Highlights
-
•
Persistent rhinovirus infections are associated with impaired cellular immunity.
-
•
The persistence of HRV infection is due to unrestricted replication of single virus strains rather than reinfections by different strains.
-
•
The reconstitution of cell-mediated immunity might be crucial for complete virus clearance.
Keywords: Hematopoietic stem cell transplant recipients, HRV persistent infection, Cellular immunity, Immunocompromised pediatric patients
Abstract
Background
HRV infections are generally self-limiting in healthy subjects, whereas in immunocompromised hosts HRV infections can lead to severe complications and persistent infections. The persistence of HRV shedding could be due to the inefficient immunological control of a single infectious episode.
Objectives
To investigate the clinical, virologic and immunologic characteristics of pediatric HSCT recipients with HRV-PI infection.
Study design
During the period 2006–2012, eight hematopoietic stem cell transplant (HSCT) recipients presented with persistent rhinovirus infection (HRV-PI, ≥30 days). Viral load and T-CD4+, T-CD8+, B and NK lymphocyte counts at the onset of infection were compared with those of fourteen HSCT recipients with acute HRV infection (HRV-AI, ≤15 days).
Results
The median duration of HRV positivity in patients with HRV-PI was 61 days (range 30–174 days) and phylogenetic analysis showed the persistence of a single HRV type in all patients (100%). In HSCT recipients with HRV-PI, T-CD4+, T-CD8+ and NK cell counts at the onset of infection were significantly lower than those observed in recipients with HRV-AI (p < 0.01), while B cell counts were similar in the two groups (p = 0.25). A decrease in HRV load was associated with a significant increase in T-CD4+, T-CD8+and NK lymphocyte counts in HRV-PI patients (p < 0.01).
Conclusions
This study suggests a role for cellular immunity in HRV clearance and highlights the importance of its recovery for the control of HRV infection in HSCT recipients.
1. Background
Human rhinoviruses (HRVs) have been previously classified into two species, HRV-A and HRV-B, while a distinct rhinovirus group (HRV-C) was recently identified in patients with acute respiratory infections (ARIs) [1], [2], [3], [4], [5], [6]. HRV-C and HRV-A are more frequently associated with ARIs in hospitalized patients and often co-circulate in near-equivalent proportions, while HRV-Bs are often under-represented [4], [6], [7].
Even though ARIs caused by HRVs are generally self-limiting in healthy subjects, in hematopoietic stem cell transplant (HSCT) recipients [8], [9] and patients with hematological diseases [10] HRV infection may lead to severe complications such as pneumonia [9], [11], [12]. In addition, in HSCT [13], [14], [15] and lung transplant recipients, persistent HRV infections have been reported [16]. The persistence of HRV shedding could be due to the inefficient immunological control of a single infectious episode [16], [17].
Schiffer et al. reported that respiratory virus infections are more frequent and severe in the first 100 days following transplant in patients receiving myeloablative vs non-myeloablative HSCT, despite an overall similar infection rate [18]. These data would suggest an important role for T-cell immunity in the control of HRV infection. However, correlates of severe or persistent HRV infections (HRV-PI) after HSCT are still largely obscure.
2. Objectives
This study investigated the clinical, virologic and immunologic characteristics of pediatric HSCT recipients with HRV-PI infection.
3. Study design
3.1. Patients and samples
From January 2006 through December 2012, 1687 respiratory samples (936 nasal swabs, 713 nasopharyngeal aspirates, 25 bronchoalveolar lavages and 13 pharyngeal swabs) from 536 pediatric HSCT recipients with acute respiratory syndromes admitted to the Pediatric Hematology–Oncology Unit, Fondazione IRCCS Policlinico San Matteo were collected both at admission and discharge. Follow-up samples were prospectively collected 7–10 days apart from positive patients to monitor viral load until the clearance of HRV or hospital discharge. In the latter case, follow-up samples were collected at subsequent outpatient clinical evaluations. Respiratory samples were routinely tested using a panel of RT-PCR and real-rime RT-PCR assays [13], [19] for human influenza virus types A and B, human metapneumovirus A and B, human respiratory syncytial virus A and B, four human coronaviruses (OC43, 229E, NL63, HKU1), human parainfluenza types 3, human adenovirus, rhinoviruses and enteroviruses.
A further characterization of HRV infection was retrospectively performed on the samples collected prospectively for acute respiratory infection screening. HRV-PI was defined as HRV shedding ≥30 days, and all patients fulfilling the criteria were included in the study. A cohort of pediatric HSCT recipients with HRV-AI (defined as virus shedding ≤15 days), matched for clinical characteristics, was selected as a control group for virologic and immunologic analysis. This retrospective study was performed according to the guidelines of the Institutional Review Board on the use of biological specimens for scientific purposes in keeping with Italian law (art.13 D.Lgs 196/2003).
3.2. Definition of clinical respiratory syndromes
Patients with rhinitis, pharyngitis, and laryngitis were classified as upper respiratory tract infection (URTI), while patients with bronchitis, bronchiolitis, and pneumonia (characterized by cough, wheezing, and/or dyspnea as well as suggestive X-ray findings) were classified as affected by lower RTI (LRTI).
3.3. Rhinovirus quantification and typing
Following RNA extraction (Nuclisens® easy MAG™ automatic extractor; BioMérieux, Lyon, France), HRV RNA was quantified by real-time RT-PCR [20]. Results were reported as HRV RNA copies/ml respiratory sample. HRV molecular typing was performed as reported [21] by sequencing a 549 nt fragment spanning part of the 5′ NCR and the VP2 gene (nt 534–1083) or using an alternative protocol that amplifies the same genomic region with different primer sets (nt 547–1087) [6]. Sequencing reactions were carried out using primer sets utilized for amplification with the BigDye Terminator Cycle-Sequencing kit (Applied Biosystems, Foster City, USA) and run in an ABI Prism 3100 DNA sequencer (Applied Biosystems, Foster City, USA). Sequences were assembled using the Sequencher software, version 4.6 (Gene Codes Corporation, Ann Arbor, USA).
3.4. Phylogenetic analysis
Nucleotide sequences were aligned using the ClustalW method and a phylogenetic tree was constructed using the neighbor-joining method and the kimura-2-parameter for simultaneously estimating distance among sequences with MEGA software (version 5.05) [22]. Bootstrap values included 1000 replicates. HRV type assignment was defined by the nearest HRV reference strains observed in the phylogenetic tree. The nucleotide sequences used in this study were deposited into GenBank under accession numbers KJ603464–KJ603520.
3.5. Immunological data
Serial T-CD4+, T-CD8+, B and NK lymphocyte counts were routinely performed in HSCT recipients (both with HRV-PI and HRV-AI), before and after transplant, while patients with hematological malignancies (HMs) not undergoing transplant were not routinely tested. T-Lymphocytes were stained with anti-CD45 PerCP-Cy5.5, anti-CD3 APC, anti-CD8 PE, anti-CD4 FITC monoclonal antibodies (mAbs); B lymphocytes were stained with anti-CD19 and anti-CD20 mAbs while NK lymphocytes, defined as CD3-/CD56+ cells, were stained with anti-CD56 and anti-CD3 mAbs (all mAbs were from Becton Dickinson, Mountain View, CA). Appropriate isotype-matched controls were included. Cytofluorimetric analysis was performed by direct immunofluorescence on a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA).
3.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software, La Jolla, CA). The Mann–Whitney U test was used to compare T-CD4+, T-CD8+, B and NK cell counts and viral load for different sampling times and patient categories. P values <0.05 were considered statistically significant.
4. Results
4.1. Patient characteristics
From January 2006 through December 2012, at least one episode of HRV infection was observed in 311/536 [58.0%] patients. HRV-PI was identified in 8/536 [1.5%] pediatric patients with a median age of five years (range, 1–11 years) (Table 1 ). Of these, five [62.5%] were recipients of an HSCT from a matched-unrelated donor (MUD) and three [37.5%] recipients of a haploidentical T-cell depleted HSCT from a partially matched family donor (PMFD). The median age of HSCT recipients with HRV-AI was four years (range, 1–14 years) (Table 1). Five patients [35.7%] underwent HSCT from a MUD, four [28.6%] received an HSCT from a matched family donor (MFD) and five [35.7%] received a haploidentical HSCT from a PMFD (after T-cell depletion in 3 case).
Table 1.
Characteristics of the pediatric HSCT recipients with HRV infection.
| Patient category | Patient ID | Gender/age (years) | Underlying disease | Type of transplant | Conditioning regimen |
Time post-HSCT (days) |
HRV duration (days) | Nearest HRV type |
Presentation |
|---|---|---|---|---|---|---|---|---|---|
| Patients with HRV-PI (n = 8) | #1 | M/6 | AML | PMFD + TCD | BU + CY + MEL | −8 | 106 | A18 | URTI |
| #2 | F/2 | ALL | MUD | BU + CY + MEL | −14 | 35 | A54 | URTI | |
| #3 | M/3 | AML | PMFD + TCD | BU + CY + MEL | 65 | 174 | C23 | URTI | |
| #4 | M/11 | ALL | MUD | BU + CY + MEL | 14 | 31 | A32 | URTI | |
| #7 | M/1 | SCID | MUD | THIO + TREO | −36 | 135 | C16 | URTI | |
| #8 | F/7 | JMML | MUD | BU + CY + MEL | 19 | 52 | A102 | URTI | |
| #9 | M/4 | ALL | PMFD + TCD | TBI + THIO + FLU | −13 | 30 | A34 | URTI | |
| #11 | M/7 | ALL | MUD | TBI + THIO + CY | −24 | 70 | B97 | URTI | |
| Patients with HRV-AI (n = 14) | #12 | F/1 | ALL | MFD | BU + CY + MEL | 272 | 10 | C19 | URTI |
| #13 | M/2 | Thal Major | MUD | THIO + TREO | 97 | 8 | A94 | URTI | |
| #14 | M/4 | Thal Major | MFD | THIO + TREO | 52 | 13 | B100 | URTI | |
| #15 | F/12 | AML | MUD | THIO + TREO | 47 | 10 | C15 | LRTI | |
| #16 | F/14 | LH | PMFD | THIO + TREO | 33 | 10 | C42 | URTI | |
| #17 | M/11 | HLH | MUD | BU + THIO + FLU | 60 | 9 | C | URTI | |
| #18 | M/2 | HLH | MUD | BU + THIO + FLU | 136 | 10 | A88 | URTI | |
| #19 | F/7 | ALL | MFD | TBI + THIO + CY | 90 | 7 | B69 | LRTI | |
| #20 | M/5 | ALL | PMFD + TCD | TBI + THIO + MEL | 56 | 12 | C16 | URTI | |
| #21 | F/3 | ALL | PMFD + TCD | TBI + THIO + FLU | 0 | 6 | A38 | URTI | |
| #22 | M/4 | CML | MUD | BU + THIO + FLU | 91 | 9 | C36 | URTI | |
| #23 | F/2 | ALL | PMFD + TCD | BU + THIO + FLU | 88 | 5 | A59 | URTI | |
| #24 | F/14 | LH | PFMD | THIO + TREO + FLU | 33 | 10 | B42 | URTI | |
| #25 | M/2 | SCA | MFD | THIO + TREO + FLU | 8 | 6 | C25 | URTI |
HRV-PI, rhinovirus persistent infection; HRV-AI, rhinovirus acute infection; mos, months; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; JMML, juvenile myelomonocytic leukemia; SCID, severe combined immunodeficiency; Thal Major, thalassemia major; LH, Hodgkin’s disease; HLH, hemophagocytic lymphohistiocytosis; SCA, sickle cell anemia; PMFD, partially matched family donor (haploidentical); MUD, matched unrelated donor; MFD, matched family donor; TCD, T-cell depletion; BU, busulfan; CY, cyclophosphamide; MEL, melphalan; THIO, thiotepa; TREO, treosulfan; TBI, total body irradiation; FLU, fludarabin; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection
4.2. Phylogenetic analysis
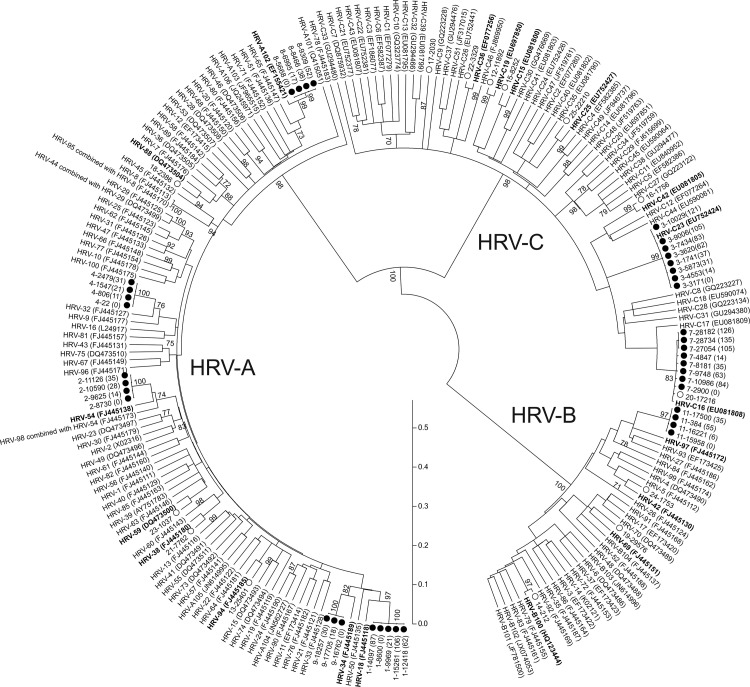
In all HRV-PI patients (100.0%) a single HRV type was present during the entire shedding period (Fig. 1 ). Overall, eight different HRV types were observed (Table 1). Of these, five [62.5%] were HRV-A, two [25.0%] were HRV-C and one [12.5%] was HRV-B. In the control group, fourteen different HRV types were identified: seven [50.0%] were HRV-C, four [28.6%] were HRV-A, and three [21.4%] were HRV-B (Table 1).
Fig. 1.
Phylogenetic tree based on the VP4/VP2nucleotide sequences. The sequences from patients with HRV-PI and HRV-AI are respectively reported with black and white circles. The sampling time from the first positive sample is reported in brackets for each patient sequence series. The HRV reference strains are reported with the accession number. The closest related HRV prototype for each patient series is reported in bold. The scale shows the evolutionary distance.
4.3. HRV infection
Among the eight patients with HRV-PI, the median duration of HRV shedding was 61 days (range 30–174 days), while in the fourteen HRV-AI patients, the median duration of HRV shedding was significantly shorter (10 days, range 5–13 days; p = 0.001). All patients with HRV-PI suffered from URTI, while, among patients with HRV-AI, 12/14 [85.7%] had an URTI and 2/14 [14.3%] had LRTI, documented by imaging. No episodes of progression to LRT were observed in the eight HRV-PI patients with URTI.
In all HSCT recipients with HRV-PI, infection occurred either during the conditioning regimen (#1, #2, #7, #9, #11) or prior to transplant engraftment (#3, #4, #8). In these patients, follow-up viral load, T-CD4+, T-CD8±, B, and NK lymphocyte counts were available. All HSCT recipients received intravenous immunoglobulin (Ig) replacement therapy (400 mg/kg) whenever IgG levels were found below 600 mg/dl after the transplant or at the moment of a positive HRV result.
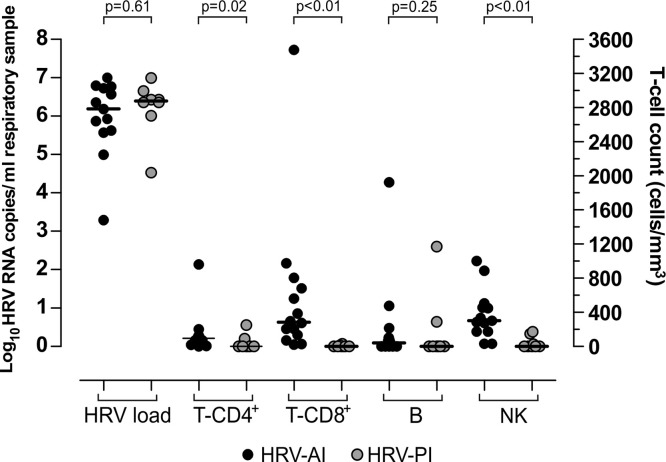
In the eight HSCT recipients with HRV-PI, the onset of HRV infection occurred at a median time of −10.5 days prior to HSCT (range −36 to 65 days); while in the group of patients with HRV-AI, infection occurred at a median of 58 days (range 0–272 days) after HSCT. Viral load as well as T-CD4+, T-CD8+, B and NK cell counts of patients with HRV-PI at the onset of infection were compared with those of patients with HRV-AI (Fig. 2 ). Viral load (median 1.5 × 106 vs 2.5 × 106 RNA copies/ml respiratory sample; p = 0.61) and B cell counts (median 0 vs 40 cells/μl; p = 0.25) were not significantly different in HSCT recipients with HRV-PI vs HRV-AI. On the contrary, the number of T-CD4+ lymphocytes (median T-CD4+ 0 vs 95 cells/μl; p = 0.02) T-CD8+ lymphocytes (median 0 vs 285 cells/μl; p < 0.01) and NK lymphocytes (median 0 vs 301 cells/μl; p < 0.01 was significantly higher in HRV-AI patients.
Fig. 2.
Comparison of HRV load, T-CD4+, T-CD8+, B and NK cell counts at the onset of infection. Comparisons was made between 8 patients with HRV-PI (grey circle) and 14 patients with HRV-AI (black circle).
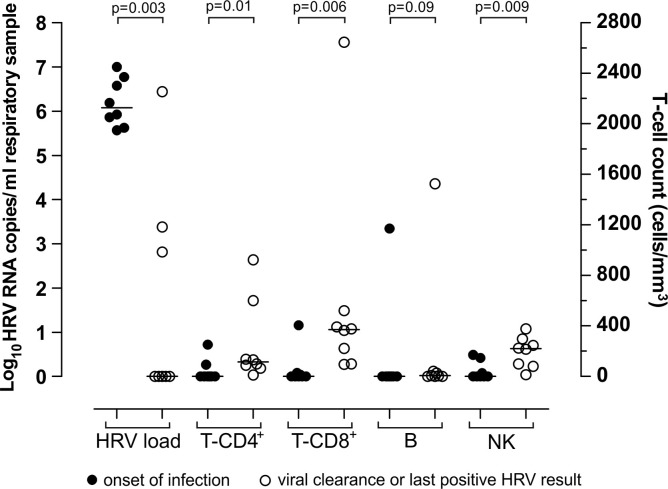
In the eight patients with HRV-PI, the increase in T-CD4+, T-CD8+ and NK cell counts was paralleled by a decrease in HRV load (Fig. 3 ). Of note, in one patient (#7) with persistently low T-CD8+ cell counts (<100 cells/μl), HRV load was still high after 135 days of infection. Among patients with HRV-PI, a significant difference in HRV load (median 1.1 × 106 vs 0 copies/ml respiratory sample; p = 0.003), T-CD4+ (median 0 vs 115.5 cells/μl; p = 0.01) T-CD8+ (median 0 vs 371 cells/μl; p = 0.006) and NK (median 0 vs 220 cells/μl; p = 0.009) cell counts was observed between the onset of infection and the last positive HRV result or viral clearance (Fig. 3). In contrast, no difference in B cell counts (median 0 vs 7 cells/μl; p = 0.09) was observed between the onset of infection and the last positive HRV result or viral clearance (Fig. 3). Notably, at the time of infection resolution in patients with HRV-PI, the number of T-CD4+, T-CD8+ and NK lymphocytes was comparable with that in patients with HRV-AI (T-CD4+ 115 vs 95, T-CD8+ 371 vs 285, NK 220 vs 301 cells/μl; p > 0.05).
Fig. 3.
Comparison of HRV load, T-CD4+, T-CD8+, B and NK cell counts in 8 pediatric HSCT recipients with HRV-PI. Comparisons were made between values at the onset of infection (black circle) and at the time of HRV clearance or the last positive HRV sample (white circle).
5. Discussion
HRV infections are self-limiting in both pediatric and adult immunocompetent individuals, typically lasting <2 weeks [5], [23], [24], [25]. Moreover, sequential HRV infections caused by different HRV genotypes have been described as a common event in healthy subjects [3], [5], [23], [24], [26]. In contrast, HRV-PIs are infrequent events and have been reported only in immunocompromised patients such as lung transplant [14], HSCT recipients [13], [15], or patients with hypogammaglobulinaemia [24], [27]. Interestingly, HRV-PIs were associated with the prolonged shedding of a single HRV genotype suggesting the lack of efficient immunologic control of an initial HRV infection event. In the present retrospective study, HRV-PIs (≥30 days) were observed in eight pediatric HSCT recipients representing a small fraction of all HSCT recipients [1.5%]. In agreement with previous observations [15], [16], [23], [26], also in our study, HRV-PIs were sustained by a single HRV genotype in all patients. The distribution of HRV species in HRV-PI and HRV-AI in this study reflects the worldwide HRV circulation, with HRV-A and HRV-C as the more prevalent HRV species observed [4], [5], [6], [7].
In this and previous reports, where HRV-PIs have all been recorded in immunocompromised patients [13], [15], [16], [28] the role of B- or T-cell immunity in controlling HRV-PI is unclear. Peltola et al. recently documented that intravenous immunoglobulin therapy does not effectively correct the defective clearance of HRV in patients with primary hypogammaglobulinaemia [24]. In accordance, our results demonstrate the presence of HRV-PI in HSCT recipients receiving intravenous immunoglobulin replacement therapy, thus suggesting that impaired T cell-mediated immunity may have contributed to delayed HRV clearance. Similarly, Gooskens et al. reported that impaired T cell-mediated immunity correlated with delayed elimination of the influenza virus in HSCT recipients [29]. In the present study, HSCT recipients with HRV-PI had absent or very low T-CD4+, T-CD8+ and NK lymphocyte counts during induction and before transplant engraftment, moreover T-CD4+, T-CD8+ and NK lymphocyte counts at the time of infection onset were significantly lower than those observed in HSCT recipients with HRV-AI. T-CD4+, T-CD8+ and NK lymphocyte counts were also significantly lower at the onset of infection with respect to the time of HRV clearance in patients with HRV-PI, and inversely correlated with HRV load. These findings also suggest that the peak HRV load at the onset of infection is an independent factor with respect to the immunological status of the patient. Finally, there was no significant difference in the T-CD4+, T-CD8+ and NK lymphocyte counts between HSCT recipients with HRV-AI and those with HRV-PI at the time of infection resolution. This finding suggests that the reconstitution of cell-mediated immunity might be crucial for complete virus clearance. Nevertheless, our study has some limitations, including the absence of virus-specific immunological analyses, the small number of cases studied and the retrospective nature of design. As HRV-PIs are infrequent events, further investigations on large cohorts are needed to clarify the respective role of innate, T cell-mediated and humoral immunity in the clearance of HRV infections.
Although HRV is one of the most frequent viruses detected in severe respiratory syndromes in HSCT recipients [12], [14], many aspects of HRV respiratory infections in patients with hematologic diseases still remain to be defined. In a recent study involving adult HSCT recipients, HRV was recognized as a significant cause of pneumonia [10]. Of note, in this study, an adult patient acquiring HRV infection during pre-transplant conditioning had persistent URTI and HRV positivity (3 months) [10]. This is consistent with our finding that in five HSCT recipients, HRV infection was acquired in the pre-transplant period; these subjects suffered only from mild URTI with no major clinical complications. However, in pediatric HSCT recipients the risk for development of LRTI cannot be excluded.
In conclusion, our data suggest that cellular immunity may play a role in the clearance of HRV infection. In addition, we demonstrated that persistence of HRV infection was due to unrestricted replication of a single virus strain, rather than sequential reinfections by different strains.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
This work was supported by the Ministero della Salute, Fondazione IRCCS Policlinico San Matteo, Ricerca Corrente (grant no. 80622; 8045801; 8069113; 8045814); Ministero della Salute, Ricerca Finalizzata (no. RF-2009-1548666).
Ethical approval
This retrospective study was performed according to the guidelines of the Institutional Review Board on the use of biological specimens for scientific purposes in keeping with Italian law (art.13 D.Lgs 196/2003).
References
- 1.Arden K.E., McErlean P., Nissen M.D., Sloots T.P., Mackay I.M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J. Med. Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kistler A., Avila P.C., Rouskin S., Wang D., Ward T., Yagi S. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau S.K., Yip C.C., Tsoi H.W., Lee R.A., So L.Y., Lau Y.L. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J. Clin. Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piralla A., Baldanti F., Gerna G. Phylogenetic patterns of human respiratory picornavirus species, including the newly identified group C rhinoviruses, during a 1-year surveillance of a hospitalized patient population in Italy. J. Clin. Microbiol. 2011;49:373–376. doi: 10.1128/JCM.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piralla A., Lilleri D., Sarasini A., Marchi A., Zecca M., Stronati M. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008–2009. Diagn. Microbiol. Infect. Dis. 2012;73:162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Wisdom A., Leitch E.C., Gaunt E., Harvala H., Simmonds P. Screening respiratory samples for detection of human rhinoviruses (HRVs) and enteroviruses: comprehensive VP4–VP2 typing reveals high incidence and genetic diversity of HRV species C. J. Clin. Microbiol. 2009;47:3958–3967. doi: 10.1128/JCM.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E.K., Williams J.V., Gebretsadik T., Carroll K.N., Dupont W.D., Mohamed Y.A. Host and viral factors associated with severity of human rhinovirus-associated infant respiratory tract illness. J. Allergy Clin. Immunol. 2011;127:883–891. doi: 10.1016/j.jaci.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S., Champlin R., Couch R., Englund J., Raad I., Malik S. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin. Infect. Dis. 1999;29:528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 9.Ison M.G., Hayden F.G., Kaiser L., Corey L., Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin. Infect. Dis. 2003;36:1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs S.E., Soave R., Shore T.B., Satlin M.J., Schuetz A.N., Magro C. Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2013;15:474–486. doi: 10.1111/tid.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parody R., Rabella N., Martino R., Otegui M., del Cuerpo M., Coll P. Upper and lower respiratory tract infections by human enterovirus and rhinovirus in adult patients with hematological malignancies. Am. J. Hematol. 2007;82:807–811. doi: 10.1002/ajh.20974. [DOI] [PubMed] [Google Scholar]
- 12.Garbino J., Soccal P.M., Aubert J.D., Rochat T., Meylan P., Thomas Y. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax. 2009;64:399–404. doi: 10.1136/thx.2008.105155. [DOI] [PubMed] [Google Scholar]
- 13.Gerna G., Piralla A., Rovida F., Rognoni V., Marchi A., Locatelli F. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J. Med. Virol. 2009;81:1498–1507. doi: 10.1002/jmv.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano F., Campbell A.P., Guthrie K.A., Kuypers J., Englund J.A., Corey L. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak A.K., Adams R.H., Shah N.C., Gustin K.E. Persistent human rhinovirus type C infection of the lower respiratory tract in a pediatric cord blood transplant recipient. Bone Marrow Transplant. 2013;48:747–748. doi: 10.1038/bmt.2012.226. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser L., Aubert J.D., Pache J.C., Deffernez C., Rochat T., Garbino J. Chronic rhinoviral infection in lung transplant recipients. Am. J. Respir. Crit. Care Med. 2006;174:1392–1399. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 17.Malcolm E., Arruda E., Hayden F.G., Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J. Clin. Virol. 2001;21:9–16. doi: 10.1016/s1386-6532(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 18.Schiffer J.T., Kirby K., Sandmaier B., Storb R., Corey L., Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica. 2009;94:1101–1108. doi: 10.3324/haematol.2008.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piralla A., Lunghi G., Percivalle E., Viganò C., Nasta T., Pugni L. FilmArray® respiratory panel performance in respiratory samples from neonatal care units. Diagn. Microbiol. Infect. Dis. 2014;79:183–186. doi: 10.1016/j.diagmicrobio.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Holloway B., Dare R.K., Kuypers J., Yagi S., Williams J.V. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J. Clin. Microbiol. 2008;46:533–539. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savolainen-Kopra C., Blomqvist S., Kaijalainen S., Jounio U., Juvonen R., Peitso A. All known human rhinovirus species are present in sputum specimens of military recruits during respiratory infection. Viruses. 2009;1:1178–1189. doi: 10.3390/v1031178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jartti T., Lee W.M., Pappas T., Evans M., Lemanske R.F., Jr., Gern J.E. Serial viral infections in infants with recurrent respiratory illnesses. Eur. Respir. J. 2008;32:314–320. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltola V., Waris M., Kainulainen L., Kero J., Ruuskanen O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin. Microbiol. Infect. 2013;19:E322–E327. doi: 10.1111/1469-0691.12193. [DOI] [PubMed] [Google Scholar]
- 25.Corne J.M., Marshall C., Smith S., Schreiber J., Sanderson G., Holgate S.T. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 26.Zlateva K.T., de Vries J.J., Coenjaerts F.E., van Loon A.M., Verheij T., Little P. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur. Respir. J. 2014;44:169–177. doi: 10.1183/09031936.00172113. [DOI] [PubMed] [Google Scholar]
- 27.Kainulainen L., Vuorinen T., Rantakokko-Jalava K., Osterback R., Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J. Allergy Clin. Immunol. 2010;126:120–126. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tapparel C., Cordey S., Junier T., Farinelli L., Van Belle S., Soccal P.M. Rhinovirus genome variation during chronic upper and lower respiratory tract infections. PLoS One. 2011;6:e21163. doi: 10.1371/journal.pone.0021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gooskens J., Jonges M., Claas E.C., Meijer A., Kroes A.C. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J. Infect. Dis. 2009;199:1435–1441. doi: 10.1086/598684. [DOI] [PubMed] [Google Scholar]