Highlights
-
•
Respiratory virus infections are the single greatest precipitants of asthma exacerbations.
-
•
Current treatment options for AE are limited and have developed little in recent years.
-
•
Development of effective anti-viral treatments remains a key target for therapeutic intervention.
-
•
Approaches include therapies that either target the virus or boost host response to the virus.
-
•
New clinical studies are needed to further our understanding of the mechanisms of virus induced asthma exacerbation.
Abstract
Asthma is the most common chronic respiratory disease and its prevalence is on the increase. Respiratory viral infections in early life have been suggested to increase the risk of development of asthma in later life and virus infection remains the single greatest precipitant of asthma exacerbations. The development of effective anti-viral treatments remains a key target for therapeutic intervention. Here we discuss the role of respiratory viral infection in asthma exacerbation and highlight current and potential anti-viral agents and their mechanisms of action.
Current Opinion in Pharmacology 2013, 13:331–336
This review comes from a themed issue on Respiratory
Edited by Alastair G Stewart
For a complete overview see the Issue and the Editorial
Available online 9th May 2013
1471-4892/$ – see front matter, © 2013 The Authors. Published by Elsevier Ltd. All rights reserved.
Introduction
Asthma is a heterogeneous airway disease characterised by airway inflammation, airway hyperreactivity, reversible bronchoconstriction and airway remodelling. Patients experience shortness of breath, fluctuations in normal breathing patterns, and also periodic episodes of wheeze and cough. Asthma is treated with inhaled corticosteroids, with and without other therapies including short or long acting bronchodilators. Asthma exacerbations (AEs) are the major cause of morbidity, mortality and healthcare costs associated with asthma [1, 2•], and are generally defined as worsening of the above symptoms accompanied by a drop in lung function prompting a GP consultation or visit to the emergency room. In extreme cases, AE can require oral corticosteroid therapy, supplemental oxygen and may result in death. Respiratory virus infections account for at least 80% of exacerbations in adults and children [3, 4, 5, 6] and among respiratory viruses human rhinoviruses (RVs) are by far the most common viruses associated [3, 6, 7].
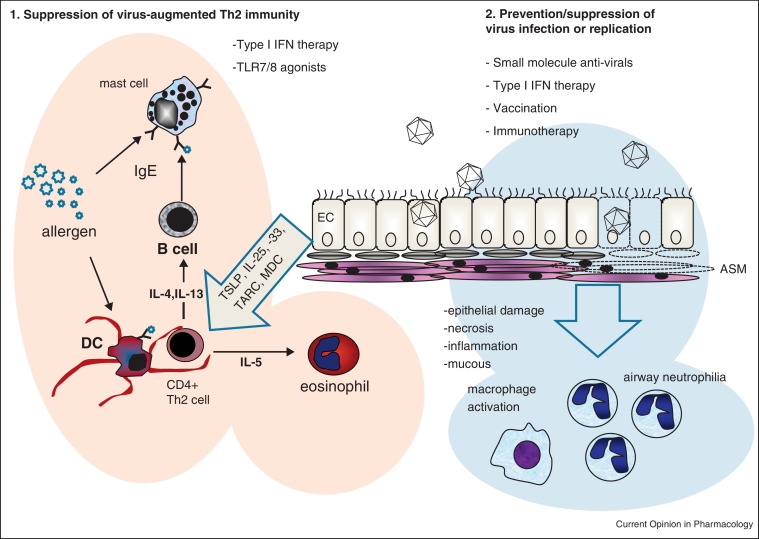
The importance of respiratory viruses as triggers of AE has therefore made them a target for therapeutic intervention. In this review we discuss the potential of two therapeutic approaches, one targeting host factors that may induce natural anti-viral immunity, such as the addition of an anti-viral cytokine, manipulation of the host's immune response such as administration of a vaccine, and secondly targeting the virus itself; including small molecule inhibitors of virus replication, and virus specific immunotherapy. These approaches are summarised in Figure 1 . Because of the overwhelmingly important role viruses play in AE, we argue that now is the time to carefully re-consider anti-viral interventions for AE.
Figure 1.
Anti-viral approaches may (1) prevent viruses acting in a synergistic or additive manner with allergens, and cause immune deviation to a Th1 rather than Th2 immune response. Anti-viral approaches may be (2) agents that prevent virus infection or replication at mucosal surfaces and prevent epithelial damage, inflammation, mucous production, activation of macrophages and attraction of neutrophils into the lung, which promote further inflammation and damage.
Respiratory viruses are potent exacerbators of asthma
Respiratory virus infections are triggers of AE. Viruses such as RVs, respiratory syncytial virus (RSV), seasonal influenza A viruses, metapneumoviruses, coronaviruses and bocaviruses may all trigger AE in adults and children. Atypical bacteria Mycoplasma pneumoniae (M. pneumoniae) and Chlamydophila pneumoniae (C. pneumoniae) are also common respiratory pathogens associated with AEs in both adults and children [8, 9, 10]. The major viruses associated with AEs are RVs, accounting for approximately 60% of all AE in all ages [6, 7]. RVs are members of the Picornaviridae, and are positive sense ssRNA viruses with genomes of 7.1–7.5 kb and can be divided into major and minor groups based on receptor utilisation. Major group RVs bind ICAM-1 while minor group RVs bind the LDL receptor. RVs may also be classified based on nucleotide sequence identity (RV-A, RV-B, RV-C). The RV-C group [11••, 12] have unique sequences at the ICAM-1 and LDL receptor binding sites, suggesting they use a unique, currently unknown receptor [13••]. RVs represent a diverse group of viruses with 100 serotypes known and an estimated further ∼60 or so group C viruses. RV-C may cause more severe AEs, although how this occurs is currently unknown [12]. In the northern hemisphere, RV infection precipitates an increase in emergency room admissions due to AEs [14], known as the ‘asthma epidemic.’ This occurs in the third week of September, after children return to school, highlighting that school age children are vectors for RV infection and their crucial role in AEs [7]. Major and minor group RV mouse models of RV infection [15••, 16] and RV induced exacerbations of airway allergen challenge [15••, 17] have also recently been developed. These animal studies mirror the human data gathered to date and support the idea that RV infection augments airways inflammation caused by allergen sensitisation and challenge, providing further evidence that respiratory viruses such as RV exacerbate asthma.
Targeting host factors
Type I IFN therapy
Recent studies have reported that impaired innate immunity to virus infections is important in the pathogenesis of AEs. Reduced capacity to induce type I interferons (IFNs) IFN-α, IFN-β or type III IFNs, the IFN-λs upon challenge with respiratory viruses or the dsRNA mimetic polyIC in bronchial epithelial cells (BECs), bronchoalveolar lavage (BAL) macrophages, dendritic cells (DCs) and peripheral blood mononuclear cells (PBMCs) from persons with asthma have recently been described [18•, 19, 20••, 21, 22•, 23, 24••, 25••, 26••]. Importantly, deficient IFN-λ was also strongly related to virus load, and AE pathogenesis and severity in vivo [22•]. The mechanism responsible for impaired IFN-α, IFN-β and IFN-λ remains poorly understood. However, the above studies advocate a role for IFN therapy in AE. Recently, a phase II placebo controlled trial of inhaled IFN-β in poorly controlled adult atopic asthmatics was performed [27]. Inhaled IFN-β, started at the reporting of a clinical cold, showed promise, reducing rates of AE in this group and increasing lung function. Virus load was studied in only a few patients, and showed trends for lower virus loads in treated patients. Therefore, inhaled IFN-β improves AE rates and associated symptoms, most likely due to a direct anti-viral activity. It is also possible that IFN-β could modulate additional processes, such as acting as an antagonist of Th2 immunity. Recent studies have shown that IFN-β and type III IFN-λ have potent Th2 antagonistic activity [28, 29•], suggesting that inhaled IFN-β may deliver a benefit on two levels in atopic asthma, firstly reducing virus replication and hence virus driven inflammation, and secondly dampening the Th2 responses to allergens. Further studies with inhaled IFN-β in AE are eagerly anticipated.
Macrolide antibiotics
Macrolides have been shown to have anti-inflammatory [30], bactericidal [31] and recently anti-viral activity [32•]. The keto-macrolide telithromycin had previously shown efficacy in AE in adult asthmatics in a phase IV clinical trial [10]. The mechanism responsible for this beneficial effect is unknown, and telithromycin could have been merely acting as an anti-inflammatory agent. In cell based assays, azithromycin, but not the closely related erythromycin or telithromycin, was shown to have anti-viral activity to RV by inducing IFN and interferon stimulated genes (ISGs) [32•]. This was a previously unknown property of azithromycin, and has since promoted the further investigation of azithromycin in phase IV clinical trials in AE which are currently ongoing.
Toll like receptor (TLR) 7/8 agonists
TLR7/8 recognise synthetic and virus encoded ssRNA and small analogues of nucleic acids including imiquimod, R848 and their derivatives. TLR7/8 are expressed on cells of myeloid or lymphoid origin including macrophages and DCs. While viruses certainly induce inflammation via recognition of ssRNA, the addition of TLR7/8 agonists in mouse models of allergen sensitisation and challenge [33, 34•] show a suppression of allergic airway inflammation. Why TLR7/8 agonists are protective in these models is not completely understood; however, it is generally accepted that TLR7/8 ligation may be useful in treating allergic diseases such as asthma. For example, recent studies of allergic airways inflammation following sensitisation and challenge to ovalbumin showed that the TLR7 agonist R848 was protective [33, 34•], reducing eosinophilic inflammation, lung function impairment and ovalbumin-specific Th2 T cell responses. Importantly, the effects of R848 were not observed in TLR7 deficient mice, showing that R848 acts as a TLR7 agonist and the protective effect is via TLR7 signalling [33].
Vaccines
The number of viruses implicated in the aetiology of asthma and their associated antigenic diversity has thus far limited development of effective vaccines.
There are a number of potential RSV vaccine candidates although none are currently licensed for use [35, 36••, 37, 38]. The creation of an effective RSV vaccine was significantly affected following the use of formalin inactivated RSV (FI-RSV) vaccine in the 1960s which led to increased morbidity and enhanced respiratory disease following infection with live virus and the subsequent deaths of 2 children [39, 40]. This phenomenon was felt to be as a result of induction of Th2 immune responses by the vaccine [41, 42].
A new development in the creation of vaccines has been the use of TLR ligands as adjuvant agents. One recent study using monophosphoryl lipid A (MPLA), a derivative of bacterial LPS, incorporated with RSV virosomes demonstrated an enhanced Th1 response with increased production of IFN-γ and decreased IL-5 in animals immunised with vaccine and then challenged with virus [36••]. The MPLA adjuvanted vaccine conferred similar protection from live RSV virus as FI-RSV vaccine but with no evidence of enhanced respiratory disease. In addition the use of MPLA led to enhanced immunogenicity of the RSV vaccine with production of higher affinity antibodies.
The only vaccines commercially available and recommended for use are against influenza where the annual vaccine has been shown to play a key role in the prevention of virus infection and its associated morbidity [43•]. This preventive approach may well have advantages over treatment of acute viral infections where current treatment options are limited.
As RV infections are implicated in the vast majority of virus induced AEs they are perhaps the most attractive target for a respiratory vaccine. However, there are >100 serotypes of RV and unlike influenza there is limited epidemiological information regarding the most important circulating serotypes [11••]. Humans are infected with RV in early life and recurrently through life and most adults have antibodies to multiple RV strains, complicating human study of antibody responses. Improved diagnostic and molecular techniques have recently allowed the identification of the RV-C group further highlighting the difficulty in selecting specific serotypes for vaccine generation [11••].
A major hurdle to the understanding of antibody production following virus infection has been a scarcity of animal models that have allowed us to study both asthma exacerbations and the subsequent effects of immunisation in greater detail [15]. A recent paper describing a novel mouse model of RV infection and immunisation has allowed study of RV mediated induction of antibody responses [44••]. This paper demonstrated the generation of strong cross-serotype IgG responses to the RV capsid protein VP1 and that multiple infections were necessary to induce neutralising antibodies [44••]. Another group have also recently shown that use of a recombinant VP1 protein was able to generate neutralising antibodies displaying cross-reactivity to distantly related RV strains [45]. These studies suggest that efforts to develop RV vaccines may be worth re-visiting.
Targeting the virus
Small molecule inhibitors of virus infection and replication
Using small molecule inhibitors of RV infection, replication or release was a popular theme in the 1980s and 1990s for the treatment of the common cold. Despite approaches showing some promise in common cold studies, the use of these anti-virals has yet to be examined in virus induced AE. Small molecule anti-virals offer the advantages of being cost effective (small molecule production and validation), and their selectivity and safety is relatively straightforward to establish. They have disadvantages in that they may be limited to specific virus types, may select for escape mutants over time and may suffer from toxicity or have side effects with continual use.
The anti-RV agent Pleconaril was used in randomised, placebo-controlled, phase II clinical trials as a treatment of the common cold [46]. Pleconaril prevents uncoating of most serotypes of RVs. Pleconaril was tested as a therapeutic agent, with infected individuals beginning therapy 1–1.5 days after experiencing clinical colds. Pleconaril showed significant improvement in mean symptoms scores and decreases in mean duration of illness. Despite promising initial results, Pleconaril was abandoned as a treatment due to side effects.
The RV 3C protease inhibitor Ruprintrivir was tested in a double blind, placebo-controlled phase II trial of experimental RV39 challenge [47]. Ruprintrivir was designed to bind irreversibly to the RV 3C active site. As a prophylaxis, Ruprintrivir reduced mean total symptom score, viral titre and nasal secretions but not the incidence or frequency of clinical colds. As a therapeutic treatment, Ruprintrivir also reduced symptom scores, nasal secretions and viral titre.
The soluble ICAM derivative Tremacamra was tested in randomised double-blind placebo-controlled studies both as a therapeutic and prophylactic intervention to RV39 challenge [48]. Tremacamra showed promise as a therapy, reducing the frequency of colds, total symptom score, nasal mucus weight, and virus induced inflammation.
Quercetin is a polyphenol which has a range of properties some of which are anti-viral. Quercetin is thought to inhibit phospho-inositol-3-kinase inhibition and inhibition of viral endocytosis, RV and poliovirus protease activity, and RNA polymerase activity of some RNA viruses. In a mouse model of RV infection, Quercetin if given during infection reduced virus titre and improved lung function. However, if given daily for 10 days finishing 40 hours before RV infection, Quercetin had little effect on virus replication and lung function [49•].
Development of all these drugs was abandoned for various reasons. However, considering the role of viruses in AE, and the available human and mouse models of virus induced AE, there has never been a better time to trial small molecule anti-virals as therapies for AE. The future is likely to witness further studies of anti-virals as a potential treatment for virus-induced AE.
Virus specific antibodies
The recombinant monoclonal antibody (MAb) Palivizumab has been licenced for use in human RSV immunoprophylaxis since 1998 [50]. It acts against an epitope in the A region of the RSV fusion protein and has been shown to reduce the rate of hospitalisations in high risk-infants when used prophylactically [50]. There is a scarcity of evidence regarding the role of Palivizumab in the treatment of acute RSV disease with evidence predominantly limited to case reports and small retrospective studies. A single dose of 15 mg/kg in children intubated with respiratory failure due to RSV was shown to reduce RSV concentration in tracheal aspirates [50] and Palivizumab has been shown to be well tolerated in adult stem cell transplant recipients [51]. However, there is a need for further large scale studies to assess the role of Palivizumab as therapy in RSV infection.
Motavizumab (MEDI-524, MedImmune) is a second generation MAb developed from Palivizumab by affinity maturation [52]. In comparison to Palivizumab, it has been shown to bind to RSV F protein 70-fold better and have an approximately 20-fold improvement in neutralisation of RSV in vitro [52] as well as being able to reduce pulmonary RSV titres up to 100-fold lower than equivalent doses of Palivizumab [52]. Motavizumab is able to inhibit viral replication in the upper respiratory tract making it a potentially attractive therapy for treatment of RSV infection [52]. A study comparing Motavizumab with Palivizumab for RSV prophylaxis showed that Motavizumab treatment resulted in 50% fewer RSV related lower respiratory tract infections needing medical attention [53]. However, this study also documented an increase in cutaneous hypersensitivity reactions and Motavizumab is not currently licenced for use in humans.
There has been a growing body of work directed towards the generation of MAbs against influenza. Two recently described MAbs, Fi6v3 and PN-SIA28, have been shown to be broadly neutralising against both group 1 and 2 influenza A subtypes [54••, 55••]. Fi6v3 antibody was generated from single cell culture of plasma cells from individuals following natural influenza A infection or vaccination and passive transfer of this MAb was protective in both mice and ferrets against H1, H3 and H5 subtypes [54••]. PN-SIA28 was identified from a single healthy donor who had a negative history for influenza in the preceding decade and it too demonstrated neutralising activity against group 1 and 2 subtypes [55••]. Both Fi6v3 and PN-SIA28 act on regions near the HA stem and have identified new mechanisms underlying virus–host interaction and new areas of interest in development of anti-viral therapies [54••, 55••]. There are currently no MAbs available for use against RV.
Concluding remarks
Current treatment options for AE are limited and have developed little in recent years. Furthermore, these treatments do not address the cause of the exacerbations, nor specific mechanisms involved in their pathogenesis. New clinical studies are needed to further our understanding of the mechanisms of virus induced AE so that targets for development of novel approaches to prevention and therapy can be identified. Anti-viral therapies may be a source of these new therapies; this review has highlighted potential therapies that target the virus or boost host response to the virus. The latter approach is based on recent studies that show an impairment in the ability of the asthmatic host to raise an effective anti-viral immune response, and there are several potential ways to restore this. Alternatively, the virus itself may be targeted, using specific anti-virals or immunotherapy. We believe the future will likely see greatly increased study of anti-viral therapies in one form or another for treatment/prevention of virus induced AE.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Masoli M., Fabian D., Holt S., Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2•.Ivanova J.I. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]; Study comparing direct health care costs between moderate/severe asthmatics with and without exacerbations. Demonstrated that moderate severe asthmatics who had exacerbations had higher total and asthma related health care costs.
- 3.Corne J.M. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan A.J. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361:1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston S.L. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston N.W. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wark P.A., Johnston S.L., Simpson J.L., Hensley M.J., Gibson P.G. Chlamydia pneumoniae immunoglobulin A reactivation and airway inflammation in acute asthma. Eur Respir J. 2002;20:834–840. doi: 10.1183/09031936.02.00192002. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham A.F., Johnston S.L., Julious S.A., Lampe F.C., Ward M.E. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11:345–349. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 10.Johnston S.L. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 11••.Palmenberg A.C. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper examining diversity of human rhinovirus (HRV) by completing the genome sequences for all known serotypes (n = 99) including newly discovered HRV-C.
- 12.Lau S.K. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Bochkov Y.A. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat Med. 2011;17:627–632. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper detailing development of a sinus organ culture and reverse genetics system allowing study of HRV-C in vitro, demonstrating a unique cell attachment site for HRV-C.
- 14.Busti A.J. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J Cardiovasc Pharmacol. 2008;51:605–610. doi: 10.1097/FJC.0b013e31817b5b5a. [DOI] [PubMed] [Google Scholar]
- 15••.Bartlett N.W. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper describing the first mouse model of RV infection.
- 16.Newcomb D.C. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagarkar D.R. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol. 2010;185:2525–2535. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Iikura K. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int Arch Allergy Immunol. 2011;155(Suppl 1):27–33. doi: 10.1159/000327262. [DOI] [PubMed] [Google Scholar]; Study suggesting increased susceptibility of asthmatics to RV infection as a consequence of impaired production of IFNα and other inflammatory cytokines.
- 19.Bufe A., Gehlhar K., Grage-Griebenow E., Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002;127:82–88. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- 20••.Wark P.A. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study demonstrating deficient innate immune response in bronchial epithelial cells from asthmatic individuals. In particular, deficiency of IFN-β in asthma shown to facilitate virus replication and cytolysis. Addition of exogenous IFN-β in vitro restored this deficit.
- 21.Gehlhar K., Bilitewski C., Reinitz-Rademacher K., Rohde G., Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 22•.Contoli M. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]; Paper demonstrating a previously unknown mechanism of susceptibility to RV infection in asthmatics as a result of deficient IFN-λ production.
- 23.Uller L. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax. 2010;65:626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 24••.Edwards M.R. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper expanding on previous reports of deficient type I and type III IFN production in mild/moderate asthmatics and highlighting impaired anti-viral immune response as a feature of severe therapy resistant asthma.
- 25••.Baraldo S. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–1314. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]; Study highlighting deficient interferon responses to RV in children with asthma irrespective of atopic status and in children with atopy but not asthma.
- 26••.Sykes A. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129:1506–1514. doi: 10.1016/j.jaci.2012.03.044. e1506. [DOI] [PubMed] [Google Scholar]; Authors demonstrate delayed and deficient rhinovirus induction of type I interferons in human BAL cells following ex vivo rhinovirus infection.
- 27.Marsden R., Ward J. 2012. Positive Phase II Asthma Clinical Trial Data. Press Release.http://www.synairgen.com/media/1536/19%20april%202012%20Phase%20II%20press%20release%20final.pdf [Google Scholar]
- 28.Koltsida O. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3:348–361. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Pritchard A.L. Innate IFNs and plasmacytoid dendritic cells constrain Th2 cytokine responses to rhinovirus: a regulatory mechanism with relevance to asthma. J Immunol. 2012;188:5898–5905. doi: 10.4049/jimmunol.1103507. [DOI] [PubMed] [Google Scholar]; Study demonstrating a link between type-I IFNs and pDC in constraining Th2 cytokine production by RV-stimulated PBMC.
- 30.Vrancic M. Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br J Pharmacol. 2011;165:1348–1360. doi: 10.1111/j.1476-5381.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard A.E. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]; Study investigating anti-viral properties of multiple macrolide antibiotics, with azithromycin demonstrating significant anti-RV activity in bronchial epithelial cells and the ability to increase production of interferon stimulated genes during RV infection.
- 33.Xirakia C. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am J Respir Crit Care Med. 2010;181:1207–1216. doi: 10.1164/rccm.200908-1255OC. [DOI] [PubMed] [Google Scholar]
- 34•.Van L.P. Treatment with the TLR7 agonist R848 induces regulatory T-cell-mediated suppression of established asthma symptoms. Eur J Immunol. 2011;41:1992–1999. doi: 10.1002/eji.201040914. [DOI] [PubMed] [Google Scholar]; Study demonstrating attenuation of allergic symptoms by R848 in a murine experimental allergic asthma model through mechanism involving Treg cells.
- 35.Swanson K.A. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Kamphuis T. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS ONE. 2012;7:e36812. doi: 10.1371/journal.pone.0036812. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study evaluating virosomal RSV vaccine adjuvanted with TLR4 ligand MPLA, demonstrating enhanced immunogenicity and skewing of immune response to a balanced Th1/Th2 pattern without adverse immune reaction.
- 37.Luongo C., Winter C.C., Collins P.L., Buchholz U.J. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J Virol. 2012;86:10792–10804. doi: 10.1128/JVI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glenn G.M. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2012;31:524–532. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Graham B.S. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am J Respir Crit Care Med. 1995;152:S63–S66. doi: 10.1164/ajrccm/152.4_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 40.Kim H.W. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 41.Waris M.E., Tsou C., Erdman D.D., Zaki S.R., Anderson L.J. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moghaddam A. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 43•.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2011;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]; Meta-analysis of effectiveness of influenza vaccines, highlighting lack of evidence of protection in adults over 65 and need for new vaccines with increased efficacy and effectiveness.
- 44••.McLean G.R. Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1. Antiviral Res. 2012;95:193–201. doi: 10.1016/j.antiviral.2012.06.006. [DOI] [PubMed] [Google Scholar]; Study characterising the generation of antibody responses to RV following infection and immunisation of mice. Identified potential of VP1 based antigens combined with adjuvants in generation of successful antibody-mediated vaccine design and development.
- 45.Edlmayr J. Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. Eur Respir J. 2011;37:44–52. doi: 10.1183/09031936.00149109. [DOI] [PubMed] [Google Scholar]
- 46.Hayden F.G. Oral pleconaril treatment of picornavirus-associated viral respiratory illness in adults: efficacy and tolerability in phase II clinical trials. Antivir Ther. 2002;7:53–65. [PubMed] [Google Scholar]
- 47.Hayden F.G. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob Agents Chemother. 2003;47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner R.B. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 49•.Ganesan S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94:258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study demonstrating quercetin, a plant flavinoid, possesses anti-rhinoviral effects. The authors show that quercetin inhibits viral infection at multiple stages, including endocytosis and viral protein synthesis.
- 50.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 51.Boeckh M. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184:350–354. doi: 10.1086/322043. [DOI] [PubMed] [Google Scholar]
- 52.Wu H. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Carbonell-Estrany X. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 54••.Corti D. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]; Study identifying a broadly neutralising monoclonal antibody with potential for use against all influenza A viruses.
- 55••.Clementi N. A human monoclonal antibody with neutralizing activity against highly divergent influenza subtypes. PLoS ONE. 2011;6:e28001. doi: 10.1371/journal.pone.0028001. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe a human monoclonal antibody able to neutralize pandemic influenza subtypes as well as other subtypes with pandemic potential.