |
| Protozoan pathogens |
Toxoplasma gondii Cryptosporidium parvum Giardia intestinalis Entamoeba histolytica Cyclospora cayetanensis Microsporidia (Enterocytozoon bieneusi, Septata intestinalis) Isospora belli Dientamoeba fragilis Blastocystis hominis |
| Cestodes and nematodes |
Taenia saginata Taenia solium Diphyllobothrium latum Hymenolepis nana Ascaris lumbricoides Trichuris trichiura Trichinella spiralis |
| Natural toxins | Ciguatera Scrombroid |
Adapted from Acheson (2009).
3.2. Water-borne diseases
The global burden of infectious water-borne diseases is considerable, and even in high-income countries, water-borne illness continues to be a concern (Murphy et al., 2014). Water-borne diseases generally occur via ingestion of water and are highly linked to the quality of drinking water. Drinking water containing pathogenic microorganisms is the main driver of the burden of water-borne diseases (David et al., 2014; Murphy et al., 2014). Important water-borne diseases include diarrhoeal diseases, cholera, shigella, typhoid, hepatitis A and E, and poliomyelitis (WHO, 2015b). Diarrhoeal diseases alone account for an estimated 3.6% of the global burden of disease, as expressed in DALYs and were responsible for of 1.5 million deaths in 2012 (WHO, 2015b).
In many countries, health systems face difficulties in the proper collection and reporting of water-borne diseases. This explains why globally reported numbers generally highly underestimate the real incidence of water-borne diseases (Leclerc et al., 2002). Good examples in data collection on the matter are found in high-income countries, such as the United States of America where the Water-borne Disease and Outbreak Surveillance System (WBDOSS) has been established that collects data on water-borne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. In the United States of America and other high-income countries (e.g. Australia and Switzerland), the monitoring systems made it possible to have a large number of studies and reports related to drinking water–associated outbreaks, including highlights of risks associated with Legionella, Cryptosporidium, Giardia intestinalis, campylobacteriosis, Salmonella spp., Listeria spp. and norovirus (Beer et al., 2015; Benedict et al., 2015; FOPH, 2018; Gibney et al., 2017; Pons et al., 2015).
In LMICs, the burden of diarrhoeal diseases is estimated at 842,000 deaths per year, including 361,000 in children under the age of 5 years (WHO, 2015b). Water-borne disease outbreaks, particularly infectious intestinal diseases, have been attributed to various pathogens, as highlighted in Table 1 (e.g. bacteria, protozoa, viruses and parasites) and drinking water system characteristics (Bless et al., 2016; Ligon and Bartram, 2016). Lack of basic hygiene and sanitation and failing infrastructure also remain as two of the greatest challenges in the global fight against water-borne disease (Ford and Hamner, 2015). Climate change is likely to exacerbate in LMICs the risks for diarrhoeal diseases and other water-borne diseases in the future (IPCC, 2018).
3.3. Climate change and health challenges
The IPCC’s recent special report on 1.5°C states that human activities are estimated to have caused approximately 1.0°C of global warming above pre-industrial levels, with a likely range of 0.8 °C–1.2°C (IPCC, 2018). Global warming is likely to reach 1.5°C between 2030 and 2052 if it continues to increase at the current rate (high confidence). The consequent increased severity and number of extreme climatic conditions will be accompanied by changes in microbial communities and species interaction (Walker, 2018).
Climate change impacts on health are considered as one of today’s greatest public health threat (DeJarnett et al., 2017). A number of publications highlight that climate change will alter globally the incidence of food-borne diseases, water-borne diseases and diarrheal diseases in particular (Lake and Barker, 2018; Levy et al., 2018; Schijven et al., 2013). In the African region, 91 million illness episodes and 137,000 death every year are attributable to food-borne diseases (WHO, 2018). Diarrheal diseases were responsible for 70% of the burden of these food-borne diseases.
It was reported that extreme temperature and precipitation will impact on enteric pathogens, particularly on faecal-oral pathogens that are present in the environment, increasing the risk of gastrointestinal and diarrhoeal diseases (Levy et al., 2018). The World Health Organization estimates that, under climate change, an additional 48,000 deaths will occur in children aged below 15 years, mainly due to diarrhoeal diseases by 2030 and 33,000 deaths by 2050 (WHO, 2014b). The impact of climate change on diarrhoeal disease is projected to be higher in Asia and Africa. In 2030, sub-Saharan Africa is projected to have the greatest burden of mortality impacts attributable to climate change, while in the 2050, it is likely to have shifted to Southeast Asia.
In countries like Mauritania, WHO estimates that around 2150 individuals, including 1700 children under the age of 5 years, die each year from diarrhoeal diseases and that nearly 90% of these deaths are directly attributed to poor quality of WASH (WHO, 2014a). The major food- and water-borne diseases in Mauritania are bacterial and protozoan diarrhoea, hepatitis A, and typhoid fever (Central Intelligence Agency, 2018). The West Africa Sahel, including Burkina Faso and Mauritania, is considered one of the world’s most vulnerable regions to climate change, as temperature increases are projected to be 1.5 times higher than in the rest of the world (USAID, 2018). For LMICs in general, and West Africa in particular, the challenges of infectious diseases under climate change trends are particularly pronounced, and there are multiple international collaboration efforts underway to deepen risk profiling and to reduce the burden in different contexts (illustrative cases from West Africa are presented below).
3.4. Illustrative case 1: Infectious diseases risks through WASH systems under climate change in LMICs
The West Africa region recorded a series of extreme events, including flooding in areas that are typically arid and rather dry (e.g. Nouakchott and Ouagadougou) (Tall et al., 2012). In the context of predominant very simple traditional facilities (latrines), non-disposed solid waste, non-treated wastewater in streets, and traditional sources of water (e.g. unprotected wells), the threat to water quality and consequently to health is important in case of repeated flooding events. This case study highlighted the health risks associated to the vulnerability of water and sanitation systems in the face of climate extreme events in West African medium-sized cities (Cissé et al., 2011; Costello et al., 2009; Sherpa et al., 2014). The case is drawn from a regional project entitled “An Ecohealth Approach to Water and Health Management in relation to Climate Change: Adaptive Strategies to Cope with Drought and Floods in Four West African Countries (Côte d’Ivoire, Mauritania, Senegal and Togo)”. The project was conducted in four secondary cities in four countries: (i) Korhogo, Côte d’Ivoire (212,000 inhabitants, located near a dam); (ii) Kaédi, Mauritania (70,612 inhabitants, located near a river); (iii) Ziguinchor, Senegal (269,000 inhabitants, located near a river); and (iv) Kara, Togo (120,000 inhabitants, located near a river). The study objective was to deepen the understanding of the vulnerabilities of community and household water systems in selected medium-sized cities in the face of more frequent rainfalls that could lead to flooding and consequently affect infectious diseases (Cissé et al., 2011).
For the Kaédi case (Cissé et al., 2016), the city is located in the southern part of Mauritania at the border with Senegal, marked by the Senegal River, at about 430 km south of Nouakchott, the capital city of Mauritania. The city has no sewerage system, and families are mainly using on-site sanitation facilities. A number of socio-economic activities occur in the river (e.g. fishing, urban agriculture) involving vulnerable groups including women. The topography is relatively flat, characterized by plains with some hills not exceeding 200 m in height. Therefore, the proximity to the river puts the city at risk of river floods and urban floods.
Most of the households interviewed (94%) reported that flooding had occured in their courtyards after heavy rains. Drinking water systems are marked by the important presence of private water wells, which – very often – are not protected (see Photo 1 ). There were more than 100 wells found in the city; 12% of households have their own wells in the yard and well water constitutes a major source of some families’ drinking water in the city (33%).
Photo 1.
Examples of water wells in Kaédi, Mauritania.
Photo source: Mbra R.K.
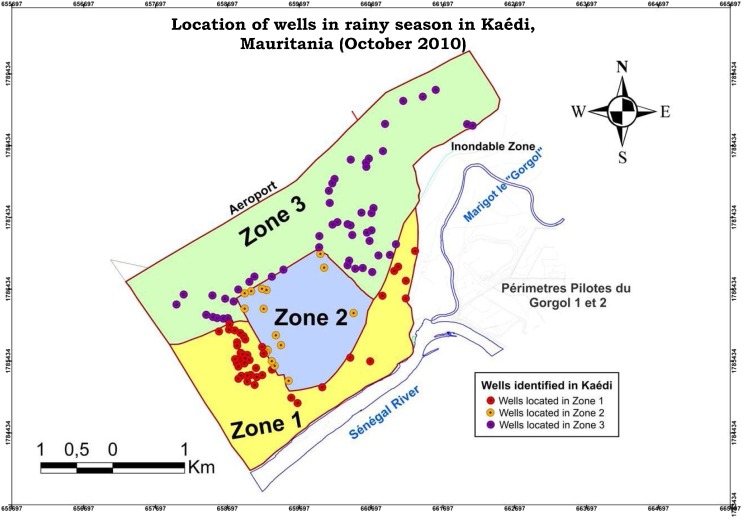
Kaédi was divided into three zones, delineated according to different perceived physical vulnerabilities to flooding, considering events that occurred in the recent past: zone 1 (more affected), zone 2 (less affected) and zone 3 (intermediary). The vulnerability assessment of the drinking water systems to floods is presented by zones in a vulnerability map (see Fig. 2 ) showing the zones with wells under higher threat of flooding and the risks that this could entail for infectious diseases.
Fig. 2.
A map of traditional water wells and their vulnerability to flooding events by zone in Kaédi, Mauritania: Zone 1 (most vulnerable to floods), zone 2 (least vulnerable) and zone 3 (medium vulnerability).
Source: Cissé et al. (2016).
The study showed that the increasing occurrence of flooding events due to climate variability and change in the context of WASH system vulnerabilities at both the household and community levels, with the predominance of very simple traditional excreta disposal facilities (latrines) and traditional sources of water (like unprotected wells) will threaten water quality and public health. This called for appropriate anticipative and adaptive management strategies, involving water and health sectors, which will be of high interest for hundreds of similar secondary cities in Africa.
3.5. Illustrative case 2: WASH effects on school-aged children’s parasitic infections in LMICs
Intestinal parasitic infections and malnutrition among school-aged children are among major interlinked public health problems in Africa (Duedu et al., 2015). This case study highlights the WASH components in an integrated school garden programme for improving the nutritional and health status of school-aged children in Burkina Faso and Nepal. The overarching goal of the project was to produce vegetables at school (Vegetables Go to School Project, 2018). The specific objectives of the Vegetables go to School project (phase I and II) were to contribute to the improved nutritional security of school-aged children in target countries (Philippines, Nepal, Bhutan, Indonesia, Burkina Faso) through school vegetable gardens linked to other school-based health, nutrition and environmental initiatives, with close participation of local communities (Vegetables Go to School Project, 2018). The project designed and implemented in all countries and “intervention schools”. Some integrated basic interventions comprised of school gardens and education activities including teachers and parents.
The rationale of the added WASH component was based on the following three assumptions (Cissé et al., 2017): (i) inadequate WASH conditions for children may lead to intestinal parasitic infections; (ii) intestinal parasitic infections may hamper children’s nutritional status and benefits from school gardens; and (iii) improving WASH conditions may therefore contribute to further expected benefits from nutrition-sensitive programmes. The component aimed at ensuring hygiene in the food chain from production to the eating table, giving particular attention to what the children do or find regarding WASH conditions after attending school (e.g. in the streets or at home). The research team implemented school gardens and integrated educational, nutritional and WASH interventions (see Table 2 ) with the assumption that these will reduce infectious diseases among children.
Table 2.
Integrated educational, nutritional, and environmental interventions to reduce intestinal parasitic infections among school-aged children.
| Categories of interventions | Interventions |
|---|---|
| Water, sanitation and hygiene (WASH) interventions |
|
| Nutrition interventions |
|
| Health interventions |
|
| Health promotion |
|
Adapted from Cissé et al. (2017).
The WASH component undertook in-depth assessments (quantitative and qualitative) in two countries (Burkina Faso and Nepal) on selected schools (same number of “intervention” and “control” schools) to measure the possible effects of well-integrated and complementary WASH interventions on top of school vegetable garden interventions on school-aged children’s health (particularly on intestinal parasitic infections) and nutritional status (Erismann et al., 2016, 2017). In Burkina Faso, a total of 360 school-aged children (182 boys and 178 girls) has complete data records (baseline and 1-year follow-up data) (Erismann et al., 2017). In Nepal, a total of 562 children aged 8–15 years had complete data records (Shrestha et al., 2017). In both Nepal and Burkina, between the baseline and the end-line, a significant decrease in total intestinal parasitic infections in both intervention and control schools was found, but most importantly in intervention schools. For example, in Burkina Faso, the prevalence of intestinal parasitic infections decreased both in intervention and control schools, but the decrease was significantly higher in the intervention schools compared to the control schools (odds ratio [OR] of the intervention effect = 0.2, 95% confidence interval [CI] = 0.1–0.5); and safe hand washing practices before eating and the use of latrines at schools were significantly higher in the intervention schools than in the control schools at the end-line (OR = 6.9, 95% CI = 1.4–34.4, and OR = 14.9, 95% CI = 1.4–153.9, respectively) (Erismann et al., 2017).
The higher decrease of intestinal parasitic infections among children in the intervention schools compared to the control schools (despite the short period between the complete implementation of the interventions and the end-line) showed promise that the effects of correctly implemented WASH interventions, integrated with other interventions, could be considerable on the nutritional status of children.
4. Conclusions and outlook
Improvements related to WASH will considerably contribute to the reduction of the total burden of infectious diseases. In LMICs, the increased frequency of floods exacerbates challenges with water pollution and this will increase the risks for food- and water-borne diseases, and various other infectious diseases, that are disproportionally affecting vulnerable groups and areas. Promoting well-targeted and integrated WASH interventions, including at school levels, constitutes an important component of needed strategies for strengthening the resilience of communities and systems at several levels in face of climate change. WASH can significantly reduce risks for water contamination in case of floods, prevent diarrhoeal diseases and parasitic infections and improve both environmental and human health.
Actions for the control of infectious diseases in the future should consider adaptation needs to face climate change impacts. This will require more integrated interventions. Old and new challenges to face need concomitant efforts on several Sustainable Development Goals (SDGs), e.g. SDG 3 (health), SDG 6 (water and sanitation), SDG 11 (cities and human settlements) and SDG 13 (climate change). There is, more than ever, a need of multi-stakeholder engagement and multi-sectorial collaborations for relevant research and integrated interventions at the school, community and household levels. This will also require strengthening the resilience of health systems (WHO, 2015a) and reducing the current adaptation gaps globally (UNEP, 2018).
Conflict of interest and ethical principle
The author has no conflicts of interest to declare.
Funding
This paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
This paper is an expanded version of a keynote ‘Water-borne and food-borne diseases: a need for further efforts on reducing environmental health exposure’ presented at the International Conference on Impact of Environmental Changes on Infectious Diseases (IECID 2017), Trieste, Italy, 17–19 May 2017.
References
- Acheson D.W.K. In: Encyclopedia of Microbiology. third ed. Schaechter M., editor. Academic Press; Oxford: 2009. Food and waterborne illnesses; pp. 365–381. [Google Scholar]
- Beer, K.D., Gargano, J.W., Roberts, V.A., Reses, H.E., Hill, V.R., Garrison, L.E., Kutty, P.R., Hilborn, E.D., Wade, T.J., Fullerton, K.E., Yoder, J.S., 2015. Outbreaks Associated With Environmental and Undetermined Water Exposures, United States, 2011–2012. [DOI] [PMC free article] [PubMed]
- Benedict, K.M., Reses, H., Vigar, M., Roth, D.M., Roberts, V.A., Mattioli, M., Cooley, L.A., Hilborn, E.D., Wade, T.J., Fullerton, K.E., Yoder, J.S., Hill, V.R., 2015. Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water, United States, 2013–2014. [DOI] [PMC free article] [PubMed]
- Bless P.J., Muela Ribera J., Schmutz C., Zeller A., Mausezahl D. Acute gastroenteritis and campylobacteriosis in swiss primary care: the viewpoint of general practitioners. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Intelligence Agency . 2018. The World Factbook.https://www.cia.gov/library/publications/the-world-factbook/geos/mr.html Available online: [Accessed] [Google Scholar]
- Cissé G., Koné B., Bâ H., Mbaye I., Koba K., Utzinger J., Tanner M. In: Resilient Cities: Cities and Adaptation to Climate Change. Otto-Zimmermann K., editor. Proceedings of the Global Forum 2010, Local Sustainability 1, Springer Science+Business Media B.V.; 2011. Ecohealth and climate change: adaptation to flooding events in riverside secondary cities in West Africa; pp. 55–67. [Google Scholar]
- Cissé G., Traoré D., Touray S., Bâ H., Keita M., Sy I., Koné B., Utzinger J., Tanner M. Vulnerabilities of water and sanitation at households and community levels in face of climate variability and change: trends from historical climate time series in a West African medium-sized town. Int. J. Global Environ. Issues. 2016;15(1/2):81–99. [Google Scholar]
- Cissé G., Erismann S., Shrestha A., Gerold J., Schindler C., Odermatt P., Utzinger J. 2017. Water, Sanitation and Hygiene Integration in School Garden Programs for Nutrition - A Must for Better Child Health. Available online: http://vgts.avrdc.org/download/resources/policy/02-VGtS-PB-02-SwissTPH-05.2017. pdf[Accessed] [Google Scholar]
- Cissé G., Menezes J.A., Confalonieri U. In: The Adaptation Gap Report 2018. UNEP, editor. United Nations Environment Programme (UNEP); Nairobi, Kenya: 2018. Climate-sensitive infectious diseases; pp. 49–59. [Google Scholar]
- Confalonieri U.E., Menezes J.A., Margonari de Souza C. Climate change and adaptation of the health sector: the case of infectious diseases. Virulence. 2015;6(6):554–557. doi: 10.1080/21505594.2015.1023985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A., Abbas M., Allen A., Ball S., Bell S., Bellamy R., Friel S., Groce N., Johnson A., Kett M., Lee M., Levy C., Maslin M., McCoy D., McGuire B., Montgomery H., Napier D., Pagel C., Patel J., de Oliveira J.A., Redclift N., Rees H., Rogger D., Scott J., Stephenson J., Twigg J., Wolff J., Patterson C. Managing the health effects of climate change: lancet and university college london institute for global health commission. Lancet. 2009;373(9676):1693–1733. doi: 10.1016/S0140-6736(09)60935-1. [DOI] [PubMed] [Google Scholar]
- David J.M., Ravel A., Nesbitt A., Pintar K., Pollari F. Assessing multiple foodborne, waterborne and environmental exposures of healthy people to potential enteric pathogen sources: effect of age, gender, season, and recall period. Epidemiol. Infect. 2014;142(1):28–39. doi: 10.1017/S0950268813000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJarnett N., Robb K., Castellanos I., Dettman L., Patel S.S. The american public health association’s 2017 year of climate change and health: time for action. Am. J. Public Health. 2017:e1–e2. doi: 10.2105/AJPH.2017.304168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S., Fisher D. Climate change and infectious diseases: the next 50 years. Ann. Acad. Med. Singapore. 2018;47(10):401–404. [PubMed] [Google Scholar]
- Devleesschauwer B., Haagsma J.A., Angulo F.J., Bellinger D.C., Cole D., Dopfer D., Fazil A., Fevre E.M., Gibb H.J., Hald T., Kirk M.D., Lake R.J., Maertens de Noordhout C., Mathers C.D., McDonald S.A., Pires S.M., Speybroeck N., Thomas M.K., Torgerson P.R., Wu F., Havelaar A.H., Praet N. Methodological framework for world health organization estimates of the global burden of foodborne disease. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0142498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duedu K.O., Karikari Y.A., Attah S.K., Ayeh-Kumi P.F. Prevalence of intestinal parasites among patients of a Ghanaian psychiatry hospital. BMC Res. Notes. 2015;8:651. doi: 10.1186/s13104-015-1634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erismann S., Diagbouga S., Odermatt P., Knoblauch A.M., Gerold J., Shrestha A., Grissoum T., Kabore A., Schindler C., Utzinger J., Cisse G. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasit. Vectors. 2016;9(1):554. doi: 10.1186/s13071-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erismann S., Diagbouga S., Schindler C., Odermatt P., Knoblauch A.M., Gerold J., Leuenberger A., Shrestha A., Tarnagda G., Utzinger J., Cisse G. School children’s intestinal parasite and nutritional status one year after complementary school garden, nutrition, water, sanitation, and hygiene interventions in Burkina Faso. Am. J. Trop. Med. Hyg. 2017;97(3):904–913. doi: 10.4269/ajtmh.16-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOPH . 2018. Current Outbreaks and Epidemics.https://www.bag.admin.ch/bag/en/home/themen/mensch-gesundheit/uebertragbare-krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien.html Available online: [Accessed] [Google Scholar]
- Ford T.E., Hamner S. A perspective on the global pandemic of waterborne disease. Microb. Ecol. 2015;76:2–8. doi: 10.1007/s00248-015-0629-0. [DOI] [PubMed] [Google Scholar]
- Ford L., Miller M., Cawthorne A., Fearnley E., Kirk M. Approaches to the surveillance of foodborne disease: a review of the evidence. Foodborne Pathog. Dis. 2015;12(12):927–936. doi: 10.1089/fpd.2015.2013. [DOI] [PubMed] [Google Scholar]
- Gibb H., Devleesschauwer B., Bolger P.M., Wu F., Ezendam J., Cliff J., Zeilmaker M., Verger P., Pitt J., Baines J., Adegoke G., Afshari R., Liu Y., Bokkers B., van Loveren H., Mengelers M., Brandon E., Havelaar A.H., Bellinger D. World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: a data synthesis. F1000Res. 2015;4:1393. doi: 10.12688/f1000research.7340.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney K.B., O’Toole J., Sinclair M., Leder K. Burden of disease attributed to waterborne transmission of selected enteric pathogens, Australia, 2010. Am. J. Trop. Med. Hyg. 2017;96(6):1400–1403. doi: 10.4269/ajtmh.16-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald T., Aspinall W., Devleesschauwer B., Cooke R., Corrigan T., Havelaar A.H., Gibb H.J., Torgerson P.R., Kirk M.D., Angulo F.J., Lake R.J., Speybroeck N., Hoffmann S. World health organization estimates of the relative contributions of food to the burden of disease due to selected foodborne hazards: a structured expert elicitation. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., Speybroeck N., Cawthorne A., Mathers C., Stein C., Angulo F.J., Devleesschauwer B., World Health Organization Foodborne Disease Burden Epidemiology Reference, G World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Devleesschauwer B., Aspinall W., Cooke R., Corrigan T., Havelaar A., Angulo F., Gibb H., Kirk M., Lake R., Speybroeck N., Torgerson P., Hald T. Attribution of global foodborne disease to specific foods: findings from a World Health Organization structured expert elicitation. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0183641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . 2018. Special Report on the Impacts of a Global Warming of 1.5 ºC.https://www.ipcc.ch/sr15/ Available online: [Accessed] [Google Scholar]
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Dopfer D., Fazil A., Fischer-Walker C.L., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Havelaar A.H., Angulo F.J. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake I.R., Barker G.C. Climate change, foodborne pathogens and illness in higher-income countries. Curr. Environ. Health Rep. 2018;5(1):187–196. doi: 10.1007/s40572-018-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc H., Schwartzbrod L., Dei-Cas E. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2002;28(4):371–409. doi: 10.1080/1040-840291046768. [DOI] [PubMed] [Google Scholar]
- Levy K., Smith S.M., Carlton E.J. Climate change impacts on waterborne diseases: moving toward designing interventions. Curr. Environ. Health Rep. 2018;5(2):272–282. doi: 10.1007/s40572-018-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives. Environ. Int. 2017;103:99–108. doi: 10.1016/j.envint.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Ligon G., Bartram J. Literature review of associations among attributes of reported drinking water disease outbreaks. Int. J. Environ. Res. Public Health. 2016;13(6) doi: 10.3390/ijerph13060527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J. Globalization, climate change, and human health. N. Engl. J. Med. 2013;369(1):96. doi: 10.1056/NEJMc1305749. [DOI] [PubMed] [Google Scholar]
- Murphy H.M., Pintar K.D., McBean E.A., Thomas M.K. A systematic review of waterborne disease burden methodologies from developed countries. J. Water Health. 2014;12(4):634–655. doi: 10.2166/wh.2014.049. [DOI] [PubMed] [Google Scholar]
- Park M.S., Kim Y.S., Lee S.H., Kim S.H., Park K.H., Bahk G.J. Estimating the burden of foodborne disease, South Korea, 2008-2012. Foodborne Pathog. Dis. 2015;12(3):207–213. doi: 10.1089/fpd.2014.1858. [DOI] [PubMed] [Google Scholar]
- Pons W., Young I., Truong J., Jones-Bitton A., McEwen S., Pintar K., Papadopoulos A. A systematic review of waterborne disease outbreaks associated with small non-community drinking water systems in Canada and the United States. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijven J., Bouwknegt M., de Roda Husman A.M., Rutjes S., Sudre B., Suk J.E., Semenza J.C. A decision support tool to compare waterborne and foodborne infection and/or illness risks associated with climate change. Risk Anal. 2013;33(12):2154–2167. doi: 10.1111/risa.12077. [DOI] [PubMed] [Google Scholar]
- Sherpa A.M., Koottatep T., Zurbrügg C., Cissé G. Vulnerability and adaptability of sanitation systems to climate change. J. Water Clim. Chang. 2014;05.4:487–495. [Google Scholar]
- Shrestha A., Sharma S., Gerold J., Erismann S., Sagar S., Koju R., Schindler C., Odermatt P., Utzinger J., Cisse G. Water quality, sanitation, and hygiene conditions in schools and households in Dolakha and Ramechhap Districts, Nepal: results from A cross-sectional survey. Int. J. Environ. Res. Public Health. 2017;14(1) doi: 10.3390/ijerph14010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall A., Mason S.J., van Aalst M., Suarez P., Ait-Chellouche Y., Diallo A.A., Braman L. Using seasonal climate forecasts to guide disaster management: the Red Cross experience during the 2008 West Africa floods. Int. J. Geophys. 2012:12. [Google Scholar]
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F., Rokni M.B., Zhou X.N., Fevre E.M., Sripa B., Gargouri N., Furst T., Budke C.M., Carabin H., Kirk M.D., Angulo F.J., Havelaar A., de Silva N. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP . United Nations Environment Programme (UNEP); Nairobi, Kenya: 2018. The Adaptation Gap Report. [Google Scholar]
- USAID . 2018. Climate Change Risk Profile – West Africa Sahel.https://www.climatelinks.org/sites/default/files/asset/document/2017%20April_USAID%20ATLAS_Climate%20Change%20Risk%20Profile%20-%20Sahel.pdf Available online: [Accessed] [Google Scholar]
- Vegetables Go to School Project . 2018. Vegetables Go to School – Growing Together in School Vegetable Gardens!http://vgts.avrdc.org/ Available online: [Accessed] [Google Scholar]
- Walker J.T. The influence of climate change on waterborne disease and Legionella: a review. Perspect. Public Health. 2018;138(5):282–286. doi: 10.1177/1757913918791198. [DOI] [PubMed] [Google Scholar]
- WHO . 2003. Climate Change and Infectious Diseases.https://www.who.int/globalchange/climate/en/chapter6.pdf Available online: [Accessed] [Google Scholar]
- WHO . 2014. Environmental Health Challenges in Mauritania.http://www.who.int/features/2013/mauritania_environmental_health/en/ Available online: [Accessed] [Google Scholar]
- WHO . Geneva; 2014. Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2015. Operational Framework for Building Climate Resilient Health Systems. [Google Scholar]
- WHO . 2015. Waterborne Diseases – MDGs-SDGs2015.https://www.who.int/gho/publications/mdgs-sdgs/MDGs-SDGs2015_chapter5_snapshot_waterborne_diseases.pdf?ua=1 Available online: [Accessed] [Google Scholar]
- WHO . 2015. WHO Estimates of the Global Burden of Foodborne Diseases.http://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/ Available online: [Accessed] [Google Scholar]
- WHO . 2016. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks. [Google Scholar]
- WHO . 2017. Inheriting a Sustainable World? Atlas on Children’s Health and the Environment.http://apps.who.int/iris/bitstream/10665/254677/1/9789241511773-eng.pdf Available online: [Accessed] [Google Scholar]
- WHO . 2018. Foodborne Diseases in WHO African Region – Fact Sheet.http://www.who.int/foodsafety/areas_work/foodborne-diseases/infographics_afro_en.pdf Available online: [Accessed] [Google Scholar]