Abstract
Emerging viral diseases represent an ongoing challenge for globalized world and bats constitute an immense, partially explored, reservoir of potentially zoonotic viruses. Caliciviruses are important human and animal pathogens and, as observed for human noroviruses, they may impact on human health on a global scale. By screening fecal samples of bats in Hungary, calicivirus RNA was identified in the samples of Myotis daubentonii and Eptesicus serotinus bats. In order to characterize more in detail the bat caliciviruses, large portions of the genome sequence of the viruses were determined. Phylogenetic analyses and molecular modeling identified firmly the two viruses as candidate members within the Caliciviridae family, with one calicivirus strain resembling members of the Sapovirus genus and the other bat calicivirus being more related to porcine caliciviruses of the proposed genus Valovirus. This data serves the effort for detecting reservoir hosts for potential emerging viruses and recognize important evolutionary relationships.
Keywords: Calicivirus, Bat, Virus, Protein modeling, Hungary, Daubenton's bat, Serotine bat, Europe
Highlights
-
•
Two novel bat caliciviruses were genetically characterized.
-
•
Mature viral capsids were molecularly modeled.
-
•
Bat caliciviruses are highly heterogeneous genetically.
-
•
The two novel viruses are genetically related to valoviruses and sapoviruses.
-
•
New sequences were most closely related to Chinese sequences.
Despite ongoing advances in biomedicine and molecular biology, infectious diseases remain a major threat to human health, economic sustainability and wildlife conservation. In the last decades bats became a possible source for several human infecting agents such as Ebola, SARS and MERS coronaviruses, Nipah virus, and Hendra virus (Wynne and Wang, 2013). With over 1250 species distributed globally, bats represent the most diverse and species rich taxa among mammals (Teeling et al., 2005). The zoonotic potential of numerous bat-harbored viruses has been described also in Europe (Kohl and Kurth, 2014). Additionally, several novel viruses have been detected recently; however, there is no data on their role in association with bat or human diseases (Kemenesi et al., 2014, Kemenesi et al., 2015a, Kemenesi et al., 2015b).
The family Caliciviridae comprises small non-enveloped RNA viruses, which are classified in five genera: Vesivirus, Lagovirus, Norovirus, Sapovirus and Nebovirus. In addition, unclassified caliciviruses have been identified in monkeys, pigs, avian species and fishes (Farkas et al., 2008, L'Homme et al., 2009, Wolf et al., 2011, Liao et al., 2014, Mikalsen et al., 2014). Calicivirus related sequences have been also reported from different bat species in Hong Kong, Hungary and New Zealand (Tse et al., 2012, Kemenesi et al., 2014, Wang et al., 2015). The common characteristic of these bat-derived calicivirus sequences is the phylogenetic divergence from other known members within the family Caliciviridae. The generation of partial or full-length genome sequence data on these recently discovered viruses is crucial to improve viral taxonomy and classification, to develop reliable diagnostic assays and to better understand the molecular mechanisms driving the evolution and host species adaptation of caliciviruses. Human and animal caliciviruses may evolve by accumulation of punctate mutations and by exchange of genetic material via recombination (Giammanco et al., 2013). Also, interspecies transmission has been documented repeatedly and the origin of some human calicivirus strains may have been triggered by a combination of various evolutionary mechanisms (Smith et al., 1998, Scheuer et al., 2013). In this regard the possible reservoir role of bats has been investigated minimally. Here we report the genomic characterization and molecular modeling based analysis for two novel calicivirus strains (BtCalV/BS58/HUN/2013 and BtCalV/M63/HUN/2013) identified from Serotine bat (Eptesicus serotinus) and Daubenton's bat (Myotis daubentonii).
Bat guano samples were collected during the swarming period in 2013 as part of a countrywide survey program as described previously (Kemenesi et al., 2014). Nucleic acid extractions were performed with DiaExtract Total RNA Isolation Kit (Diagon Ltd., Hungary). 3′ RACE PCRs were fulfilled on both samples with SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen), according to the manufacturer's instructions with using the previously described forward primer by Jiang et al. (1999). 5′ RACE PCR was also performed with 5′/3′ RACE Kit, 2nd Generation (Roche). In order to obtain sequences upstream the RNA dependent RNA polymerase (RdRp) region we performed a two-step RT-PCR protocol. cDNA synthesis was carried out with the Transcriptor First Strand cDNA Synthesis Kit (Roche) using random hexamer oligonucleotide mixture. PCR assays were implemented with Thermo Scientific Phusion High-Fidelity PCR Master Mix following the protocol provided by the manufacturer, using previously published generic calicivirus forward primer (L'Homme et al., 2009) with RdRp specific reverse primer (Jiang et al., 1999).
High throughput sequencing was performed on PCR amplicons to obtain primary sequence data (Mihalov-Kovács et al., 2015). Briefly, an Ion Torrent compatible library was prepared applying the NEBNext® Fast DNA Fragmentation & Library Prep Set for Ion Torrent (New England Biolabs) with the Ion Torrent Xpress barcode adapters (Life Technologies). The emulsion PCR to obtain clonally amplified fragments was carried out using the Ion OneTouch™ 200 Template Kit (Life Technologies) on a OneTouch version 2 equipment (Life Technologies) as recommended by the manufacturer. Templated beads were enriched using an Ion OneTouch™ ES pipetting robot (Life Technologies). The 200 bp sequencing protocol was performed on a 316 chip (Life Technologies) using the Ion Torrent PGM (Life Technologies) semiconductor sequencing equipment. Trimmed sequence reads were used for de novo assembly utilizing the MIRA (version 3.9.17) (Chevreux et al., 1999). Additional bioinformatic analyses, validation with remapping were performed using the CLC Genomics Workbench (version 6.5.1; http://www.clcbio.com) and the DNAStar (version 12; http://www.dnastar.com). GenBank accession numbers for BtCalV/BS58/HUN/2013 and BtCalV/M63/HUN/2013 are KJ652318 and KJ652319, respectively.
Basic sequence alignments and sequence manipulations were fulfilled with ClustalX v2.1 and GeneDoc v2.7 softwares. Multiple evolutionary models were tested on our sequence alignments with PhyML v3.0 software in order to designate the best phylogenetic model for our data set. The phylogenetic trees were constructed with MEGA v5.0 software using the Maximum-Likelihood method, based on the General Time Reversible model with Gamma Distribution (GTR + G). Number of bootstraps for simulations was 1000.
The 3D structure models of mature BtCalV/M63/HUN/2013 (aa. 317 to 831) and BtCalV/BS58/HUN/2013 (aa. 268 to 746) bat calicivirus VP1 capsid protein (CP) structures were generated with I-TASSER (Zhang, 2008, Roy et al., 2010). The models were built using the recently identified bat VP1 CP amino acid sequences. The following templates were used to thread the VP1 CP structure of the bat calicivirus BtCalV/M63/HUN/2013: PDB ID codes 3M8L (Feline calicivirus (FCV) capsid protein) and 2GH8 (X-ray structure of a native calicivirus). The structure of Norwalk virus capsid (PDB ID code 1IHM) was used for modeling of the VP1 capsid protein of the bat calicivirus BtCalV/BS58/HUN/2013. The raw protein model structures were refined with the MacroModel energy minimization module of the Schrödinger Suite (Schrödinger, 2015) to eliminate the steric conflicts between the side-chain atoms. Pairwise protein sequence alignments were calculated with the NeedleP tool of the SRS bioinformatics software package. The T = 3 virion models were created with the Oligomer Generator application of VIPERdb (available at http://viperdb.scripps.edu/oligomer_multi.php). Prior to virion model generations the asymmetric units were constructed with Schrödinger Suite using the coordinates of subunit A, B and C of FCV and Norwalk virus in vdb convention format. Molecular graphics and sequence alignment visualization were prepared using VMD v1.9.1 (Humphrey et al., 1996) and the Multiple Sequence Viewer of the Schrödinger Suite, respectively.
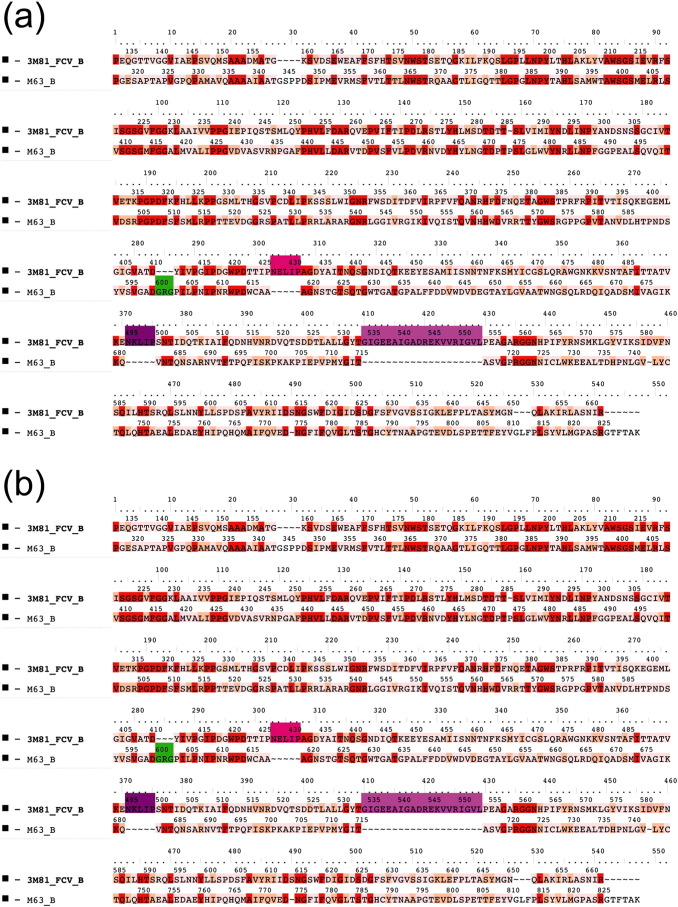
Since 5′ RACE PCR protocols were not successful for the two samples, we obtained a 3278 and 3130 nt long partial genome sequences, respectively, for the strains BtCalV/M63/HUN/2013 and BtCalV/BS58/HUN/2013. The sequenced genomic fragment spanned the partial RNA-dependent RNA polymerase (RdRp) gene (M63, 879 nt; BS58, 841 nt) and the whole coat protein coding region (1545 nt and 1436 nt) along with the complete VP2 protein coding region (656 nt and 668 nt). The calicivirus conserved aa motifs GLPSG and YGDD (Smiley et al., 2002) were identified in the RdRp region. A third calicivirus RdRp motif DYXXWDST, was also well conserved in both viruses, exhibiting the following aa sequence DFSKWDST (Wolf et al., 2011).
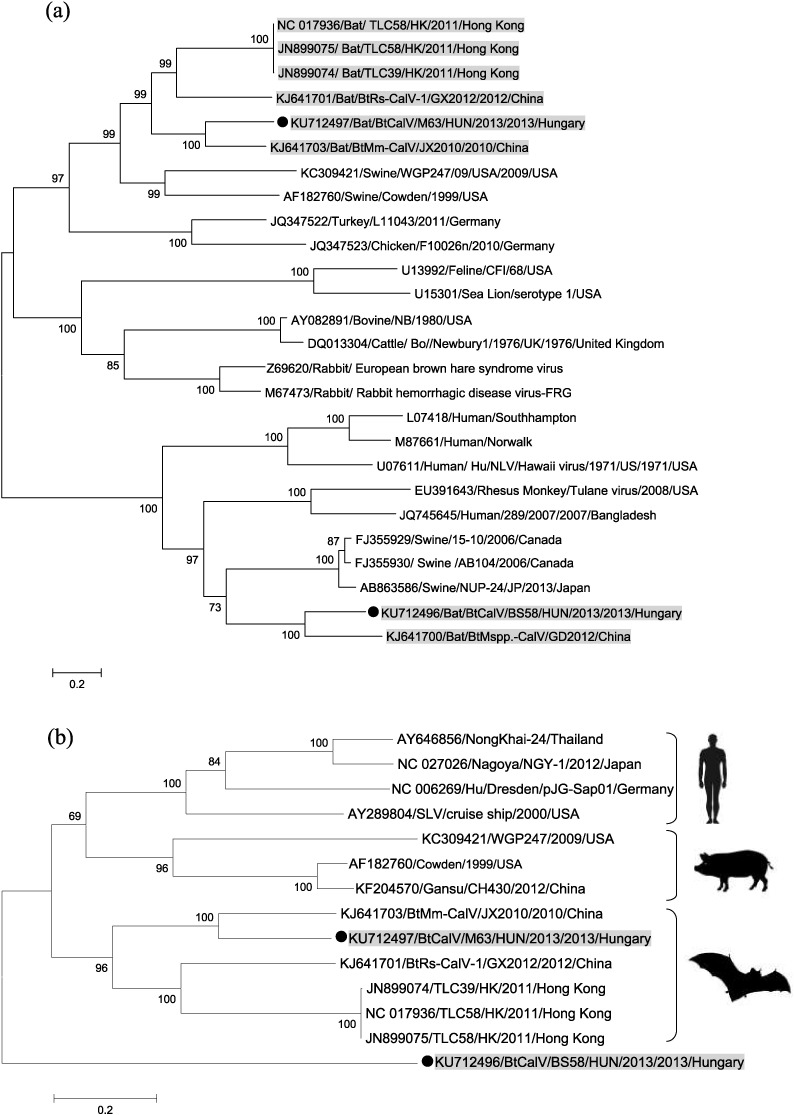
Strain BtCalV/M63/HUN/2013, detected in a M. daubentonii bat, was grouped with other bat-derived sapoviruses and showed the closest relationship with a Chinese sequence (GenBank: KJ641703), detected from the feces of a Myotis myotis bat (Fig. 2a), with 67% nt and 67% aa identity in the RdRp and 66% nt and 61% aa identity in the capsid gene. Strain BtCalV/BS58/HUN/2013, derived from an E. serotinus bat species, was grouped with a bat calicivirus and with porcine caliciviruses of the proposed genus Valovirus. The closest phylogenetic relationship was described with a Chinese bat calicivirus strain, BtMspp.-CalV/GD2012 (GenBank: KJ641700), detected in an undefined Myotis species. Identity was 63 nt and 60% aa in the RdRp and 63% nt and 56% aa in the capsid gene between strain BS58/HUN/2013 and strain BtMspp.-CalV/GD2012 (Table 1 ). This geographically distant evolutionary relationship among European and Asian origin bat viruses has already been described when picornaviruses and parvoviruses detected in European Miniopterus schreibersii bats were characterized (Kemenesi et al., 2015a, Kemenesi et al., 2015b). Our new results with related caliciviruses in Myotis and Eptesicus bats, widens the list of bat species harboring genetically related viruses in large geographic distances, which can be a result of ancient evolutionary history of bats and their viruses and presumably could be a result of biogeographical history of these bat species.
Fig. 2.
Phylogenetic tree of the two novel bat calicivirus sequences and their relationship with other known genera within the family Caliciviridae (a) and relationship with sapoviruses (b) identified from human, swine and bats. Phylogenetic trees were constructed based on a partial sequence of 3130 nucleotides, incorporating the whole capsid region of the viruses. Strains reported in this study are marked with black dots, and all bat derived calicivirus strains are marked with gray background. The phylogenetic trees were constructed with MEGA v5.0 software using the Maximum-Likelihood method, based on the General Time Reversible model with Gamma Distribution (GTR + G). Number of bootstraps for simulations was 1000.
Table 1.
Amino acid and nucleic acid sequence homology patterns between the examined sequences. Greatest homologies are emphasized in bold.
| M63 (2493 nt) |
BS58 (2238 nt) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RdRp (951 nt) |
Capsid (1542 nt) |
RdRp (804 nt) |
Capsid (1434 nt) |
||||||
| nt (%) | aa (%) | nt (%) | aa (%) | nt (%) | aa (%) | nt (%) | aa (%) | ||
| Bat calicivirus | KJ641703 | 67 | 67 | 66 | 61 | 34 | 25 | 28 | 25 |
| KJ641700 | 33 | 23 | 29 | 20 | 63 | 60 | 63 | 56 | |
| Bat Sapovirus | JN899074 | 52 | 43 | 50 | 40 | 35 | 23 | 28 | 23 |
| Bat calicivirus | KJ641701 | 50 | 45 | 54 | 47 | 33 | 25 | 28 | 23 |
| Swine Sapovirus | KC309421 | 42 | 35 | 44 | 37 | 31 | 24 | 28 | 24 |
| Chicken calicivirus | JQ347523 | 40 | 31 | 35 | 27 | 33 | 27 | 28 | 24 |
| Norovirus Norwalk | M87661 | 33 | 24 | 30 | 21 | 42 | 32 | 37 | 29 |
| Recovirus | EU391643 | 31 | 23 | 31 | 24 | 44 | 39 | 38 | 29 |
| Valovirus | FJ355929 | 31 | 25 | 27 | 19 | 49 | 37 | 47 | 38 |
| Vesivirus | U13992 | 33 | 29 | 25 | 16 | 33 | 28 | 22 | 27 |
| Lagovirus | M67473 | 37 | 32 | 31 | 22 | 35 | 26 | 28 | 23 |
| Nebovirus | AY82891 | 36 | 31 | 31 | 22 | 32 | 24 | 29 | 23 |
| Human Sapovirus | AY646856 | 49 | 42 | 43 | 40 | 37 | 27 | 35 | 25 |
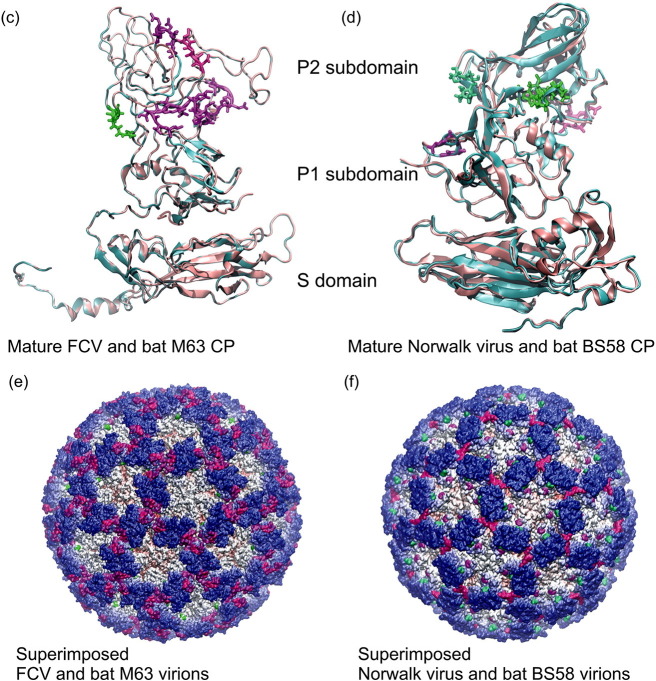
In depth structural analysis of the capsid of caliciviruses has been reported in previous studies (Ossiboff et al., 2010, Prasad et al., 1999). There was 27.3% aa identity, and 42.3% aa similarity between the template FCV capsid protein (PDB ID: 3M8L) and the mature CP of the bat calicivirus strain BtCalV/M63/HUN/2013. Likewise, there was 34.7% aa identity and 47.6% similarity, between the template Norwalk virus CP (PDB ID: 1IHM) and the CP of the bat calicivirus strain BtCalV/BS58/HUN/2013. These identity and similarity values allowed reliable homology modeling and reconstruction of the capsid and virion structure of the two viruses to be made. The mature calicivirus capsid protein has three main domains: S, P1 and P2 (Fig. 1c and d). The S (i.e. shell) domain is composed of a canonical eight-stranded β-barrel structure. This domain forms the inner part of the virion and it has the most conserved structure within caliciviruses. The P1 and P2 subdomains build up the middle and the outer protruding part of the virion, respectively. The hypervariable regions, the primary targets of neutralizing antibodies, are located on the P2 subdomain (Ossiboff et al., 2010). The basic structure together with the recognized main domains was predicted to be conserved in the two Hungarian bat caliciviruses. However in the P2 region we detected three deletions and an insertion (Fig. 1a and c) in the CP of the bat calicivirus BtCalV/M63/HUN/2013 when compared to the FCV template sequence. In the case of the strain BtCalV/BS58/HUN/2013, we found two insertions and two deletions on the P2 subdomain (Fig. 1b and d) with respect to the template structure. Insertions and/or deletions in the P2 domain are common even among caliciviruses of the same genus and/or genotype (Pinto et al., 2012, Medici et al., 2015). These small structural and sequential differences may play important roles in the P2 subdomain dual functions. The protruding regions of the virion may be involved as antigens in the humoral immune response (Fig. 1e and f). Furthermore, this variable part of the P2 subdomain may take part in protein–protein interactions between the CP and its functional cell receptor. Additionally, these regions are responsible for the fine tuning of cell-virus interaction during cell entry (Ossiboff et al., 2010).
Fig. 1.
Structure comparison of mature calicivirus (ORF2, VP1 segment) CP proteins and virions. Structure based amino acid sequence alignments of the newly reported bat calicivirus capsid proteins and the template FCV and Norwalk calicivirus VP1 proteins (a) and (b). The background of the sequence alignments reflects the homology levels of the two–two related capsid protein sequences: identical amino acids are red, similar aas. are light orange while different aas. are light pink. The main structural differences are indicated by shades of magenta and green color codes on the sequence alignment and on the superimposed CP structures. The template calicivirus VP1 protein structures are illustrated in cyan cartoon representation, while the new bat calicivirus VP1 model structure are pink (c) and (d). Molecular surface representation of the superimposed template and the newly described bat calicivirus virions (e) and (f). The molecular surface is colored by radial extension of the amino acids from the virion center. Dark blue represents the most protruding CP parts. The structural differences were colored in the same way as for the capsid monomers. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In conclusion, genome sequencing, phylogenetic analysis and homology modeling allowed a more precise characterization of two novel bat caliciviruses. One virus could be firmly assigned to the Sapovirus genus. Sapoviruses are enteric viruses associated with gastro-enteritis in humans and with enteritis in pigs (Oka et al., 2015). These viruses have been also identified in diarrheic dogs and cats (Soma et al., 2015, Li et al., 2011) and display an impressive genetic heterogeneity (Oka et al., 2015). Interestingly, genetic heterogeneity is also displayed by the few bat sapoviruses identified thus far, thus posing a challenge for the design of specific diagnostic tools. Based on our phylogenetic analyses species-specific patterns are clear among sapoviruses of different animal origin (Fig. 2b). However, high phylogenetic diversity can be observed within these species-related clusters with several separate branches, which indicate a long-term evolutionary diversification. Neither amino acid and nucleic acid sequence homology patterns between the examined sequences (Table 1.) nor the phylogenetic results indicate evidence for possible recombination event related to our sequences.
The other bat calicivirus was genetically more similar to porcine caliciviruses of the putative novel genus Valovirus. Valoviruses are orphan viruses, detected mostly in finisher pigs with a low prevalence (L'Homme et al., 2009, Wang et al., 2011, Di Martino et al., 2011). Antibodies specific for valoviruses have been detected in 63 (10.3%) of 614 porcine serum samples in a previous study (Di Martino et al., 2012), indicating that these viruses circulate in pigs, although their pathogenic role is unclear. Although these viruses were described in asymptomatic swine from several geographically distant countries, such as Japan and Canada (Sato et al., 2014, L'Homme et al., 2009), the circumstances of their worldwide distribution should be extensively examined in future. The genomic organization of the bat calicivirus was not identical to the organization observed in valoviruses. The closest phylogenetic relatedness was observed with valoviruses, however the novel bat calicivirus sequence (BtCalV/BS58/HUN/2013) clearly formed a separate branch.
The limited number of studies describing bat caliciviruses is providing evidence that these viruses are highly heterogeneous genetically. It is possible that as data will accumulate, this picture will be confirmed and the interactions between caliciviruses from bats and from other animal species, including humans, will be more evident. Also, this will help clarify whether caliciviruses may have a pathogenic role in bats and will help reveal the evolutionary pathways of these viruses across the various bat species and geographic areas.
Acknowledgments
Research activity of Ferenc Jakab was supported by the TÁMOP 4.2.4. A/2-11-1-2012 0001 — National Excellence Program elaborating and operating an inland student and researcher personal support system. The project was subsidized by the European Union and co-financed by the European Social Fund. Krisztián Bányai was supported by the “Momentum program”. Ákos Gellért and Szilvia Marton were the recipients of a János Bolyai fellowship from the Hungarian Academy of Sciences. The licensing of the Schrödinger Suite software package was funded from the OTKA grant under agreement no.:108793. The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs, Hungary.
References
- Chevreux B., Wetter T., Suhai S. Computer Science and Biology. GCB; Hannover, Germany: 1999. Genome sequence assembly using trace signals and additional sequence information; pp. 45–56. (Proceedings of the German Conference on Bioinformatics, GCB'99). [Google Scholar]
- Di Martino B., Martella V., Di Profio F., Ceci C., Marsilio F. Detection of St-Valerien-like viruses in swine, Italy. Vet. Microbiol. 2011;149:221–224. doi: 10.1016/j.vetmic.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Ceci C., Martella V., Lavazza A., Massirio I., Marsilio F. Seroprevalence of St-Valerien-like caliciviruses in Italian swine. J. Gen. Virol. 2012;93:102–105. doi: 10.1099/vir.0.036236-0. [DOI] [PubMed] [Google Scholar]
- Farkas T., Sestak K., Wei C., Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J. Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giammanco G.M., De Grazia S., Tummolo F., Bonura F., Calderaro A., Buonavoglia A., Martella V., Medici M.C. Norovirus GII.4/Sydney/2012 in Italy, winter 2012–2013. Emerg. Infect. Dis. 2013;19:1348–1349. doi: 10.3201/eid1908.130619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., Schulten K. VMD — visual molecular dynamics. J. Mol. Graphs. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jiang X., Huang P.W., Zhong W.M., Farkas T., Cubitt D.W., Matson D.O. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Kemenesi G., Dallos B., Görföl T., Boldogh S., Estók P., Kurucz K., Kutas A., Földes F., Oldal M., Németh V., Martella V., Bányai K., Jakab F. Molecular survey of RNA viruses in Hungarian bats: discovering novel astroviruses, coronaviruses, and caliciviruses. Vector Borne Zoonotic Dis. 2014;12:846–855. doi: 10.1089/vbz.2014.1637. [DOI] [PubMed] [Google Scholar]
- Kemenesi G., Dallos B., Görföl T., Estók P., Boldogh S., Kurucz K., Oldal M., Marton S., Bányai K., Jakab F. Genetic diversity and recombination within bufaviruses: detection of a novel strain in Hungarian bats. Infect. Genet. Evol. 2015;33:288–292. doi: 10.1016/j.meegid.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenesi G., Zhang D., Marton S., Dallos B., Görföl T., Estók P., Boldogh S., Kurucz K., Oldal M., Kutas A., Bányai K., Jakab F. Genetic characterization of a novel picornavirus detected in Miniopterus schreibersii bats. J. Gen. Virol. 2015;96:815–821. doi: 10.1099/jgv.0.000028. [DOI] [PubMed] [Google Scholar]
- Kohl C., Kurth A. European bats as carriers of viruses with zoonotic potential. Viruses. 2014;8:3110–3128. doi: 10.3390/v6083110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Homme Y., Sansregret R., Plante-Fortier E., Lamontagne A.M., Ouardani M., Lacroix G., Simard C. Genomic characterization of swine caliciviruses representing a new genus of Caliciviridae. Virus Genes. 2009;39:66–75. doi: 10.1007/s11262-009-0360-3. [DOI] [PubMed] [Google Scholar]
- Li L., Pesavento P.A., Shan T., Leutenegger C.M., Wang C., Delwart E. Viruses in diarrhoeic dogs include novel kobuviruses and sapoviruses. J. Gen. Virol. 2011;92:2534–2541. doi: 10.1099/vir.0.034611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q., Wang X., Wang D., Zhang D. Complete genome sequence of a novel calicivirus from a goose. Arch. Virol. 2014;159:2529–2531. doi: 10.1007/s00705-014-2083-6. [DOI] [PubMed] [Google Scholar]
- Medici M.C., Tummolo F., Calderaro A., Chironna M., Giammanco G.M., De Grazia S., Arcangeletti M.C., De Conto F., Chezzi C., Martella V. Identification of the novel Kawasaki 2014 GII.17 human norovirus strain in Italy. Euro Surveill. 2015;20(35):30011. doi: 10.2807/1560-7917.ES.2015.20.35.30010. [DOI] [PubMed] [Google Scholar]
- Mihalov-Kovács E., Gellért Á., Marton S., Farkas S.L., Fehér E., Oldal M., Jakab F., Martella V., Bányai K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infect. Dis. 2015;21:660–663. doi: 10.3201/eid2104.141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikalsen A.B., Nilsen P., Frøystad-Saugen M., Lindmo K., Eliassen T.M., Rode M., Evensen O. Characterization of a novel calicivirus causing systemic infection in atlantic salmon (Salmo salar L.): proposal for a new genus of caliciviridae. PLoS One. 2014;9:e107132. doi: 10.1371/journal.pone.0107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Wang Q., Katayama K., Saif L.J. Comprehensive review of human sapoviruses. Clin. Microbiol. Rev. 2015;28:32–53. doi: 10.1128/CMR.00011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossiboff R.J., Zhou Y., Lightfoot P.J., Prasad B.V., Parker J.S. Conformational changes in the capsid of a calicivirus upon interaction with its functional receptor. J. Virol. 2010;84:5550–5564. doi: 10.1128/JVI.02371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto P., Wang Q., Chen N., Dubovi E.J., Daniels J.B., Millward L.M., Buonavoglia C., Martella V., Saif L.J. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One. 2012;2 doi: 10.1371/journal.pone.0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- Roy A., Kucukural A., Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato G., Ido H., Kiuchi M., Kataoka M., Katayama K., Tohya Y. Characterization of St-Valerien-like virus genome detected in Japan. J. Vet. Med. Sci. 2014;76:1045–1050. doi: 10.1292/jvms.13-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer K.A., Oka T., Hoet A.E., Gebreyes W.A., Molla B.Z., Saif L.J., Wang Q. Prevalence of porcine noroviruses, molecular characterization of emerging porcine sapoviruses from finisher swine in the United States, and unified classification scheme for sapoviruses. J. Clin. Microbiol. 2013;51:2344–2353. doi: 10.1128/JCM.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger L. LLC; New York, NY: 2015. Schrödinger Suite, Schrödinger. [Google Scholar]
- Smiley J.R., Chang K.O., Hayes J., Vinjé J., Saif L.J. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J. Virol. 2002;76:10089–10098. doi: 10.1128/JVI.76.20.10089-10098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.W., Skilling D.E., Cherry N., Mead J.H., Matson D.O. Calicivirus emergence from ocean reservoirs: zoonotic and interspecies movements. Emerg. Infect. Dis. 1998;4:13–20. doi: 10.3201/eid0401.980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma T., Nakagomi O., Nakagomi T., Mochizuki M. Detection of Norovirus and Sapovirus from diarrheic dogs and cats in Japan. Microbiol. Immunol. 2015;59:123–128. doi: 10.1111/1348-0421.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling E.C., Springer M.S., Madsen O., Bates P., O'brien S.J., Murphy W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307:580–584. doi: 10.1126/science.1105113. [DOI] [PubMed] [Google Scholar]
- Tse H., Chan W.M., Li K.S., Lau S.K., Woo P.C., Yuen K.Y. Discovery and genomic characterization of a novel bat sapovirus with unusual genomic features and phylogenetic position. PLoS One. 2012;4 doi: 10.1371/journal.pone.0034987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Scheuer K., Ahang Z., Gebreyes W.A., Molla B.Z., Hoet A.E., Saif L.J. Characterization and prevalence of a new porcine calicivirus in swine, United States. Emerg. Infect. Dis. 2011;17:1103–1106. doi: 10.3201/eid1706.101756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Moore N.E., Murray Z.L., McInnes K., White D.J., Tompkins D.M., Hall R.J. Discovery of novel virus sequences in an isolated and threatened bat species, the New Zealand lesser short-tailed bat (Mystacina tuberculata) J. Gen. Virol. 2015;96:2442–2452. doi: 10.1099/vir.0.000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Reetz J., Otto P. Genetic characterization of a novel calicivirus from a chicken. Arch. Virol. 2011;156:1143–1150. doi: 10.1007/s00705-011-0964-5. [DOI] [PubMed] [Google Scholar]
- Wynne J.W., Wang L.F. Bats and viruses: friend or foe? PLoS Pathog. 2013;10 doi: 10.1371/journal.ppat.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]