Abstract
The available literature concerning utilization management in the clinical microbiology laboratory is relatively limited compared with that for high-volume, automated testing in the central Core Laboratory. However, the same strategies employed elsewhere in the clinical laboratory operation can be applied to utilization management challenges in microbiology, including decision support systems, application of evidence-based medicine, screening algorithms and gatekeeper functions. The results of testing in the microbiology laboratory have significant effects on the cost of clinical care, especially costs related to antimicrobial agents and infection control practices. Consequently many of the successful utilization management interventions described in clinical microbiology have targeted not just the volume of tests performed in the laboratory, but also the downstream costs of care. This article will review utilization management strategies in clinical microbiology, including specific examples from our institution and other healthcare organizations.
Keywords: Utilization management, Microbiology, Antimicrobial stewardship, Decision support, MALDI-TOF mass spectroscopy
Highlights
-
•
The literature on utilization management in the microbiology laboratory is limited.
-
•
Utilization management strategies employed in core laboratory can be applied to microbiology.
-
•
Many utilization interventions in microbiology target the use of antimicrobial agents.
1. Introduction
Clinical microbiology includes bacteriology and antimicrobial susceptibility testing, virology, parasitology, mycobacteriology, mycology, serology and molecular microbiology. Unlike the core laboratory (chemistry/hematology), where the majority of testing is performed on highly automated analyzers, most testing in microbiology is performed manually or on semi-automated platforms. Many microbiology tests also require interpretation by a skilled microbiology technologist, including visual interpretation of culture results and microscopic examinations. For these reasons the unit cost of microbiology testing is usually greater than that for routine automated testing. The results of microbiology tests have a significant impact on the overall cost of clinical care, most notably in the use and selection of antimicrobial therapy. Therefore, when approaching utilization management in microbiology, it is important to consider not only the cost of testing within the microbiology laboratory but also the downstream costs resulting from clinical decisions based on the test results.
The published literature on utilization management in microbiology is relatively limited when compared to reports on managing utilization of routine automated testing in the chemistry and hematology laboratories. This article will outline a number of utilization management interventions in microbiology that have been reported in the literature. We will also describe several unpublished initiatives that have proven successful in our institution. The specific interventions to be discussed are outlined in Table 1 , and will be described in more detail in the text that follows. In a number of cases, the initiative's success arose not only from a reduction in laboratory testing per se, but rather also from its impact in the clinical care arena (for example, a reduction in antibiotic use or hospital length of length-of-stay). This observation highlights the importance of the clinical microbiology director in forming collaborative, interdepartmental teams to improve quality and reduce the cost of medical care.
Table 1.
Examples of utilization management initiatives in clinical microbiology (see text for details).
| 1. Decision support: test selection for cytomegalovirus testing |
| 2. Reducing blood culture contamination |
| 3. Proper formatting of microbiology reports to avoid misinterpretation |
| 4. Use of MALDI-TOF mass spectroscopy for rapid identification of pathogens |
| 5. Antimicrobial stewardship of carbapenems and other expensive antimicrobial agents |
| 6. Rapid point-of-care testing for influenza A and Group A streptococcus: impact on test ordering and antibiotic utilization |
| 7. Rapid molecular diagnostic testing for patients previously colonized with methicillin resistant Staphylococcus aureus (MRSA) |
| 8. Use of screening methods to reduce low-yield urine cultures |
| 9. Restricting stool examinations in hospital acquired diarrhea |
| 10. Rapid testing for respiratory viruses: impact on inpatient bed management |
| 11. Application of evidence based medicine: discontinuation of fungal blood cultures |
| 12. Selection and oversight of molecular diagnostics in microbiology |
2. Examples of utilization management initiatives in microbiology
2.1. Decision support: cytomegalovirus assays
Tests for cytomegalovirus (CMV) include antigenemia testing, viral load testing by quantitative polymerase chain reaction (qPCR), viral genotyping, shell vial culture, or serologic tests for the detection of a host immunologic response (CMV IgM and IgG antibody tests and antibody avidity tests). For an individual patient, the most appropriate test depends on the clinical indication. It is difficult for clinicians to keep up-to-date with esoteric tests in rapidly evolving specialties, especially when there are numerous tests that can be ordered. In these situations, the use of a decision support tool is an effective mechanism to assist physicians in proper test selection, potentially avoiding inappropriate test selection. As one example, Fig. 1 shows a screen display from the on-line laboratory handbook at the Massachusetts General Hospital. When the clinician types “cytomegalovirus” or “CMV” into the handbook's search function, the available tests and their appropriate indication are presented. In addition, the same decision-support information is provided in the electronic physician order entry (POE) system when a clinician views CMV-related test options. An advantageous feature of this approach is that when new tests become available, or outdated ones are removed from the test menu, the decision-support function can be updated accordingly. For example, the MGH microbiology laboratory recently discontinued the CMV antigenemia assay in favor of the CMV qPCR test. The information provided in the on-line handbook makes it clear that the preferred test has changed. This approach can be applied to many other tests in microbiology, particularly in areas such as molecular microbiology where new assays are supplanting more traditional assays at a rapid rate. The topic of decision support is covered in more detail in another chapter of this special edition.
Fig. 1.
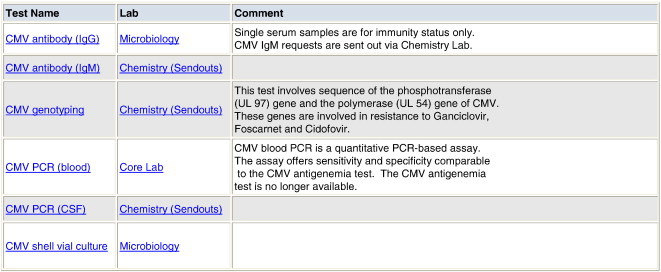
Laboratory on-line handbook display for query on cytomegalovirus testing at the Massachusetts General Hospital.
2.2. Reducing blood culture contamination and repeated orders
The problem of contamination of blood cultures from improper or poor technique is well known. It has been estimated that up to 5% of positive blood cultures may represent contaminants [1], resulting in significant increases in resource utilization. Consequently many hospitals have engaged in ongoing efforts to reduce blood culture contamination by improving staff training, or designating specific types of employees to collect the blood culture specimens. For example, blood cultures collected by medical house officers are more likely to be contaminated than those collected by phlebotomists [2]. In a retrospective study, Bates et al. studied the impact of contaminated blood cultures on hospital length-of-stay and hospital charges [3]. In patients with falsely positive blood cultures, there was a 4.5-day increase in the median length-of-stay and an increase in hospital charges of 33.4%. False-positive episodes were associated with increased pharmacy charges for intravenous antibiotics (39% increase) and laboratory charges (20% increase). In another study, Segal and Chamberlain assessed the impact of false-positive blood cultures in a pediatric emergency department [4]. The authors reported an increase in phone calls, return visits to the emergency department, unnecessary laboratory tests, inappropriate antibiotic administration and hospital admissions. Finally, Tabriz et al. evaluated the practice of repeating blood cultures serially [5]. Blood cultures were repeated in 31.6% of cases and amounted to approximately one-third of all blood cultures handled in the laboratory. The results of the repeated cultures showed no growth in 83.4% of cases, the same pathogen in 9.1% of cases, and a new pathogen or contaminant in 2.5% and 5.0% of cases respectively. The authors concluded that repeating blood cultures provides little additional yield and that guidelines for when to repeat blood cultures might decrease utilization.
2.3. Proper formatting of microbiology reports to avoid misinterpretation
Laboratory reports that are not optimally designed can lead to confusion among clinicians, with the potential for misdiagnosis or unnecessary requests for additional testing. Ackerman et al. evaluated the interpretation of 5 typical microbiology reports by physicians in a teaching hospital [6]. The investigators found that reports were often misinterpreted. For example, one report of “isolation of a gram negative rod from sputum was misinterpreted by 4 out of 5 physicians.” The reasons for misinterpretation were reported to be the use of jargon, unfamiliar names of bacterial species, or ill-defined reporting conventions, and the omission of a clear-cut conclusion in many reports. The misunderstandings resulted in both inappropriate use of antibiotics and orders for unnecessary testing in the laboratory. This study highlights the importance of developing clear, concise, standardized reporting formats in microbiology, and the need for the laboratory to work closely with physicians in designing and communicating microbiology reports.
2.4. Rapid identification of bacteria using matrix-assisted laser desorption-ionization time of flight mass spectroscopy (MALDI-TOF MS) to improve clinical decision making and guide antibiotic use
It has long been known that rapid bacterial identification and susceptibility testing lead to more appropriate use of antibiotics and a reduction in antimicrobial utilization [7]. In the past, rapid identification and susceptibility testing were mainly accomplished using automated instrumentation to perform conventional tests. More recently, manufacturers have developed MALDI-TOF MS for rapid organism identification, a method that has been demonstrated to reduce turnaround time for the identification of bacteria and yeasts by 1.45 days compared with conventional methods [8]. Another advantage of MALDI-TOF MS is its simplicity and the relatively low cost of consumables [9]. For example, one study performed at a large, academic medical center demonstrated potential cost savings to the laboratory (for reagents and labor) of $100,000 per year [8]. Although MALDI-TOF MS does not provide antimicrobial susceptibility data, rapid organism identification may help clinicians earlier to select an effective empirical antimicrobial strategy [10].
2.5. Antimicobial stewardship of high-cost, broad-spectrum antimicrobial agents such as carbapenems
Carbapenems including imipenem, meropenem, ertapenem and doripenem are broad spectrum antimicrobial agents active against many gram-positive, gram-negative and anaerobic organisms. They are highly effective when used appropriately but are also very expensive relative to potential alternative agents. Indiscriminant use of carbapenems will also contribute to antimicrobial resistance, decreasing the effectiveness of the drug class. Many hospitals have established antimicrobial formularies in the pharmacy to assist in management of expensive antibiotic drugs. In our institution some antibiotics are restricted and can only be obtained after approval from the division of infectious diseases. However, once approved there was no requirement or formal mechanism in place to re-evaluate the ongoing need for a restricted antibiotic once the results of microbiology culture and antimicrobial susceptibility testing became available. The clinical pathology staff in our microbiology laboratory recently began an antimicrobial stewardship program related to carbapenems. Each day, a microbiology fellow and laboratory director review the clinical history, culture results and susceptibility test results for all patients newly started on a carbapenem, to determine appropriate versus inappropriate use of the drugs. If objective data indicate that a patient's infection can be treated using a non-restricted agent, an email is sent to the clinician as shown in the example below.
“Dear Dr.________
Your patient ________ is currently receiving a restricted antibiotic: ertapenem. The use of this restricted drug is being monitored by the MGH Antimicrobial Stewardship Program.
Recent culture and antimicrobial susceptibility data from your patient reveal that the organism(s) is/are susceptible to other, non-restricted antibiotics (see sensitivity report below). Given these data, if clinically appropriate, please consider discontinuing the restricted carbapenem and/or changing to a non-restricted antimicrobial option. This may help reduce both the development of future resistance to these broad spectrum drugs and costs of therapy. If you have not already done so, you may request an infectious disease consult in order to obtain assistance on the choice of antimicrobial agents”.
After implementation of this stewardship effort, a decrease in carbapenem use was observed, and carbapenems have been removed from the “top 10” list of money spent on antimicrobials. The microbiology group is now extending the program to include other high cost antimicrobials.
2.6. Use of rapid point-of-care testing to support clinical decision making and reduce resource utilization
Point-of-care testing (POCT) performed at the patient's bedside provides rapid, real-time results. In some cases this facilitates clinical decision making and improves the efficiency of clinical operations [11]. A number of rapid point-of-care tests are available for the diagnosis of infectious diseases [12]. Among these are single use, visually read, lateral flow tests for influenza A and B. In a randomized, prospective controlled study by Bonner et al., the use of a rapid POC influenza test in a pediatric emergency department was associated with a significant reduction in laboratory tests ordered (complete blood count, blood cultures, urinalysis and urine cultures), a decrease in chest radiographs performed and a reduction in emergency department length-of-stay [13]. In another study of pediatric patients presenting to the emergency department with acute pharyngitis, the authors compared antibiotic use in patients who received a rapid streptococcus A test to those who were managed by conventional throat culture alone. They reported a decrease in antibiotic use of 50% when the rapid test was employed (22.45% versus 41.38%) and concluded that the rapid test significantly reduced unnecessary prescription of antibiotics [14].
2.7. Use of around-the-clock methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) testing to screen previously colonized patients before readmission to the hospital
Patients who are colonized with MRSA require contact precautions when admitted to the hospital. This entails placing them in a private room or cohorting the patient in a semi-private room with another colonized patient. In addition, hospital staff must wear gloves and gowns, and use dedicated equipment when interacting with the patient. Some of these patients also have a longer hospital length of stay due to delays in discharge to other health care facilities. Collectively, these features of MRSA colonization result in greater use of hospital resources compared with non-MRSA colonized patients. Because MRSA colonization can be transient, protocols have been put in place to identify previously MRSA colonized patients who are no longer colonized, and can be managed without contact precautions. In our institution, patients with a history of MRSA are eligible for discontinuation of contact precautions if the last documentation of MRSA colonization or infection was greater than 90 days prior, and if they screen negative for MRSA by one of two methods [15]:
-
1.
Three MRSA-negative nasal swab cultures on specimens collected at least 24 h apart
-
2.
A negative MRSA PCR test performed on a nasal swab.
For either screening method, the patient cannot have been received antibiotics active against MRSA during the 48 h prior to screening. Discontinuation of contact precautions on the basis of a single negative MRSA PCR is faster than screening using the culture-based method, and could result in an increase in discontinuation of contact precautions because of a reduction in the number of samples needed [15].
2.8. Use of screening urinalysis to reduce urine cultures in non-complicated adult community acquired lower urinary tract infection
Uncomplicated urinary tract infection is very common, especially among women. Various strategies have been employed to make (or rule out) a diagnosis of community acquired UTI in adult women without the need for urine culture. Some sources suggest that uncomplicated UTI in outpatients can be diagnosed and managed without culture, unless the patient fails treatment or has had recurrent UTIs [16], [17]. Others have even suggested that suspected UTI can be managed over the telephone in women with typical symptoms of cystitis and without vaginal symptoms or major co-morbidities [18]. This approach eliminates both office visit and any subsequent laboratory testing. Another approach is to limit urine culture utilization by pre-screening urine using various methods. For example, dipstick urinalysis for leukocyte esterase and nitrites has a high negative predictive value, and may be used to exclude bacteriuria without a culture step when the results are negative. Studies have also demonstrated that urine can be screened for significant bacteriuria prior to culture using automated urine sediment examination [19] or flow cytometry [20].
2.9. Restricting stool examinations in hospital-acquired diarrhea
Diarrhea is a common complaint among hospitalized patients. Although it is common for clinicians to request a routine stool culture and ova and parasites (O&P) examination for patients with diarrhea, these tests are designed to detect agents of community-acquired rather than hospital-acquired infection. A number of studies have indicated that routine stool culture or stool O&P examination is usually not warranted in adult patients who develop diarrhea more than three days after admission to the hospital [21], [22], [23]. In contrast, testing for Clostridium difficile should be considered, as this is a major cause of nosocomial diarrheal illness. New molecular diagnostic tests offer the promise of providing rapid and reliable testing for C. difficile. Highly sensitive molecular testing could potentially permit rapid rule-out of C. difficile, obviating the need for unnecessary antibiotics and contact precautions in many patients.
2.10. Use of viral respiratory virus panels to assist in utilization management of inpatient beds
Upper respiratory viral infections in the United States are frequently caused by rhinoviruses, coronaviruses, influenza A and B, parainfluenza, respiratory syncytial virus, adenovirus or metapneumovirus. While many of these illnesses can be managed on an outpatient basis, patients with severe illness or major comorbidities are often admitted to the hospital. Usually they present first to the emergency department, where the initial challenge is to confirm the presence of a respiratory viral infection and then to identify the specific offending virus in order to direct specific therapy (if available) and aid in hospital bed assignment. Often, respiratory viral infections occur in seasonal epidemics (especially influenza A and B), resulting in hospital overcrowding and a shortage of hospital beds. When this occurs, managing the availability of hospital beds becomes a priority. In cases where the offending virus has been identified, contact and/or droplet precautions must be instituted to prevent transmission between patients as shown in Table 2 . Patients on contact or droplet precautions must be placed in a private room. Alternatively, if two patients are infected with the same respiratory virus, they can be cohorted together in one hospital room. In our hospital, we offer rapid molecular diagnostic testing for influenza A and B for patients who require hospital admission for a flu-like illness. Testing is performed 24 h per day, 7 days per week, in order to facilitate bed management and timely initiation of anti-viral therapy. We also offer a respiratory viral panel by direct immunofluorescence for the many viruses listed above, 7 days per week during peak respiratory virus season. The ability to identify the specific virus causing the infection greatly assists in managing our inpatient beds and maintaining effective infection control measures. The savings to the hospital from improved bed management during epidemics greatly exceeds the cost of the testing in the laboratory.
Table 2.
Contact and droplet precaution guidelines for patients with respiratory viral infections at the Massachusetts General Hospital.
| Influenza | Parainfluenza | Adenovirus | RSV | |
|---|---|---|---|---|
| Contact | No | Yes | Yes | Yes |
| Droplet | Yes | No | Yes | No |
Key: RSV: respiratory syncytial virus.
3. Application of evidence based medicine: an example related to fungal blood cultures
Many clinical laboratories offer “fungal blood cultures” that employ specialized media designed to enhance the detection of yeast or mold fungemia. However, it is well-established that specialized fungal blood culture media are not superior to routine blood culture media for the detection of Candida fungemia (candidemia) [24], [25]. Thus, the main rationale for the use of fungal blood cultures is to improve detection of cryptococcal yeast, endemic fungi (Histoplasma capsulatum, Coccidioides spp., etc) and filamentous fungi (e.g., Aspergillus spp.) in the blood. Until recently, our institution offered fungal blood cultures using Myco/F Lytic bottles (Becton Dickinson) designed for use with the BACTEC automated culture monitoring system. This approach was costly, not only in terms of reagent costs but also in terms of technical labor. The highly-enriched and non-selective culture medium needed to be incubated for up to 30 days before a negative result could be obtained, and this frequently promoted the growth and eventual detection of skin contaminants that would not have been detected using routine blood culture bottles and a 5 to 7 day incubation period. Thus, we reviewed our experience with this approach to understand whether or not it provided a significant clinical benefit.
We reviewed the results of all fungal blood cultures over a 44 month period. During this time period, 5544 Myco/F Lytic fungal blood cultures were performed. Our review revealed the following:
-
•
No dimorphic fungi were recovered by fungal blood culture.
-
•
Mold (Fusarium sp.) was recovered twice from a single patient using fungal blood culture. However, in this case Fusarium was also recovered from 2 sets of routine blood cultures (2 out of 4 bottles), several days prior to recovery from fungal blood culture.
-
•
Cryptococcus neoformans was recovered from 3 patients by fungal blood culture. Two of the patients had cryptococcal meningitis, and in both cases the organism was detected in numerous other ways (CSF and blood cryptococcal antigen tests, CSF gram stain, routine and fungal CSF cultures, and routine blood cultures). The third patient had cryptococcal fungemia (but not meningitis); a cryptococcal antigen test on blood was positive, and the organism was recovered twice from routine blood cultures.
Based on these findings, we concluded that fungal blood cultures had failed to detect any dimorphic fungi, molds or cryptococcal yeast that were not otherwise detected by routine blood culture. Furthermore, blood culture is not a useful method for the detection of invasive infection caused by molds or dimorphic fungi. Therefore, blood culture specifically designed for the detection of fungi was discontinued at our institution.
3.1. Microbiology testing by molecular methods
Many microbiology tests suffer from drawbacks that limit their clinical utility. For example, results from traditional culture methods may take several days to become available, and antibiotic treatment prior to specimen collection may degrade sensitivity. Serologic studies often cannot distinguish current from past infection unless acute and convalescent specimens are available. Techniques for specialized cultures such as for viruses are beyond the capability of most hospital laboratories. Finally many microbiology tests do not yield quantitative results which may be desirable in some situations. Recent advances in molecular diagnostic techniques for microbiology offer promise to resolve some of these issues. Some molecular tests, such as those for influenza A and B, hepatitis B virus DNA and HIV viral load are requested in sufficient volume that many hospital laboratories are able to offer the tests in-house. In these examples, the molecular diagnostic test is either superior to alternative testing methods or provides unique information of clinical importance (e.g. viral load). However, in many cases the volume of requests for molecular microbiology tests is too low to warrant performing the test in the hospital laboratory. Typically these tests are sent to outside reference laboratories for analysis which, over time, can prove very expensive. Intuitively a molecular diagnostic test is expected to be highly sensitive and specific, but this is not always the case. In some situations, conventional microbiology tests are more appropriate [26]. For this reason, it is important for the clinical microbiology director to work with infectious disease specialists to scrutinize the send out budget for potential inappropriate test ordering. Further, such analysis may reveal opportunities for in-sourcing the testing at significant savings. For example, our microbiology laboratory recently in-sourced nucleic-acid testing for Epstein Bar Virus (EBV) saving over $100,000 per year and providing superior turnaround time.
3.2. Conclusions
Tests performed in the clinical microbiology laboratory are ripe for utilization management efforts. Clinicians are frequently confused about which test to order, and appropriate test selection can be guided through decision support mechanisms and gatekeeper functions. The diagnostic yield of routine cultures can be improved by facilitating proper specimen collection and transport, or screening specimens prior to culture. Clinical decision making, particularly in selecting appropriate antibiotics and implementing (or discontinuing) infection control measures, can be aided by efforts to reduce turnaround time and by antimicrobial stewardship efforts directed by microbiologists. Finally, errors can be prevented by carefully formatting reports to avoid miscommunication.
References
- 1.Zwang O., Albert R. Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med. 2006;1:272–276. doi: 10.1002/jhm.115. [DOI] [PubMed] [Google Scholar]
- 2.Rao G., Crook M., Tillyer M. Pathology tests: is the time for demand management ripe at last. J Clin Pathol. 2003;56:243–248. doi: 10.1136/jcp.56.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates D., Goldman L., Lee T. Contaminant blood cultures and resource utilization: the true consequences of false positive results. JAMA. 1991;265:365–369. [PubMed] [Google Scholar]
- 4.Segal G., Chamberlain J. Resource utilization and contaminated blood cultures in children at risk for occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:469–473. doi: 10.1001/archpedi.154.5.469. [DOI] [PubMed] [Google Scholar]
- 5.Tabriz M., Riederer K., Baran J., Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect. 2004;10:624–627. doi: 10.1111/j.1469-0691.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman V., Obbink d, Pritchard R., Lee B. Consumer survey on microbiology reports. Lancet. 1979;313:199–202. doi: 10.1016/s0140-6736(79)90593-2. [DOI] [PubMed] [Google Scholar]
- 7.Kerremans J., Verboom P., Hallart-van-Roijen L., Goessens W., Verbrugh H., Vos M. Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. J Antimicrob Chemother. 2008;61:428–435. doi: 10.1093/jac/dkm497. [DOI] [PubMed] [Google Scholar]
- 8.Tan K.E., Ellis B.C., Lee R., Stamper P.D., Zhang S.X., Carroll K.C. Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol. 2012;50(10):3301. doi: 10.1128/JCM.01405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel R. MALDI-TOF mass spectroscopy: transformative proteomics for clinical microbiology. Clin Chem. 2013;59:340–342. doi: 10.1373/clinchem.2012.183558. [DOI] [PubMed] [Google Scholar]
- 10.Clerc O., Prod'hom G., Vogne C., Bizzini A., Calandra T., Greub G. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis. 2013;56(8):1101–1107. doi: 10.1093/cid/cis1204. [DOI] [PubMed] [Google Scholar]
- 11.Lee-lewandrowski E., Lewandrowski K. Implementing point-of-care testing to improve outcomes. J Hosp Admin. 2013;2:125–132. [Google Scholar]
- 12.Dekker J. Infectious disease testing at the point-of-care. Point Care. 2012;11:85–89. [Google Scholar]
- 13.Bonner A., Monroe K., Talley L., Klasner A., Kimberlin D. Impact of the rapid diagnosis of influenza on physician decision making and patient management in the pediatric emergency: results of a randomized, prospective controlled study. Pediatrics. 2003;112:363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 14.Ayanroug S., Waseem M., Quee F., Humphrey A., Reynolds T. Impact of rapid streptococcal test on antibiotic use in a pediatric emergency department. Pediatr Emerg Care. 2009;11:748–750. doi: 10.1097/PEC.0b013e3181bec88c. [DOI] [PubMed] [Google Scholar]
- 15.Shenoy E.S., Kim J.-Y., Rosenberg E.S. Discontinuation of contact precautions for methicillin-resistant Staphylococcus aureus: a randomized controlled trial comparing passive and active screening with culture and polymerase chain reaction. Clin Infect Dis. 2013;57(2):176–184. doi: 10.1093/cid/cit206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamm W.E., Hooten T.M. Management of urinary tract infections in adults. N Engl J Med. 1993;329:1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M.L., Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38:1150–1158. doi: 10.1086/383029. [DOI] [PubMed] [Google Scholar]
- 18.Wright O., Safranek S. Urine dipstick for diagnosing urinary tract infection. Am Fam Physician. 2006;73:129–132. [PubMed] [Google Scholar]
- 19.Falbo R., Sala M.R., Signorelli S., Venturi N., Signorini S., Brambilla P. Bacteriuria screening by automated whole-field-image-based microscopy reduces the number of necessary urine culture. J Clin Microbiol. 2012;50(4):1427–1429. doi: 10.1128/JCM.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S., Kim Y., Lee S. Evaluation of the Sysmex UF-100 urine cell analyzer as a screening test to reduce the need for cultures for community-acquired urinary tract infection. Am J Clin Pathol. 2007;128:922–925. doi: 10.1309/4606EC29U50DVAFY. [DOI] [PubMed] [Google Scholar]
- 21.Decre D., Barbut F., Petit J. Role of the microbiology laboratory in the diagnosis of nosocomial diarrhea. Pathol Biol. 2000;48:733–744. [PubMed] [Google Scholar]
- 22.Rao G., Ozerek A., Jeans A. Rational testing for faeces in the investigation of sporadic hospital-acquired diarrhoea. J Hosp Infect. 2001;47:79–83. doi: 10.1053/jhin.2000.0845. [DOI] [PubMed] [Google Scholar]
- 23.Mathieu A., Tachet A., Pariente A. When should a stool culture be done in adults with nosocomial infections. Presse Med. 2005;34:81–84. doi: 10.1016/s0755-4982(05)88232-6. [DOI] [PubMed] [Google Scholar]
- 24.Kirby J.E., Delaney M., Qian Q., Gold H.S. Optimal use of Myco/F Lytic and standard BACTEC blood culture bottles for detection of yeast and mycobacteria. Arch Pathol Lab Med. 2009;133:93–96. doi: 10.5858/133.1.93. [DOI] [PubMed] [Google Scholar]
- 25.CLSI . CLSI document M47-A. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. Principles and procedures for blood cultures; approved guideline. [Google Scholar]
- 26.Forbes B. Introducing a molecular test into the clinical laboratory: development, evaluation, and validation. Arch Pathol Lab Med. 2003;127:1106–1111. doi: 10.5858/2003-127-1106-IAMTIT. [DOI] [PubMed] [Google Scholar]


