Abstract
To design an alternative vaccine for control of infectious bronchitis in chickens, three recombinant duck enteritis viruses (rDEVs) expressing the N, S, or S1 protein of infectious bronchitis virus (IBV) were constructed using conventional homologous recombination methods, and were designated as rDEV-N, rDEV-S, and rDEV-S1, respectively. Chickens were divided into five vaccinated groups, which were each immunized with one of the rDEVs, covalent vaccination with rDEV-N & rDEV-S, or covalent vaccination with rDEV-N & rDEV-S1, and a control group. An antibody response against IBV was detectable and the ratio of CD4+/CD8+ T-lymphocytes decreased at 7 days post-vaccination in each vaccinated group, suggesting that humoral and cellular responses were elicited in each group as early as 7 days post-immunization. After challenge with a homologous virulent IBV strain at 21 days post-immunization, vaccinated groups showed significant differences in the percentage of birds with clinical signs, as compared to the control group (p < 0.01), as the two covalent-vaccination groups and the rDEV-S group provided better protection than the rDEV-N- or rDEV-S1-vaccinated group. There was less viral shedding in the rDEV-N & rDEV-S- (2/10) and rDEV-N & rDEV-S1- (2/10) vaccinated groups than the other three vaccinated groups. Based on the clinical signs, viral shedding, and mortality rates, rDEV-N & rDEV-S1 covalent vaccination conferred better protection than use of any of the single rDEVs.
Keywords: Infectious bronchitis virus, Recombinant duck enteritis virus, Protective efficacy
Highlights
-
•
Infectious bronchitis virus (IBV) causes a respiratory disease in domestic chickens worldwide.
-
•
Duck enteritis virus (DEV) was used as viral vaccine vector in chickens.
-
•
Recombinant DEV (rDEV) expressing IBV N, S, or S1 conferred protection against IBV.
-
•
Covalent vaccination with rDEV-N & rDEV-S1 conferred higher level of protection against IBV.
1. Introduction
Infectious bronchitis (IB) is a highly contagious viral disease of the upper respiratory and urogenital tracts of chickens that is caused by the infectious bronchitis virus (IBV). The disease is prevalent in nearly all countries with an intensive poultry industry, causing respiratory and renal diseases in chickens of all ages. It also reduces the quality and quantity of eggs produced by mature hens, causing heavy economic losses to the poultry industry. In addition, high mortality often occurs in young chickens infected with nephropathogenic strains as a result of renal pathology (Cavanagh and Gelb, 2008).
The IBV genome consists of a linear, single-stranded, positive-sense RNA, which encodes four major structural proteins, which include the spike (S) glycoprotein, the membrane (M) glycoprotein, the nucleocapsid (N) phosphoprotein, and the envelope or small membrane (E) protein. The N phosphoprotein is conserved among different IBV serotypes and can induce high titers of cross-reactive antibodies and cell-mediated immunity that protects chickens from acute infection, thus it is used as a target protein in designing vaccines against IB (Williams et al., 1992, Collisson et al., 2000, Seo et al., 1997). The S glycoprotein is responsible for receptor binding and membrane fusion (Hofmann et al., 2004), and consists of the N-terminal S1 and C-terminal S2 subunits (Bosch et al., 2003). Most of the conformation-dependent, neutralizing antigenic, and serotype-specific determinants in IBV have been mapped to S1, while other immunodominant regions are located in the N-terminal regions of S2 (Koch et al., 1990, Kusters et al., 1989, Lenstra et al., 1989). In addition, interactions between the S1 and S2 subunits might affect the conformation of the S1 subunit, thereby accounting for differences in serologic protection (Callison et al., 1999). The M glycoprotein of coronaviruses gives the virion envelope its shape. It has been reported that the S glycoprotein interacts with the transmembrane region of the M glycoprotein and the cytoplasmic tail of the IBV E protein is responsible for its interaction with the IBV M glycoprotein (Cavanagh, 2007).
Measures to control IB in poultry rely primarily on vaccination. Multiple live attenuated IBV vaccines are most often required because of poor cross protection between vaccines produced from different IBV serotypes (Liu et al., 2009, Liu et al., 2014). Live attenuated IBV vaccines do not provide adequate protection throughout the lifetime of layers or breeders, whereas inactivated vaccines convey certain advantages, such as slow antigen release and long-lasting immunity throughout the laying period. Unfortunately, inactivated IBV vaccines are not effective when used alone, as birds require one or a series of vaccinations with live-attenuated IBV vaccines (live priming) prior to administration of an inactivated vaccine (Cook et al., 2012). Conventional live IBV vaccines are attenuated by multiple serial passages in embryonated eggs (Gelb and Cloud, 1983, Jackwood et al., 2003, Bijlenga et al., 2004, Huang and Wang, 2006), although this is a time-consuming process.
Genetically engineered vaccines present an alternative to inactivated and attenuated vaccines. In previous studies, a multivalent DNA vaccine expressing S1, N, and M conferred 85% protection (Yang et al., 2009), while a multivalent DNA vaccine combined with an inactivated vaccine booster conferred complete protection (Yan et al., 2013). In addition, a recombinant Newcastle disease virus expressing the S2 protein of IBV was shown to provide broad protection against IBV challenge (Toro et al., 2014).
Duck enteritis virus (DEV) causes duck plague, an acute, contagious, and lethal disease that affects birds of all ages of the order Anseriformes (Davison et al., 1993). DEV is a member of the family Herpesviridae with a genome approximately 158 kb in size (Li et al., 2009). Because certain DEV genes are not essential for viral replication in vitro (Wang and Osterrieder, 2011, Liu et al., 2011), DEV has been used as a replicating vaccine vector in chickens to provide rapid protection against the H5N1 influenza virus (Liu et al., 2013a, Liu et al., 2013b). In our current study, we used DEV as a viral vector to construct three recombinant viruses expressing the N, S, and S1 proteins of IBV, and evaluated their protective efficacy in chickens against virulent IBV challenge.
2. Materials and methods
2.1. Viruses and cells
The nephropathogenic IBV strain ck/CH/LDL/091022 is an LX4-type (QX-like) strain that was first isolated in China in 2009 (Sun et al., 2011). The DEV Clone-03 was isolated from a commercial vaccine by plaque assay (Li et al., 2006, Liu et al., 2007). Primary chicken embryo fibroblasts (CEFs) were used for DEV propagation (Li et al., 2006).
2.2. Embryos and chickens
Specific pathogen-free (SPF) white leghorn chickens, chicken embryo eggs and duck embryo eggs were obtained from Harbin Veterinary Research Institute (HVRI; Harbin, China). The birds were maintained in isolators under negative pressure and provided with food and water ad libitum. All experiments were performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People's Republic of China, and the study protocols were approved by the Committee of the Ethics of Animal Experiments of the HVRI.
2.3. Plasmid construction
DEV genomic DNA was extracted as previously described (Prigge et al., 2004). The left and right homologous arms of the transfer vector were amplified by polymerase chain reaction (PCR) using primers VL1, VL2, VR1, and VR2. The enhanced green fluorescent protein (EGFP) cassette was amplified by primers R1 and R2 (Table 1 ) from the pEGFP-N1 plasmid and cloned into the pMD18 T-simple plasmid to produce pT-EGFP. The left and right arm PCR products were inserted through the ClaI and BlnI restriction sites and the MluI and AvaIII restriction sites, respectively, of the pT-EGFP plasmid to produce pUS10-EGFP. Complementary DNA (cDNA) of the N, S, and S1 genes of the virulent IBV strain ck/CH/LDL/091022 was synthesized from the viral genomic RNA by reverse transcription (RT)-PCR (Sun et al., 2011). The pUS10-N, pUS10-S, and pUS10-S1 plasmids were produced by inserting the N, S, and S1 PCR products, respectively, between the XhoI and NotI restriction sites flanking the EGFP open reading frame (ORF) in the pUS10-EGFP plasmid.
Table 1.
Primers used in this study.
| Primer | Primer sequence | Template |
|---|---|---|
| VL1 | 5′-ATCGATTGACGATGAGCGATCGGAAT-3′ | Left arm of transfer vector |
| VL2 | 5′-CCTAGGGTTGCGCGTTGTGTATAAGT-3′ | |
| VR1 | 5′-ACGCGTGACTCTGACTGATACTCTAC-3′ | Right arm of transfer vector |
| VR2 | 5′-ATGCATCTAATCGGTTATTTGCTGCT-3′ | |
| R1 | 5′-ATGCATCGCGACGCGTTAGTTATTAATAGTAATCAA-3′ | EGFP cassette |
| R2 | 5′-ATCGATGGGCCTAGGACAAACCACAACTAGAATGCA-3′ | |
| N1F | 5′-CTCGAGATGGCGAGCGGTAAAGTATCTGGA-3′ | IBV N gene |
| N1R | 5′-GCGGCCGCTCAAAGTTCATTTTCACCA-3′ | |
| SF | 5′-CTCGAGATGTTGGGGAAGTCACTGTTTTTA-3′ | IBV S gene |
| SR | 5′-GCGGCCGCTTAAACAGACTTTTTAGGTC-3′ | |
| S1F | 5′-CTCGAGATGTTGGGGAAGTCACTGTTTTTAGTGACCATT-3′ | IBV S1 gene |
| S1R | 5′-GCGGCCGCTTAACGCCTACGACGATGT-3′ | |
| DF | 5′-ACTTATACACAACGCGCAAC-3′ | Flanking sequence of DEV US10 ORF |
| DR | 5′-GCACACATAAAGTAATATACAAACC-3′ |
EGFP, enhanced green fluorescence protein; IBV, infectious bronchitis virus.
2.4. Construction of recombinant viruses
The strategy for the construction of the recombinant DEVs (rDEVs) is depicted in Fig. 1 A. Briefly, the genomic DNA of DEV and the pUS10-EGFP transfer vector were cotransfected into CEFs using TurboFect transfection reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). rDEV containing EGFP (rDEV-EGFP) with deletion of the complete US10 gene was selected by plaque assay and used as the parental virus for constructing rDEVs expressing the N, S, and S1 proteins of IBV. The selection of rDEV-N, rDEV-S, and rDEV-S1 was conducted using fluorescence microscopy, and plaques without green fluorescence were purified by plaque assay.
Fig. 1.
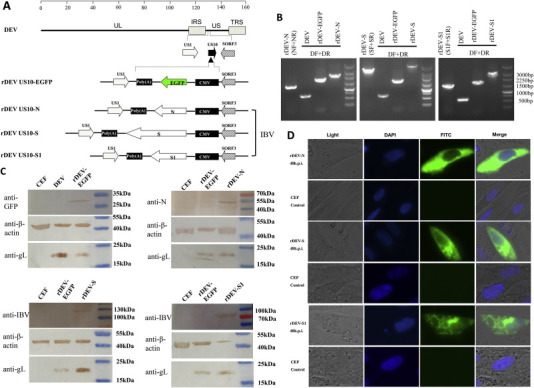
Construction and identification of recombinant duck enteritis viruses (DEVs) expressing the N, S, or S1 protein of infectious bronchitis virus (IBV). (A) Schematic of the insertion of the N, S, and S1 genes of IBV into the DEV genome. rDEV-EGFP was used as the parental virus for construction of rDEV-N, rDEV-S, and rDEV-S1. (B) PCR identification by specific primers and differentiated primers. Primer pairs NF and NR, SF and SR, and S1F and S1R were used to amplify specific genes from corresponding rDEVs. The differentiated primer pair DF and DR, corresponding to flanking regions of the US10 ORF of DEV, was used to analyze the purity and genetic stability of rDEVs. There were differences in the fragment lengths amplified from the WT DEV and each rDEVs (DEV Clone-03, 637 bp; rDEV-EGFP, 1727 bp; rDEV-N, 2217 bp; rDEV-S, 4502 bp; and rDEV-S1, 2610 bp). (C) Western blot analysis of the expression of the N, S, and S1 proteins of IBV from the rDEVs. The expression of chicken β-actin and the gL protein of DEV were used as internal references of CEFs infected with the rDEVs. (D) Immunofluorescence detection of the N, S, and S1 proteins of IBV in rDEV-infected CEFs. For detection of the N, S, and S1 proteins, rDEV-infected CEFs were fixed using 4% paraformaldehyde at 48 h postinfection. DAPI was used to stain the nuclei and uninfected CEFs were used as a control.
2.5. Identification of the rDEVs
N1F, N1R, SF, SR, S1F, and S1R gene-specific primers (Table 1) were used to confirm the identity of rDEV-N, rDEV-S, and rDEV-S1 by PCR. In addition, the primer pair DF and DR, corresponding to the flanking sequence of the DEV US10 gene (Table 1), were used to differentiate wild-type (WT) DEV from the rDEVs. Western blotting was performed to detect the expression of IBV proteins from the rDEVs (Han et al., 2013). Rabbit anti-GFP IgG (Sigma–Aldrich Corporation, St. Louis, MO, USA), mouse anti-IBV N protein monoclonal antibody (4F10) (Han et al., 2013), and chicken anti-IBV serum were used as primary antibodies for detection of EGFP, N, S, and S1 expressed from rDEV-EGFP, rDEV-N, rDEV-S, and rDEV-S1, respectively. In addition, an anti-chicken β-actin monoclonal antibody of mouse origin (Sigma–Aldrich Corporation) and mouse anti-DEV gL serum (prepared in our laboratory) were used to detect reference proteins and the efficacious replication of DEV or rDEVs in CEFs. Horseradish peroxidase-conjugated anti-rabbit IgG, anti-mouse IgG, or anti-chicken IgG (Sigma–Aldrich Corporation) were used as secondary antibodies. An indirect immunofluorescence assay was performed to detect protein expression in infected CEFs. Briefly, CEFs were infected with rDEVs at multiplicity of infection (MOI) of 0.001 and then fixed with 4% paraformaldehyde at 48 h postinfection. The antibodies described in Section 2.5 were used as primary antibodies, while fluorescein isothiocyanate (FITC)-conjugated anti-mouse or anti-chicken IgG (Sigma–Aldrich Corporation) was used as the secondary antibody. Expression of foreign proteins in recombinant viruses was observed by fluorescent microscopy.
2.6. Growth kinetics and stability of the rDEVs
To examine the growth kinetics of CEFs infected with rDEV-EGFP, rDEV-N, rDEV-S, rDEV-S1, or DEV Clone-03 at an MOI of 0.001, the infected CEFs and supernatants were harvested at 12, 24, 48, 72 and 96 h postinfection, and then titrated according to the method of Reed and Muench (1938). To evaluate genetic stability, rDEVs were passaged 20 times in CEFs. After passages 5, 10, 15 and 20, the identity of each rDEV was confirmed by PCR as described in Section 2.5.
2.7. Animal experiment design and sample collection
A total of 150 four-week-old SPF chickens were divided into six groups of 25 birds each. Five groups of 25 chickens were inoculated intramuscularly with 106 pfu of rDEV-N, rDEV-S, rDEV-S1, or received covalent vaccinations with rDEV-N & rDEV-S or rDEV-N & rDEV-S1. The remaining 25 chickens were immunized with Dulbecco's modified Eagle's medium, as a negative control. Oropharyngeal and cloacal swabs were collected on post-vaccination days 3 and 6 for detection of the replication of the rDEVs in chickens. Peripheral blood samples from five vaccinated chickens were collected in sodium heparin-coated tubes to analyze the cellular immune responses to the rDEVs on post-vaccination days 3, 7, 14, and 21, and on post-challenge day 5. On post-vaccination day 21, 10 chickens, including five that were stable for cellular immune analysis, were selected from each group and challenged with 106 50% egg infectious dose (EID50) of the virulent IBV strain ck/CH/LDL/091022 by the oculonasal route. On post-challenge day 5, oropharyngeal swabs were collected from all chickens in each group to evaluate shedding of IBV. Serum samples were collected from the remaining 15 chickens in each group to monitor levels of anti-IBV antibodies at 4 and 5 weeks post-vaccination. Chickens were monitored daily for clinical signs of infection. Snicks (abnormal respiratory sounds) made by each of the birds were counted by three individuals over a 2-min period. Birds were checked individually for tracheal rales, nasal discharge, watery eyes, and wheezing. The percentage of birds that died or exhibited clinical signs was recorded daily for 20 days following IBV challenge.
2.8. rDEV replication in chickens after vaccination
To determine whether the rDEVs had the ability to replicate in chickens, CEFs and 9-day-old SPF embryonated chicken and duck eggs were used to re-isolate the viruses from the oropharyngeal and cloacal swabs of the vaccinated chickens. Swabs maintained in phosphate-buffered saline (PBS) were centrifuged instantaneously at 8000 × g and the supernatants were filtered using a 0.45-μM Syringe Filter (Millipore Corporation, Billerica, MA, USA) and inoculated in CEFs and chicken or duck embryos through the chorioallantoic membrane, respectively. Three blind passages were conducted for each swab sample. Samples cultured for 5 days were detected by PCR using primers P1 and P2 (Li et al., 2006).
2.9. Serum antibody detection
Serum samples were analyzed for the presence of anti-IBV antibodies using an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with homologous IBV strain ck/CH/LDL/091022, which was concentrated and purified by differential centrifugation and sucrose gradient ultracentrifugation. Standard positive and negative sera, secondary antibodies, substrates, and stop solution were provided with the IBV antibody ELISA Kit (IDEXX Corporation, Westbrook, ME, USA). Titers were automatically calculated using a microplate reader at an optical density of 605 nm. Each serum titer was detected in triplicate, calculated based on the mean serum-to-positive (S/P) ratios (de Wit et al., 1998, Liu et al., 2006), the CP value was determined as 0.25 and an S/P ratio ≥ 0.25 was considered positive.
2.10. Analysis of CD4+, CD8+, and CD3+ T-lymphocytes
Lymphocytes were isolated from peripheral blood by Ficoll–Hypaque density gradient centrifugation using the Chicken Lymphocyte Separation Kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), according to the manufacturer's instructions. Peripheral blood mononuclear cells were isolated from each blood sample, adjusted to a concentration of 1 × 106 cells/100 μL, and co-stained with mouse anti-chicken CD3-SPRD, mouse anti-chicken CD8-PE, and mouse anti-chicken CD4-FITC (SouthernBiotech, Birmingham, AL, USA) antibodies for 1 h at room temperature. Flow cytometry was performed using the BD FACSAria cell sorter (BD Biosciences, Franklin Lake, NJ, USA) to calculate the percentages of CD4+, CD8+, and CD3+ T-lymphocytes.
2.11. Virus detection by real-time RT-PCR
Viral shedding was quantified based on IBV RNA levels in the oropharyngeal secretions of chickens challenged with strain ck/CH/LDL/091022. The oropharyngeal swabs contained in PBS were treated and detected as described previously (Jones et al., 2011, Cao et al., 2011, Cao et al., 2012). All samples were tested in triplicate and the data were analyzed using the LightCycler 480 software, version 1.5 (Roche Diagnostics, Basel, Switzerland).
2.12. Statistical analysis
The antibody titers of vaccinated chickens, the ratio of CD4+/CD8+ T-lymphocytes, the growth kinetics of recombinant viruses, percentages of birds showing clinical signs, and mortality rates were statistical analyzed using multiple t-tests (GraphPad Prism6; GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as means ± standard deviations.
3. Results
3.1. Molecular and serological characterization of rDEVs
Plaques formed by rDEV-EGFP exhibited green fluorescence, whereas those formed by rDEV-N, rDEV-S, and rDEV-S1 did not (data not shown). The PCR screening and DNA sequencing data confirmed that the N, S, and S1 cDNAs from IBV were properly inserted into the DEV genome with the deletion of the entire US10 gene. The primer pair DF and DR, corresponding to the flanking regions of the US10 gene ORF, were used to differentiate the WT from the rDEVs. Single fragments of varying lengths were amplified from the WT DEV Clone-03, rDEV-EGFP, rDEV-N, rDEV-S, or rDEV-S1, indicating that the rDEVs had been purified (Fig. 1B).
Western blotting analysis of the rDEV-EGFP-infected cells revealed a band of approximately 27 kDa, which corresponded to the size of EGFP. Specific bands corresponding to the N (50 kDa), S (128 kDa), and S1 (71 kDa) proteins of IBV were detected in the cells infected with rDEV-N, rDEV-S, and rDEV-S1, respectively. The gL protein of DEV was detected in cells infected with WT DEV Clone-03 or rDEVs, indicating efficient DEV replication as a viral vector in CEFs (Fig. 1C). The immunofluorescence analysis showed that the N, S, and S1 proteins were expressed in CEFs infected with rDEV-N, rDEV-S, and rDEV-S1, respectively, whereas the mock-infected CEFs exhibited no response to the anti-IBV or anti-IBV-N monoclonal antibodies (Fig. 1D).
3.2. Growth kinetics and genetic stability of the rDEVs
Generally, the growth trends of rDEV-EGFP, rDEV-N, rDEV-S, and rDEV-S1 were consistent with that of WT DEV Clone-03, which showed that the viruses reached the highest titer at 72 h and decreased at 96 h postinfection (Fig. 2 A). However, statistical analysis demonstrated that the titers of the rDEVs at each time point significantly differed from that of the WT DEV Clone-03 (*p < 0.05, **p < 0.01). The primer pair DF and DR was used to confirm the identity of three rDEVs at passages 5, 10, 15, and 20. However, no 637-bp or 1727-bp fragment (existed in WT DEV or rDEV-EGFP, respectively) was detected, demonstrating that three rDEVs expressing IBV proteins were genetically stable without reversion (Fig. 2B).
Fig. 2.

Growth kinetics and evaluation of the genetic stability of recombinant duck enteritis viruses (rDEVs). (A) Growth kinetics of the rDEVs. Compared with the WT DEV Clone-03, the rDEVs with the US10 deletion exhibited statistically significant lower viral titers (**p < 0.01, *p < 0.05). Viral titers are shown in TCID50/100 μL. (B) The genetic stability of the rDEVs expressing the major structural proteins of infectious bronchitis virus (IBV). The identity of the rDEVs was confirmed by PCR using the differentiated primer pair DF and DR following the 5th, 10th, 15th, and 20th passages. No reversion occurred during the passage from the rDEVs to the WT DEV Clone-03 (DEV Clone-03, 637 bp; rDEV-N, 2217 bp; rDEV-S, 4502 bp; rDEV-S1, 2610 bp).
3.3. Replication of rDEVs in chickens after immunization
Oropharyngeal and cloacal swabs were collected post-vaccination for the detection of rDEVs in chickens. PCR detection showed that viral isolation was negative from the CEFs, as well as the chicken and duck embryo cultures, suggesting that the vaccinated chickens do not shed rDEVs from the respiratory and gastrointestinal tracts.
3.4. Vaccination with the rDEVs induced protective immunity against virulent IBV
Chickens were challenged with 106 EID50 of the virulent IBV strain ck/CH/LDL/091022 at 21 days post-vaccination. The percentage of chickens that showed clinical signs in the rDEV-N, rDEV-S, rDEV-S1, rDEV-N & rDEV-S, rDEV-N & rDEV-S1, and control groups was 30%, 20%, 40%, 20%, 20%, and 80% (p < 0.01, very significant difference, each group compared with the control group only), respectively (Table 2 ). Birds began to exhibit ruffled feathers and snicks at 5 days after challenge with virulent IBV, and developed depression and huddle, dark combs, and death as time went on. Mortality rates in the rDEV-N, rDEV-S, rDEV-S1, rDEV-N & rDEV-S, rDEV-N & rDEV-S1, and control groups were 30%, 10%, 30%, 20%, 10% and 40%, respectively (p > 0.05, no significant difference) (Table 2). Gross lesions were confined primarily to the kidneys. The renal parenchyma of the affected birds was pale, swollen, and mottled, while the renal tubules and urethras were distended due to accumulation of uric acid crystals.
Table 2.
Efficacy of recombinant duck enteritis virus (rDEV) vaccines against the virulent ck/CH/LDL/091022 strain of infectious bronchitis virus (IBV).
| Vaccine | Dose (pfu) | Viral shedding (%)a |
Clinical signs (%)b | Survival (Mortality [%]) | Seroconversion post-vaccination (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |||||
| rDEV-N | 106 | 2/10 (20)c | 3/10(30)c | 7/10 (30) | 21/25 (84) | 19/25 (76) | 10/25 (40) | 7/15 (46.7) | 7/15 (46.7) |
| rDEV-S | 106 | 3/10 (30)c | 2/10(20)c | 9/10 (10) | 23/25 (92) | 13/25 (52) | 4/25 (16) | 1/15 (6.7) | 1/15 (6.7) |
| rDEV-S1 | 106 | 4/10 (40)c | 4/10(40)c | 7/10 (30) | 23/25 (92) | 7/25 (28) | 2/25 (8) | 2/15 (13.3) | 1/15 (6.7) |
| rDEV-N & rDEV-S | 106 | 2/10 (20)c | 2/10(20)c | 8/10 (20) | 23/25(92) | 16/25(64) | 13/25(52) | 7/15(46.7) | 7/15(46.7) |
| rDEV-N & rDEV-S1 | 106 | 2/10 (20)c | 2/10(20)c | 9/10 (10) | 25/25(100) | 25/25(100) | 21/25(84) | 11/15(73.3) | 11/15(73.3) |
| Control | – | 9/10 (90) | 8/10(80) | 6/10 (40) | 0/25 (0) | 0/25 (0) | 0/25 (0) | 0/15 (0) | 0/15 (0) |
Number of chickens shedding IBV on day 5 post-challenge.
Clinical signs (%) are presented as the percentage of birds showed clinical signs out of the total number of vaccinated chickens in the group after virulent IBV challenge.
p < 0.01, very significant difference, each group was only compared to the control group.
We evaluated viral shedding using qRT-PCR to quantify the IBV RNA in oropharyngeal swabs. In the control group, 90% of the chickens shed viruses, while challenged chickens in the rDEV-N, rDEV-S, rDEV-S1, rDEV-N & rDEV-S, and rDEV-N & rDEV-S1 groups showed relatively lower viral shedding rates of only 20%, 30%, 40%, 20%, and 20%, respectively (p < 0.01, each group compared to the control group only).
3.5. Antibody responses to IBV in chickens immunized with rDEVs
The antibody titers of anti-N, -S, and -S1 of vaccinated chickens were measured weekly using an ELISA with plates coated with the homologous virus ck/CH/LDL/091022. In general, all groups showed relatively high antibody titers and seroconversion rates at week 1 post-vaccination, which then decreased gradually. At week 1 post-vaccination, 84% (21/25) of the chickens vaccinated with rDEV-N were seropositive, although seropositivity gradually decreased from week 2 (76%) to week 5 (46.7%). In the rDEV-S vaccinated group, 92% (23/25) of the chickens exhibited seroconversion at week 1, but this percentage decreased rapidly afterward to 52% at week 2 and 16% at week 3 post-vaccination. In the rDEV-S1 group, 92% (23/25) of the chickens were seropositive, but this rate decreased sharply to 28% at week 2 and to 8% at week 3. The antibody levels and seroconversion rates of the rDEV-N & rDEV-S covalent-vaccination group were similar to those of the rDEV-N and rDEV-S group. The antibody titers of the rDEV-N & rDEV-S1 covalent-vaccination group were higher than all other groups and all of the chickens in this group were IBV antibody-positive at weeks 1 (100%) and 2 (100%), although these rates then slowly decreased from week 3 post-vaccination (84%). None of the chickens in the control group exhibited an antibody response (Table 2).
There were significant differences (**p < 0.01, *p < 0.05) in antibody titers of each single vaccinated rDEV group, as compared to the corresponding covalent-vaccination group (Fig. 3 ), suggesting that the covalent rDEV-N & rDEV-S1 vaccine enhanced the humoral response.
Fig. 3.
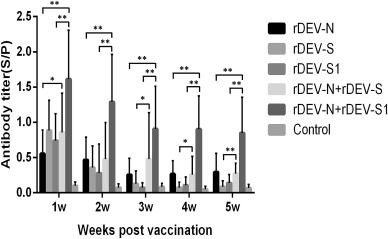
Antibody responses of chickens vaccinated with recombinant duck enteritis viruses expressing the N, S or S1 gene of infectious bronchitis virus (IBV). Sera from chickens immunized with rDEV-N, rDEV-S, rDEV-S1, rDEV-N + rDEV-S, rDEV-N + rDEV-S1, or the control were tested for the presence of antibodies against IBV at 1 week (n = 25), 2 weeks (n = 25), 3 weeks (n = 25), 4 weeks (n = 15), and 5 weeks (n = 15) post-vaccination. There were very significant differences in the antibody titers of the vaccinated groups as compared to that of the control group (p < 0.01). All single vaccinated groups were compared to the corresponding covalent vaccinated group. Significant differences in antibody titers are indicated with dashes.
3.6. Cellular immune responses induced by rDEVs vaccination
CD4+ and CD8+ T-lymphocytes are important parameters of cell-mediated immune responses in vaccinated chickens. To evaluate the cellular response induced by rDEVs in vaccinated chickens, the percentages of CD4+CD3+ and CD8+CD3+ T-lymphocytes, respectively, in peripheral blood were analyzed by flow cytometry, which showed that the ratio of CD4+ and CD8+ T-lymphocytes decreased at day 7 post-vaccination in each vaccinated group, and slightly increased from day 14 post-vaccination, as compared with that of birds in the control group (Table 3 ). There was a significant difference (p < 0.05) in the CD4+/CD8+ ratio of the rDEV-N group at day 7 post-vaccination, as compared to the control group. There was no significant difference among the three single-vaccination groups and the two covalent-vaccination groups (p > 0.05) (Table 3).
Table 3.
Ratio of CD4+/CD8+ T-lymphocytes after vaccination and challenge.
| Group | Ratio of CD4+ and CD8+ T-lymphocytes |
||||
|---|---|---|---|---|---|
| 3 dpva | 7 dpv | 14 dpv | 21 dpv | 5 dpc | |
| rDEV-N | 0.80 ± 0.06b | 0.64 ± 0.06a | 0.72 ± 0.08 | 0.72 ± 0.09 | 0.72 ± 0.12 |
| rDEV-S | 0.92 ± 0.13 | 0.74 ± 0.10 | 0.85 ± 0.18 | 0.85 ± 0.14 | 0.89 ± 0.20 |
| rDEV-S1 | 0.82 ± 0.12 | 0.71 ± 0.08 | 0.82 ± 0.09 | 0.80 ± 0.14 | 0.80 ± 0.14 |
| rDEV-N & rDEV-S | 0.93 ± 0.16 | 0.73 ± 0.8.4 | 0.96 ± 0.24 | 0.81 ± 0.25 | 0.90 ± 0.33 |
| rDEV-N & rDEV-S1 | 0.82 ± 0.14 | 0.76 ± 0.08 | 0.87 ± 0.20 | 0.83 ± 0.15 | 0.89 ± 0.22 |
| Control | 0.87 ± 0.20 | 0.86 ± 0.17 | 0.92 ± 0.24 | 0.80 ± 0.19 | 0.86 ± 0.44 |
Days post-vaccination (dpv) or post-challenge (dpc). Statistically significant differences (p < 0.05) (compared to the control group).
Data are expressed as means ± standard deviations.
4. Discussion
The IBV genome codes for four structural proteins, which play different roles in immune protection of vaccinated chickens. The N protein is an immunodominant antigen that induces high titers of cross-reactive antibodies. The M glycoprotein elicits low titers of antibodies with limited cross-reactivity, whereas the S1 glycoprotein induces production of serotype-specific and cross-reactive antibodies (Ignjatovic and Galli, 1993). The S1 glycoprotein induces virus neutralizing and cross-reactive antibodies and cell-mediated immune responses (Ignjatovic and Galli, 1994, Ignjatovic and Galli, 1995). Moreover, Ignjatovic and Galli (1993) reported that immunization of chickens with purified M glycoproteins did not induce protection against virulent IBV challenge, whereas immunization with the S1 glycoprotein prevented replication of nephropathogenic IBV in the kidneys, but not the tracheas, of immunized chickens. Hence, the N, S, and S1 proteins of IBV were chosen as the target proteins to construct rDEVs in order to evaluate the immune protection of these proteins against the virulent IBV strain ck/CH/LDL/091022.
In the current study, the US10 gene of DEV was replaced with the N, S, or S1 gene of IBV, respectively, to produce rDEVs that expressed the N, S, and S1 proteins of IBV, respectively. Virus titers of rDEVs decreased 10-fold at 72 h postinfection compared to that of the WT DEV Clone-03. However, it was reported that virus titers of the UL44 gene deletion rDEV decreased by 50–100-fold, as compared to the parental virus (Wang and Osterrieder, 2011), suggesting that gene deletion of DEV does affect viral replication, while the influence depends on the deletion site in the viral genome.
The antibody levels of the chickens in the rDEV-N & rDEV-S1 covalent-vaccination groups were higher than those of all other vaccinated groups. At week 3 post-vaccination, the antibody levels in the rDEV-S and rDEV-S1 groups decreased sharply, whereas the covalent-vaccination groups had higher antibody levels. Single rDEV-N and single rDEV-S1 vaccination invoked weak antibody responses, while covalent vaccination with these two rDEVs induced strong antibody responses. The high antibody titers were related to the lower mortality in the rDEV-N & rDEV-S1 covalent-vaccination group, indicating that covalent vaccination with the IBV structural proteins N and S1 provided better protection against challenge with virulent IBV strains. The IBV S glycoprotein consists of the S1 and S2 subunits. The S1 subunit induces efficient cellular immunity (Zhang et al., 2014), while the N protein mainly induces cross-reactive antibodies (Ignjatovic and Galli, 1993), which may be one of the reasons for the difference in antibody levels among the rDEV-N, rDEV-S and rDEV-S1 groups. To evaluate the cellular immune responses induced by the rDEVs, we detected the percentages of CD4+ CD3+ and CD8+CD3+ T-lymphocytes in peripheral blood samples collected from vaccinated chickens on post-vaccination days 3, 7, 14, and 21. The results showed that CD8+ T-lymphocytes increased and the causative ratio of CD4+/CD8+ T-lymphocytes decreased at day 7 post-vaccination in each vaccinated group, suggesting the induction of cytotoxic T lymphocytes (CTLs). Reportedly, CD4+ T-lymphocytes induce and enhance the immune response by secreting cytokines, while most CTLs were shown to be CD8+ cells, which play a major role in the control of viral infection. Collisson et al. (2000) reported that IBV-specific CTL activity was dependent on the S and N proteins of IBV, which was consistent with the results of the present study. Moreover, chickens infected with IBV Gray strain showed CTL responses as early as 3 days and peaked at 10 days postinfection (Collisson et al., 2000), consistent with our results on the detection of CD8+ T-lymphocytes. In this study, the antibody responses and increases in CD8+ T-lymphocytes levels were detected as early as 7 days post-vaccination, suggesting efficacious delivery of the foreign protein as a vaccine vector to the host, which elicited both humoral and cellular immune responses.
Chen et al. (2010) reported partial protection of chickens against a recombinant fowlpox virus expressing the IBV S1 protein with only 25% of birds showing clinical signs. In this study, clinical signs were detected in a significantly lower percentage of birds that were vaccinated with rDEV-S, rDEV-S1, or rDEV-N after challenge with virulent strain ck/CH/LDL/091022 when compared with the control group, suggesting vaccination with these three viruses conferred better protection. In addition, a lower percentage of birds vaccinated with rDEV-S showed clinical signs after challenge when compared with birds vaccinated with the other two viruses, demonstrating that better protection was conferred by vaccination with rDEV-S. Furthermore, although covalent vaccination with rDEV-N & rDEV-S or rDEV-N & rDEV-S1 provided the same clinical protection (20% clinical signs) as chickens in the rDEV-S group, vaccination with the covalent vaccines offered better protection in regard to viral shedding of virulent IBV. We did not test a live IBV vaccine in the present study because no commercial LX4-type (QX-like) vaccine is currently available. In our previous study, we found that the H120 commercial vaccine did not provide efficacious protection against the ck/CH/LDL/091022 strain of IBV, resulting in a morbidity rate of 40% (Sun et al., 2011), which was higher than that observed in chickens vaccinated with rDEV-S (20%), rDEV-N (30%), DEV-N & rDEV-S (20%), or rDEV-N & rDEV-S1 (20%). The mortality induced by IBV ck/CH/LDL/091022 infection in the control group in this study was higher than that reported by Sun et al. (2011), which may be due to the higher dosages of the challenged viruses used in that experiment as compared to the present study (106 vs. 104.8 EID50 per chicken, respectively).
In a previous study, immunization with a recombinant fowlpox that coexpressed the S1 protein of IBV and chicken interleukin 18 provided complete protection against IBV infection in chickens (Chen et al., 2010). Therefore, the coexpression of immunomodulatory factors, such as cytokines, may improve the efficacy of rDEVs to induce protection against IBV infection. Hence, future studies are warranted to determine whether the use of combinations of our rDEV vaccines or boosting with a live or inactivated vaccine may induce better efficacious protection against IBV infection in poultry. In addition, the protection provided by our rDEV vaccine against other IBV serotypes, such as the Massachusetts serotype, should also be evaluated.
Acknowledgments
This work was supported by grants from the China Agriculture Research Systerm (No. CARS-41-K12), National “Twelfth Five-Year” Plan for Science & Technology Support (2015BAD12B03), and Special Fund for Agro-scientific Research in the Public Interest (No. 201303033).
Glossary
- IBV
infectious bronchitis virus
- DEV
duck enteritis virus
- rDEV
recombinant duck enteritis virus
- CEF
chicken embryo fibroblast
- RT-qPCR
RT and real-time PCR
- ELISA
enzyme-linked immunosorbent assay
- SPF
specific-pathogen-free
References
- Bijlenga G., Cook J.K.A., Gelb J., Jr., de Wit J.J. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Van Der Zee R., De Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callison S.A., Jackwood M.W., Hilt D.A. Infectious bronchitis virus S2 gene sequence variability may affect S1 subunit specific antibody binding. Virus Genes. 1999;19:143–151. doi: 10.1023/A:1008179208217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Han Z., Shao Y., Geng H., Kong X., Liu S. Proteomic analysis of chicken embryonic trachea and kidney tissues after infection in ovo by avian infectious bronchitis coronavirus. Proteome Sci. 2011;9:11. doi: 10.1186/1477-5956-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Han Z., Shao Y., Liu X., Sun J., Yu D., Kong X., Liu S. Proteomics analysis of differentially expressed proteins in chicken trachea and kidney after infection with the highly virulent and attenuated coronavirus infectious bronchitis virus in vivo. Proteome Sci. 2012;10:24. doi: 10.1186/1477-5956-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Gelb J. Infectious bronchitis. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. (EDS), Diseases of Poultry. Wiley-Blackwell Publishing; Iowa: 2008. pp. 117–135. [Google Scholar]
- Chen H.Y., Yang M.F., Cui B.A., Cui P., Sheng M., Chen G., Wang S.J., Geng J.W. Construction and immunogenicity of a recombinant fowlpox vaccine coexpressing S1 glycoprotein of infectious bronchitis virus and chicken IL-18. Vaccine. 2010;28:8112–8119. doi: 10.1016/j.vaccine.2010.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson E.W., Pei J.W., Dzielawaa J., Seob S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Davison S., Converse K.A., Hamir A.N., Eckroade R.J. Duck viral enteritis in Muscovy ducks in Pennsylvania. Avian Dis. 1993;37:1142–1146. [PubMed] [Google Scholar]
- de Wit J.J., de Jong M.C.M., Pijpers A., Verheijden J.H.M. Transmission of infectious bronchitis virus within vaccinated and unvaccinated groups of chickens. Avian Pathol. 1998;27:464–471. doi: 10.1080/03079459808419370. [DOI] [PubMed] [Google Scholar]
- Gelb J., Jr., Cloud S.S. Effect of serial embryo passage of an Arkansas-type avian infectious bronchitis virus isolate on clinical response, virus recovery, and immunity. Avian Dis. 1983;27:679–687. [PubMed] [Google Scholar]
- Han Z., Zhao F., Shao Y., Liu X., Kong X., Song Y., Liu S. Fine level epitope mapping and conservation analysis of two novel linear B-cell epitopes of the avian infectious bronchitis coronavirus nucleocapsid protein. Virus Res. 2013;171:54–64. doi: 10.1016/j.virusres.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Hattermann K., Marzi A., Gramberg T., Geier M., Krumbiegel M., Kuate S., Uberla K., Niedrig M., Pöhlmann S. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004;78:6134–6142. doi: 10.1128/JVI.78.12.6134-6142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-P., Wang C.-H. Development of attenuated vaccines from Taiwanese infectious bronchitis virus strains. Vaccine. 2006;24:785–791. doi: 10.1016/j.vaccine.2005.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. Structural proteins of avian infectious bronchitis virus: role in immunity and protection. Adv. Exp. Med. Biol. 1993;342:449–453. doi: 10.1007/978-1-4615-2996-5_71. [DOI] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. The S1 glycoprotein but not N or M proteins of avian infectiousbronchitis virus induces protection in vaccinated chickens. Arch. Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. Immune responses to structural proteins of avian infectious bronchitis virus. Avian Pathol. 1995;24:313–332. doi: 10.1080/03079459508419072. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Hilt D.A., Brown T.P. Attenuation, safety, and efficacy of an infectious bronchitis virus GA98 serotype vaccine. Avian Dis. 2003;47:627–632. doi: 10.1637/6094. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Ellis R.J., Cox W.J., Errington J., Fuller C., Irvine R.M., Wakeley P.R. Development and validation of RT-PCR tests for the detection and S1 genotyping of infectious bronchitis virus and other closely related gammacoronaviruses within clinical samples. Transbound. Emerg. Dis. 2011;58:411–420. doi: 10.1111/j.1865-1682.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Hartog L., Kant A., van Roozelaar D.J. Antigenic domains on thepeplomer protein of avian infectious bronchitis virus: correlation with biologicalfunctions. J. Gen. Virol. 1990;71:1929–1935. doi: 10.1099/0022-1317-71-9-1929. [DOI] [PubMed] [Google Scholar]
- Kusters J.G., Niesters H.G., Lenstra J.A., Horzinek M.C., van der Zeijst B.A. Phylogeny of antigenic variants of avian coronavirus IBV. Virology. 1989;169:217–221. doi: 10.1016/0042-6822(89)90058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra J.A., Kusters J.G., Koch G., van der Zeijst B.A. Antigenicity of thepeplomer protein of infectious bronchitis virus. Mol. Immunol. 1989;26:7–15. doi: 10.1016/0161-5890(89)90014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu S., Kong X. Characterization of the genes encoding UL24, TK and gH proteins from duck enteritis virus (DEV): a proof for the classification of DEV. Virus Genes. 2006;33:221–227. doi: 10.1007/s11262-005-0060-6. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang B., Ma X., Wu J., Li F., Ai W., Song M., Yang H. Molecular characterization of the genome of duck enteritis virus. Virology. 2009;391:151–161. doi: 10.1016/j.virol.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen P., Jiang Y., Deng G., Shi J., Wu L., Lin Y., Bu Z., Chen H. Recombinant duck enteritis virus works as a single-dose vaccine in broilers providing rapid protection against H5N1 influenza infection. Antivir. Res. 2013;97:329–333. doi: 10.1016/j.antiviral.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen P., Jiang Y., Wu L., Zeng X., Tian G., Ge J., Kawaoka Y., Bu Z., Chen H. A duck enteritis virus-vectored bivalent live vaccine provides fast and complete protection against H5N1 avian influenza virus infection in ducks. J. Virol. 2011;85:10989–10998. doi: 10.1128/JVI.05420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Chen J., Han Z., Zhang Q., Shao Y., Kong X., Tong G. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006;35:394–399. doi: 10.1080/03079450600920984. [DOI] [PubMed] [Google Scholar]
- Liu S., Chen S., Li H., Kong X. Molecular characterization of the herpes simplex virus 1 (HSV-1) homologues, UL25 to UL30, in duck enteritis virus (DEV) Gene. 2007;401:88–96. doi: 10.1016/j.gene.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Liu S., Xu Q., Han Z., Liu X., Li H., Guo H., Sun N., Shao Y., Kong X. Origin and characteristics of the recombinant novel avian infectious bronchitis coronavirus isolate ck/CH/LJL/111054. Infect. Genet. Evol. 2014;23:189–195. doi: 10.1016/j.meegid.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang X., Gong Li, Yan B., Li C., Han Z., Shao Y., Li H., Kong X. Altered pathogenicity, immunogenicity, tissue tropism and 3-7 kb region sequence of an avian infectious bronchitis coronavirus strain after serial passage in embryos. Vaccine. 2009;27:4630–4640. doi: 10.1016/j.vaccine.2009.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wei S., Liu Y., Fu P., Gao M., Mu X., Liu H., Xing M., Ma B., Wang J. Recombinant duck enteritis virus expressing the HA gene from goose H5 subtype avian influenza virus. Vaccine. 2013;31:5953–5959. doi: 10.1016/j.vaccine.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Prigge J.T., Majerciak V., Hunt H.D., Dienglewicz R.L., Parcells M.S. Construction and characterization of Marek's disease viruses having green fluorescent protein expression tied directly or indirectly to phosphoprotein 38 expression. Avian Dis. 2004;48:471–487. doi: 10.1637/7110. [DOI] [PubMed] [Google Scholar]
- Reed I.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Seo S.H., Wang L., Smith R., Collisson E.W. The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 1997;71:7889–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Han Z., Ma H., Zhang Q., Yan B., Shao Y., Xu J., Kong X., Liu S. Phylogenetic analysis of infectious bronchitis coronaviruses newly isolated in China, and pathogenicity and evaluation of protection induced by Massachusetts serotype H120 vaccine against QX-like strains. Avian Pathol. 2011;40:43–54. doi: 10.1080/03079457.2010.538037. [DOI] [PubMed] [Google Scholar]
- Toro H., Zhao W., Breedlove C., Zhang Z., Yub Q. Infectious bronchitis virus S2 expressed from recombinant virus confers broad protection against challenge. Avian Dis. 2014;58:83–89. doi: 10.1637/10641-081613-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wang J., Osterrieder N. Generation of an infectious clone of duck enteritis virus (DEV) and of a vectored DEV expressing hemagglutinin of H5N1 avian influenza virus. Virus Res. 2011;159:23–31. doi: 10.1016/j.virusres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Williams A.K., Wang L., Sneed L.W., Collisson E.W. Comparative analyses of the nucleocapsid genes of several strains of infectious bronchitis virus and other coronaviruses. Virus Res. 1992;25:213–222. doi: 10.1016/0168-1702(92)90135-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Zhao Y., Hu Y., Qiu J., Lei W., Ji W., Li X., Wu Q., Shi X., Li Z. Protection of chickens against infectious bronchitis virus with a multivalent DNA vaccine and boosting with an inactivated vaccine. J. Vet. Sci. 2013;14:53–60. doi: 10.4142/jvs.2013.14.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Wang H.N., Wang X., Tang J.N., Gao R., Li J., Guo Z.C., Li Y.L. Multivalent DNA vaccine enhanced protection efficacy against infectious bronchitis virus in chickens. J. Vet. Med. Sci. 2009;71:1585–1590. doi: 10.1292/jvms.001585. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen X.W., Tong T.Z., Ye Y., Liao M., Fan H.Y. BacMam virus-based surface display of the infectious bronchitis virus (IBV) S1 glycoprotein confers strong protection against virulent IBV challenge in chickens. Vaccine. 2014;32:664–670. doi: 10.1016/j.vaccine.2013.12.006. [DOI] [PubMed] [Google Scholar]


