Abstract
This review presents the applications of intestinal cell models of human and pig origin in food and nutritional sciences and highlights their potential as in vitro platforms for preclinical research. Intestinal cell models are used in studies of bioavailability, adsorption and transport in nutritional or toxicological settings, allergic effects of food components, as well as probiotics and/or host–pathogen gut interactions. In addition, this review discusses the advantages of using specialized and functional cell models over generic cancer-derived cell lines.
Introduction
Cell models are gaining a growing interest among the scientific research community, resulting in their wider use in the pharmaceutical and cosmetic industry in particular. They are becoming more realistic and representative of the in vivo physiology and therefore offer a suitable alternative for in vivo animal testing. This is due to several reasons. By means of modern miniaturization and automation techniques, cell culture models can support massive screening and cost effectiveness in contrast to the more expensive animal trials with limited screening capacity. Another driving force behind their growing popularity is the generally negative view of animal testing by the wide public. In effect, cell models are in line with the three R paradigm (Reduce, Refine, Replace). In this spirit, the EU will ban the marketing of cosmetic products developed using repeated dose animal experimentation by 2013, which raises a profound need for efficient alternative methods. The new EU regulation on chemicals (Regulation (EC) No 1907/2006 of the European Parliament and of the Council, 2006 - REACH) demands toxicological data for all chemicals, forcing industry into testing a large panel of potentially hazardous substances. Additionally, cell line models simplify biological systems to an easier and more manageable platform for research. The establishment of standardized and validated cell culture based methods for toxicology, food borne diseases, bioavailability and metabolism studies in the field of food science and nutrition can bring intestinal cell models to a new era due to their simplicity, reliability and reproducibility.
The present review aims to overview the current applications of intestinal cell models in food science and nutrition and to discuss the advantages that novel, specialised cell lines can offer.
Intestine –morphology & functionality
The intestine is a tubular structure extending from the gastric pylorus to the anus and acts as a highly selective barrier and communication organ between the nutritional environment and the mammalian metabolism. Histologically, the small intestine can be divided into the duodenum, jejunum and ileum, although precise boundaries between these three segments are not observed macroscopically or microscopically (Cencic & Langerholc, 2010). Due to folds and invaginations, the presence of millions of villi on the apical surface amplifies the mucosal surface area to approximately 400 m2. In contrast, the large intestine, is wider, shorter (100–150 cm) and has a smoother surface (Klein, Cohn, & Alpers, 2006). The intestinal epithelium is continuously renewing itself at an unprecedented rate, which on the downside makes it susceptible to malignant transformations (Radtke & Clevers, 2005).
The ingested food composed of complex molecules is digested through physical and chemical processes followed by absorption of the refined macro- and micronutrients, which mainly takes place in the duodenum and upper jejunum. Absorption is mainly performed by enterocytes using active transporters, passive diffusion, and transcytosis (Shimizu, 2010). During this process, nutrients are metabolized into a form suitable for transport to target organs or even metabolized by phase I and phase II metabolic enzymes.
The gastrointestinal tract is the body’s largest endocrine organ and releases more than 20 different regulatory peptide hormones that influence a number of physiological processes including the regulation of appetite, which has raised interest as target for combating obesity (Murphy & Bloom, 2006). The intestine houses a major part of the body’s immune system (GALT - gut-associated lymphoid tissues) and is contributing to the defence of the host against bacterial, viral, parasitic, and food component antigens that are constantly present in the intestinal lumen. Cells of the innate immune system include dendritic (antigen sampling) and intra-epithelial Paneth, M (antigen transport), cup and Goblet cells (secretion of defensive peptides and mucus), macrophages and myelomonocytic cells, as well as cells of the adaptive immune system that regulates antigen-specific immune responses (Klein, et al., 2006).
Each epithelial cell maintains intimate association with its neighbours and seals the surface of the gut with tight junctions (Macdonald & Monteleone, 2005), which act as a physical and biological barrier to prevent passage of larger molecules and microorganisms from gut lumen to the underlying connective tissue (lamina propria). This barrier is of pivotal role for maintaining body health and homeostasis. Finally, the adult human intestine is host to approximately 100 trillion microorganisms, which endow us with functional features that we have not had to evolve ourselves (Backhed, Ley, Sonnenburg, Peterson, & Gordon, 2005).
Limitations of established cell line models and their alternatives
Complexity and need for well-characterized cell lines
The intestinal wall is composed of several cell types that form the epithelial barrier (mainly enterocytes), accompanied by immune cells on the basolateral side of the epithelia. A perfect in vitro cell model should be a direct substitute for the in vivo environment, reflecting both the natural responses as well as the complex physiology of the intestine.
Therefore, cell lines involved in single or co-culture models must be sufficiently characterised prior to its successful use. This characterization includes epithelial markers (cytokeratins), brush border enzymes (digestive enzymes, etc.), proper formation of tight junctions and polarization (ZO-1, occludins and claudins; transepithelial electrical resistance-TEER/transepithelial electrical potential-TEEP), molecular transporters, internal metabolism (cytochromes P450 -CYPs) and responsiveness to endo/paracrine hormones or inflammation molecules (lipopolysaccharide-LPS, cytokines). Depending on the model, cells in different activation/differentiation state can be used. For example, immature non-activated immune cells are useful in host–bacterial intestinal interaction models, while activated cells can be applied to study inflammatory intestinal disorders (Sergent, Piront, Meurice, Toussaint, & Schneider, 2010).
Limitations of cancer-derived and virus immortalized cell lines
Primary cell cultures are rarely used in models due to limitations with repeatability and long-term studies because of their short life span. Most of the current cell line intestinal models are using transformed cell lines (Table 1 ), isolated from cancer affected individuals. Among them, only Caco-2, the most widely used and established cell line has been studied in detail. Despite the non-specificity of the existing cancer-derived cell lines, as well as their other limitations such as altered glycosylation, unresponsiveness to hormones/cytokines and inadequate expression of proteins defining epithelial character (Peracaula, Barrabes, Sarrats, Rudd, & de Llorens, 2008), they have been extensively used in specific applications of intestinal simulation. Additionally, cell passage dependent protein expression or other functional changes should be documented (e.g. for Caco-2 there are inter-laboratory variations in transepithelial resistance as much as 20-fold) (Hidalgo, 2001). A common alternative to cancer-derived cell lines is cell immortalization by infection with viruses such as Epstein–Barr, SV40 or transfection with anti-apoptotic proteins, but metabolic and morphological changes are often observed (Q. Chen, Liang, Fromm, & Overbeek, 2004). In addition, cells of non-human or non-intestinal origin are commonly accepted as the model for the human intestine.
Table 1.
Available cell models of porcine and human gut. Only the initial or topic-relevant references are listed.
| Cell | Origin | Characteristics and applications | References |
|---|---|---|---|
| Caco-2 | Human colon adenocarcinoma | Most widely studied and applied model, differentiation into enterocyte-like cells, under-expression of P450 enzymes | (Sambuy, et al., 2005) |
| HT-29 | Human colon adenocarcinoma | Mucin producing goblet cells | (Chen, Drabkowski, Hay, Macy, & Peterson, 1987) |
| LS180 | Human colon carcinoma | Mucin expression | (Masago, et al., 2010) |
| CaSki | Human small intestine metastatic cervical cancer | Contains human papilloma virus | (Pattillo, et al., 1977) |
| Intestine 407 | Human small intestine/HeLa | Mixed cell line with HeLa (cervix adenocarcinoma epithelial cell line), contains human papilloma virus | (Henle & Deinhardt, 1957) |
| T84 | Human colonic carcinoma (lung metastasis) | Ion transport studies | (Wasserman, et al., 1988) |
| TC-7, PD7, PF11, PD10, C2BBe1 | Caco-2 subclones | Similar to Caco-2 | (Sambuy, et al., 2005) |
| HIEC-6 | Human small intestine Normal |
Transport and physiology studies | (Beaulieu, 1997) |
| H4 | Human small foetal intestine Normal |
Studies of the infant gut development Expression of cytokines upon activation |
(Nanthakumar, Fusunyan, Sanderson, & Walker, 2000) |
| H4-1 | Human small foetal intestine Normal |
Studies of the infant gut development, host–pathogen interactions, anticancer drug tests, develops moderate to high transepithelial electrical resistance | (Cencic & Langerholc, 2010) |
| LLC-PK1 | Pig kidney epithelial cells Normal |
Differentiation, formation of tight junctions, transport studies | (Li, Chung, & Shim, 2002) |
| IPEC-J2 | Neonatal pig small intestine Normal |
Studies of the neonatal gut development | (Berschneider, 1989) |
| IPEC-J2-3 | Neonatal pig small intestine Normal |
Studies of the neonatal gut development Develops high transepithelial resistance |
(Nissen et al., 2009) |
| IPEC-J2-9 | Neonatal pig small intestine Normal |
Studies of the neonatal gut development Mucin producing cell line |
(Cencic & Langerholc, 2010) |
| PSI-1 | Pig mature small intestine Normal |
Develops high transepithelial resistance Host–pathogen interactions, immunological studies, probiotic research |
(Cencic and Langerholc, 2010, Nissen et al., 2009) |
| CLAB | Pig enterocytes from small intestine Normal |
Studies of paracellular transport, host–pathogen interactions, immunological studies, probiotic research Mucin secretion |
(Cencic and Langerholc, 2010, Nissen et al., 2009) |
| TLT | Human monocyte/macrophage Normal | Intestinal functional model (in combination with human epithelial cell lines) | (Cencic and Langerholc, 2010, Maragkoudakis et al., 2010) |
| PoM | Pig monocyte/macrophage Normal | Intestinal functional model (in combination with pig epithelial cell lines) | (Cencic & Langerholc, 2010) |
Alternatives to one-dimensional model set ups
Currently, most in vitro intestinal models are cultivated as one-dimensional (1D) monolayers on plastic surfaces, where the establishment of a functional, epithelial character is not defined, possibly resulting in misleading results (Wagner, Krul, Moberly, Alpers, & Schonfeld, 1992). On the other hand, growing epithelial cells on microporous membranes (e.g. insert filters, Transwell models) leads to spontaneous cell differentiation and polarization (Tremblay, et al., 2006), enabling basolateral feeding of epithelia similar to the in vivo setting (Fig. 1 ). Additionally, microporous membranes facilitate bioavailability and transport studies through the polarised epithelial monolayer. To achieve better functionality, epithelial cells growing on microporous membranes can be co-cultured with same-species derived monocyte/macrophages growing in the lower compartment or on the microporous membrane using an inverted setting (Fig. 1). In this way the in vivo environment of the intestine can be simulated more accurately and the chemical crosstalk (chemokine and cytokine release) between the intestinal and immune cells in response of an antigenic stimulus can be studied in detail (Westendorf, Fleissner, Hansen, & Buer, 2010). Furthermore, since intestinal epithelia is not only populated by enterocytes, they can be co-cultured with other more-specialized cell types on microporous membranes, like mucus-producing Goblet cells (Mahler, Shuler, & Glahn, 2009). Mucins, mainly present in the colon, physically protect the epithelia from commensal bacteria (Dharmani, Srivastava, Kissoon-Singh, & Chadee, 2009) and could represent a diffusion barrier for larger proteins and lipophilic substances (Larhed, Artursson, & Bjork, 1998).
Fig. 1.
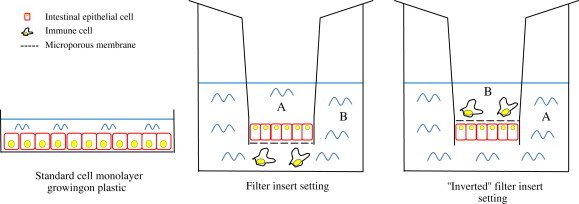
Experimental intestinal cell model settings. Epithelial cells can be grown on plastic or on microporous membranes (filter inserts) in the presence of immune cells, leading to better cell polarization and differentiation due more accurate simulation of the in vivo intestinal environment (A = apical; B = basolateral). Cells can be grown in a conventional manner with apical side upwards, or alternatively, the filter with polarized cells can be inverted, so that the top of the membrane is equivalent to the basolateral side. In both conventional and inverted setting co-cultured immune cells, substances and microorganisms can be added to apical or basolateral side, enabling functional studies described in the main text on both sides of the epithelia. The inverted setting is particularly appropriate for mechanistic studies of the basolateral side of the epithelia with underlying (immune) cells, since gravity forces the cells to make closer contacts.
Functional cell line models
A good alternative to cancer-derived or virus-immortalized cells are normal cell lines, prepared by a limiting dilution technique of primary cells isolated from healthy dissected tissue (Coller & Coller, 1986), where tissue characteristics are generally better preserved. Using this method several complete functional intestinal epithelial models of the gut of various animal species were developed (Cencic & Langerholc, 2010) in the frame of the PathogenCombat project (EU-FP6 programme). Examples include the human foetal small intestinal cell line H4 (Nanthakumar, Fusunyan, Sanderson, & Walker, 2000) which has been subcloned to obtain a small intestinal epithelial cell line capable of TEER and TEEP formation (Table 1). Similarly, the IPEC-J2 cell line, originally derived from jejunal epithelia isolated from a neonate piglet (Schierack, et al., 2006) was subcloned to obtain cultures of enterocyte-like and mucin producing cell types.
Out of all the animal-derived models obtained, the pig intestinal cell model is of increased interest. Due to its similarity with the human gut, it represents a good platform for the simulation of various human intestinal applications (Lunney, 2007, Nissen et al., 2009, Pipenbaher et al., 2009). This pig functional intestinal cell model is a co-culture composed of the CLAB (adult mucin secreting enterocyte-like) or PSI (partially differentiated cryptic enterocyte-like) intestinal lines and of the PoM monocytes/macrophages that can be differentiated into dendritic cells. All of these cell lines were characterized with respect to relevant molecular markers, responses to common activators, functional characteristics as well as host–pathogen interactions (Maragkoudakis et al., 2010, Nissen et al., 2009, Pogacar et al., 2010), providing valuable tools for future in vivo gut research.
It is important to stress here the potential future applications of intestinal stem cells (Umar, 2010), which can be differentiated into all cell types populating intestinal epithelia. Controlled differentiation could be used for establishment of new specialised functional cell models, but apart from differentiation studies (Simon-Assmann, Turck, Sidhoum-Jenny, Gradwohl, & Kedinger, 2007) no such applications have been described in the literature yet.
Applications of intestinal models in food, health and nutritional science
Bioavailability and membrane transport of nutrients and drugs
Bioavailability is directly linked to nutritional efficiency, since from the total nutrients intake from food usually a lower amount is up-taken and being accessible for metabolic functions in the host. Bioavailability includes bioaccessibility and bioactivity, although these two definitions are usually used indistinguishably. Bioaccessibility includes events that take place during food digestion from mouth to colon, which transform nutrients into potentially bioaccessible material that can be absorbed through the epithelia and/or pre-systemically metabolically changed. Bioactivity describes transport to the target tissue, interactions with biomolecules and physiological response as a consequence of the ingested nutrient (Fernandez-Garcia, Carvajal-Lerida, & Perez-Galvez, 2009).
There are several approaches using cell models for estimation of nutrient bioavailability. Most of them rely on measuring transport through intestinal epithelia as the bottleneck in the process. In such experiments, epithelial cells growing on a microporous membrane form a barrier between the apical and basolateral compartments. When substances are added to the apical side, time dependent concentration on the basolateral side is measured, usually by HPLC. Alternatively, reverse transport can be measured by adding substances to the basolateral side. Intestinal cell models have also been used for bioavailability studies of drugs. The experimental layout based on microporous membranes generally gives a good correlation with in vivo pharmacological data and can differentiate between slow, intermediate and fast absorbed substances.
Most of the bioavailability studies were done with Caco-2, TC7 and T84 cells, especially to find nutrition formulas dealing with micronutrient (iron, zinc) deficiencies (Beiseigel et al., 2007, Sharp, 2005), plant polyphenols (Gao, Jiang, Yin, & Hu, 2010), flavonoids (Tian, Yang, Yang, & Wang, 2009) and other bioactive substances (Madgula, et al., 2008). Existing intestinal models for permeability studies have several drawbacks. Most of the used cell lines require prolonged cultivation before they polarize and differentiate into enterocyte-like cells (up to 21 d for Caco-2, Markowska, et al., 2001), although shorter times have been reported for LLC-PK1 (Da Violante, et al., 2004). In addition, inaccurate bioavailability results can be obtained because of non-specific drug binding to plastic devices observed at lipophilic compounds. Protocols, media composition, in vitro digestion of food matrix prior to application onto the cells, pH, culturing conditions as well as cell passage number are not standardized, which is reflected by largely different permeability values obtained by different laboratories for the same substances (Ingels & Augustijns, 2003). Apart from technical problems, another issue concerns the suitability of in vitro intestinal cell models in reflecting in vivo permeability. Most of the existing cell line models are cancer-derived, with significantly different gene expression as of the normal human intestinal cells (Hayeshi, et al., 2008). Changes in expression of transporters and metabolic enzymes in cell models can lead to under- or overestimation of intestinal epithelial permeability.
Transport across the epithelia is key in understanding bioavailability. Various transporters and carriers are known and they represent important factors that affect intestinal drug absorption, nutrient bioavailability and they constitute the first defence against xenobiotic uptake (Wacher, Silverman, Zhang, & Benet, 1998). P glycoprotein (Pgp) was the first multidrug resistance ATP-binding cassette (ABC)-transporter to be identified (Riordan & Ling, 1979). Other members of the family are multidrug resistance transporters MRP1 and MRP2, primarily transporting intracellularly formed drug conjugates (Borst & Elferink, 2002).
The most common disadvantage of existing cell lines is a lack of expression of certain influx and efflux pumps. There were attempts to restore them by transfection, although loss of intracellular regulation, synergistic and antagonistic effects can take place (Tang, Horie, & Borchardt, 2002). Besides the transporters, some cell lines are more appropriate for measuring passive paracellular permeability than others, depending on the formation of tight junctions between polarized cells. Typical TEER in the jejunum is estimated in the range of 25–40 Ω∗cm−2 with paracellular pore radius 9.0 ± 0.2 Å. The TEER of Caco-2 cell line was reported to be significantly smaller, varying from 80 to 1420 Ω∗cm−2 with pore size 3.7 ± 0.1 Å (Tavelin, et al., 2003) and as a result a lack of correlation to in vivo data has been observed for paracellularly transported compounds (Hidalgo, 2001). In contrast, the PSI cell line with TEER values reaching 7000 Ω∗cm−2 is a particularly suitable model to study active transport, while the CLAB cell line (reaching 50–100 Ω∗cm−2), is more fitted for paracellular transport studies. From human models, the H4 cell line is superior to Caco-2, since TER values reach 200–400 Ω∗cm−2 and are physiologically close to the normal tissue (Nanthakumar, et al., 2000).
Metabolism
Metabolism in the intestinal epithelia is attributed to phase I and phase II metabolizing enzymes. Phase I enzymes are primarily CYPs, which transform lipophilic exogenous substrates into more polar products, but also contribute to oxidative metabolism of cytokines, fatty acids and endogenous hormones (Nebert, 1991). CYP3A is the most common member of the superfamily expressed in the human small intestine, present in mature enterocytes lining the villus (Yang, Tucker, & Rostami-Hodjegan, 2004). Many carcinogens are activated by CYPs into highly reactive electrophiles, since they can react with targets such as DNA. Therefore phase I metabolites are often conjugated by phase II enzymes (glutation S-transferases, sulfotransferases and UDP-glucoronosyltransferases) before excretion from the cells by efflux transporters (Nies, Schwab, & Keppler, 2008).
Intestinal metabolism can lead to a reduced bioavailability of substances, especially if the metabolites are substrates for apical efflux pumps. Numerous natural food compounds (some indoles and flavonoids) and contaminants (polycyclic aromatic hydrocarbons, toxic heavy metals) have been reported to change levels of metabolizing enzymes (Sergent, et al., 2008). Furthermore, synergistic or antagonistic effects of substances in complex food matrixes on intestinal metabolism can lead to significant differences in bioavailability of isolated compounds.
Lack of metabolizing enzymes is a significant disadvantage in most of the intestinal models. Caco-2 cell-based models in addition to other disadvantages fail to express appreciable quantities of CYP3A (Paine, et al., 1997). Most of the other existing intestinal models in this context have not been characterized in detail, but non transformed cell lines are expected to be superior and closer to the in vivo situation.
Toxicity studies
Intestinal cell models can be applied in the risk assessment analysis of toxic substances that can be found in the food chain, such as toxins of microbial origin or chemicals. In most cases, functional cell models have not been utilized for such kind of studies, which are restricted mainly to the use of Caco-2, with the disadvantages that have been already described.
Biological toxins
Clostridium botulinum is a Gram + anaerobic food pathogen which is responsible for botulism, a serious paralytic disease caused by the neurotoxin that the organism is producing. Guinea pig intestinal epithelium cells have been used for the identification of functional subunits of the botulinum toxin, as well as for the study of the toxin subcomponents binding and passage across the intestinal barrier (Fujinaga, et al., 2004). The binding mode of the type A toxin has also been investigated using an intestinal cell line of human origin as a model, Intestine 407 (Kojima, et al., 2005). In addition, well established cell lines models have been applied to study the botulinum toxin, such as HT-29 cells for the study of the receptor and internalization transporter of the type C toxin (Nishikawa, et al., 2004) or Caco-2 cells for the role of non-toxic components in the permeation capacity of the type D toxin (Niwa, et al., 2007).
Cell line models have also been applied to study mycotoxin toxicity and interactions with the intestinal barrier. HT-29 and Caco-2 cell models were used to elucidate the permeability and absorption of Ochratoxin A, which is a potent teratogenic, mutanogenic and carcinogenic mycotoxin (Maresca, Mahfoud, Pfohl-Leszkowicz, & Fantini, 2001). Similar cell models were also used to investigate the effects of Patulin on the intestinal barrier (Mahfoud, Maresca, Garmy, & Fantini, 2002), which is another potent mycotoxin that apart from being mutagenic, carcinogenic and teratogenic, can also induce intestinal injuries.
Chemical toxic compounds
Butyltins are toxic compounds widespread in the environment due to their use in industrial production of biocides and polymer stabilizers. Oral exposure to butyltins leads to their interaction with intestinal barrier. As a result, Caco-2 cell cultures have been used in vitro to study the butyltin permeability of the intestinal barrier (Azenha, Evangelista, Martel, & Vasconcelos, 2004). Caco-2 cell cultures have also been used to investigate the mutagenic potential of melanoidins which are formed during household cooking procedures (through the Maillard reaction) and may present a potential health threat (Glosl, et al., 2004).
In addition, intestinal cell models have also been applied in pesticide absorption studies, since toxic residues can be detected in food and water, and therefore the oral route would be the major mode of exposure in humans and animals. For example, the permeability to the neonicotinoid imidacloprid has been investigated in Caco-2 cell lines, in order to assess the risk to mammals due to chronic exposure (Brunet, Maresca, Fantini, & Belzunces, 2004). Other examples include the in vitro cytotoxicity studies of pesticides such as the insecticides phenylpyrazole, using Caco-2 cells (Vidau, Brunet, Badiou, & Belzunces, 2009) or clorpyrifos, which is also intended for domestic use, using Caco-2 and TC7 cells (Tirelli, et al., 2007), as well the widespread anti-fungal pesticide imazalil, using Caco-2 cells (Sergent, et al., 2009).
Allergies
Food related allergies are widespread and constitute a major problem for the consumer, since it can result in a much reduced quality of life, apart from the serious clinical implications involved. The prevalence of food allergy has been estimated to range between 3 and 4% of the adult population, while it can reach up to 6% in children (Sicherer & Sampson, 2006). Ingested food allergen components come in contact with the intestinal epithelium and cell models have been used as in vitro platforms for the investigation of the mechanism of action of allergen transport across the intestinal barrier.
One of the most common food related allergies is the cow milk allergy, mainly associated with β-lactoglobulin, causing cutaneous, intestinal or even respiratory symptoms. Intestinal cell models of Caco-2 have been used to study the effects of the various milk-processing treatments to reduce allergenicity of β-lactoglobulin, such as trypsin digestion, reduction/alkylation, microbial fermentation and heat denaturation (Bernasconi, Fritsche, & Corthesy, 2006).
Wheat allergy can cause atopic dermatitis in children and anaphylaxis in adults. The mechanism of intestinal translocation of wheat allergenic proteins has already been investigated in Caco-2 cell models (Bodinier, et al., 2007), as well as for allergenic albumin proteins from Brazil nut and sesame seeds (Moreno, Rubio, Olano, & Clemente, 2006), and ovomucoid allergenic protein from egg white (Mine & Zhang, 2003).
Apart from allergic components of foods, intestinal cell line models have been utilized also to elucidate the mode of action of the transport of allergic components across the intestinal barrier. For example, the permeability of the T84 human intestinal monolayer was found to be up-regulated by higher temperatures, leading to intestinal barrier dysfunction and intact protein absorption (P. C. Yang, He, & Zheng, 2007). Immunoglobulin E (IgE), along with CD23, an antibody involved in allergic reactions may facilitate transepithelial antigen sampling (Li, et al., 2006). Similarly, allergenic proteins such as β-lactoglobulin and ovalbumin were able to permeate the intestinal monolayer via energy-independent paracellular pathways in the presence of excessive interleukin-4 (IL-4) concentrations (Mochizuki, Satsu, Totsuka, & Shimizu, 2009).
Probiotic applications and inflammation models
The applications of probiotics on cell lines usually include studies on adhesion, immunomodulation as well as bacterial or viral host – pathogen interactions. The adhesion ability on intestinal cell lines is one of the criteria proposed by FAO/WHO for the characterization of a strain as probiotic, since transient or permanent colonization of the intestinal epithelium allows the probiotic strain to exert its beneficial effect (FAO/WHO, 2002).
The adhesion ability of various potential probiotics has been investigated using mainly established cell lines like Caco-2 (Maragkoudakis, et al., 2006). However, in contrast to previous applications, specialised animal and human cell lines have been characterised and utilized in order to evaluate probiotic strains. Examples include adhesion studies on cell lines of human, porcine, bovine, ovine, chicken and fish origin, for both farm animal and human probiotic applications (Huang & Adams, 2003; Maragkoudakis et al., 2010, Nissen et al., 2009; Pan et al. 2008).
The immunomodulatory effects of potential probiotic bacteria are mainly focused on both inflammatory and anti-inflammatory cytokine release from intestinal cell lines, as well as chemical substances of immunological significance, such as nitric oxide (NO) induced by the attachment of potential probiotic bacteria (Hunter et al. 2009; Lammers et al. 2002; Pipenbaher et al., 2009; Riedel et al. 2006). In most of these experiments the two-compartment (intestinal epithelial – macrophage) cell model was applied; alternatively, probiotic effects were measured in both cell lines (van Hoffen et al. 2010; Zoumpopoulou, Tsakalidou, Dewulf, Pot, & Grangette, 2009).
The ability of certain potentially probiotic bacteria to induce release of anti-inflammatory cytokines, mainly interleukin-10 (IL-10), has been exploited in the field of intestinal bowel diseases such as Crohn’s disease and colitis (Foligne, Grangette, & Pot, 2005). Although the majority of such studies are performed in vivo using murine models of chemically induced colitis (Grangette, et al., 2005), the preliminary screening of potentially probiotic bacteria for cytokine release induction is done in single or two-compartment functional cell line models, as described previously. However, cell models have been directly used in other fields of intestinal pathogenesis, in order to elucidate the mechanism of inflammatory disease progression (Biasi, et al., 2009), carcinogenic transformation (Rafa, et al., 2010) and intestinal mucosa barrier disruption (Rodriguez-Lagunas, Martin-Venegas, Moreno, & Ferrer, 2010).
Host–pathogen interactions studies
After ingestion, food borne pathogens invade the mammalian body via interactions with the host intestinal tract. Reaching the intestinal tract, food pathogens do not only interact with the host intestinal barrier but also with commensal microorganisms that colonise intestinal epithelia. Most food pathogens must cross the intestinal epithelial barrier to exert their pathological effects and to interact with mucosa-associated lymphoid tissue (Arulampalam, Greicius, & Pettersson, 2006).
Various pathogens have developed several mechanisms to overcome the epithelial barrier, including disruption of barrier function, opening of paracellular pathways, transcytosis or induction of cell movement through neutrophil recruitment. Cell line models have been used to study such host–pathogen interactions. For example, infection of the T84 cell line with enteropathogenic Escherichia coli leads to a decrease in TEER, caused by the EspF protein (McNamara, et al., 2001). MDCK cells (despite being dog kidney cells) were used as an intestinal model were used to determine the entry mechanism of Yersinia pseudotuberculosis, which interacts with β-integrins expressed on M cells in the areas of cell contacts (Tafazoli, Holmstrom, Forsberg, & Magnusson, 2000). Salmonella typhimurium infection involves a signal transduction pathway in epithelia that results in the recruitment of neutrophils (Criss, Silva, Casanova, & McCormick, 2001), identified by using the MDCK cell line. Possible mechanisms of Campylobacter jejuni invasion, translocation and pathogenesis in the pig functional cell model were in part elucidated (Pogacar et al., 2010). Cell cultures can also be used for studies of viruses that enter the host via the alimentary tract and either infect or destroy epithelial cells (coronaviruses, rotaviruses). Some viruses cross the mucosal layer and cause systemic infection, as in the case of poliovirus. In the latter case, M cells have been indicated as transporters of the virus across the mucosal barrier in a Caco-2 cell model derived M cells (Ouzilou, et al., 2002). In addition, the Hepatitis E virus can be efficiently propagated in pig cell cultures and tick-borne encephalitis virus in mammalian intestinal and macrophage cell lines (Cencic & Langerholc, 2010).
Along the digestive tract pathogens also interact with beneficial bacteria. Commensal microbiota and probiotics have been demonstrated in vivo and in vitro to prevent pathogen infection by a mechanism of competition for the cell surface binding sites or secretion of antimicrobial agents (Lebeer, Vanderleyden, & De Keersmaecker, 2010). Lactobacillus species have been shown to reduce the infection of rotavirus (Maragkoudakis, et al., 2009) as well as strengthen the epithelial barrier and increase NO production in epithelial intestinal models of pigs and other farm animals (Nissen, et al., 2009). Finally, significant efforts have been made to find clinically relevant probiotic strains to prevent infection of Caco-2 and HT-29 cell lines by enteropathogenic strains of E. coli, S. typhimurium and others (Candela, et al., 2008).
Co-cultures of eukaryotic cells with bacteria and pathogens provide significant difficulties because of their widely varying growth rates, which leads to rapid deterioration of the eukaryotic cells. Additionally, microbial biofilms on the cells can develop over time. To answer this question, more complex experimental set-ups have been developed, such as the micro-fluidic device (Kim, Hegde, & Jayaraman, 2010).
Conclusion
The large number of cell model studies in food science and nutrition in the literature indicates the importance of intestinal cell lines for in vitro research in this field. The main advantage of intestinal cell models is their simplicity, inter-laboratory repeatability and large-scale testing capacity. In addition, they constitute an indispensable tool for the elucidation of intestinal absorption mechanisms. Moreover, they present a more economic and ethical alternative to animal testing. Although not frequently used so far, specialised and functional intestinal cell models hold several advantages over generic cell lines, as they can simulate more closely the in vivo environment, without however sacrificing their simplicity and reliability. In the future, we expect that the development of more improved and specialised cell models and experimental set-ups, together with automation (such as high-throughput screening) and miniaturization, will bring cell line platforms to a higher level, appropriate for cost effective and large-scale analysis in food and nutritional research.
Acknowledgments
This review is part of the dissemination activities of the E.U. funded project entitled «Control and prevention of emerging and future pathogens at cellular and molecular level throughout the food chain» (PathogenCombat, FP6-007081). The authors would like to thank Dr. Sandra Caldeira for the critical reading of the document.
References
- Arulampalam V., Greicius G., Pettersson S. The long and winding road to gut homeostasis. Current Opinion in Gastroenterology. 2006;22:349–353. doi: 10.1097/01.mog.0000231806.65030.ed. [DOI] [PubMed] [Google Scholar]
- Azenha M.A., Evangelista R., Martel F., Vasconcelos M.T. Estimation of the human intestinal permeability of butyltin species using the Caco-2 cell line model. Food and Chemical Toxicology. 2004;42:1431–1442. doi: 10.1016/j.fct.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Progress in Histochemistry and Cytochemistry. 1997;31:1–78. doi: 10.1016/s0079-6336(97)80001-0. [DOI] [PubMed] [Google Scholar]
- Beiseigel J.M., Hunt J.R., Glahn R.P., Welch R.M., Menkir A., Maziya-Dixon B.B. Iron bioavailability from maize and beans: a comparison of human measurements with Caco-2 cell and algorithm predictions. The American Journal of Clinical Nutrition. 2007;86:388–396. doi: 10.1093/ajcn/86.2.388. [DOI] [PubMed] [Google Scholar]
- Bernasconi E., Fritsche R., Corthesy B. Specific effects of denaturation, hydrolysis and exposure to Lactococcus lactis on bovine beta-lactoglobulin transepithelial transport, antigenicity and allergenicity. Clinical & Experimental Allergy. 2006;36:803–814. doi: 10.1111/j.1365-2222.2006.02504.x. [DOI] [PubMed] [Google Scholar]
- Berschneider H.M. 1989. Abstract of the annual meeting of the American gastroenterological association. [Google Scholar]
- Biasi F., Mascia C., Astegiano M., Chiarpotto E., Nano M., Vizio B. Pro-oxidant and proapoptotic effects of cholesterol oxidation products on human colonic epithelial cells: a potential mechanism of inflammatory bowel disease progression. Free Radical Biology & Medicine. 2009;47:1731–1741. doi: 10.1016/j.freeradbiomed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Bodinier M., Legoux M.A., Pineau F., Triballeau S., Segain J.P., Brossard C. Intestinal translocation capabilities of wheat allergens using the Caco-2 cell line. Journal of Agricultural and Food Chemistry. 2007;55:4576–4583. doi: 10.1021/jf070187e. [DOI] [PubMed] [Google Scholar]
- Borst P., Elferink R.O. Mammalian ABC transporters in health and disease. Annual Review of Biochemistry. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Brunet J.L., Maresca M., Fantini J., Belzunces L.P. Human intestinal absorption of imidacloprid with Caco-2 cells as enterocyte model. Toxicology and Applied Pharmacology. 2004;194:1–9. doi: 10.1016/j.taap.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Candela M., Perna F., Carnevali P., Vitali B., Ciati R., Gionchetti P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. International Journal of Food Microbiology. 2008;125:286–292. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Cencic A., Langerholc T. Functional cell models of the gut and their applications in food microbiology–a review. International Journal of Food Microbiology. 2010;141(Suppl 1):S4–S14. doi: 10.1016/j.ijfoodmicro.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Liang D., Fromm L.D., Overbeek P.A. Inhibition of lens fiber cell morphogenesis by expression of a mutant SV40 large T antigen that binds CREB-binding protein/p300 but not pRb. The Journal of Biological Chemistry. 2004;279:17667–17673. doi: 10.1074/jbc.M311678200. [DOI] [PubMed] [Google Scholar]
- Chen T.R., Drabkowski D., Hay R.J., Macy M., Peterson W., Jr. WiDr is a derivative of another colon adenocarcinoma cell line, HT-29. Cancer Genetics and Cytogenetics. 1987;27:125–134. doi: 10.1016/0165-4608(87)90267-6. [DOI] [PubMed] [Google Scholar]
- Coller H.A., Coller B.S. Poisson statistical analysis of repetitive subcloning by the limiting dilution technique as a way of assessing hybridoma monoclonality. Methods in Enzymology. 1986;121:412–417. doi: 10.1016/0076-6879(86)21039-3. [DOI] [PubMed] [Google Scholar]
- Criss A.K., Silva M., Casanova J.E., McCormick B.A. Regulation of Salmonella-induced neutrophil transmigration by epithelial ADP-ribosylation factor 6. The Journal of Biological Chemistry. 2001;276:48431–48439. doi: 10.1074/jbc.M106969200. [DOI] [PubMed] [Google Scholar]
- Da Violante G., Zerrouk N., Richard I., Frendo J.L., Zhiri A., Li-Khuan R. Short term Caco-2/TC7 cell culture: comparison between conventional 21-d and a commercially available 3-d system. Biological & Pharmaceutical Bulletin. 2004;27:1986–1992. doi: 10.1248/bpb.27.1986. [DOI] [PubMed] [Google Scholar]
- Dharmani P., Srivastava V., Kissoon-Singh V., Chadee K. Role of intestinal mucins in innate host defense mechanisms against pathogens. Journal of Innate Immunity. 2009;1:123–135. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Garcia E., Carvajal-Lerida I., Perez-Galvez A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutrition Research. 2009;29:751–760. doi: 10.1016/j.nutres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Foligne B., Grangette C., Pot B. Probiotics in IBD: mucosal and systemic routes of administration may promote similar effects. Gut. 2005;54:727–728. [PMC free article] [PubMed] [Google Scholar]
- Fujinaga Y., Inoue K., Watarai S., Sakaguchi Y., Arimitsu H., Lee J.C. Molecular characterization of binding subcomponents of Clostridium botulinum type C progenitor toxin for intestinal epithelial cells and erythrocytes. Microbiology. 2004;150:1529–1538. doi: 10.1099/mic.0.26805-0. [DOI] [PubMed] [Google Scholar]
- Gao S., Jiang W., Yin T., Hu M. Highly variable contents of phenolics in St. John’s Wort products affect their transport in the human intestinal Caco-2 cell model: pharmaceutical and biopharmaceutical rationale for product standardization. Journal of Agricultural and Food Chemistry. 2010;58:6650–6659. doi: 10.1021/jf904459u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glosl S., Wagner K.H., Draxler A., Kaniak M., Lichtenecker S., Sonnleitner A. Genotoxicity and mutagenicity of melanoidins isolated from a roasted glucose-glycine model in human lymphocyte cultures, intestinal Caco-2 cells and in the Salmonella typhimurium strains TA98 and TA102 applying the AMES test. Food and Chemical Toxicology. 2004;42:1487–1495. doi: 10.1016/j.fct.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Grangette C., Nutten S., Palumbo E., Morath S., Hermann C., Dewulf J. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proceedings of the National Academy of Sciences U S A. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayeshi R., Hilgendorf C., Artursson P., Augustijns P., Brodin B., Dehertogh P. Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. European Journal of Pharmaceutical Science. 2008;35:383–396. doi: 10.1016/j.ejps.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Henle G., Deinhardt F. The establishment of strains of human cells in tissue culture. The Journal of Immunology. 1957;79:54–59. [PubMed] [Google Scholar]
- Hidalgo I.J. Assessing the absorption of new pharmaceuticals. Current Topics in Medicinal Chemistry. 2001;1:385–401. doi: 10.2174/1568026013395010. [DOI] [PubMed] [Google Scholar]
- Huang Y., Adams M.C. An in vitro model for investigating intestinal adhesion of potential dairy propionibacteria probiotic strains using cell line C2BBe1. Letters in Applied Microbiology. 2003;36:213–216. doi: 10.1046/j.1472-765x.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- Hunter C.J., Williams M., Petrosyan M., Guner Y., Mittal R., Mock D. Lactobacillus bulgaricus precents intestinal epithelial cell injury caused by enterobacter sakazakii-induced nitric oxide in both in vitro and in the newborn rat model of necrotizing enterocolitis. Infection and Immunity. 2009;77:1031–1043. doi: 10.1128/IAI.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ingels F.M., Augustijns P.F. Biological, pharmaceutical, and analytical considerations with respect to the transport media used in the absorption screening system, Caco-2. Journal of Pharmaceutical Sciences. 2003;92:1545–1558. doi: 10.1002/jps.10408. [DOI] [PubMed] [Google Scholar]
- Kim J., Hegde M., Jayaraman A. Co-culture of epithelial cells and bacteria for investigating host-pathogen interactions. Lab on a Chip. 2010;10:43–50. doi: 10.1039/b911367c. [DOI] [PubMed] [Google Scholar]
- Klein S., Cohn S.M., Alpers D.H. Alimentary tract in nutrition. In: Shils M.E., Shike M., Ross C.A., Caballero B., Cousins R.J., editors. Modern nutrition in health and disease (10th ed.) Lippincot Williams @ Wilkins; Phildelphia, PA: 2006. pp. 1115–1142. [Google Scholar]
- Kojima S., Eguchi H., Ookawara T., Fujiwara N., Yasuda J., Nakagawa K. Clostridium botulinum type A progenitor toxin binds to Intestine-407 cells via N-acetyllactosamine moiety. Biochemical and Biophysical Research Communications. 2005;331:571–576. doi: 10.1016/j.bbrc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Lammers K.M., Helwig U., Svennen E., Rizello F., Venturi A., Caramelli E. Effect of probiotic strains on interleukin 8 production by HT29/19A cells. The American Journal of Gastroenterology. 2002;97:1182–1186. doi: 10.1111/j.1572-0241.2002.05693.x. [DOI] [PubMed] [Google Scholar]
- Larhed A.W., Artursson P., Bjork E. The influence of intestinal mucus components on the diffusion of drugs. Pharmaceutical Research. 1998;15:66–71. doi: 10.1023/a:1011948703571. [DOI] [PubMed] [Google Scholar]
- Lebeer S., Vanderleyden J., De Keersmaecker S.C. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nature Reviews Microbiology. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- Li H., Chung S.J., Shim C.K. Characterization of the transport of uracil across Caco-2 and LLC-PK1 cell monolayers. Pharm Res. 2002;19:1495–1501. doi: 10.1023/a:1020456632737. [DOI] [PubMed] [Google Scholar]
- Li H., Nowak-Wegrzyn A., Charlop-Powers Z., Shreffler W., Chehade M., Thomas S. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131:47–58. doi: 10.1053/j.gastro.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Lunney J.K. Advances in swine biomedical model genomics. International Journal of Biological Sciences. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald T.T., Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- Madgula V.L., Avula B., Choi Y.W., Pullela S.V., Khan I.A., Walker L.A. Transport of Schisandra chinensis extract and its biologically-active constituents across Caco-2 cell monolayers - an in-vitro model of intestinal transport. Journal of Pharmacy and Pharmacology. 2008;60:363–370. doi: 10.1211/jpp.60.3.0012. [DOI] [PubMed] [Google Scholar]
- Mahfoud R., Maresca M., Garmy N., Fantini J. The mycotoxin patulin alters the barrier function of the intestinal epithelium: mechanism of action of the toxin and protective effects of glutathione. Toxicology and Applied Pharmacology. 2002;181:209–218. doi: 10.1006/taap.2002.9417. [DOI] [PubMed] [Google Scholar]
- Mahler G.J., Shuler M.L., Glahn R.P. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. The Journal of Nutritional Biochemistry. 2009;20:494–502. doi: 10.1016/j.jnutbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Maragkoudakis P.A., Chingwaru W., Gradisnik L., Tsakalidou E., Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. International Journal of Food Microbiology. 2010;141(Suppl 1):S91–S97. doi: 10.1016/j.ijfoodmicro.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragkoudakis P.A., Mountzouris K.C., Psyrras D., Cremonese S., Fischer J., Cantor M.D., Tsakalidou E. Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. International Journal of Food Microbiology. 2009;130:219–226. doi: 10.1016/j.ijfoodmicro.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Maragkoudakis P.A., Zoumpopoulou G., Miaris C., Kalantzopoulos G., Pot B., Tsakalidou E. Probiotic potential of Lactobacillus strains isolated from dairy products. International Dairy Journal. 2006;16:189–199. [Google Scholar]
- Maresca M., Mahfoud R., Pfohl-Leszkowicz A., Fantini J. The mycotoxin ochratoxin A alters intestinal barrier and absorption functions but has no effect on chloride secretion. Toxicology and Applied Pharmacology. 2001;176:54–63. doi: 10.1006/taap.2001.9254. [DOI] [PubMed] [Google Scholar]
- Markowska M., Oberle R., Juzwin S., Hsu C.P., Gryszkiewicz M., Streeter A.J. Optimizing Caco-2 cell monolayers to increase throughput in drug intestinal absorption analysis. Journal of Pharmacological and Toxicological Methods. 2001;46:51–55. doi: 10.1016/s1056-8719(01)00161-7. [DOI] [PubMed] [Google Scholar]
- Masago M., Takaai M., Sakata J., Horie A., Ito T., Ishida K. Membrane transport mechanisms of quinidine and procainamide in renal LLC-PK1 and intestinal LS180 cells. Biological & Pharmaceutical Bulletin. 2010;33:1407–1412. doi: 10.1248/bpb.33.1407. [DOI] [PubMed] [Google Scholar]
- McNamara B.P., Koutsouris A., O’Connell C.B., Nougayrede J.P., Donnenberg M.S., Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. Journal of Clinical Investigation. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine Y., Zhang J.W. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. International Archives of Allergy and Immunology. 2003;130:135–142. doi: 10.1159/000069009. [DOI] [PubMed] [Google Scholar]
- Mochizuki T., Satsu H., Totsuka M., Shimizu M. Transepithelial transport of macromolecular substances in IL-4 treated human intestinal T84 cell monolayers. Bioscience, Biotechnology, and Biochemistry. 2009;73:2422–2426. doi: 10.1271/bbb.90383. [DOI] [PubMed] [Google Scholar]
- Moreno F.J., Rubio L.A., Olano A., Clemente A. Uptake of 2S albumin allergens, Ber e 1 and Ses i 1, across human intestinal epithelial Caco-2 cell monolayers. Journal of Agricultural and Food Chemistry. 2006;54:8631–8639. doi: 10.1021/jf061760h. [DOI] [PubMed] [Google Scholar]
- Murphy K.G., Bloom S.R. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Nanthakumar N.N., Fusunyan R.D., Sanderson I., Walker W.A. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proceedings of the National Academy of Sciences U S A. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D.W. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Molecular Endocrinology. 1991;5:1203–1214. doi: 10.1210/mend-5-9-1203. [DOI] [PubMed] [Google Scholar]
- Nies A.T., Schwab M., Keppler D. Interplay of conjugating enzymes with OATP uptake transporters and ABCC/MRP efflux pumps in the elimination of drugs. Expert Opinion on Drug Metabolism and Toxicology. 2008;4:545–568. doi: 10.1517/17425255.4.5.545. [DOI] [PubMed] [Google Scholar]
- Nishikawa A., Uotsu N., Arimitsu H., Lee J.C., Miura Y., Fujinaga Y. The receptor and transporter for internalization of Clostridium botulinum type C progenitor toxin into HT-29 cells. Biochemical and Biophysical Research Communications. 2004;319:327–333. doi: 10.1016/j.bbrc.2004.04.183. [DOI] [PubMed] [Google Scholar]
- Nissen L., Chingwaru W., Sgorbati B., Biavati B., Cencic A. Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: a functional study in the small intestinal cell model. International Journal of Food Microbiology. 2009;135:288–294. doi: 10.1016/j.ijfoodmicro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Niwa K., Koyama K., Inoue S., Suzuki T., Hasegawa K., Watanabe T. Role of nontoxic components of serotype D botulinum toxin complex in permeation through a Caco-2 cell monolayer, a model for intestinal epithelium. FEMS Immunology and Medical Microbiology. 2007;49:346–352. doi: 10.1111/j.1574-695X.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- Ouzilou L., Caliot E., Pelletier I., Prevost M.C., Pringault E., Colbere-Garapin F. Poliovirus transcytosis through M-like cells. Journal of General Virology. 2002;83:2177–2182. doi: 10.1099/0022-1317-83-9-2177. [DOI] [PubMed] [Google Scholar]
- Paine M.F., Khalighi M., Fisher J.M., Shen D.D., Kunze K.L., Marsh C.L. Characterization of interintetinal and intraintestinal variations in human CYP3A-dependent metabolism. Journal of Pharmacology and Experimental Therapeutics. 1997;283:1552–1562. [PubMed] [Google Scholar]
- Pan X., Wu T., Zhang L., Song Z., Tang H., Zhao Z. In vitro evaliation of adherence and antimicrobial properties of a candidate probiotic Clostridum butyricum CB2 for farmed fish. Journal of Applied Microbiology. 2008;105:1623–1629. doi: 10.1111/j.1365-2672.2008.03885.x. [DOI] [PubMed] [Google Scholar]
- Pattillo R.A., Hussa R.O., Story M.T., Ruckert A.C., Shalaby M.R., Mattingly R.F. Tumor antigen and human chorionic gonadotropin in CaSki cells: a new epidermoid cervical cancer cell line. Science. 1977;196:1456–1458. doi: 10.1126/science.867042. [DOI] [PubMed] [Google Scholar]
- Peracaula R., Barrabes S., Sarrats A., Rudd P.M., de Llorens R. Altered glycosylation in tumours focused to cancer diagnosis. Disease Markers. 2008;25:207–218. doi: 10.1155/2008/797629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipenbaher N., Moeller P.L., Weingartl H., Dolinšek J., Jakobsen M., Cenic A. Nitric oxide (NO) production in mammalian non-tumorigenic epithelial cells of the small intestine and macrophages induced by individual strains of lactobacilli and bifidobacteria. International Dairy Journal. 2009;19:166–171. [Google Scholar]
- Pogacar M.S., Klancnik A., Mozina S.S., Cencic A. Attachment, invasion, and translocation of Campylobacter jejuni in pig small-intestinal epithelial cells. Foodborne Pathogens and Disease. 2010;7:589–595. doi: 10.1089/fpd.2009.0301. [DOI] [PubMed] [Google Scholar]
- Radtke F., Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Rafa L., Dessein A.F., Devisme L., Buob D., Truant S., Porchet N. REG4 acts as a mitogenic, motility and pro-invasive factor for colon cancer cells. International Journal of Oncology. 2010;36:689–698. doi: 10.3892/ijo_00000544. [DOI] [PubMed] [Google Scholar]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council (2006). In: T. E. P. a. t. C. o. t. E. Union (Ed.), Official Journal of the European Union (Vol. L396, pp. 1–849).
- Riedel C.U., Foata F., Philippe D., Adolfsson O., Eikmanss B., Blum S. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kB activation. World Journal of Gastroenterology. 2006;12:3729–3735. doi: 10.3748/wjg.v12.i23.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J.R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. The Journal of Biological Chemistry. 1979;254:12701–12705. [PubMed] [Google Scholar]
- Rodriguez-Lagunas M.J., Martin-Venegas R., Moreno J.J., Ferrer R. PGE2 promotes Ca2+-mediated epithelial barrier disruption through EP1 and EP4 receptors in Caco-2 cell monolayers. American Journal of Physiology - Cell Physiology. 2010;299:C324–C334. doi: 10.1152/ajpcell.00397.2009. [DOI] [PubMed] [Google Scholar]
- Sambuy Y., De Angelis I., Ranaldi G., Scarino M.L., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biology and Toxicology. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- Schierack P., Nordhoff M., Pollmann M., Weyrauch K.D., Amasheh S., Lodemann U. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochemistry and Cell Biology. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Sergent T., Dupont I., Jassogne C., Ribonnet L., van der Heiden E., Scippo M.L. CYP1A1 induction and CYP3A4 inhibition by the fungicide imazalil in the human intestinal Caco-2 cells-comparison with other conazole pesticides. Toxicology Letters. 2009;184:159–168. doi: 10.1016/j.toxlet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Sergent T., Piront N., Meurice J., Toussaint O., Schneider Y.J. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chemico-Biological Interactions. 2010;188:659–667. doi: 10.1016/j.cbi.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Sergent T., Ribonnet L., Kolosova A., Garsou S., Schaut A., De Saeger S. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chemistry and Toxicology. 2008;46:813–841. doi: 10.1016/j.fct.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Sharp P. Methods and options for estimating iron and zinc bioavailability using Caco-2 cell models: benefits and limitations. International Journal for Vitamin and Nutrition Research. 2005;75:413–421. doi: 10.1024/0300-9831.75.6.413. [DOI] [PubMed] [Google Scholar]
- Shimizu M. Interaction between food substances and the intestinal epithelium. Bioscience, Biotechnology, and Biochemistry. 2010;74:232–241. doi: 10.1271/bbb.90730. [DOI] [PubMed] [Google Scholar]
- Sicherer S.H., Sampson H.A. 9. Food allergy. Journal of Allergy and Clinical Immunology. 2006;117:S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P., Turck N., Sidhoum-Jenny M., Gradwohl G., Kedinger M. In vitro models of intestinal epithelial cell differentiation. Cell Biology and Toxicology. 2007;23:241–256. doi: 10.1007/s10565-006-0175-0. [DOI] [PubMed] [Google Scholar]
- Tafazoli F., Holmstrom A., Forsberg A., Magnusson K.E. Apically exposed, tight junction-associated beta1-integrins allow binding and YopE-mediated perturbation of epithelial barriers by wild-type Yersinia bacteria. Infection and Immunity. 2000;68:5335–5343. doi: 10.1128/iai.68.9.5335-5343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Horie K., Borchardt R.T. Are MDCK cells transfected with the human MRP2 gene a good model of the human intestinal mucosa? Pharmaceutical Research. 2002;19:773–779. doi: 10.1023/a:1016192413308. [DOI] [PubMed] [Google Scholar]
- Tavelin S., Taipalensuu J., Soderberg L., Morrison R., Chong S., Artursson P. Prediction of the oral absorption of low-permeability drugs using small intestine-like 2/4/A1 cell monolayers. Pharmaceutical Research. 2003;20:397–405. doi: 10.1023/a:1022699920043. [DOI] [PubMed] [Google Scholar]
- Tian X.J., Yang X.W., Yang X., Wang K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. International Journal of Pharmacology. 2009;367:58–64. doi: 10.1016/j.ijpharm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Tirelli V., Catone T., Turco L., Di Consiglio E., Testai E., De Angelis I. Effects of the pesticide clorpyrifos on an in vitro model of intestinal barrier. Toxicology in Vitro. 2007;21:308–313. doi: 10.1016/j.tiv.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Auclair J., Delvin E., Levy E., Menard D., Pshezhetsky A.V. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. Journal of Cellular Biochemistry. 2006;99:1175–1186. doi: 10.1002/jcb.21015. [DOI] [PubMed] [Google Scholar]
- Umar S. Intestinal stem cells. Current Gastroenterology Reports. 2010;12:340–348. doi: 10.1007/s11894-010-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoffen E., Korthagen N.M., de Kivit S., Shouten B., Bardoel B., Duivelshof A., Knol J. Exposure of intestinal epithelial cells to uv-killed Lactobacillus GG but not Bifidobacterium Breve enhances the effector immune response in vitro. International Archives of Allergy and Immunology. 2010;152:159–168. doi: 10.1159/000265537. [DOI] [PubMed] [Google Scholar]
- Vidau C., Brunet J.L., Badiou A., Belzunces L.P. Phenylpyrazole insecticides induce cytotoxicity by altering mechanisms involved in cellular energy supply in the human epithelial cell model Caco-2. Toxicology in Vitro. 2009;23:589–597. doi: 10.1016/j.tiv.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Wacher V.J., Silverman J.A., Zhang Y., Benet L.Z. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. Journal of Pharmaceutical Sciences. 1998;87:1322–1330. doi: 10.1021/js980082d. [DOI] [PubMed] [Google Scholar]
- Wagner R.D., Krul E.S., Moberly J.B., Alpers D.H., Schonfeld G. Apolipoprotein expression and cellular differentiation in Caco-2 intestinal cells. American Journal of Physiology. 1992;263:E374–E382. doi: 10.1152/ajpendo.1992.263.2.E374. [DOI] [PubMed] [Google Scholar]
- Wasserman S.I., Barrett K.E., Huott P.A., Beuerlein G., Kagnoff M.F., Dharmsathaphorn K. Immune-related intestinal Cl- secretion. I. Effect of histamine on the T84 cell line. American Journal of Physiology. 1988;254:C53–C62. doi: 10.1152/ajpcell.1988.254.1.C53. [DOI] [PubMed] [Google Scholar]
- Westendorf A.M., Fleissner D., Hansen W., Buer J. T cells, dendritic cells and epithelial cells in intestinal homeostasis. International Journal of Medical Microbiology. 2010;300:11–18. doi: 10.1016/j.ijmm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Yang J., Tucker G.T., Rostami-Hodjegan A. Cytochrome P450 3A expression and activity in the human small intestine. Clinical Pharmacology & Therapeutics. 2004;76:391. doi: 10.1016/j.clpt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Yang P.C., He S.H., Zheng P.Y. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. Journal of Gastroenterology and Hepatology. 2007;22:1823–1831. doi: 10.1111/j.1440-1746.2006.04710.x. [DOI] [PubMed] [Google Scholar]
- Zoumpopoulou G., Tsakalidou E., Dewulf J., Pot B., Grangette C. Differential Crosstalk between epithelial cells, dendritic cells and bacteria in a co-culture model. International Journal of Food Microbioloby. 2009;131:40–51. doi: 10.1016/j.ijfoodmicro.2008.12.037. [DOI] [PubMed] [Google Scholar]


